Abstract
Congenital erythropoietic porphyria (CEP) is a rare autosomal recessive disorder characterized by photosensitivity and by hematologic abnormalities in affected individuals. CEP is caused by mutations in the uroporphyrinogen synthase (UROS) gene. In three reported cases, CEP has been associated with a specific X-linked GATA1 mutation. Disease-causing mutations in either gene result in absent or markedly reduced UROS enzymatic activity. This in turn leads to the accumulation of the nonphysiologic and photoreactive porphyrinogens, uroporphyrinogen I and coproporphyrinogen I, which damage erythrocytes and elicit a phototoxic reaction upon light exposure.
The clinical spectrum of CEP depends on the level of residual UROS activity, which is determined by the underlying pathogenic loss-of-function UROS mutations. Disease severity ranges from non-immune hydrops fetalis in utero to late-onset disease with only mild cutaneous involvement. The clinical characteristics of CEP include exquisite photosensitivity to visible light resulting in bullous vesicular lesions which, when infected lead to progressive photomutilation of sun-exposed areas such as the face and hands. In addition, patients have erythrodontia, brownish discoloration of teeth, and can develop corneal scarring. Chronic transfusion-dependent hemolytic anemia is common and leads to bone marrow hyperplasia, which further increases porphyrin production.
Management of CEP consists of strict avoidance of exposure to visible light with sun-protective clothing, sunglasses, and car and home window filters. Adequate care of ruptured vesicles and use of topical antibiotics is indicated to prevent superinfections and osteolysis. In patients with symptomatic hemolytic anemia, frequent erythrocyte cell transfusions may be necessary to suppress hematopoiesis and decrease marrow production of the phototoxic porphyrins. In severe transfection-dependent cases, bone marrow or hematopoietic stem cell transplantation has been performed, which is curative.. Therapeutic approaches including gene therapy, proteasome inhibition, and pharmacologic chaperones are under investigation.
Keywords: Porphyria, phototoxicity, cutaneous lesions heme biosynthetic pathway, hemolysis
1. Introduction
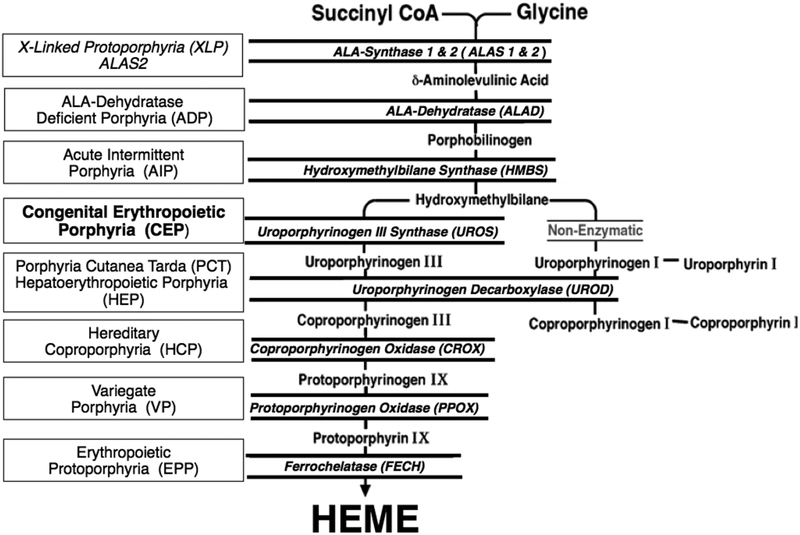
The inherited porphyrias are a diverse group of inborn errors of heme biosynthesis, each resulting from the deficient activity of a specific enzyme in the pathway [1–3] [Fig 1]. Porphyrias are classified as either hepatic or erythropoietic, according to whether the excess production of porphyrin precursors and porphyrins occurs primarily in the liver or in the erythron. Patients with the erythropoietic porphyrias have elevated bone marrow and erythrocyte porphyrins, and their clinical manifestations usually include anemia and/or cutaneous photosensitivity causing mild to severe dermatological involvement. The erythropoietic porphyrias include congenital erythropoietic porphyria (CEP), erythropoietic protoporphyria (EPP), and X-linked protoporphyria (XLP). While EPP and XLP have a very similar clinical presentation, CEP has a distinct phenotype and typically presents with significantly more severe cutaneous involvement and debilitating complications [1–3].
Fig 1.
The human biosynthetic pathway and the porphyrias resulting from the indicated heme biosynthetic enzyme defect. There are eight enzymatic steps in the conversion of glycine and succinyl-CoA to heme. The heme biosynthetic enzymes are italicized, their substrates and products are indicated, and the resulting porphyrias are shown in boxes. Note that there are two aminolevulinic acid synthase (ALAS) isozymes: a housekeeping enzyme, ALAS1, encoded by a gene that is regulated by negative feedback repression by heme, and an erythroid specific enzyme, ALAS2, that is regulated by the iron response proteins and erythroid transcription binding proteins.
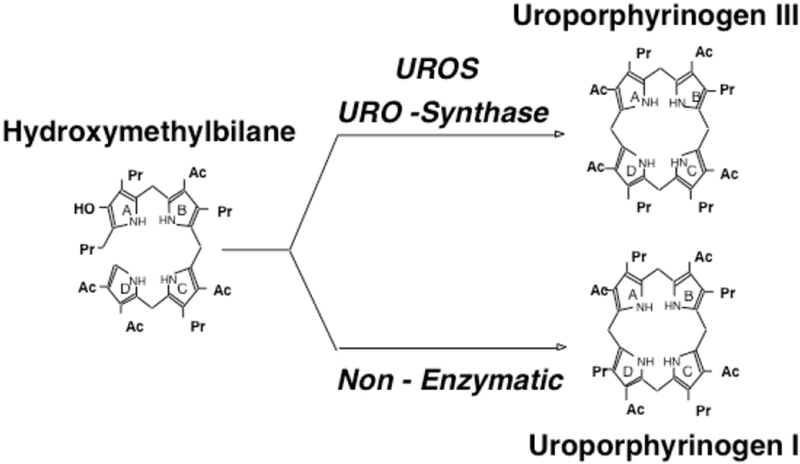
CEP is a rare, autosomal recessive, panethnic disease, resulting from deficient activity of the fourth enzyme of the heme biosynthetic pathway, uroporphyrinogen III synthase (UROS), which is encoded by the uroporphyrinogen III synthase (UROS) gene [1–4]. The enzyme deficiency results in the accumulation of the non-physiologic porphyrinogen I isomers, uroporphyrinogen I and coproporphyrinogen I in bone marrow erythroid precursors and erythrocytes [Fig 2]. The isomer I porphyrinogens undergo auto-oxidation to the corresponding porphyrins, which are photo-activated, damage erythrocytes, and are deposited in tissues and bones. Since they are photocatalytic compounds, exposure of the skin to sunlight and other sources of long-wave ultraviolet light elicits a phototoxic reaction, resulting in blistering and vesicle formation as well as increased friability of the skin [1, 5].
Fig 2.
Uroporphyrinogen synthase (UROS) normally converts hydroxymethylbilane (HMB) to uroporphyrinogen III. When the UROS activity is markedly decreased, HMB is non-enzymatically converted to uroporphyrinogen I, which is then enzymatically converted to oproporphyrinogen I by uroporphyrinogen decarboxylase. The accumulated uroporphyrinogen I and coproporphyrinogen I are oxidized to their respective porphyrins, which are photoactive and cause the sun/light-induced hemolysis and cutaneous manifestations of CEP.
In most cases, CEP is caused by UROS mutations and follows an autosomal recessive inheritance pattern. One specific mutation in the GATA1 gene, located on the X chromosome, has also been linked to the CEP phenotype in 3 male individuals [6, 7]. So far, about 250 CEP patients have been described in the literature [8] and their clinical manifestations are markedly heterogeneous, ranging from non-immune hydrops fetalis to milder, later-onset forms characterized by mild cutaneous involvement without hematologic symptoms in adult life [1, 9]. Severely affected patients are transfusion-dependent throughout life, have secondary hypersplenism and significant cutaneous involvement. Severe complications such as secondary skin infections with subsequent bone resorption and photomutilation, leading to loss of digits and facial features are common [10]. Chronic infections have caused osteomyelitis in some CEP patients [11, 12]. Successful bone marrow (BMT) or hematopoietic stem cell (HSCT) transplantation is the only curative approach. Management otherwise consists of strict avoidance of sun and light exposure as well as erythrocyte transfusions to maintain the hematocrit >35 to decrease reticulocytosis in transfusion-dependent patients [13, 14].
2. Pathophysiology
2.1. The Enzymatic Defect
The pathogenesis of CEP is readily explained by markedly deficient, but not absent, UROS activity. Consistent with its autosomal recessive inheritance, a very low level (≤1% of normal) appears to be sufficient for life. A UROS knockout mouse model was a fetal lethal in the early embryo, indicating that the absence of UROS activity is not compatible with life [15]. Deficient UROS activity leads to the accumulation of the enzyme’s substrate, hydroxymethylbilane (HMB), most of which is converted non-enzymatically to uroporphyrinogen I [Fig 2]. Although uroporphyrinogen I can undergo decarboxylation by uroporphyrinogen decarboxylase (UROD) to form hepta-, hexa- and pentacarboxyl-porphyrinogen I, and, finally, coproporphyrinogen I, further metabolism cannot proceed because the next enzyme in the pathway, coproporphyrinogen oxidase (CPOX), is stereospecific for the III isomer. The isomer I porphyrins are non-physiologic, phototoxic, cannot be metabolized to heme, and are pathogenic when they accumulate in large amounts [10, 16, 17].
Uroporphyrin I accumulation in bone marrow, erythrocytes, plasma and urine is the biochemical hallmark of the disease. Large amounts of isomer I porphyrinogens in bone marrow erythroid precursors (especially normoblasts and reticulocytes) and erythrocytes undergo auto-oxidation to the corresponding porphyrins, which damage erythrocytes and are deposited in tissues and bones. Photosensitivity occurs because these porphyrins are photocatalytic and cytotoxic compounds [16]. Exposure of the skin to sunlight and other sources of long-wave ultraviolet light results in blistering and vesicle formation and increased friability of the skin [5]. Damage to the erythrocytes leads to hemolysis, which is almost always present, but may not be accompanied by anemia if erythroid hyperplasia is sufficient to compensate for the increased rate of erythrocyte destruction. The degree of compensation may vary over time but in severe cases, chronic hemolysis and ineffective erythropoiesis may result in transfusion dependence [10].
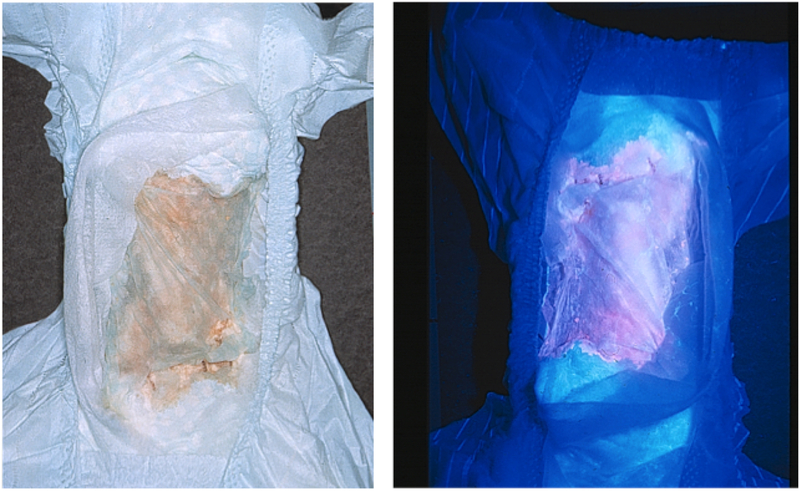
Accumulated porphyrins are excreted in large amounts in urine and feces and pink to dark-reddish urine is typically noted in the diaper shortly after birth [Fig 3]. Urinary porphyrin concentrations are markedly increased (100–1000 times normal; up to 50–100 mg/d) and consist mostly of the uroporphyrin I and coproporphyrin I isomers, with lesser increases in hepta-, hexa- and pentacarboxylporphyrins I isomers [18]. Although type I urinary porphyrin isomers predominate, type III isomers also are increased. Fecal porphyrins are also markedly increased with a predominance of coproporphyrin I. Fecal isocoproporphyrins are not increased [19]. As opposed to the acute hepatic porphyrias, there is no elevation of urinary aminolevulinic acid (ALA) or porphobilinogen (PBG).
Fig 3.
Diaper of an infant with CEP. The patient’s urine has stained the diaper a reddish color due to the accumulated uroporphyrin I and coproporphyrin I (left). The accumulated porphyrins fluoresce when illuminated with ultraviolet light (right) [95].
2.2. The Genetic Defect
With one notable exception, CEP results from biallelic UROS mutations. Since 2007, three male patients presenting with CEP symptoms have been reported with a specific mutation in GATA1, an important erythroid transcription factor [6, 7]. Since the GATA1 gene is located on the X chromosome, the affected males developed CEP manifestations, while the female heterozygotes were mostly asymptomatic [6, 7, 10]. In a small percentage of CEP patients (>10%), a disease-causing mutation has not been detected in the UROS or GATA1 genes, raising the possibility that additional gene(s) may play a role in CEP pathogenesis [8].
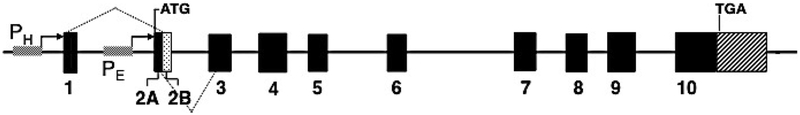
UROS Gene
The UROS gene is located on chromosome 10q26.2. The gene is ~34 kb long and contains 10 exons, including two non-coding untranslated exons 1 and 2A, and nine coding exons 2B to 10 [20][Fig 4]. There are two promoters, a housekeeping promoter upstream of exon 1 and an erythroid-specific promoter upstream of exon 2A, which generate housekeeping and erythroid-specific transcripts, respectively. The housekeeping transcript contains the 5′ untranslated exon 1 fused to coding exons 2B through 10 and is present in all tissues and cells, while the erythroid-specific transcript contains the 5′-untranslated exon 2A fused to coding exons 2B through 10 and is present in fetal and adult erythropoietic tissues. Since the start codon is located in exon 2B, which is common to both the housekeeping and erythroid UROS messages, the enzymes encoded by both promoters are identical.
Fig 4.
Organization of the UROS gene. The UROS gene has unique housekeeping (PH) and erythroid-specific (PE) promoters. The dotted lines indicate the exons transcribed by each promoter. However, both promoters encode the same enzyme polypeptide.
Expression studies revealed ubiquitous presence of the housekeeping form, although at relatively low levels. This can be explained by the absence of TATA and SP1 sites needed for initiation of transcriptional activity in the housekeeping-specific promoter region upstream of exon 1. The erythroid-specific promoter upstream of exon 2A contains erythroid transcription factor binding sites including GATA1 and CP2 and high levels of erythroid expression of UROS were detected in fetal liver, bone marrow, and fetal spleen [20–22].
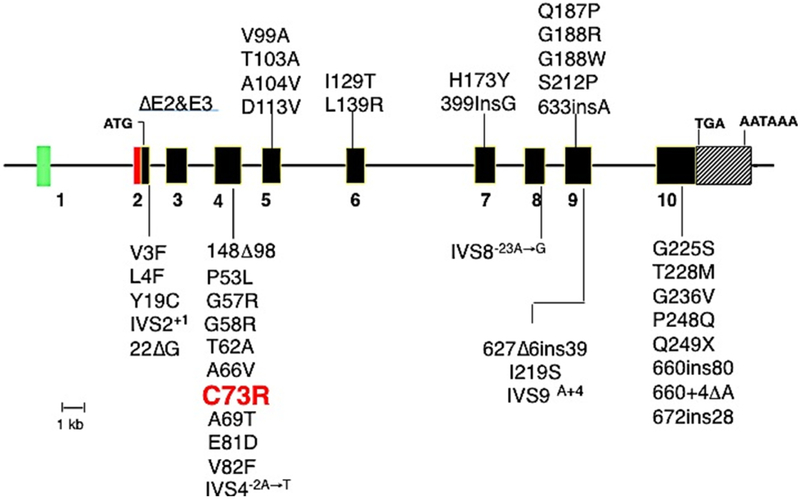
As of July 2018, 51 pathogenic UROS mutations have been listed in the Human Gene Mutation Database (HGMD, http://www.hgmd.cf.ac.uk/ac/index.php)[23][Fig 5]. Most (59%) of these mutations are missense variants (n=30), followed in frequency by regulatory mutations (n=6; 12%) and mutations affecting splice sites (n=4; 8%). In addition, rare nonsense mutations, small and large insertions as well as deletions, and more complex rearrangements have been reported.
Fig 5.
Molecular lesions in the UROS gene causing CEP. Coding exons are shown as solid black rectangles. ATG is the initiation of translation codon of the housekeeping and erythroid transcripts. Missense and nonsense mutations are indicated by the one-letter amino acid code and codon position, e.g. Q155X = glutamine codon (Q) at position 155 replaced by a termination codon (X). Deletions are indicated by Δ and insertions by ins. Note that the mutation encoding C73R is the most common lesion found in ~30–35% of reported patients [10].
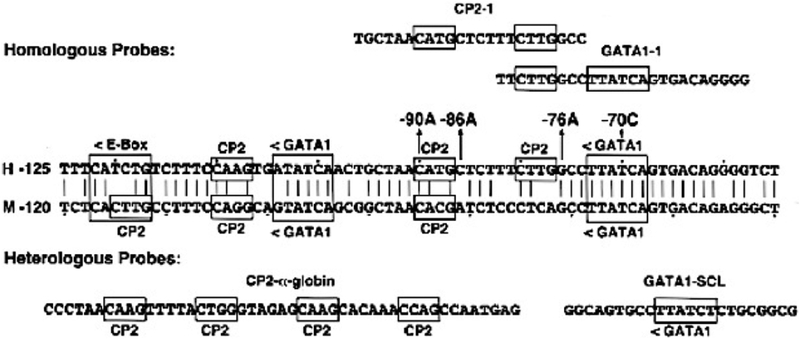
The most common UROS mutation is the missense mutation c.217C>T (p.C73R) [8, 10, 24], which was present in ~30% of disease-causing alleles in a series of 40 patients [12]. Of note, this mutation does not occur at a CpG dinucleotide, a known hotspot for mutations [25] nor was it a founder mutation as most probands were not related. The p.C73R enzyme was shown to have markedly decreased enzymatic stability (<1% of wild-type) due to its unfolding, instability, and rapid degradation [26, 27]. Other missense mutations such as those encoding p.S47P, p.V99A, and p.T228M were found to be more stable with higher enzyme activity [28, 29]. Among the regulatory mutations, four particular variants were found clustered in a 20 bp region of the erythroid-specific promoter [22] [Fig 6]. Two of those mutations, −70T>C and −90C>A, are located in a putative GATA-1 consensus binding site and in a putative CP2 binding motif, respectively, which leads to significant impairment of erythroid-specific heme biosynthesis. The other two erythroid-specific promoter mutations, −76G>A and −86C>A, are not directly interfering with the transcriptional binding elements, and therefore, have less of a deleterious effect [22].
Fig 6.
Partial sequence of the human and murine UROS erythroid-specific promoters showing the GATA1 and CP2 transcription factor binding sites. The location and orientation (<, >) of the GATA1, E-box, and CP2 erythroid binding elements are indicated, as are the four novel promoter mutations causing CEP (−70C, −76A, −86A and −90A). Dots are placed every tenth nucleotide. The homologous CP2 site from the α-globin gene promoter and the GATA1 site from the human stem cell leukemia (SCL) gene promoter are shown.
GATA1 Gene
The GATA1 gene encodes the erythroid-specific transcription factor GATA1, which plays a critical role in the normal development of hematopoietic cell lineages [30]. As such, GATA1 regulates the expression of numerous erythroid-specific genes, such as erythropoietin receptor, α- and ß-globins, ALAS2, and UROS, during erythroid differentiation. GATA1 is located on chromosome Xp11.23 [31] and disorders caused by GATA1 mutations therefore are inherited as X-linked traits.
GATA1 is a zinc finger protein and consists of three functional domains: an N-terminal activation domain, an N-terminal zinc finger, and a C-terminal zinc finger. While the N-terminal zinc finger plays a crucial role in the formation of complexes with cofactors and stabilization of DNA binding [32], the C-terminal zinc finger is responsible for the recognition of the GATA consensus sequence and binding to DNA [33, 34].
Germline as well as somatic mutations in GATA1 are known to cause hematologic disorders. Somatic GATA1 mutations are associated mainly with acute megakaryoblastic anemia and a transient myeloproliferative disorder in children with trisomy 21 [35]. Germline mutations are less common and cause several different phenotypes such as thrombocytopenia with beta-thalassemia, thrombocytopenia with/without dyserythropoietic anemia, or anemia with/without neutropenia and thrombocytopenia. The disease-causing germline mutations cluster in the region that forms the protein’s N-terminal zinc finger and result in altered affinity of GATA1 for either its cofactor, Friend of GATA1 (FOG1), or with palindromic DNA GATA recognition sites [36]. Since there are two GATA1 sites in the UROS erythroid-specific promoter region, it is conceivable that mutations leading to an altered structure of the GATA1 N-terminal zinc finger could interfere with GATA1 binding and hence alter UROS expression [22].
So far, one GATA1 missense mutation encoding p.R216W was detected in three unrelated males who presented with the typical features of CEP as well as beta-thalassemia and thrombocytopenia [6, 7]. The highly conserved R216 residue is located in the N-terminal zinc finger and a different substitution at the same residue (R216Q) results in thalassemia with thrombocytopenia without any CEP symptoms [37].
3. Clinical Features
The age at onset and clinical severity of CEP are highly variable, ranging from non-immune hydrops fetalis due to severe hemolytic anemia in utero to milder, later-onset forms, which have only cutaneous lesions in adult life [38]. In most cases, photosensitivity develops in early infancy and one of the earliest signs is the presence of reddish urine in the diaper which fluoresces with a Woods lamp [Fig 3]. Due to the hematological complications and an increased risk of infection, overall life expectancy may be markedly diminished in more severely affected patients [10]. In addition, long-term damage, such as loss of digits and facial cartilage or contractures of the hands, can have a significant impact on patients’ quality of life, psychiatric well-being, and functional status with regard to daily life and ability to work.
Several cases of late-onset CEP have been reported in patients older than 50 years of age who presented with cutaneous CEP symptoms and increased porphyrin metabolite excretion, albeit at a lower level than in patients with the classic infantile-onset CEP presentation. In several cases, the occurrence of CEP symptoms was associated with the presence of myelodysplastic syndrome (MDS) and neither germline nor somatic mutations in UROS or GATA1 were detected [39–41]. However, low-level mosaicism in the bone marrow may not be picked up by sequencing methods, but may be sufficient to cause an accumulation of porphyrin metabolites resulting in an attenuated phenotype [39–41].
Recently, one 60 year-old male patient was described with late-onset cutaneous porphyria symptoms and a urinary porphyrin metabolite pattern consistent with CEP. He was heterozygous for the common UROS mutation encoding p.C73R, but a second mutation was not detected. He did not have MDS or another underlying hematologic abnormality. While the exact pathophysiology was unclear in the absence of a second disease-causing mutation, acquired mosaicism in the bone marrow affecting the UROS gene was hypothesized to be the underlying cause [42].
3.1. Dermatologic Involvement
Cutaneous photosensitivity usually begins shortly after birth and is characterized by increased friability and blistering of the epidermis on the hands and face and other sun-exposed areas. Bullae and vesicles containing serous fluid which fluoresces due to their porphyrin content. Blisters are prone to rupture and become infected. Recurrent vesicle formation and secondary infection can lead to cutaneous scarring and deformities, as well as to loss of digits and facial features such as the eyelids, nose and ears [Fig 7]. The skin may be thickened, with areas of hypo- and hyper-pigmentation and hypertrichosis of the face and extremities [43]. Adult-onset patients have milder clinical symptoms and often exhibit only the skin manifestations of the disease [44–49]. Photosensitivity symptoms are provoked mainly by visible light (400–410 nm Soret wavelength) and to a lesser degree by wavelengths in the long-wave UV region. Affected individuals are also sensitive to sunlight that passes through window glass that does not filter long-wave UVA or visible light as well as to light from artificial light sources [50].
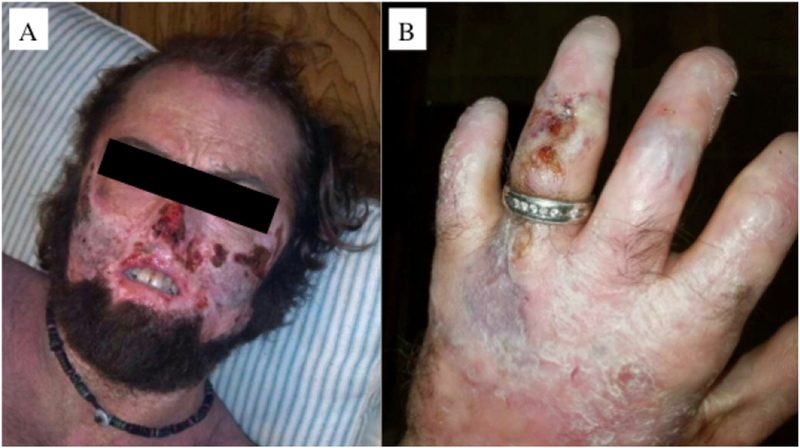
Fig 7.
Male 34 year-old CEP patient with severe cutaneous manifestations of face (left) and hand (right). Note the extensive skin erosions in the face, destruction of the nasal cartilage, sclerodermoid changes of the mouth, and erythrodontia. The patient also has significant shortening of the digits due to photomutilation and contractures of the joints, along with erosions and scleroderma-like thickening of the skin.
3.2. Hematological Involvement
Mild to severe hemolysis is accompanied by anisocytosis, poikilocytosis, polychromasia, basophilic stippling, reticulocytosis, increased nucleated red cells, absence of haptoglobin, increased unconjugated bilirubin and increased fecal urobilinogen. Plasma iron turnover also is increased [51]. Hemolysis presumably results from the accumulated uroporphyrin I in erythrocytes. Secondary splenomegaly develops in response to the increased uptake of abnormal erythrocytes from the circulation, which may contribute to the anemia and also may result in leucopenia and thrombocytopenia. The latter is sometimes associated with significant bleeding and splenectomy may be beneficial in such cases. Anemia due to hemolysis can be severe. Erythroid hyperplasia and markedly ineffective erythropoiesis usually accompany hemolytic anemia in transfusion-dependent patients [18, 47, 52].
In the three male patients with CEP due to GATA1 mutations, hematologic abnormalities including dyserythropoietic anemia, ß-thalassemia intermedia, thrombocytopenia, and hereditary persistence of fetal hemoglobin have been described [6, 7].
3.3. Other Clinical Features
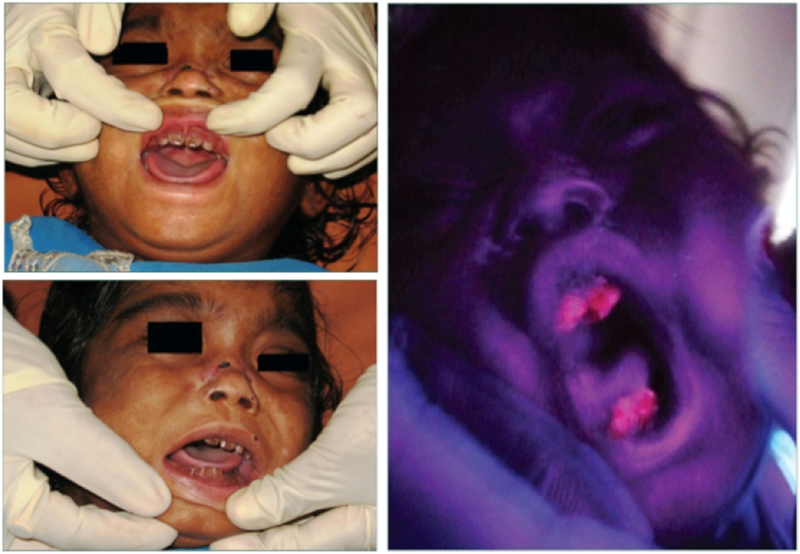
Deposition of porphyrins can cause corneal ulcers and scarring, which can ultimately can lead to blindness. Other ocular manifestations can include scleral necrosis, necrotizing scleritis, seborrheic blepharitis, keratoconjunctivitis, sclerokeratitis, and ectropion [53–55]. Porphyrins deposited in the teeth produce a reddish-brown color, termed erythrodontia. The teeth may fluoresce on exposure to long-wave ultraviolet light [Fig 8].
Fig 8.
Photographs showing the brownish color of the teeth on an infant with CEP in daylight (left) and fluorescing when illuminated with ultraviolet light from a Woods lamp (right) [96].
Deposition of porphyrins in bone causes osteopenia due to demineralization [14, 18, 40, 56]. The risk for osteopenia and osteoporosis is further increased by vitamin D deficiency, which individuals with CEP are prone to due to avoidance of sun exposure. Porphyrin accumulation in the bone can also cause expansion of the bone marrow, which can lead to hyperplastic bone marrow observed on biopsy [1, 43].
4. Diagnosis
The diagnosis of CEP may be suspected in utero, at birth, in infancy or childhood, or even in adulthood. CEP should be considered in the differential diagnosis of non-immune hydrops fetalis, in which case the amniotic fluid surrounding the fetus will be pink, dark-red or brown, and should examined for porphyrins [57]. Neonates with pink to dark-red urine-stained diapers [Fig 3] should immediately have diagnostic studies. CEP also should be considered in children or adults who have porphyrinuria or skin blistering following exposure to sunlight or other sources of long-wave ultraviolet light. In some cases, the disease is less severe and presents in adult life with mild anemia and/or skin lesions.
The biochemical diagnosis of CEP is established by detection of markedly elevated levels of uroporphyrin I and coproporphyrin I in urine, erythrocytes, or amniotic fluid as well as high fecal coproporphyrin I concentrations. Once the diagnosis of CEP is made or suspected based on quantitative porphyrin analyses, sequencing of the UROS gene should be performed to confirm the diagnosis. Knowing the disease-causing mutations permits genotype-phenotype correlations and provides information with respect to expected disease severity, which in turn may help guide management decisions [10].
5. Genotype-Phenotype Correlation
A number of factors are responsible for the phenotypic variability among CEP patients, most importantly the amount of residual UROS activity, which depends on a patient’s specific UROS mutations. When correlating genotype with clinical manifestations, it becomes apparent that mutations with absent or very low residual enzyme activity tend to have a more severe clinical picture with increasing degrees of hemolytic anemia, organomegaly, osteopenia, and cutaneous involvement [10, 58].
In an effort to determine the effect of different UROS mutations on residual enzyme activity in vitro, prokaryotic expression and gene promoter–reporter systems with wild-type and mutant UROS constructs have been used [Table 1]. For different missense mutations, the residual enzyme activity ranged from essentially non-detectable to about 35% of the mean activity expressed by the wild-type allele [10, 38, 59–65].
Table 1.
Expression of UROS Mutations in E. Coli
| Mutation | % of Mean Normal Activity | Reference Number |
|---|---|---|
| V3F | <1.0 | 58 |
| L4F | 1.8 | 61 |
| Y19C | 1.1 | 61 |
| P53L | <1.0 | 37 |
| T62A | <1.0 | 37 |
| A66V | 14.5 | 37 |
| A69T | 1.4 | 59 |
| C73R | <1.0 | 37,9 |
| E81D | 30 | 59 |
| V82F | 35.8 | 61 |
| V99A | 5.6 | 61 |
| A104V | 7.7 | 61 |
| H173Y | <1.0 | 62 |
| Q187P | <1.0 | 62 |
| G188R | 4.3 | 60 |
| G188W | 1.7 | 59 |
| 633insA | 1.2 | 61 |
| S212P | <1.0 | 63 |
| I219S | 1.3 | 59 |
| G225S | 1.2 | 61 |
| T228M | <1.0 | 37 |
| P248Q | <1.0 | 62 |
| Q249X | 1.1 | 61 |
| 148del98 | <2.0 | 64 |
| 660ins80 | <2.0 | 64 |
The most common UROS mutation which encodes p.C73R, exhibited < 1% of the enzyme activity expressed by the wild-type allele [62][Table 2]. This finding is reflected in the clinical presentation of individuals homozygous for this mutation, who present with the most severe phenotype including non-immune hydrops fetalis and/or transfusion dependency from birth. Patients heteroallelic for the mutation encoding p.C73R and a mutation that expressed little residual activity, such as the lesion encoding p.A69T, also resulted in a severe or moderately severe phenotype. Patients heteroallelic for mutations that expressed higher residual activities such as the mutations encoding p.V82F (35% of normal activity), p.A104V (7.7% of normal activity) and p.A66V (14.5% of normal activity) had milder forms of CEP, even if the allelic mutation encoded p.C73R or did not express detectable activity (e.g., nonsense and frameshift mutations) [10, 60]. For example, a teenage boy who was biallelic for mutations encoding p.C73R and p.A66V, only had mild cutaneous involvement [58].
Table 2.
Congenital Erythropoietic Porphyria: Types of UROS Mutations
| Mutation Types | Number | % of Total |
|---|---|---|
| Missense | 30 | 58.8 |
| Nonsense | 1 | 2.0 |
| Splicing | 4 | 7.8 |
| Frameshift: | ||
| Small Deletions | 2 | 3.9 |
| Large Deletions | 2 | 3.9 |
| Small Insertions | 2 | 3.9 |
| Small Indels | 1 | 2.0 |
| Large Insertions/Duplications | 2 | 3.9 |
| Complex Rearrangements | 1 | 2 |
| Regulatory | 6 | 11.8 |
| Total | 51 | 100 % |
Human Gene Mutation Database 2018.2: www.hgmd.org, [21]
The effect of the four erythroid promoter mutations on transcription was also assessed in vitro using a luciferase assay [22][Fig 6]. Results revealed approximately 8% residual enzyme activity of two of the promoter mutations (−70C, −90A) and 43% residual activity of the other two promoter mutations (−86A, −76A) when compared to wild-type. This difference is explained by the observation that −70C and −90A directly alter GATA1 and CP2 binding, respectively, while the −86A and −76A mutations do not interfere with these transcriptional binding elements and therefore have less effect on erythroid-specific heme biosynthesis. Genotype–phenotype correlations for CEP probands with erythroid promoter mutations revealed that a proband with a promoter mutation that resulted in low activity (−70C) on one allele and the mutation encoding p.C73R on the other allele had severe non-immune hydrops fetalis. In contrast, another proband who was heterozygous for the mutation encoding p.C73R and a promoter mutation with more activity (−76A) had a mild cutaneous disease phenotype [22].
In addition to residual enzyme activity, the CEP phenotype is also likely influenced by variants in other genes of the heme biosynthesis pathway [66–68]. It was found that the presence of an exon 11 gain-of-function mutation in the erythroid-specific ALAS2 gene, which can independently cause X-linked protoporphyria (XLP), can significantly increase disease severity. A female patient with CEP due to compound heterozygous UROS mutations encoding p.C73R and p.R248Q had a much more severe phenotype than her three affected siblings. She was found to carry a heterozygous ALAS2 exon 11 gain-of-function mutation in addition to the two UROS mutations. The ALAS2 mutation was not detected in her siblings. This likely explains the more severe disease manifestations in her due to increased erythroid heme synthesis, which in turn leads to excess erythroid protoporphyrins and hence symptoms of XLP [69]. As additional mutations are identified and expressed, more information will become available to further establish genotype/phenotype correlations.
6. Management
At this time, the only curative treatment approach for CEP is BMT or HSCT, which is associated with significant morbidity and mortality. The indication for transplantation, therefore, needs to be considered carefully, weighing the risks of the procedure against the risks associated with potential complications of CEP. Therefore, knowing the patient’s UROS genotype is helpful to predict disease severity, particularly when the mutation has been expressed in vitro. If the patient is not transplanted, the most important aspect of disease management is the avoidance of sun/light exposure.
6.1. Hematopoietic Stem Cell Transplantation
BMT or HSCT is the only curative approach for CEP which have been performed successfully. When successful, BMT and HSCT have resulted in marked reduction in porphyrin levels to normal if fully engrafted and patients have not experienced cutaneous blistering after sun exposure without protection [61, 70–78].
6.2. Sun Avoidance and Skin Protection
In patients who have not undergone BMT or HSCT, exposure to sunlight, ultraviolet light as well as light emitted by fluorescent sources, should be avoided. Sunscreen lotions containing zinc oxide or titanium oxide can be beneficial, but do not replace strict avoidance of sun and light exposure [79]. Most individuals with CEP try to adjust their daily life in order to reduce light exposure as much as possible, which in some cases includes choosing a profession which allows work during the night. This leads to a very restricted family and social life and quality of life is often significantly decreased.
Skin trauma should be avoided and the use of skin antiseptics should be encouraged to prevent bacterial superinfections that can complicate cutaneous blisters and result in scarring and mutilation. Further measures in an effort to minimize bacterial colonization if affected areas and decrease the risk for cutaneous complications could include bleach baths as well as topical treatment with hypochlorous acid solution [80]. Severe infections such as cellulitis and bacteremia may have to be treated with intravenous antibiotics and long-term oral antibiotic therapy to suppress chronic skin infections may be required.
Pharmacologic photoprotection has been explored in erythropoietic protoporphyria (EPP), which is the third most common porphyria [81]. While beta-carotene has been reported to provide some photoprotective effect in EPP patients, it has not proven to be beneficial in CEP [81, 82]. Recently, afamelanotide (Scenesse®) – a synthetic melanocortin stimulating hormone analog - was approved for EPP patients by the European Medicines Agency in 2014. This hormone is implanted subcutaneously and promotes melanin synthesis via the melanocortin-1 receptor. Beneficial effects have been reported in clinical trials with EPP patients [83], however its use in CEP patients has not been investigated thus far and it is unclear if the CEP cutaneous complications can be prevented. One recent case report describes an adult patient with severe CEP who underwent treatment with afamelanotide over a period of one year. While improved tolerance of sun exposure was reported by the individual, no protective effect was observed in already scarred areas of hands and face [84].
Newborns with a known diagnosis of CEP who develop hyperbilirubinemia should not receive photodynamic therapy to avoid its harmful photosensitizing effects and subsequent severe cutaneous blistering and scarring [19].
6.3. Bone Marrow Suppression
In cases of severe hemolysis, frequent blood transfusions may be necessary. Chronic transfusions (every 2–4 weeks) can suppress erythropoiesis and and decrease porphyrin production, which reduces porphyrin levels and photosensitivity [14]. Such therapy is likely to be successful if the hematocrit remains above 32% and parenteral or oral chelators can be administered to reduce the resulting iron overload [85]. Treatment with hydroxyurea to reduce the bone marrow porphyrin synthesis has been used in some severe CEP cases and may be considered [86]. Splenectomy may be considered for patients with splenomegaly and hemolytic anemia, thrombocytopenia and leukopenia. Removing the spleen may correct blood dyscrasia, improve the red blood cell lifespan and substantially reduce transfusion requirements in some patients. It may indirectly also lead to reduced photosensitivity [14].
Efforts to reduce porphyrin levels by administration of hydroxychloroquine, by plasmapheresis, and by intravenous hematin have not shown a clear benefit. Oral charcoal may increase fecal loss of porphyrins [87] and may be considered for patients who are not transfusion-dependent and have milder disease. It seems less successful in more severe cases [88, 89]. Iron deficiency induced by treatment with deferasirox led to better erythroid differentiation in the bone marrow and improved hemolysis as well as photosensitivity in one patient [90].
6.4. Treatment of Other Manifestations
To avoid ocular complications and damage to the eyelids, wrap-around sun glasses should be worn. Corneal ulcers, scleritis, and blepharitis should be treated with topical antibiotics. In patients with ectropion, corrective surgery of the eyelid to help protect the cornea from injury may be indicated [76]. To avoid demineralization of the bones, vitamin D supplementation is indicated and bisphosphonates can be considered in individuals with osteoporosis [76].
7. Conclusion and Future Therapeutic Prospects
CEP is an inborn error of heme biosynthesis characterized by the deficient activity of UROS, which leads to massive porphyrin I isomer accumulation in erythroid cells, and causes chronic hemolysis and severe cutaneous photosensitivity. The clinical presentation is heterogeneous and disease severity is associated with the amount of residual UROS activity. Treatment is mainly symptomatic and consists of diligent light protection and erythrocyte transfusions for the hemolytic anemia. BMT or HSCT is the only curative approach for CEP, but it is associated with morbidity and mortality, and is therefore, currently performed primarily in severe transfusion-dependent patients. Other treatment modalities have been or are currently being investigated, but to date, no clinical trials have been performed in humans.
Gene therapy in a murine CEP model (Urosmut248/mut248) using genetically modified hematopoietic stem cells (HSC) showed correction of enzymatic, metabolic, and phenotypic improvements in CEP mice as well as improved survival of the corrected erythrocytes [91]. To circumvent some of the difficulties of HSCT gene therapy, induced pluripotent stem cells (iPSC), derived from a CEP patient’s keratinocytes, were corrected by lentiviral transduction of a therapeutic vector containing the UROS cDNA. Corrected iPSC clones free of potentially oncogenic reprogramming genes were obtained and erythroid cells derived from these corrected iPSC cells showed correction of the UROS defect. However, no clinical trials have been performed in humans [92].
Recently, another approach has been investigated which attempts to increase the activity of UROS mutant enzymes encoded by missense mutations. Altered protein folding has been shown to lead to thermodynamic instability and premature degradation of via the proteasome [27, 93]. The use of proteasome inhibitors in UROS-deficient mice (Urosmut248/mut248) resulted in a partial metabolic correction with a significant decrease in porphyrin accumulation and improvement of cutaneous photosensitivity. However, the lack of improvement of the hematologic phenotype and potential neurotoxicity at higher doses were limiting factors of this approach [94]. In vitro studies aiming at stabilizing the UROS mutation at the p.C73 hotspot have restored catalytic activity as well as improved kinetic stability of the enzyme [27]. These observations suggest that pharmacologic and molecular approaches such as gene therapy or chaperone-based enzyme stabilization may be viable future therapeutic options.
While promising treatment approaches are under investigation, improved understanding of the underlying pathophysiology of CEP on a molecular and biochemical level is necessary in order to optimize current therapeutic modalities, establish new treatment concepts, and advance the field so that more therapeutic options become available to CEP patients in the near future.
Table 3.
Genotype/Phenotype Correlations in Congenital Erythropoietic Porphyria:
| Phenotype | Genotype | Residual Activity Expressed in E.coli Alleles/Total |
|
|---|---|---|---|
| Hydrops Fetalis/Newborn Demise: | |||
| C73R/C73R | <0.l / <0.l | = <0.1 | |
| Transfusion Dependent: | |||
| Severe | C73R/T228M | <0.1 / <0.1 | = <0.1 |
| Transfusion Independent: | |||
| Moderate | T62A/E249X | <0.1 / 1.8 | = 1.8 |
| G225S/T228L | 2.1 / <0.1 | = 2.1 | |
| L4F/C73R | 2.9 / <0.1 | = 2.9 | |
| L4F/Deletion | 2.9 / 0 | = 2.9 | |
| L4F/IVS2+1 | 2.9 / ? | = 2.9 | |
| Mild | Y19C/G225S | 2.7 / 2.1 | = 4.8 |
| V99A/Ins211A | 3.7 / 1.7 | = 5.4 | |
| C73R/A104V | <1.0 / 5.6 | = 5.6 | |
| A66V/C73R | 14.5 / <0.1 | = 14.5 | |
| L4F/V82F | 2.9 / 24.2 | = 27.1 | |
Desnick, RJ & Astrin, [10]
Acknowledgements:
This work was supported in part by the Porphyrias Consortium (U54DK083909), which is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through collaboration between NCATS and the NIDDK. This work was also supported in part by the Department of Genetics and Genomic Sciences of the Icahn School of Medicine at Mount Sinai.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors report no relevant conflicts
Contributor Information
Angelika L. Erwin, Email: Angelika.Erwin@gmail.com.
Robert J. Desnick, Email: Robert.Desnick@mssm.edu.
Literature:
- [1].Anderson KE, Sassa S, Bishop DF, Desnick RJ, Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyrias In: Metabolic The and Molecular Bases of Inherited Disease, 8th (ed), Scriver CR, Beaudet AL, Sly WS and Valle D, (eds), New York (NY), McGraw-Hill, (2014) 2961–3062. http://ommbid.mhmedical.com/content.aspx?bookid=971&Sectionid=62638866. [Google Scholar]
- [2].Puy H, Gouya L, Deybach JC, Porphyrias. Lancet 375 (2010) 924–937. [DOI] [PubMed] [Google Scholar]
- [3].Balwani M, Desnick RJ, The porphyrias: advances in diagnosis and treatment. Blood 120 (2012) 4496–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Romeo G, Levin EY, Uroporphyrinogen 3 cosynthetase in human congenital erythropoietic porphyria. Proc Natl Acad Sci U S A 63 (1969) 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bickers DR, Frank J, The Porphyrias. In: Fitzpatrick’s Dermatology in General Medicine, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K (eds), 8th (ed), McGraw-Hill, New York (NY), Chapter 132, (2012) 1679 https://accessmedicine.mhmedical.com/Content.aspx?bookId=392§ionId=41138853. [Google Scholar]
- [6].Phillips JD, Steensma DP, Pulsipher MA, Spangrude GJ, Kushner JP, Congenital erythropoietic porphyria due to a mutation in GATA1: the first trans-acting mutation causative for a human porphyria. Blood 109 (2007) 2618–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Di Pierro E, Russo R, Karakas Z, Brancaleoni V, Gambale A, Kurt I, Winter SS, Granata F, Czuchlewski DR, Langella C, Iolascon A, Cappellini MD, Congenital erythropoietic porphyria linked to GATA1-R216W mutation: challenges for diagnosis. Eur J Haematol 94 (2015) 491–497. [DOI] [PubMed] [Google Scholar]
- [8].Di Pierro E, Brancaleoni V, Granata F, Advances in understanding the pathogenesis of congenital erythropoietic porphyria. Br J Haematol 173 (2016) 365–379. [DOI] [PubMed] [Google Scholar]
- [9].Desnick RJ, Glass IA, Xu W, Solis C, Astrin KH, Molecular genetics of congenital erythropoietic porphyria. Semin Liver Dis 18 (1998) 77–84. [DOI] [PubMed] [Google Scholar]
- [10].Desnick RJ, Astrin KH, Congenital erythropoietic porphyria: advances in pathogenesis and treatment. Br J Haematol 117 (2002) 779–795. [DOI] [PubMed] [Google Scholar]
- [11].Schulenburg-Brand D, Katugampola R, Anstey AV, Badminton MN, The cutaneous porphyrias. Dermatol Clin 32 (2014) 369–384, ix. [DOI] [PubMed] [Google Scholar]
- [12].Katugampola RP, Badminton MN, Finlay AY, Whatley S, Woolf J, Mason N, Deybach JC, Puy H, Ged C, de Verneuil H, Hanneken S, Minder E, Schneider-Yin X, Anstey AV, Congenital erythropoietic porphyria: a single-observer clinical study of 29 cases. Br J Dermatol 167 (2012) 901–913. [DOI] [PubMed] [Google Scholar]
- [13].Erwin A, Balwani M, Desnick RJ, Congenital Erythropoietic Porphyria, in: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (Eds.), GeneReviews((R)), Seattle (WA), 2013. [Google Scholar]
- [14].Piomelli S, Poh-Fitzpatrick MB, Seaman C, Skolnick LM, Berdon WE, Complete suppression of the symptoms of congenital erythropoietic porphyria by long-term treatment with high-level transfusions. N Engl J Med 314 (1986) 1029–1031. [DOI] [PubMed] [Google Scholar]
- [15].Bensidhoum ML, Lemeur M, Dierich M, Costet A, Raymond P, Daniel S, De Verneuil JY, Ged CH, The Disruption of Mouse Uroporphyrinogen III Synthase (uros) Gene is Fully Lethal. Transgenics 2 (1998) 275–280. [Google Scholar]
- [16].Poh-Fitzpatrick MB, Porphyrin-sensitized cutaneous photosensitivity: pathogenesis and treatment. Clin Dermatol 3 (1985) 41–82. [DOI] [PubMed] [Google Scholar]
- [17].Shoolingin-Jordan PM, Porphobilinogen deaminase and uroporphyrinogen III synthase: structure, molecular biology, and mechanism. J Bioenerg Biomembr 27 (1995) 181–195. [DOI] [PubMed] [Google Scholar]
- [18].Fritsch C, Bolsen K, Ruzicka T, Goerz G, Congenital erythropoietic porphyria. J Am Acad Dermatol 36 (1997) 594–610. [DOI] [PubMed] [Google Scholar]
- [19].Verstraeten L, Van Regemorter N, Pardou A, de Verneuil H, Da Silva V, Rodesch F, Vermeylen D, Donner C, Noel JC, Nordmann Y, et al. , Biochemical diagnosis of a fatal case of Gunther’s disease in a newborn with hydrops foetalis. Eur J Clin Chem Clin Biochem 31 (1993) 121–128. [DOI] [PubMed] [Google Scholar]
- [20].Aizencang G, Solis C, Bishop DF, Warner C, Desnick RJ, Human uroporphyrinogen-III synthase: genomic organization, alternative promoters, and erythroid-specific expression. Genomics 70 (2000) 223–231. [DOI] [PubMed] [Google Scholar]
- [21].Aizencang GI, Bishop DF, Forrest D, Astrin KH, Desnick RJ, Uroporphyrinogen III synthase. An alternative promoter controls erythroid-specific expression in the murine gene. J Biol Chem 275 (2000) 2295–2304. [DOI] [PubMed] [Google Scholar]
- [22].Solis C, Aizencang GI, Astrin KH, Bishop DF, Desnick RJ, Uroporphyrinogen III synthase erythroid promoter mutations in adjacent GATA1 and CP2 elements cause congenital erythropoietic porphyria. J Clin Invest 107 (2001) 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stenson PD, Mort M, Ball EV, Evans K, Hayden M, Heywood S, Hussain M, Phillips AD, Cooper DN, The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet 136 (2017) 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Frank J, Wang X, Lam HM, Aita VM, Jugert FK, Goerz G, Merk HF, Poh-Fitzpatrick MB, Christiano AM, C73R is a hotspot mutation in the uroporphyrinogen III synthase gene in congenital erythropoietic porphyria. Ann Hum Genet 62 (1998) 225–230. [DOI] [PubMed] [Google Scholar]
- [25].Cooper DN, Youssoufian H, The CpG dinucleotide and human genetic disease. Hum Genet 78 (1988) 151–155. [DOI] [PubMed] [Google Scholar]
- [26].Fortian A, Castano D, Gonzalez E, Lain A, Falcon-Perez JM, Millet O, Structural, thermodynamic, and mechanistical studies in uroporphyrinogen III synthase: molecular basis of congenital erythropoietic porphyria. Adv Protein Chem Struct Biol 83 (2011) 43–74. [DOI] [PubMed] [Google Scholar]
- [27].ben Bdira F, Gonzalez E, Pluta P, Lain A, Sanz-Parra A, Falcon-Perez JM, Millet O, Tuning intracellular homeostasis of human uroporphyrinogen III synthase by enzyme engineering at a single hotspot of congenital erythropoietic porphyria. Hum Mol Genet 23 (2014) 5805–5813. [DOI] [PubMed] [Google Scholar]
- [28].Ged C, Megarbane H, Chouery E, Lalanne M, Megarbane A, de Verneuil H, Congenital erythropoietic porphyria: report of a novel mutation with absence of clinical manifestations in a homozygous mutant sibling. J Invest Dermatol 123 (2004) 589–591. [DOI] [PubMed] [Google Scholar]
- [29].Maakaron JE, Abdel Malak O, Itani S, Cappellini MD, Di Pierro E, Brancaleoni V, Granata F, Taher AT, A puzzling mutation in congenital erythropoietic porphyria and an association with beta-thalassaemia trait. Br J Dermatol 167 (2012) 697–699. [DOI] [PubMed] [Google Scholar]
- [30].Calligaris R, Bottardi S, Cogoi S, Apezteguia I, Santoro C, Alternative translation initiation site usage results in two functionally distinct forms of the GATA-1 transcription factor. Proc Natl Acad Sci U S A 92 (1995) 11598–11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Caiulo A, Nicolis S, Bianchi P, Zuffardi O, Bardoni B, Maraschio P, Ottolenghi S, Camerino G, Giglioni B, Mapping the gene encoding the human erythroid transcriptional factor NFE1-GF1 to Xp11.23. Hum Genet 86 (1991) 388–390. [DOI] [PubMed] [Google Scholar]
- [32].Ghirlando R, Trainor CD, GATA-1 bends DNA in a site-independent fashion. J Biol Chem 275 (2000) 28152–28156. [DOI] [PubMed] [Google Scholar]
- [33].Martin DI, Orkin SH, Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev 4 (1990) 1886–1898. [DOI] [PubMed] [Google Scholar]
- [34].Yang HY, Evans T, Distinct roles for the two cGATA-1 finger domains. Mol Cell Biol 12 (1992) 4562–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gurbuxani S, Vyas P, Crispino JD, Recent insights into the mechanisms of myeloid leukemogenesis in Down syndrome. Blood 103 (2004) 399–406. [DOI] [PubMed] [Google Scholar]
- [36].Cantor AB, GATA transcription factors in hematologic disease. Int J Hematol 81 (2005) 378–384. [DOI] [PubMed] [Google Scholar]
- [37].Campbell AE, Wilkinson-White L, Mackay JP, Matthews JM, Blobel GA, Analysis of disease-causing GATA1 mutations in murine gene complementation systems. Blood 121 (2013) 5218–5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Warner CA, Yoo HW, Roberts AG, Desnick RJ, Congenital erythropoietic porphyria: identification and expression of exonic mutations in the uroporphyrinogen III synthase gene. J Clin Invest 89 (1992) 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sarkany RP, Ibbotson SH, Whatley SD, Lawrence CM, Gover P, Mufti GJ, Murphy GM, Masters GS, Badminton MN, Elder GH, Erythropoietic uroporphyria associated with myeloid malignancy is likely distinct from autosomal recessive congenital erythropoietic porphyria. J Invest Dermatol 131 (2011) 1172–1175. [DOI] [PubMed] [Google Scholar]
- [40].Kontos AP, Ozog D, Bichakjian C, Lim HW, Congenital erythropoietic porphyria associated with myelodysplasia presenting in a 72-year-old man: report of a case and review of the literature. Br J Dermatol 148 (2003) 160–164. [DOI] [PubMed] [Google Scholar]
- [41].Cernik C, Haller N, Mostow EN, Adult-onset erythropoietic porphyria in the setting of MDS. Arch Dermatol 145 (2009) 948–949. [DOI] [PubMed] [Google Scholar]
- [42].Aguilera P, Badenas C, Whatley SD, To-Figueras J, Late-onset cutaneous porphyria in a patient heterozygous for a uroporphyrinogen III synthase gene mutation. Br J Dermatol 175 (2016) 1346–1350. [DOI] [PubMed] [Google Scholar]
- [43].Poh-Fitzpatrick MB, The erythropoietic porphyrias. Dermatol Clin 4 (1986) 291–296. [PubMed] [Google Scholar]
- [44].Fityan A, Fassihi H, Sarkany R, Congenital erythropoietic porphyria: mild presentation with late onset associated with a mutation in the UROS gene promoter sequence. Clin Exp Dermatol 41 (2016) 953–954. [DOI] [PubMed] [Google Scholar]
- [45].Deybach JC, de Verneuil H, Phung N, Nordmann Y, Puissant A, Boffety B, Congenital erythropoietic porphyria (Gunther’s disease): enzymatic studies on two cases of late onset. J Lab Clin Med 97 (1981) 551–558. [PubMed] [Google Scholar]
- [46].Murphy A, Gibson G, Elder GH, Otridge BA, Murphy GM, Adult-onset congenital erythropoietic porphyria (Gunther’s disease) presenting with thrombocytopenia. J R Soc Med 88 (1995) 357P–358P. [PMC free article] [PubMed] [Google Scholar]
- [47].Pain RW, Welch FW, Woodroffe AJ, Handley DA, Lockwood WH, Erythropoietic uroporphyria of Gunther first presenting at 58 years with positive family studies. Br Med J 3 (1975) 621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kramer S, Viljoen E, Meyer AM, Metz J, The anaemia of erythropoietic prophyria with the description of the disease in an elderly patient. Br J Haematol 11 (1965) 666–675. [DOI] [PubMed] [Google Scholar]
- [49].Rao SU, Dar NR, Abbas M, Mumtaz J, Late onset erythropoietic porphyria (Gunther’s disease). J Coll Physicians Surg Pak 21 (2011) 564–566. [PubMed] [Google Scholar]
- [50].Fritsch C, Lang K, Bolsen K, Lehmann P, Ruzicka T, Congenital erythropoietic porphyria. Skin Pharmacol Appl Skin Physiol 11 (1998) 347–357. [DOI] [PubMed] [Google Scholar]
- [51].Schmid R, Schwartz S, Sundberg RD, Erythropoietic (congenital) porphyria: A rare abnormality of the normoblasts. Blood 10 (1955) 416–428. [PubMed] [Google Scholar]
- [52].Weston MJ, Nicholson DC, Lim CK, Clark KG, Macdonald A, Henderson MA, Williams R, Congenital erythropoietic uroporphyria (Gunther’s disease) presenting in a middle aged man. Int J Biochem 9 (1978) 921–926. [DOI] [PubMed] [Google Scholar]
- [53].Venkatesh P, Garg SP, Kumaran E, Tewari HK, Congenital porphyria with necrotizing scleritis in a 9-year-old child. Clin Exp Ophthalmol 28 (2000) 314–318. [DOI] [PubMed] [Google Scholar]
- [54].Siddique SS, Gonzalez-Gonzalez LA, Thakuria P, Chang PY, Foster CS, Scleral necrosis in a patient with congenital erythropoietic porphyria. Cornea 30 (2011) 97–99. [DOI] [PubMed] [Google Scholar]
- [55].Oguz F, Sidal M, Bayram C, Sansoy N, Hekim N, Ocular involvement in two symptomatic congenital erythropoietic porphyria. Eur J Pediatr 152 (1993) 671–673. [DOI] [PubMed] [Google Scholar]
- [56].Laorr A, Greenspan A, Severe osteopenia in congenital erythropoietic porphyria. Can Assoc Radiol J 45 (1994) 307–309. [PubMed] [Google Scholar]
- [57].Daikha-Dahmane F, Dommergues M, Narcy F, Gubler MC, Dumez Y, Gauthier E, Nordmann Y, Nessmann C, Terrasse G, Muller F, Congenital erythropoietic porphyria: prenatal diagnosis and autopsy findings in two sibling fetuses. Pediatr Dev Pathol 4 (2001) 180–184. [DOI] [PubMed] [Google Scholar]
- [58].Warner CA, Poh-Fitzpatrick MB, Zaider EF, Tsai SF, Desnick RJ, Congenital erythropoietic porphyria. A mild variant with low uroporphyrin I levels due to a missense mutation (A66V) encoding residual uroporphyrinogen III synthase activity. Arch Dermatol 128 (1992) 1243–1248. [DOI] [PubMed] [Google Scholar]
- [59].Takamura N, Hombrados I, Tanigawa K, Namba H, Nagayama Y, de Verneuil H, Yamashita S, Novel point mutation in the uroporphyrinogen III synthase gene causes congenital erythropoietic porphyria of a Japanese family. Am J Med Genet 70 (1997) 299–302. [DOI] [PubMed] [Google Scholar]
- [60].Shady AA, Colby BR, Cunha LF, Astrin KH, Bishop DF, Desnick RJ, Congenital erythropoietic porphyria: identification and expression of eight novel mutations in the uroporphyrinogen III synthase gene. Br J Haematol 117 (2002) 980–987. [DOI] [PubMed] [Google Scholar]
- [61].Tezcan I, Xu W, Gurgey A, Tuncer M, Cetin M, Oner C, Yetgin S, Ersoy F, Aizencang G, Astrin KH, Desnick RJ, Congenital erythropoietic porphyria successfully treated by allogeneic bone marrow transplantation. Blood 92 (1998) 4053–4058. [PubMed] [Google Scholar]
- [62].Xu W, Warner CA, Desnick RJ, Congenital erythropoietic porphyria: identification and expression of 10 mutations in the uroporphyrinogen III synthase gene. J Clin Invest 95 (1995) 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fontanellas A, Bensidhoum M, Enriquez de Salamanca R, Moruno Tirado A, de Verneuil H, Ged C, A systematic analysis of the mutations of the uroporphyrinogen III synthase gene in congenital erythropoietic porphyria. Eur J Hum Genet 4 (1996) 274–282. [DOI] [PubMed] [Google Scholar]
- [64].Tanigawa K, Bensidhoum M, Takamura N, Namba H, Yamashita S, de Verneuil H, Ged C, A novel point mutation in congenital erythropoietic porphyria in two members of Japanese family. Hum Genet 97 (1996) 557–560. [DOI] [PubMed] [Google Scholar]
- [65].Boulechfar S, Da Silva V, Deybach JC, Nordmann Y, Grandchamp B, de Verneuil H, Heterogeneity of mutations in the uroporphyrinogen III synthase gene in congenital erythropoietic porphyria. Hum Genet 88 (1992) 320–324. [DOI] [PubMed] [Google Scholar]
- [66].Whatley SD, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton MN, Elder GH, Holme SA, Anstey AV, Parker M, Corrigall AV, Meissner PN, Hift RJ, Marsden JT, Ma Y, Mieli-Vergani G, Deybach JC, Puy H, C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am J Hum Genet 83 (2008) 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Balwani M, Doheny D, Bishop DF, Nazarenko I, Yasuda M, Dailey HA, Anderson KE, Bissell DM, Bloomer J, Bonkovsky HL, Phillips JD, Liu L, Desnick RJ, Porphyrias N Consortium of the National Institutes of Health Rare Diseases Clinical Research, Loss-of-function ferrochelatase and gain-of-function erythroid-specific 5-aminolevulinate synthase mutations causing erythropoietic protoporphyria and x-linked protoporphyria in North American patients reveal novel mutations and a high prevalence of X-linked protoporphyria. Mol Med 19 (2013) 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bishop DF, Tchaikovskii V, Nazarenko I, Desnick RJ, Molecular expression and characterization of erythroid-specific 5-aminolevulinate synthase gain-of-function mutations causing X-linked protoporphyria. Mol Med 19 (2013) 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].To-Figueras J, Ducamp S, Clayton J, Badenas C, Delaby C, Ged C, Lyoumi S, Gouya L, de Verneuil H, Beaumont C, Ferreira GC, Deybach JC, Herrero C, Puy H, ALAS2 acts as a modifier gene in patients with congenital erythropoietic porphyria. Blood 118 (2011) 1443–1451. [DOI] [PubMed] [Google Scholar]
- [70].Thomas C, Ged C, Nordmann Y, de Verneuil H, Pellier I, Fischer A, Blanche S, Correction of congenital erythropoietic porphyria by bone marrow transplantation. J Pediatr 129 (1996) 453–456. [DOI] [PubMed] [Google Scholar]
- [71].Harada FA, Shwayder TA, Desnick RJ, Lim HW, Treatment of severe congenital erythropoietic porphyria by bone marrow transplantation. J Am Acad Dermatol 45 (2001) 279–282. [DOI] [PubMed] [Google Scholar]
- [72].Shaw PH, Mancini AJ, McConnell JP, Brown D, Kletzel M, Treatment of congenital erythropoietic porphyria in children by allogeneic stem cell transplantation: a case report and review of the literature. Bone Marrow Transplant 27 (2001) 101–105. [DOI] [PubMed] [Google Scholar]
- [73].Dupuis-Girod S, Akkari V, Ged C, Galambrun C, Kebaili K, Deybach JC, Claudy A, Geburher L, Philippe N, de Verneuil H, Bertrand Y, Successful match-unrelated donor bone marrow transplantation for congenital erythropoietic porphyria (Gunther disease). Eur J Pediatr 164 (2005) 104–107. [DOI] [PubMed] [Google Scholar]
- [74].Taibjee SM, Stevenson OE, Abdullah A, Tan CY, Darbyshire P, Moss C, Goodyear H, Heagerty A, Whatley S, Badminton MN, Allogeneic bone marrow transplantation in a 7-year-oldgirl with congenital erythropoietic porphyria: a treatment dilemma. Br J Dermatol 156 (2007) 567–571. [DOI] [PubMed] [Google Scholar]
- [75].Faraci M, Morreale G, Boeri E, Lanino E, Dallorso S, Dini G, Scuderi F, Cohen A, Cappelli B, Unrelated HSCT in an adolescent affected by congenital erythropoietic porphyria. Pediatr Transplant 12 (2008) 117–120. [DOI] [PubMed] [Google Scholar]
- [76].Katugampola RP, Anstey AV, Finlay AY, Whatley S, Woolf J, Mason N, Deybach JC, Puy H, Ged C, de Verneuil H, Hanneken S, Minder E, Schneider-Yin X, Badminton MN, A management algorithm for congenital erythropoietic porphyria derived from a study of 29 cases. Br J Dermatol 167 (2012) 888–900. [DOI] [PubMed] [Google Scholar]
- [77].Zix-Kieffer I, Langer B, Eyer D, Acar G, Racadot E, Schlaeder G, Oberlin F, Lutz P, Successful cord blood stem cell transplantation for congenital erythropoietic porphyria (Gunther’s disease). Bone Marrow Transplant 18 (1996) 217–220. [PubMed] [Google Scholar]
- [78].Kauffman L, Evans DI, Stevens RF, Weinkove C, Bone-marrow transplantation for congenital erythropoietic porphyria. Lancet 337 (1991) 1510–1511. [DOI] [PubMed] [Google Scholar]
- [79].Mathews-Roth MM, Carotenoids in erythropoietic protoporphyria and other photosensitivity diseases. Ann N Y Acad Sci 691 (1993) 127–138. [DOI] [PubMed] [Google Scholar]
- [80].Howard M, Hall A, Ramsay D, Beneficial use of a novel topical hypochlorous acid preparation for chronic dematoses at risk of secondary infection. Australas J Dermatol 57 (2016) 326–327. [DOI] [PubMed] [Google Scholar]
- [81].Balwani M, Bloomer J, Desnick R, Erythropoietic Protoporphyria, Autosomal Recessive, in: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (Eds.), GeneReviews((R)), Seattle (WA), 1993. [PubMed] [Google Scholar]
- [82].Badminton MN, Elder GH, Management of acute and cutaneous porphyrias. Int J Clin Pract 56 (2002) 272–278. [PubMed] [Google Scholar]
- [83].Langendonk JG, Balwani M, Anderson KE, Bonkovsky HL, Anstey AV, Bissell DM, Bloomer J, Edwards C, Neumann NJ, Parker C, Phillips JD, Lim HW, Hamzavi I, Deybach JC, Kauppinen R, Rhodes LE, Frank J, Murphy GM, Karstens FPJ, Sijbrands EJG, de Rooij FWM, Lebwohl M, Naik H, Goding CR, Wilson JHP, Desnick RJ, Afamelanotide for Erythropoietic Protoporphyria. N Engl J Med 373 (2015) 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Howard M, Hall A, Ramsay D, Congenital erythropoietic porphyria (Gunther disease) - long-term follow up of a case and review. Dermatol Online J 23 (2017). [PubMed] [Google Scholar]
- [85].Poh-Fitzpatrick MBP, S.; Seaman C; Skolnick LM, Congenital erythropoietic porphyria: complete suppression of symptoms by long-term high-level transfusion with deferoxamine infusion iron rescue, in: Orfanos RSHGCE (Ed.), Dermatology in Five Continents, Springer-Verlag, Berlin, 1988. [Google Scholar]
- [86].Guarini L, Piomelli S, Poh-Fitzpatrick MB, Hydroxyurea in congenital erythropoietic porphyria. N Engl J Med 330 (1994) 1091–1092. [DOI] [PubMed] [Google Scholar]
- [87].Tishler PV, Winston SH, Rapid improvement in the chemical pathology of congenital erythropoietic porphyria with treatment with superactivated charcoal. Methods Find Exp Clin Pharmacol 12 (1990) 645–648. [PubMed] [Google Scholar]
- [88].Minder EI, Schneider-Yin X, Moll F, Lack of effect of oral charcoal in congenital erythropoietic porphyria. N Engl J Med 330 (1994) 1092–1094. [DOI] [PubMed] [Google Scholar]
- [89].Gorchein A, Guo R, Lim CK, Raimundo A, Pullon HW, Bellingham AJ, Porphyrins in urine, plasma, erythrocytes, bile and faeces in a case of congenital erythropoietic porphyria (Gunther’s disease) treated with blood transfusion and iron chelation: lack of benefit from oral charcoal. Biomed Chromatogr 12 (1998) 350–356. [DOI] [PubMed] [Google Scholar]
- [90].Egan DN, Yang Z, Phillips J, Abkowitz JL, Inducing iron deficiency improves erythropoiesis and photosensitivity in congenital erythropoietic porphyria. Blood 126 (2015) 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Robert-Richard E, Moreau-Gaudry F, Lalanne M, Lamrissi-Garcia I, Cario-Andre M, Guyonnet-Duperat V, Taine L, Ged C, de Verneuil H, Effective gene therapy of mice with congenital erythropoietic porphyria is facilitated by a survival advantage of corrected erythroid cells. Am J Hum Genet 82 (2008) 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bedel A, Taillepierre M, Guyonnet-Duperat V, Lippert E, Dubus P, Dabernat S, Mautuit T, Cardinaud B, Pain C, Rousseau B, Lalanne M, Ged C, Duchartre Y, Richard E, de Verneuil H, Moreau-Gaudry F, Metabolic correction of congenital erythropoietic porphyria with iPSCs free of reprogramming factors. Am J Hum Genet 91 (2012) 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Blouin JM, Bernardo-Seisdedos G, Sasso E, Esteve J, Ged C, Lalanne M, Sanz-Parra A, Urquiza P, de Verneuil H, Millet O, Richard E, Missense UROS mutations causing congenital erythropoietic porphyria reduce UROS homeostasis that can be rescued by proteasome inhibition. Hum Mol Genet 26 (2017) 1565–1576. [DOI] [PubMed] [Google Scholar]
- [94].Blouin JM, Duchartre Y, Costet P, Lalanne M, Ged C, Lain A, Millet O, de Verneuil H, Richard E, Therapeutic potential of proteasome inhibitors in congenital erythropoietic porphyria. Proc Natl Acad Sci U S A 110 (2013) 18238–18243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Blasco Morente G, Martinez Peinado C, Martinez Garcia E, Tercedor Sanchez J, [Wood’s lamp in congenital erythropoietic porphyria]. An Pediatr (Barc) 81 (2014) 403–404. [DOI] [PubMed] [Google Scholar]
- [96].Bhavasar R, Santoshkumar G, Prakash BR, Erythrodontia in congenital erythropoietic porphyria. J Oral Maxillofac Pathol 15 (2011) 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]