Abstract
Objectives:
We investigated the impact and timing of smoking cessation on developing rheumatoid arthritis (RA) and serologic phenotypes.
Methods:
We investigated smoking cessation and RA risk in the Nurses’ Health Study (NHS, 1976–2014) and the NHSII (1989–2015). Smoking exposures and covariates were obtained by biennial questionnaires. Self-reported RA was confirmed by medical record review for ACR/EULAR criteria. Cox regression estimated HRs and 95%CIs for RA serologic phenotypes (all, seropositive, seronegative) by smoking status, intensity, pack-years, and years since cessation.
Results:
Among 230,732 women, we identified 1,528 incident RA cases (63.4% seropositive) during 6,037,151 person-years of follow-up. Compared to never smoking, current smoking increased risk for all RA (multivariable HR 1.47, 95%CI 1.27–1.72) and seropositive RA (HR 1.67, 95%CI 1.38–2.01), but not seronegative RA (HR 1.20, 95%CI 0.93–1.55). Increasing smoking pack-years was associated with increased trend of risk for all RA (p<0.0001) and seropositive RA (p<0.0001). With increasing duration of smoking cessation, a decreased trend was observed in risk for all RA (p=0.009) and seropositive RA (p=0.002). Compared to recent quitters (<5 years), those who quit ≥30 years ago had HR of 0.63 (95%CI 0.44–0.90) for seropositive RA. However, a modestly elevated RA risk was still detectable 30 years after quitting smoking (all RA: HR 1.25, 95%CI 1.02–1.53; seropositive RA: HR 1.30, 95%CI 1.01–1.68; reference: never smoking).
Conclusions:
These results confirm smoking as a strong risk factor for seropositive RA and demonstrate for the first time that a behavior change of sustained smoking cessation could delay or even prevent seropositive RA.
Keywords: smoking, behavior change, rheumatoid arthritis, epidemiology
INTRODUCTION
Although the etiology of rheumatoid arthritis (RA) remains obscure, previous studies implicate smoking as an important and potentially modifiable risk factor in the development of RA(1–6). Previous epidemiologic studies have identified cigarette smoking as one of the most important lifestyle risk factors for the development of RA, particularly for seropositive RA, defined as presence of rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP)(5, 7). Smoking may affect seropositive RA risk by inducing local tissue inflammation, promoting citrullination, and forming neoepitopes resulting in autoimmunity(8). Smoking also induces immune cells to secrete pro-inflammatory cytokines resulting in a systemic inflammatory state(8–10). While smoking cessation may decrease the level of systemic inflammation, other components of the immune system may be permanently altered after autoimmunity is established once a threshold of smoking is reached(11). While there is strong evidence that ever smokers (current or past) have a higher risk for seropositive RA than never smokers, it is unclear whether smoking cessation actually reduces the risk for past smokers, perhaps to the level of a never smoker after long-term cessation.
Previous studies have investigated the association between smoking status, intensity, duration, and pack-years with RA risk(2, 3, 5, 7, 12–14). However, only a few studies have investigated whether smoking cessation might reduce RA risk(5, 15–17). A previous investigation in the Nurses’ Health Study (NHS) suggested that the RA risk for past smokers was similar to the risk of never smokers by 20 years after smoking cessation(5). However, a true elevation of RA risk after long-term sustained smoking cessation may have been difficult to detect given sample size and follow-up limitations. Another study in Sweden suggested a reduced risk for RA with increased duration of smoking cessation, but these results were not statistically significant(15).
To investigate the association between smoking cessation and RA risk, repeated measures of smoking in a large sample size with lengthy follow-up prior to RA diagnosis are required. Therefore, we used two longitudinal cohorts of female nurses, the NHS and the NHSII, to investigate smoking cessation and RA risk using up to 38 years of prospective follow-up. The aims of this study were to investigate the association between smoking cessation and the risk of RA overall, and by serologic phenotype. We also aimed to determine when and to what extent smoking cessation may reduce the elevated risk of developing RA overall, and by serologic phenotype. We hypothesized that smoking cessation would reduce seropositive RA risk, but that residual RA risk would remain elevated compared to never smokers for many years since smoking cessation.
METHODS
Study population
The NHS and NHSII are prospective cohort studies of US female registered nurses. Participants completed baseline and biennial questionnaires regarding lifestyle, health behaviors, medications, and diseases. The NHS began in 1976 and enrolled 121,700 nurses aged 30–55 years; the NHSII was established in 1989 and enrolled 116,430 nurses aged 25–42 years. Both cohorts have >90% follow-up response rates and only 5% of person-time has been lost to follow-up(18).
For this analysis, we excluded participants that reported RA and other connective tissue diseases (CTD) at baseline, were missing baseline smoking information, or only answered the baseline questionnaire. After exclusions, 117,182 NHS participants followed 1976–2014 and 113,550 NHSII participants followed 1989–2015 were analyzed. All participants provided informed consent and this study was approved by the Partners HealthCare institutional review board.
Smoking exposures
On the baseline questionnaire, women reported smoking status (never/past/ current) and age at which they began to smoke. Current smokers were asked the number of cigarettes typically smoked per day, and past smokers provided the age at which they stopped smoking and the number of cigarettes smoked per day before quitting. On subsequent questionnaires, women reported smoking status and intensity (1–14, 15–24, ≥25 cigarettes/day). Smoking pack-years were derived by multiplying packs of cigarettes smoked per day (20 cigarettes per pack) with number of years smoked. Since smokers often stop and restart smoking, all smoking exposure variables were time-updated.
Identification of incident RA
RA cases were identified by a two-stage procedure. Participants who self-reported a new diagnosis of RA were contacted by mailing the CTD Screening Questionnaire (CSQ)(19). Medical records of participants with positive CSQ were requested and reviewed independently by two rheumatologists to identify RA cases meeting the 1987 American College of Rheumatology (ACR) or 2010 ACR/European League Against Rheumatism RA classification criteria(20). In addition to components of these classification criteria, dates of symptom onset/diagnosis and clinical laboratory results of RF and anti-CCP were collected. Cases therefore had confirmed incident RA with documented serologic phenotype from medical records. For women who were diagnosed with RA prior to the clinical use of anti-CCP in the early 2000s, serologic phenotype was determined by RF from medical record review. A subset of cases with blood banked prior to or after the date of RA onset had anti-CCP tested for research purposes, so we re-classified a few women as seropositive (n=23). Thus, participants were classified as seropositive if RF or anti-CCP were positive and seronegative if both RF and anti-CCP (if available) were negative.
Covariates
We selected covariates as potential confounders associated with cigarette smoking and RA based on previous studies(2, 7, 12). Time-updated sociodemographic covariates included age, race, and household income (categorized by quartile of US Census tract-based median household income at zip code level). Potential time-updated reproductive confounders were oral contraceptive use (categorized as ever or never users), parity/total breastfeeding duration, menopausal status, and postmenopausal hormone (PMH) use. We used a combined “parity/total breastfeeding duration” variable categorized as: nulliparous, parous/0-<1 month, parous/1–11 months, or parous/≥12 months, and a combined variable for menopausal status and PMH use: premenopausal, postmenopausal/never, or postmenopausal/ever. We defined time-updated sedentary physical activity as <3 MET-hours/week(21) and categorized time-updated Body mass index (BMI) as: <25.0, 25.0 to <30.0, or ≥30.0 kg/m2. Alcohol consumption (also time-updated) was assessed by a semi-quantitative food frequency questionnaire(22). We calculated cumulative average alcohol as a long-term measure of intake and categorized as none to <5, 5 to <10 and ≥10 grams/day. Missing data on physical activity, BMI, and alcohol were carried forward one cycle and then a missing indicator variable was created for data missing beyond one cycle.
Statistical analysis
We pooled data from the NHS and NHSII into a single analysis for statistical efficiency given exposures with many categories, planned subgroup analyses, and analyses for RA serologic phenotypes. We reported age-adjusted descriptive statistics for covariates across smoking status categories (never/past/current) for the NHS in 1988 and the NHSII in 1989 since these were the first cycles at similar calendar periods.
Person-years of follow-up for each woman accrued from the date of return of the baseline questionnaire to the date of censoring, whichever came first: RA diagnosis, reported other CTD not confirmed as RA, date of death, or end of follow-up for this analysis (June 1, 2014 for the NHS and June 1, 2015 for the NHSII). If participants were missing smoking data during a questionnaire cycle, we did not include these person-years in the analysis.
We used Cox proportional hazards models to test for the association between time-updated smoking intensity by status, pack-years, and smoking cessation for RA risk. We analyzed smoking status as never (reference), past, current as well as a 5-level smoking intensity variable of never (reference), past, current 1–14, current 15–24, and current ≥25 cigarettes/day. Pack-years of smoking were categorized as never (reference), >0–10, >10–20, >20–30, >30–40, or >40 pack-years. Grouped by smoking status and years since quitting, smoking cessation was analyzed as never (reference), past, in ordinal categories of years since cessation (≥30, 20-<30, 10-<20, 5-<10, or 0-<5 years ago), and current. Base models were adjusted for age, cohort, and questionnaire cycle (each cohort pooled by similar calendar times; e.g., the 1988 cycle in the NHS was pooled with the 1989 cycle in the NHSII). After examining the associations of each possible covariate with smoking status and the all RA outcome separately, the multivariable model was additionally adjusted for oral contraceptive use, parity/breastfeeding, menopausal status/PMH use, BMI, sedentary physical activity, median household income, and alcohol intake. We performed similar analyses for RA serologic phenotypes. We also analyzed additional subgroups among ever (reference: current smoking) and past smokers (reference: 0 to <5 years since quitting) to further investigate smoking cessation and RA risk.
We used restricted cubic splines with three knots to visualize risk for RA serologic phenotypes by pack-years (among the entire study sample; reference=never (0) pack-years) and years since quitting smoking (among only past smokers; reference≤2 years since cessation) adjusted for covariates(23). Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. Using higher numbers of knots in the cubic spline curves had similar results.
We tested the proportional hazards assumption by including interaction terms between smoking exposures and follow-up time, using likelihood ratio tests to compare nested models with and without interaction terms. The proportional hazards assumption was met in all analyses. Two-sided p<0.05 was considered statistically significant in all analyses. All analyses were performed using SAS v.9.4.
RESULTS
Characteristics of participants
Among 117,182 women in the NHS (1976–2014) and 113,550 women in the NHSII (1989–2015), we identified a total of 1,528 incident RA cases (1,002 in the NHS, 526 in the NHSII). There were 969 (63.4%) seropositive RA cases and 559 (36.6%) seronegative RA cases during 6,037,151 person-years of follow-up. Table 1 displays age-adjusted characteristics of the NHS and the NHSII study participants categorized by smoking status and at a similar calendar time (1988 and 1989, respectively). Women in the NHS were older in 1988 (mean age 54.3 [SD 7.2] years) compared to women in the NHSII in 1989 (mean age 34.4 [SD 4.7] years). There were more smokers in the NHS (18.8% current smokers, and 35.8% past smokers) than in the NHSII (13.4% current smokers and 21.3% past smokers). Within both cohorts, sedentary physical activity and alcohol consumption were higher among smokers than non-smokers, particularly for current smokers.
Table 1.
Age-standardized characteristics of participants in the Nurses’ Health Study in 1988 and Nurses’ Health Study II in 1989 categorized by smoking status.
| NHS (n=98,497)* | NHS II (n=113,550) | |||||
|---|---|---|---|---|---|---|
| Never | Past | Current | Never | Past | Current | |
| Participants, n (%) | 44,776 (45.5) | 35,238 (35.8) | 18,483 (18.8) | 74,181 (65.3) | 24,155 (21.3) | 15,214 (13.4) |
| Age in years, mean (SD)** | 54.2 (7.3) | 54.5 (7.1) | 53.9 (6.9) | 34.0 (4.7) | 35.2 (4.5) | 34.8 (4.6) |
| White race, % | 92.7 | 94.6 | 94.7 | 91.7 | 94.9 | 93.2 |
| Median household income quartile, % | ||||||
| Q1 – lowest | 29.0 | 24.4 | 26.0 | 24.3 | 21.2 | 28.4 |
| Q2 | 24.9 | 23.2 | 24.5 | 25.5 | 23.0 | 25.4 |
| Q3 | 23.3 | 25.2 | 25.6 | 25.1 | 26.0 | 24.4 |
| Q4 – highest | 22.7 | 27.2 | 23.9 | 25.1 | 29.9 | 21.7 |
| Body mass index category, % | ||||||
| <25.0 kg/m2 | 54.0 | 54.6 | 63.3 | 70.3 | 70.4 | 69.5 |
| 25.0 to <30.0 kg/m2 | 29.6 | 29.5 | 26.3 | 18.3 | 18.6 | 19.2 |
| ≥30.0 kg/m2 | 16.4 | 15.9 | 10.3 | 11.4 | 11.0 | 11.3 |
| Sedentary physical activity (<3 METs/week), % | 19.6 | 19.0 | 27.3 | 14.7 | 14.0 | 18.0 |
| Parity/breastfeeding duration in months, % | ||||||
| Nulliparous | 5.2 | 5.2 | 5.6 | 30.1 | 28.0 | 33.9 |
| Parous/none to <1 | 25.8 | 30.2 | 35.4 | 11.8 | 12.0 | 16.1 |
| Parous/1–11 | 27.2 | 27.8 | 26.5 | 22.1 | 24.2 | 22.6 |
| Parous/≥12 | 17.6 | 14.1 | 11.0 | 26.2 | 25.3 | 14.0 |
| Menopausal status/postmenopausal hormone use, % | ||||||
| Premenopausal | 38.9 | 36.6 | 35.4 | 94.3 | 94.3 | 91.6 |
| Postmenopausal/never | 28.0 | 27.3 | 32.6 | 2.8 | 2.8 | 4.0 |
| Postmenopausal/ever | 33.1 | 36.0 | 31.9 | 2.9 | 2.9 | 4.3 |
| Cumulative average alcohol intake (grams/day): | ||||||
| None to <5 | 71.4 | 53.9 | 52.8 | 84.2 | 71.0 | 67.3 |
| 5 to <10 | 9.8 | 15.3 | 12.5 | 8.7 | 14.5 | 13.8 |
| ≥10 | 10.7 | 23.5 | 28.4 | 6.0 | 13.7 | 18.0 |
There were n=117,182 women in the NHS at baseline in 1976.
Not age-standardized.
Missing values not shown.
Smoking status/intensity and RA risk
Compared with women who never smoked, the multivariable-adjusted hazard ratio (HR) for developing RA was 1.36 (95%CI 1.22–1.53) among past smokers and 1.46 (95%CI 1.26–1.70) among current smokers (Supplemental Table 1), adjusted for age, questionnaire period, cohort, oral contraceptive use, parity/breastfeeding, menopausal status/PMH use, BMI, sedentary physical activity, median household income, and alcohol intake. When we further examined the intensity of smoking, current smokers who smoked ≥25 cigarettes per day had a 92% increased risk for seropositive RA compared to never smokers (multivariable HR 1.92, 95%CI 1.39–2.66) (Table 2). There was no significant association of smoking intensity with seronegative RA.
Table 2.
Hazard ratios for rheumatoid arthritis serologic phenotypes by smoking status and intensity.
| Never | Past | Current, 1–14 cigarettes/day | Current, 15–24 cigarettes/day | Current, ≥25 cigarettes/day | |
|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| All RA | |||||
| Cases/person-years | 675/3,262,927 | 597/1,946,910 | 80/316,907 | 111/330,369 | 65/180,038 |
| Age-adjusted model | 1.00 (Ref) | 1.35(1.20,1.51) | 1.19(0.94,1.50) | 1.58(1.28,1.94) | 1.69(1.30,2.19) |
| Multivariable model* | 1.00 (Ref) | 1.36(1.21,1.53) | 1.23(0.97,1.55) | 1.60(1.30,1.97) | 1.69(1.30,2.20) |
| Seropositive RA | |||||
| Cases/person-years | 415/3,254,327 | 386/1,940,936 | 52/315,778 | 73/329,167 | 43/179,214 |
| Age-adjusted model | 1.00 (Ref) | 1.44(1.25,1.66) | 1.29(0.97,1.73) | 1.75(1.35,2.25) | 1.86(1.35,2.58) |
| Multivariable model* | 1.00 (Ref) | 1.48(1.28,1.71) | 1.36(1.01,1.82) | 1.80(1.39,2.34) | 1.92(1.39,2.66) |
| Seronegative RA | |||||
| Cases/person-years | 260/3,254,901 | 211/1,940,814 | 28/315,756 | 38/328,645 | 22/178,974 |
| Age-adjusted model | 1.00 (Ref) | 1.20(1.00,1.44) | 1.03(0.70,1.53) | 1.32(0.94,1.88) | 1.42(0.91,2.21) |
| Multivariable model* | 1.00 (Ref) | 1.18(0.98,1.43) | 1.03(0.69,1.53) | 1.31(0.92,1.86) | 1.36(0.86,2.12) |
Multivariable models were adjusted for age, questionnaire period, cohort, oral contraceptive use (ever, never), parity/breastfeeding in months (nulliparous, parous/<1 month, parous/1–11 months, parous/≥12 months), menopausal status/postmenopausal hormone use (premenopausal, postmenopausal/never, postmenopausal/ever), body mass index category (underweight/normal, overweight, obese), sedentary physical activity, median household income (quartiles), alcohol intake (none/<5 g/day, 5 to <10 g/day, ≥10 g/day).
Pack-years and RA risk
To further investigate the association between smoking and RA risk, we investigated the relationship between pack-years of smoking and RA risk using restricted cubic splines models (Figure 1). Compared to women who never smoked (0 pack-years), there was a statistically significant increasing trend for developing RA with increasing number of pack-years smoked up to 35 pack-years with the HR plateauing at about HR of 1.8 for all RA (p<0.0001) and HR of 2.3 for seropositive RA (p<0.0001).
Figure 1.
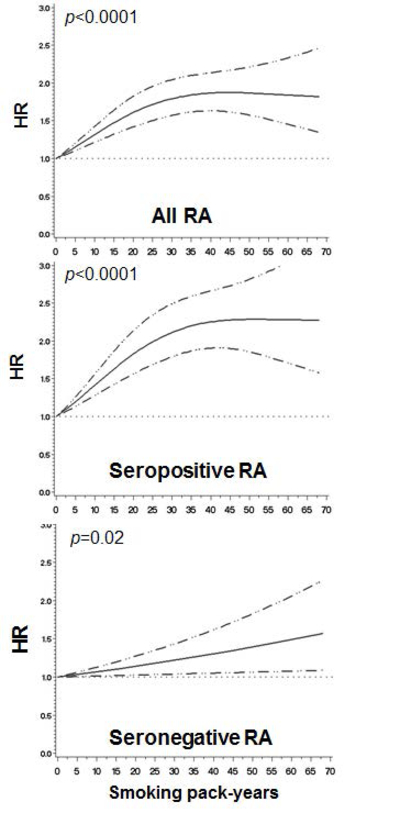
Among all women (reference: 0 pack-years), restricted cubic spline curves showing hazard ratios and 95% confidence bounds for rheumatoid arthritis serologic phenotypes by smoking pack-years. P values are for trend. Curves are adjusted for the covariates listed in Table 2. See Supplemental Table 2 for analysis by categories of smoking pack-years.
Supplemental Table 2 presents categories of pack-years and RA risk. Compared to never smokers, there was no risk for any RA serologic phenotype for >0 to 10 pack-years. However, those who smoked 10 to 20 pack-years had significantly increased risk for all RA (HR 1.38, 95%CI 1.17–1.64) and seropositive RA (HR 1.54, 95%CI 1.25–1.89), but not for seronegative RA. Those who smoked >40 pack-years had nearly two-fold risk of developing all RA (HR 1.83, 95%CI 1.52–2.20) and seropositive RA (HR 2.25, 95%CI 1.80–2.82), but not seronegative RA (HR 1.27, 95%CI 0.92–1.74).
Smoking cessation and RA risk
Table 3 shows the associations of smoking cessation with RA, comparing current and past smokers by years since quitting to the reference group of never smokers. Compared to never smokers, past smokers that quit 0 to <5 years ago had a significantly increased risk for all RA (HR 1.57, 95%CI 1.26–1.95) and seropositive RA (HR 1.99, 95%CI 1.54–2.58), but not for seronegative RA (HR 0.98, 95%CI 0.65–1.49). This point of estimate of increased risk of developing RA started to fall among past smokers who quit 10 to <20 years ago (HR 1.37, 95%CI 1.15–1.64) for all RA and who quit 5 to <10 years ago (HR 1.79, 95%CI 1.36–2.37) for seropositive RA. However, modestly increased RA risk was still detectable even 30 years after quitting smoking for both all RA (HR 1.25, 95% 1.02–1.53) and seropositive RA (HR 1.30, 95%CI 1.01–1.68).
Table 3.
Hazard ratios for rheumatoid arthritis serologic phenotypes by smoking status and years since cessation.
| Never | Past, quit ≥30 years ago | Past, quit 20 to <30 years ago | Past, quit 10 to <20 years ago | Past, quit 5 to <10 years ago | Past, quit 0 to <5 years ago | Current | |
|---|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| All RA | |||||||
| Cases/person-years | 675/3,262,927 | 123/397,373 | 129/445,185 | 160/551,108 | 90/262,059 | 94/282,440 | 257/836,060 |
| Age-adjusted model | 1.00 (Ref) | 1.22(1.00,1.50) | 1.17(0.97,1.42) | 1.36(1.14,1.62) | 1.65(1.32,2.06) | 1.58(1.27,1.96) | 1.45(1.25,1.68) |
| Multivariable model* | 1.00 (Ref) | 1.25(1.02,1.53) | 1.19(0.99,1.45) | 1.37(1.15,1.64) | 1.63(1.30,2.04) | 1.57(1.26,1.95) | 1.47(1.27,1.72) |
| Seropositive RA | |||||||
| Cases/person-years | 415/3,254,327 | 77/396,579 | 82/443,916 | 99/549,283 | 58/261,118 | 69/281,311 | 169/832,888 |
| Age-adjusted model | 1.00 (Ref) | 1.26(0.97,1.62) | 1.21(0.95,1.53) | 1.42(1.13,1.77) | 1.78(1.35,2.35) | 1.95(1.51,2.53) | 1.60(1.33,1.93) |
| Multivariable model* | 1.00 (Ref) | 1.30(1.01,1.68) | 1.25(0.98,1.59) | 1.45(1.16,1.81) | 1.79(1.36,2.37) | 1.99(1.54,2.58) | 1.67(1.38,2.01) |
| Seronegative RA | |||||||
| Cases/person-years | 260/3,254,901 | 46/396,731 | 47/443,877 | 61/549,236 | 32/261,042 | 25/281,204 | 88/832,099 |
| Age-adjusted model | 1.00 (Ref) | 1.17(0.84,1.63) | 1.12(0.82,1.53) | 1.28(0.97,1.70) | 1.45(1.00,2.10) | 1.03(0.68,1.55) | 1.22(0.95,1.56) |
| Multivariable model* | 1.00 (Ref) | 1.18(0.85,1.64) | 1.11(0.81,1.53) | 1.25(0.94,1.67) | 1.41(0.97,2.04) | 0.98(0.65,1.49) | 1.20(0.93,1.55) |
Multivariable models were adjusted for age, questionnaire period, cohort, oral contraceptive use (ever, never), parity/breastfeeding in months (nulliparous, parous/<1 month, parous/1–11 months, parous/≥12 months), menopausal status/postmenopausal hormone use (premenopausal, postmenopausal/never, postmenopausal/ever), body mass index category (underweight/normal, overweight, obese), sedentary physical activity, median household income (quartiles), alcohol intake (none/<5 g/day, 5 to <10 g/day, ≥10 g/day).
Among the subset of past smokers, Figure 2 shows the restricted cubic spline curves for the association between RA and years since smoking cessation (reference: 0–2 years since quitting). There was a trend showing a statistically significant decreasing risk for developing RA with increasing years since smoking cessation for both all RA (p=0.009) and seropositive RA (p=0.002), but not for seronegative RA (p=0.78).
Figure 2.
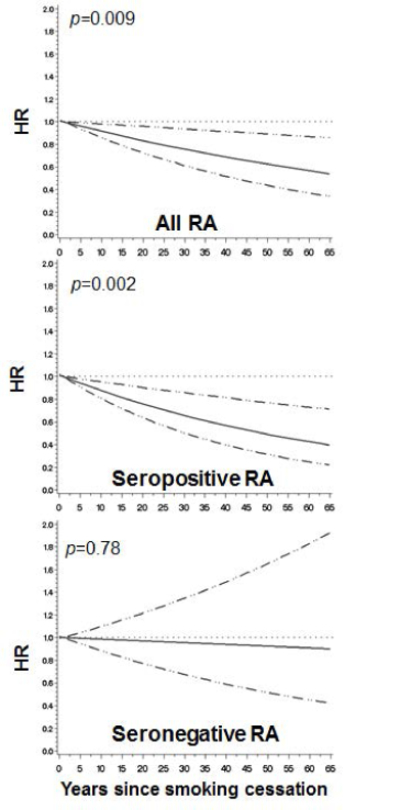
Among the subset of past smokers (reference: 0–2 years since quitting), restricted cubic spline curves showing hazard ratios and 95% confidence bounds for rheumatoid arthritis serologic phenotypes by years since smoking cessation. P values are for trend. Curves are adjusted for the covariates listed in Table 2.
Table 4 presents categories of years since smoking cessation and RA risk. Women that quit smoking ≥30 years ago had a suggestive reduced risk (HR 0.78, 95%CI 0.58–1.05) for all RA compared to those that quit 0-<5 years ago. The risk for seropositive RA was significantly reduced by 37% (HR 0.63, 95%CI 0.44–0.90) for women that quit smoking ≥30 years ago compared to those that quit 0-<5 years. There was no association of any category of time since smoking cessation and seronegative RA risk.
Table 4.
Among past smokers (reference: 0 to <5 years since quitting), hazard ratios for rheumatoid arthritis serologic phenotypes by smoking status and years since cessation.
| Past, quit ≥30 years ago | Past, quit 20 to <30 years ago | Past, quit 10 to <20 years ago | Past, quit 5 to <10 years ago | Past, quit 0 to <5 years ago | |
|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| All RA | |||||
| Cases/person-years | 123/397,373 | 129/445,185 | 160/551,108 | 90/262,059 | 94/282,440 |
| Age-adjusted model | 0.75(0.56,1.00) | 0.75(0.57,0.99) | 0.86(0.67,1.12) | 1.09(0.81,1.46) | 1.00 (Ref) |
| Multivariable model* | 0.78(0.58,1.05) | 0.78(0.59,1.02) | 0.88(0.68,1.14) | 1.09(0.81,1.46) | 1.00 (Ref) |
| Seropositive RA | |||||
| Cases/person-years | 77/396,579 | 82/443,916 | 99/549,283 | 58/261,118 | 69/281,311 |
| Age-adjusted model | 0.62(0.44,0.88) | 0.62(0.45,0.86) | 0.72(0.53,0.98) | 0.93(0.65,1.32) | 1.00 (Ref) |
| Multivariable model* | 0.63(0.44,0.90) | 0.63(0.45,0.88) | 0.73(0.53,0.99) | 0.91(0.64,1.30) | 1.00 (Ref) |
| Seronegative RA | |||||
| Cases/person-years | 46/396,731 | 47/443,877 | 61/549,236 | 32/261,042 | 25/281,204 |
| Age-adjusted model | 1.11(0.66,1.86) | 1.12(0.68,1.83) | 1.27(0.79,2.04) | 1.54(0.91,2.62) | 1.00 (Ref) |
| Multivariable model* | 1.20(0.71,2.02) | 1.17(0.71,1.93) | 1.30(0.81,2.09) | 1.57(0.92,2.66) | 1.00 (Ref) |
Multivariable models were adjusted for age, questionnaire period, cohort, oral contraceptive use (ever, never), parity/breastfeeding in months (nulliparous, parous/<1 month, parous/1–11 months, parous/≥12 months), menopausal status/postmenopausal hormone use (premenopausal, postmenopausal/never, postmenopausal/ever), body mass index category (underweight/normal, overweight, obese), sedentary physical activity, median household income (quartiles), alcohol intake (none/<5 g/day, 5 to <10 g/day, ≥10 g/day).
DISCUSSION
In this large prospective study of women, we observed that sustained smoking cessation reduced seropositive RA risk compared to recent quitters, suggesting that this behavior change can delay or even prevent the onset of seropositive RA. Seropositive RA risk was reduced by 37% for those who sustained smoking cessation for ≥30 years compared to those who recently quit smoking. Further, we showed an increased risk for RA, particularly for the seropositive phenotype, for past and current smokers, particularly for current smokers with high intensity or those with many pack-years and a strong dose-response, confirming prior studies (4, 7, 24–26). We didn’t find an association for smoking with seronegative RA risk despite large sample size and lengthy follow-up, suggesting that seropositive and seronegative RA may be distinct phenotypes with distinct risk factors.
Many previous studies have investigated smoking status and RA risk. In a large meta-analysis that included 11 studies(7), 13,885 RA cases among a total of 593,576 individuals, current smokers had an odds ratio (OR) of 1.64 for seropositive RA compared to never smokers. Recent results from the French E3N cohort study prospectively followed 71,248 women since 1990 and found that past smokers (HR 1.32, 95%CI 1.06–1.64) and current smokers (HR 1.57, 95%CI 1.13–2.19) had increased RA risk compared to never smokers(1). Our group previously investigated smoking only in the NHS using follow-up from 1976 to 2002 and demonstrated that current smokers had HR of 1.46 (95%CI 1.20–1.79) for all RA and HR of 1.58 (95%CI 1.21–2.06) for seropositive RA compared to never smokers(5). Our study findings are consistent with and extend these prior findings. We confirmed a strong association between smoking status and seropositive RA risk, but no clear association with seronegative RA.
Stolt et al investigated smoking intensity and RA risk and reported a OR of 2.4 (95%CI 1.5–3.7) for RA among current smokers who smoked ≥20 cigarettes per day compared to never smokers(16). A recent meta-analysis of 3 cohort and 7 case-control studies demonstrated a dose-response relationship between smoking pack-years and risk of RA, showing a statistically significant increased risk for developing RA with increasing pack-years up to 20 years, when the HR plateaued at about 2.0 compared to never smokers(2). We observed a similar relationship between smoking pack-years and risk for all RA and extended those findings by also investigating seropositive RA with the HR plateauing at about 2.3 after about 30 pack-years compared to never smoking.
Since smoking status, intensity, and pack-years are all associated with RA risk, particularly seropositive RA, this implies that a behavior change of smoking cessation might reduce RA risk. Some previous studies have also investigated smoking cessation and RA risk. The Swedish Mammography Cohort study(15) followed 34,101 women from 1997 to 2010 with a baseline questionnaire on smoking behaviors, and identified 219 incident RA cases. Past smokers who quit smoking ≥15 years had increased RA risk (HR 1.99, 95%CI 1.23–3.20) compared to never smokers, suggesting that residual elevated RA risk remained even after sustained cessation. When only analyzing past smokers, there was a suggestion that RA risk was reduced with increasing time since cessation compared to recent quitters. However, findings in that study were limited due to low number of events and only a single baseline assessment of smoking. In the Swedish Epidemiological Investigations of RA (EIRA) analyzing 679 cases 847 controls(16), past smokers who quit smoking ≥20 years had similar RA risk (OR 1.0, 95%CI 0.5–1.9) compared to never smokers. However, this case-control study may have been limited by recall bias and there were few cases in the sustained smoking cessation group so there may have been limited power to detect a true difference.
Similarly, the previous study only analyzing the NHS suggested that past smokers who quit smoking ≥20 years had similar RA risk (HR 1.14, 95%CI 0.88–1.48) compared to never smoking (5). In our current study, while RA risk decreased with time since cessation, a modestly elevated RA risk was detectable 30 years after quitting smoking (all RA: HR 1.25, 95%CI 1.02–1.53; seropositive RA: HR 1.30, 95%CI 1.01–1.68; reference: never smoking). By extending follow-up in the NHS and adding the NHSII cohort, our current study is better powered to detect a modest statistical difference among women with long-term sustained cessation. Therefore, our study extends previous findings and provides evidence that women who smoke may have modestly elevated RA risk for decades. This suggests that secular trends in smoking cessation may be followed by a decrease in RA incidence in future decades. While smoking cessation may not decrease RA risk to the level of a never smoker, our findings provide evidence that a behavior change of smoking cessation may delay or even prevent the onset of seropositive RA. These results could provide rationale for a smoking intervention trial among active smokers to prevent the formation of RA-related autoantibodies or to prevent the progression to RA among those at elevated risk for seropositive RA.
We observed that the risk of RA among recent quitters (0-<5 years since smoking cessation) was higher than current smokers, perhaps due to many of the recent quitters being heavy smokers (>40 pack-years). These recent quitters may be more likely to start smoking again so may not have actually had sustained smoking cessation and may be similar to current smokers. Moreover, recent quitters may have been decided to quit smoking due to early symptoms of RA or other serious health conditions. The population attributable risk for RA from smoking is 14%(27) and may contribute up to 35% of ACPA+ RA risk(28). Smoking may interact with shared epitope genes to increase seropositive RA risk(4, 29, 30).
Although the biologic mechanisms linking smoking with increased risk for developing RA are still not clear, components in cigarette smoke, such as nicotine, hydrocarbons, and carbon monoxide, are known to have aberrant effects on the immune system(9, 31). Smoking causes impaired T-cell function(32, 33) and humoral immunity(34, 35) and raises systemic levels of inflammatory markers such as interleukin-6 and C-reactive protein(36, 37). Smoking has also been shown to increase levels of citrullinated proteins and expression of peptidyl arginine deiminase 2 in pulmonary alveoli(38). Evidence has accumulated showing that, in the presence of the HLA shared epitope genes, cigarette smoking may trigger immune responses against citrullinated proteins(11, 30, 39). The observed associations between smoking status/intensity as well as the dose-response between pack-years of smoking with RA risk in our study are compatible with this triggering mechanism. Moreover, the detectable elevated RA risk 30 years after smoking cessation suggests that, in some individuals, the immune system may be permanently altered perhaps with resultant autoimmunity established once a threshold of smoking is reached and progression to RA occurring many years later.
A major strength of our study is using two large cohorts to prospectively identify incident RA cases with up to 38 years during over 6 million person-years of follow-up. We had detailed data on smoking exposures including status, intensity, cumulative pack-years, and years since smoking cessation as well as information on important potential confounders such as alcohol intake and reproductive factors updated prospectively every 2 years, allowing for time-updated analyses. Further, women who self-reported CTD including unconfirmed RA were censored at time of self-report to ensure that the analyzed sample was free of RA or other CTD. We identified cases by medical record review to ensure that all fulfilled accepted criteria and were truly incident while allowing for subphenotyping based on serologic phenotype.
However, our study does have some limitations. Our study population, consisting of mostly healthy, well-educated, white US women working in nursing professions at baseline, may not be representative of the general population. The detailed smoking data was by self-report so may have the potential for recall bias. However, self-report of smoking has been reported to be valid and these repeated measures were collected prospectively prior to RA onset so a differential bias between cases and non-cases is unlikely(40). As smoking was only assessed every 2 years, we might not have captured the intervening smoking behavior changes. In addition, there may be potential for misclassification by RA serologic phenotype. The serologic phenotype of our incident RA cases was determined by the combination of RF and anti-CCP tests obtained through routine clinical care. Many cases were diagnosed prior to the early 2000s when anti-CCP testing began to be used widely in the US. Thus, for earlier RA cases medical records only contained data on RF. It is therefore possible that some RA cases diagnosed before the early 2000s who were RF-negative may have actually been anti-CCP-positive but classified as seronegative in our study. Since RF and CCP are correlated, we expect that the misclassification of seropositivity of RA cases is relatively uncommon. We previously found that only about 2% of our seronegative RA cases were initially misclassified based on negative RF but positive anti-CCP assays among a subset who provided blood samples in 1989 and had this tested for research purposes(41).
In conclusion, we found that past smokers had significantly reduced risk for seropositive RA by time since sustained smoking cessation, providing evidence that this behavior change may decrease or even prevent the onset of RA. We detected a slightly elevated risk for seropositive RA even 30 years after smoking cessation compared to never smokers suggesting that a minority may have permanent immune alterations even after smoking cessation. We found no association of smoking with seronegative RA, suggesting a different pathogenesis than seropositive RA. Our study findings provide evidence that a behavior change of sustained smoking cessation may reduce seropositive RA risk.
Supplementary Material
Significance & Innovations.
We used data from two large prospective cohorts with up to 38 years during over 6 million person-years of follow-up, consisting of detailed assessment of smoking behavior change and 1,528 incident RA cases confirmed by medical record review.
Compared to never smokers, a slightly elevated risk for seropositive RA was still detectable for past smokers even 30 years after smoking cessation.
Among past smokers, the risk for seropositive RA was significantly reduced by 37% (HR 0.63, 95%CI 0.44–0.90) for women that quit smoking ≥30 years compared to women that quit smoking for 0 to <5 years.
Our findings demonstrate that a behavior change of sustained smoking cessation may reduce seropositive RA risk.
ACKNOWLEDGEMENTS
We thank the participants of the NHS and NHSII for their dedicated participation in this longitudinal study as well as the staff members at the Channing Division of Network Medicine (Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School).
Funding/Support: This work was supported by the National Institutes of Health (grant numbers K23 AR069688, L30 AR066953, R01 AR049880, K24 AR052403, UM1 CA186107, UM1 CA176726, P30 AR070253, and P30 AR072577). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Disclosures: All authors declare no financial disclosures.
REFERENCES
- 1.Seror R, Henry J, Gusto G, Aubin H-J, Boutron-Ruault M-C, Mariette X. Passive smoking in childhood increases the risk of developing rheumatoid arthritis. Rheumatology 2018:key219-key. [DOI] [PubMed]
- 2.Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther 2014;16(2):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooy DP, van Nies JA, Kapetanovic MC, Kristjansdottir H, Andersson ML, Forslind K, et al. Smoking as a risk factor for the radiological severity of rheumatoid arthritis: a study on six cohorts. Ann Rheum Dis 2014;73(7):1384–7. [DOI] [PubMed] [Google Scholar]
- 4.Karlson EW, Deane K. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum Dis Clin North Am 2012;38(2):405–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med 2006;119(6):503 e1–9. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson D, Shepstone L, Moots R, Lear JT, Lynch MP. Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Ann Rheum Dis 2001;60(3):223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2010;69(1):70–81. [DOI] [PubMed] [Google Scholar]
- 8.Chang K, Yang SM, Kim SH, Han KH, Park SJ, Shin JI. Smoking and rheumatoid arthritis. Int J Mol Sci 2014;15(12):22279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sopori M Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002;2(5):372–7. [DOI] [PubMed] [Google Scholar]
- 10.Onozaki K Etiological and biological aspects of cigarette smoking in rheumatoid arthritis. Inflamm Allergy Drug Targets 2009;8(5):364–8. [DOI] [PubMed] [Google Scholar]
- 11.Klareskog L, Padyukov L, Alfredsson L. Smoking as a trigger for inflammatory rheumatic diseases. Curr Opin Rheumatol 2007;19(1):49–54. [DOI] [PubMed] [Google Scholar]
- 12.Hedstrom AK, Klareskog L, Alfredsson L. Exposure to passive smoking and rheumatoid arthritis risk: results from the Swedish EIRA study. Ann Rheum Dis 2018;77(7):970–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med 2002;112(6):465–71. [DOI] [PubMed] [Google Scholar]
- 14.Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum 1999;42(5):910–7. [DOI] [PubMed] [Google Scholar]
- 15.Di Giuseppe D, Orsini N, Alfredsson L, Askling J, Wolk A. Cigarette smoking and smoking cessation in relation to risk of rheumatoid arthritis in women. Arthritis Res Ther 2013;15(2):R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis 2003;62(9):835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Ann Rheum Dis 1990;49(12):980–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 19.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5(4):297–302. [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69(2):243–9. [DOI] [PubMed] [Google Scholar]
- 23.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 24.Klareskog L, Padyukov L, Ronnelid J, Alfredsson L. Genes, environment and immunity in the development of rheumatoid arthritis. Curr Opin Immunol 2006;18(6):650–5. [DOI] [PubMed] [Google Scholar]
- 25.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 2004;50(10):3085–92. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan E, Sokka T, Hannonen P. Smoking-gender interaction and risk for rheumatoid arthritis. Arthritis Res Ther 2003;5(3):R158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparks JA, Chen CY, Hiraki LT, Malspeis S, Costenbader KH, Karlson EW. Contributions of familial rheumatoid arthritis or lupus and environmental factors to risk of rheumatoid arthritis in women: a prospective cohort study. Arthritis Care Res (Hoboken) 2014;66(10):1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallberg H, Ding B, Padyukov L, Bengtsson C, Ronnelid J, Klareskog L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis 2011;70(3):508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis 2010;69(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Jiang X, Cui J, Lu B, Costenbader KH, Sparks JA, et al. Interactions between amino acid-defined major histocompatibility complex class II variants and smoking in seropositive rheumatoid arthritis. Arthritis Rheumatol 2015;67(10):2611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George J, Levy Y, Shoenfeld Y. Smoking and immunity: an additional player in the mosaic of autoimmunity. Scand J Immunol 1997;45(1):1–6. [DOI] [PubMed] [Google Scholar]
- 32.Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, et al. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol 2004;30(2):202–11. [DOI] [PubMed] [Google Scholar]
- 33.Hughes DA, Haslam PL, Townsend PJ, Turner-Warwick M. Numerical and functional alterations in circulatory lymphocytes in cigarette smokers. Clin Exp Immunol 1985;61(2):459–66. [PMC free article] [PubMed] [Google Scholar]
- 34.Moszczynski P, Zabinski Z, Moszczynski P Jr., Rutowski J, Slowinski S, Tabarowski Z. Immunological findings in cigarette smokers. Toxicol Lett 2001;118(3):121–7. [DOI] [PubMed] [Google Scholar]
- 35.Burton RC. Smoking, immunity, and cancer. Med J Aust 1983;2(9):411–2. [DOI] [PubMed] [Google Scholar]
- 36.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol 2002;89(9):1117–9. [DOI] [PubMed] [Google Scholar]
- 37.Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol 1997;17(10):2167–76. [DOI] [PubMed] [Google Scholar]
- 38.Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008;67(10):1488–92. [DOI] [PubMed] [Google Scholar]
- 39.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006;54(1):38–46. [DOI] [PubMed] [Google Scholar]
- 40.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994;84(7):1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bengtsson C, Malspeis S, Orellana C, Sparks JA, Costenbader KH, Karlson EW. Association Between Menopausal Factors and the Risk of Seronegative and Seropositive Rheumatoid Arthritis: Results From the Nurses’ Health Studies. Arthritis Care Res (Hoboken) 2017;69(11):1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


