Abstract
BACKGROUND:
Many prognostic factors influence overall survival (OS) of patients with glioblastoma. Despite gross total resection and Stupp protocol adherence, many patients have poor survival. Perfusion magnetic resonance imaging may assist in diagnosis, treatment monitoring, and prognostication.
METHODS:
This retrospective study of 36 patients with glioblastoma assessed influence of preoperative magnetic resonance imaging parameters reflecting tumor cell density and vascularity and patient age on OS.
RESULTS:
The area under curve based on optimal receiver operating characteristic curves for the perfusion parameters normalized relative tumor blood volume (n_rTBV) and normalized relative tumor blood flow (n_rTBF) were 0.92 and 0.89, respectively, and the highest among all imaging parameters and age. OS showed strongly negative correlations with corrected n_rTBV (R = −0.70; P < 0.001) and n_rTBF (R = −0.67; P < 0.001). The Cox model, which included age and imaging parameters, demonstrated that n_rTBV and n_rTBF were most predictive of OS, with hazard ratios of 5.97 (P = 0.0001) and 8.76 (P = 0.0001), respectively, compared with 1.63 (P = 0.19)for age. Eighteen patients with corrected n_rTBV ≤2.5 (best cutoff value) had a median OS of 15.1 months (95% confidence interval (CI), 11.34–21.25) compared with 2.8 months (95% CI, 1.48–4.03; P< 0.001) for 18 patients with corrected n_rTBV >2.5. Twenty-four patients with n_rTBF ≤2.79 had a median OS of 12 months (95% CI, 10.46–17.9) compared with 2.8 months for 12 patients with n_rTBF >2.79 (95% CI, 1.31–4.2; P < 0.001).
CONCLUSIONS:
The dominant predictors of OS are normalized perfusion parameters n_rTBV and n_rTBF. Preoperative perfusion imaging may be used as a surrogate to predict glioblastoma aggressiveness and survival independent of treatment.
Keywords: Anatomic MRI, Diffusion MRI, Glioblastoma, OS, Overall survival, Perfusion MRI, TBF, TBV, Tumor blood flow, Tumor blood volume
INTRODUCTION
Many prognostic factors, such as patient age and performance status, tumor vascularity, cell density, methylguanine-DNA methyltransferase (MGMT) methylation status, and extent of resection, influence the overall survival (OS) of patients with glioblastoma.1–6 OS of these patients has been linked with various magnetic resonance imaging (MRI) parameters, including perfusion parameters, diffusion parameters, and the combination of both.7–10 Perfusion parameters include relative cerebral blood volume (rCBV), relative cerebral blood flow (rCBF), and permeability (volume transfer constant), whereas diffusion parameters include mean or minimum apparent diffusion coefficient (ADC) values.
Dynamic susceptibility contrast (DSC) and dynamic contrast enhancement (DCE) are common modalities used in perfusion MRI for investigating vascularity of a tumor.11,12 Both methods rely on the administration of a contrast agent, such as gadolinium, and track the bolus so that the perfusion parameter maps can be obtained. Based on a nondiffusible tracer kinetic principle, the DSC method calculates values of rCBV and rCBF from a T2-weighted or a T2*-weighted signal decreasing time curve of each voxel.13 It has been shown that the tumor’s rCBV (i.e., relative tumor blood volume [rTBV]) is an indicator of tumor angiogenesis and a surrogate for tumor malignancy, especially for high-grade gliomas such as glioblastoma.14 The DCE method applies the model of Tofts and Kermode15 on a T1-weighted signal increasing time curve of each voxel to calculate the value of the volume transfer constant, which indicates the tumor’s vascular permeability. Studies employing the DCE method on brain tumors have shown that permeability of a tumor is closely correlated to the tumor grade.16,17 Additionally, tumor cell density can be measured with ADC, with increasing density corresponding with increased malignancy and lower ADC values.18,19 All 3 methods provide information regarding the biologic behavior of glioblastomas and consequently help predict the OS of patients with glioblastomas.
The preoperative diffusion, perfusion, and anatomic MRI parameters may be surrogates of malignant potential of a glioblastoma and may forecast the OS of these patients independently of the treatment received. However, each parameter may have different degrees of impact on OS. The primary objective of this study was to assess several perfusion, diffusion, and anatomic MRI parameters reflecting tumor cell density and vascularity and evaluate their impact on OS of patients with glioblastoma.
MATERIALS AND METHODS
Data Acquisition and Processing
We performed a retrospective study of MRI data of 36 patients with primary glioblastoma. The research protocol (#1611340464) was approved by the West Virginia University Institutional Review Board. Consecutive patients with appropriate preoperative MRI were taken from a glioblastoma database that includes patients from 2009 to the present. Only patients treated after 2015 had routine isocitrate dehydrogenase-1 or MGMT methylation testing performed. All patients were considered eligible regardless of whether they had surgery, biopsy, or any other criterion.
Patients were divided into an older (≥65 years old [mean 74 ± 5.42 years old]; n = 20) and a younger (<65 years old [mean 53.3 ± 11.99 years old]; n = 16) group. MRI was performed using a 1.5T and/or 3.0T MRI scanner (Siemens Healthcare, Erlangen, Germany) with 20 channel head coils. MRI data were acquired from June 2015 to April 2017 by using the following pulse sequences: T1 (before and after contrast administration) with repetition time/ echo time = 550 ms/8 ms; T2 (fluid attenuated inversion recovery [FLAIR]) with repetition time/inversion time/echo time = 2200 ms/1750 ms/80 ms; DSC, acquired by a gradient echo echo-planar imaging pulse sequence with repetition time/echo time = 1500 ms/40 ms; and either diffusion-weighted imaging using a spin echo echo-planar imaging sequence with 2 Stejskal-Tanner gradients and repetition time/echo time/b value = 7600 ms/110 m/ 0 or 1000, or diffusion tensor imaging with 25 gradient directions and repetition time/echo time = 8000 ms/108 ms.
The perfusion and diffusion data were analyzed offline using Olea Sphere software (Olea Medical, La Ciotat, France) with which the outcome parameters were defined. As vessel leakage generally occurs in glioblastoma, we used a corrected rTBV (rTBV_c) value obtained from the software to select a region of interest (ROI)—an area based on rTBV_c maximum average value in a slice of the tumor volume. The area with the maximum value (e.g., maximum blood volume) in a slice of the tumor was chosen as the ROI. This particular slice also had the maximum mean parameter value for the tumor from all the slices containing tumor. As such, only a portion of the tumor was measured (and values would vary depending on the ROI). To counteract this limitation, we obtained normalized values; the normalization of each parameter was done with the following equation: mean value in tumor ROI/ mean value in contralateral mirror area of tumor ROI. For example, the normalized corrected relative tumor blood volume (n_rTBV_c) was obtained by dividing the tumor side rTBV_c by the control (non-tumor) side rTBV_c. As another example, the FLAIR region within the tumor with the largest average maximum value was compared with the mirror region in the opposite hemisphere. If the contralateral, nontumor ROI was in a ventricle or vessel, a manually drawn ROI with the same size of the tumor ROI was placed in the adjacent white matter area. The same ROIs were applied to all perfusion, diffusion, and anatomic images to obtain the corresponding normalized parameter values. The various MRI parameters (measured by comparing the parameter averages and standard deviations between the tumor and nontumor hemispheres) were measured independently of one another. Adjustment for multiple comparisons was not used on P values in our data analysis, as this was a hypothesis-generating study.
Statistical Analyses
The statistical analyses included Pearson correlations, Kaplan-Meier survival, and receiver operating characteristic (ROC) curves. Correlations between MRI parameters were quantified using Pearson co-efficients. The Kaplan-Meier survival distribution was calculated for all MRI parameters, for which the optimal cutoff to define high and low was obtained from a regression tree analysis. The goal of this method was to create a model that predicts the survival outcomes based on the deviance and constructs a classification tree by the MRI parameters (high vs. low). A log-rank test was used to compare survival distributions between high and low groups. A Cox proportional hazards model was used to assess the combinatorial effects of each imaging parameter and age ≥65 years on the OS. The perfusion, diffusion, and anatomic parameters were compared between the tumor and nontumor hemispheres using a paired t test. ROC curves and their corollary, the AUC, were used to examine the ability of all independent variables (including age) to predict survival. χ2 test was used to assess for differences in MGMT status, extent of resection, and Stupp protocol adherence (i.e., temozolomide and concomitant radiotherapy) between older (≥65 years old) and younger (<65 years old) patients as well as between patients with high (n_rTBV_c ≥2.5) and low (n_rTBV_c <2.5) tumor blood volume. Postoperative MRI was performed generally within 48 hours of surgery and reviewed for extent of resection by the primary surgeon when applicable. iPlan software (Brainlab AG, Munich, Germany) was used to calculate volumes. Complete or >98% of tumor resection was considered total resection; <98% of tumor resection was considered subtotal resection.20 All statistical tests were 2-sided, and P < 0.05 was considered statistically significant. Patients were not stratified based on any known prognostic factor except age to see if perfusion was an independent predictor of survival.
RESULTS
There were no significant differences in MGMT status, extent of resection, and Stupp protocol adherence between older and younger (<65 years old) patients and between patients with high and low (n_rTBV_c <2.5, the best cutoff value) tumor blood volume (Tables 1 and 2). OS for all patients was 290 ± 272 days. OS for the older and the younger age groups was 178 ± 216 days and 420 ± 285 days, respectively. Representative MRI, including T1 postcontrast, T2 FLAIR, corrected rCBV, rCBF, ADC, and fractional anisotropy maps, are shown in Figure 1 and include images from a patient in the older age group (Figure 1A-F) and a patient in the younger age group (Figure 1G-L).
Table 1.
Comparison of Factors Impacting Suvival of Patients with Glioblastoma Betweeb Younger and Older Age Groups
| Factor | Patients <65 Years Old |
Patients ≥65 Years Old |
P Value |
|---|---|---|---|
| Patients | 16 | 20 | |
| Male patients | 11 (69%) | 11 (55%) | |
| Age at diagnosis, years, median | 55 | 74 | |
| MGMT status | 0.534 | ||
| Insufficient data | 13 (81%) | 17 (85%) | |
| Positive | 0 | 1 (5%) | |
| Negative | 3 (19%) | 2 (10%) | |
| Extent of resection | 0.637 | ||
| Insufficient data | 1 (6%) | 2 (10%) | |
| Biopsy | 3 (19%) | 7 (35%) | |
| Subtotal | 7 (44%) | 6 (30%) | |
| Total | 5 (31%) | 5 (25%) | |
| Stupp protocol adherence | 0.082 | ||
| Insufficient data | 2 (13%) | 12 (60%) | |
| Not done | 1 (6%) | 2 (10%) | |
| Incomplete | 2 (13%) | 3 (15%) | |
| Complete | 11 (69%) | 3 (15%) | |
MGMT, methylguanine-DNA methyltransferase.
Table 2.
Comparison of Factors Impacting Survival of Patients with Glioblastoma Between Patients with High and Low Normalized Corrected Relative Tumor Blood Volume Based on Cutoff Value of 2.5.
| Factor | n_rTBV_C <2.5 | n_rTBV_C ≥2.5 | P Value |
|---|---|---|---|
| Patients | 18 | 18 | |
| Male patients | 11 (61%) | 11 (61%) | |
| Age at diagnosis, years, median | 59 | 72 | |
| MGMT status | 0.513 | ||
| Insufficient data | 14 (78%) | 16 (89%) | |
| Positive | 1 (6%) | 0 | |
| Negative | 3 (17%) | 2 (11%) | |
| Extent of resection | 0.478 | ||
| Insufficient data | 2 (11%) | 2 (11%) | |
| Biopsy | 3 (17%) | 6 (33%) | |
| Subtotal | 7 (39%) | 6 (33%) | |
| Total | 6 (33%) | 4 (22%) | |
| Stupp protocol adherence | 0.452 | ||
| Insufficient data | 4 (22%) | 10 (56%) | |
| Not done | 1 (6%) | 2 (11%) | |
| Incomplete | 3 (17%) | 2 (11%) | |
| Complete | 10 (56%) | 4 (22%) | |
n_rTBV_c, normalized corrected relative tumor blood volume; MGMT, methylguanine-DNA methyltransferase.
Figure 1.
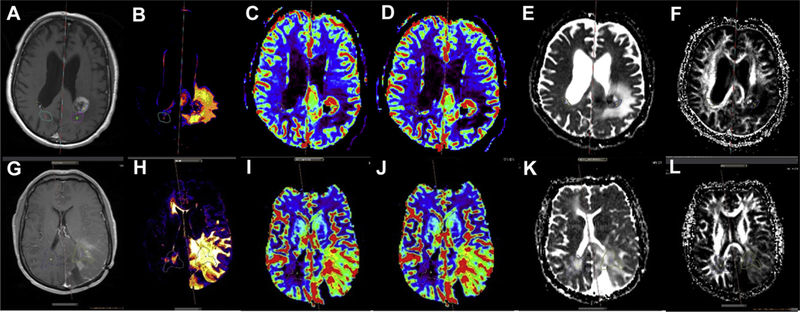
(A—F) T1-postcontrast (A), T2 fluid attenuated inversion recovery (B), corrected relative cerebral blood volume (C), relative cerebral blood flow (D), apparent diffusion coefficient (E), and fractional anisotropy (F) maps for a patient with glioblastoma in the older age group. (G—L) T1-postcontrast (G), T2 fluid attenuated inversion recovery (H), corrected relative cerebral blood volume (I), relative cerebral blood flow (J), apparent diffusion coefficient (K), and fractional anisotropy (L) maps for a patient with glioblastoma in the younger age group.
Table 3 lists the average and standard deviation values of perfusion, diffusion, and anatomic parameters in the tumor and nontumor sides and further stratifies these values based on the 2 age groups. Within our patient sample, there were significant differences in most imaging parameters—with the exception of T1 and K2—between the tumor and nontumor hemispheres. Among younger patients, all parameters—except FLAIR, T1, and K2—were significantly different between the hemispheres. Among older patients, only rTBV_c, rTBV, relative tumor blood flow (rTBF), and temporal maximum intensity projection (tMIP) were significantly different between the hemispheres.
Table 3.
Averages and Standard Deviation Values of Perfusion, Diffusion, and Anatomic Parameters in Tumor and Nontumor Hemispheres Among Patients According to Age Groups.
| rTBV_c | rTBV | rTBF | tMIP | ADC (mean) | ADC (minimum) | FA | FLAIR | T1 | K2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | ||||||||||
| T | 3.93 ± 1.46 | 3.93 ± 2.34 | 50.13 ± 16.14 | 0.40 ± 0.20 | 134.61 ± 35.51 | 67.88 ± 24.80 | 0.19 ± 0.09 | 388.69 ± 171.52 | 525.57 ± 175.70 | 46.12 ± 363.34 |
| NT | 1.66 ± 0.76 | 2.2 ± 1.42 | 23.13 ± 9.79 | 0.18 ± 0.10 | 102.78 ± 36.98 | 46.13 ± 23.15 | 0.33 ± 0.10 | 388.69 ± 171.52 | 449.01 ± 177.17 | 14.19 ± 26.61 |
| P value | 1.71 × 10−12 | 0.00021 | 3.66 × 10−13 | 7.85 × 10−8 | 0.001 | 0.00048 | 8.92 × 10−6 | 0.008 | 0.08 | 0.09 |
| Younger patients | ||||||||||
| T | 4.14 ± 1.21 | 5.22 ± 2.21 | 52.10 ± 16.53 | 0.43 ± 0.20 | 130.2 ± 28.21 | 130.21 ± 28.21 | 0.18 ± 0.056 | 401.07 ± 207.20 | 591.17 ± 154.01 | 184.79 ± 278.29 |
| NT | 1.94 ± 0.66 | 2.81 ± 1.52 | 26.43 ± 10.29 | 0.22 ± 0.12 | 99.03 ± 42.47 | 99.03 ± 42.48 | 0.35 ± 0.12 | 296.08 ± 176.62 | 535.65 ± 185.67 | 125.02 ± 120.06 |
| P value | 5.14 × 10−7 | 0.0012 | 1.08 × 10−5 | 0.001 | 0.025 | 0.025 | 0.002 | 0.133 | 0.380 | 0.437 |
| Older patients | ||||||||||
| T | 4.14 ± 1.97 | 3.93 ± 3.10 | 51.03 ± 25.83 | 0.39 ± 0.21 | 389.47 ± 672.18 | 120.21 ± 20.65 | 0.21 ± 0.12 | 344.89 ± 141.87 | 416.05 ± 168.66 | 115.91 ± 357.42 |
| NT | 1.54 ± 0.83 | 1.71 ± 0.95 | 21.2 ± 11.67 | 0.15 ± 0.18 | 309.32 ± 527.34 | 109.03 ± 52.48 | 0.29 ± 0.08 | 284.91 ± 115.92 | 339.93 ± 132.10 | 1.602 ± 79.91 |
| P value | 3.44 × 10−6 | 0.004 | 3.33 × 10−5 | 3.18 × 10−5 | 0.7069 | 0.056 | 0.0678 | 0.151 | 0.166 | 0.171 |
rTBV_c, corrected relative tumor blood volume; rTBV, relative tumor blood volume; rTBF, relative tumor blood flow; tMIP, temporal maximum intensity projection; ADC, apparent diffusion coefficient; FA, fractional anisotropy; FLAIR, fluid attenuated inversion recovery; T, tumor; NT, nontumor.
Figure 2 shows the correlation plots from the perfusion data (rTBV and rTBF), diffusion data (ADC and fractional anisotropy), and anatomic data (T1 and FLAIR) versus OS for all 36 patients. It further stratifies the correlation plots based on the 2 age groups. Within our patient sample, OS showed strongly negative correlations with n_rTBV_c (R = _0.70; P < 0.001) and n_Rtbf (R = _0.67; P < 0.001), a moderately positive correlation with normalized apparent diffusion coefficient (n_ADC) (R = 0.38; P = 0.03), a moderately negative correlation with normalized fluid attenuated inversion recovery (n_FLAIR) (R = −0.39; P = 0.043), and weak correlations with normalized T1 (n_T1) (R = −0.33; P = 0.07) and normalized fractional anisotropy (n_FA) (R = 0.27; P = 0.17). Among older patients, OS showed strongly negative correlations with n_rTBV_c (R = −0.66; P = 0.0016) and n_rTBF (R = −0.66; P = 0.0014) and weak correlations with n_ADC (R = 0.40; P = 0.096), n_FLAIR (R = −0.45; P = 0.053), n_T1 (R = −0.47; P = 0.069), and n_FA (R = 0.0085; P = 0.98). Among younger patients, OS showed strongly negative correlations with n_rTBV_c (R = −0.64; P = 0.007) and n_rTBF (R = −0.61; P = 0.012) and weak correlations with n_ADC (R = 0.27; P = 0.34), n_FLAIR (R = 0.14; P = 0.74), n_T1 (R = −0.13; P = 0.66), and n_FA (R = 0.026; P = 0.93).
Figure 2.
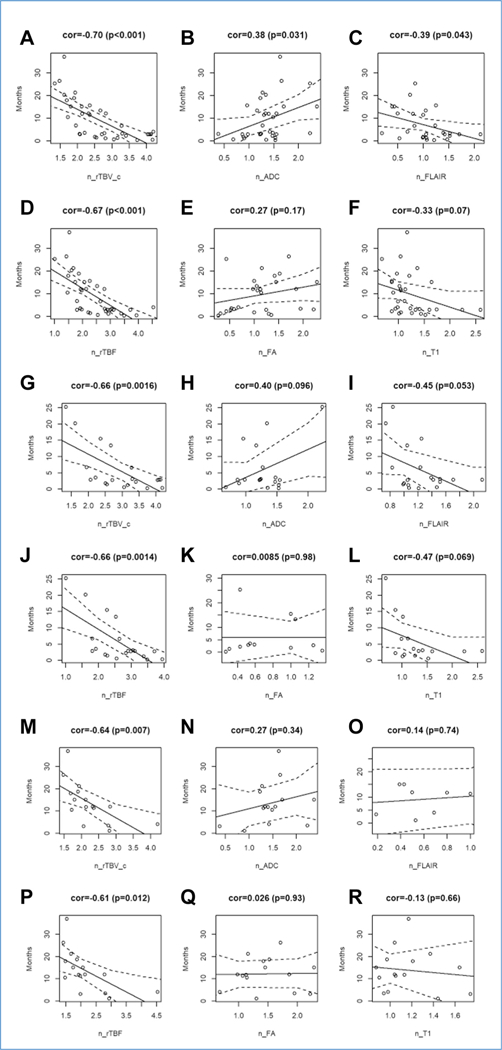
(A—R) Correlation plots for normalized corrected relative tumor blood volume, normalized apparent diffusion coefficient, normalized fluid attenuated inversion recovery, normalized relative tumor blood flow, normalized fractional anisotropy, and normalized T1 versus overall survival for all 36 patients (A—F) and the older (G—L) and younger (M— R) age subgroups. cor, correlation; n_rTBV_c, normalized corrected relative tumor blood volume; n_ADC, normalized apparent diffusion coefficient; n_ FLAIR, normalized fluid attenuated inversion recovery; n_rTBF, normalized relative tumor blood flow; n_FA, normalized fractional anisotropy; n_T1, normalized T1
Figure 3 depicts the Kaplan-Meier survival analyses. The Kaplan-Meier analysis on all 36 patients showed that the median OS was 7.5 months with a 16-month OS of 20%. Eighteen patients with n_rTBV_c ≤2.5 (the best cutoff value) had a median OS of 15.1 months (confidence interval [CI], 11.34–21.25), whereas the 18 patients with n_rTBV_c >2.5 had a median OS of 2.8 months (95% CI, 1.48–4.03; P < 0.001). Twenty-four patients with n_rTBF ≤2.79 had a median OS of 12 months (95% CI, 10.46–17.9), whereas the 12 patients with n_rTBF ≤2.79 had a median OS of 2.8 months (95% CI, 1.31–4.2; P < 0.001). Twenty-five patients with n_ADC ≤1.54 had a median OS of 3.11 months (95% CI, 2.85–11.8), whereas the 8 patients with n_ADC >1.54 had a median OS of 15.07 months (95% CI, 11.97–37; P – 0.0099). Nineteen patients with n_FA ≤1.4 had a median OS of 3.57 months (95% CI, 2.85–13.4), whereas the 8 patients with n_FA >1.4 had a median OS of 15.07 months (95% CI, 11.97–37; P = 0.0745). Ten patients with n_FLAIR ≤0.91 had a median OS of 11.89 months (95% CI, 4.03—∞), whereas the 17 patients with n_FLAIR >0.91 had a median OS of 2.85 months (95% CI, 1.77–6.69; P = 0.0111). Lastly, 23 patients with n_T1 ≤1.4 had a median OS of 11.34 months (95% CI, 4.03– 17.9), whereas the 8 patients with n_T1 >1.4 had a median OS of 2.84 months (95% CI, 2.79— ∞; P < 0.005).
Figure 3.
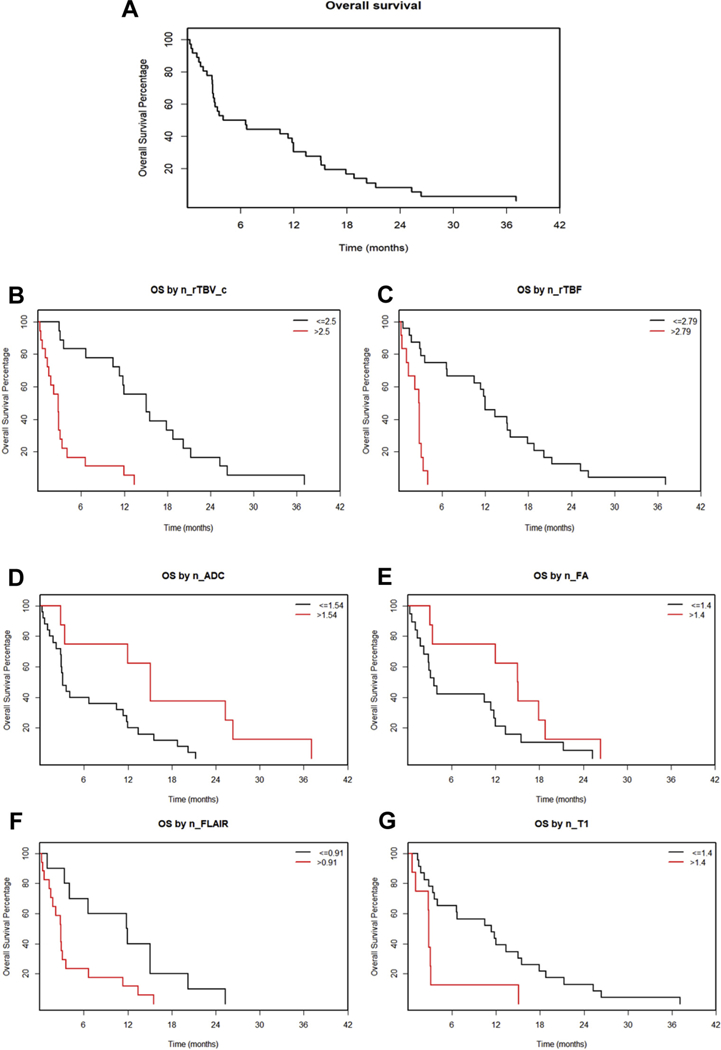
(A–G) Kaplan-Meier overall survival (OS) plot (A) and Kaplan-Meier survival curves based on the best cutoff value—found using a regression tree analysis—of normalized values of the imaging parameters (B–G). OS versus normalized corrected relative tumor blood volume for 36 patients (B), OS versus normalized relative tumor blood flow for 36 patients (C), OS versus normalized apparent diffusion coefficient for 33 patients (D), OS versus normalizedfractional anisotropy for 27 patients (E), OS versus normalized fluid attenuated inversion recovery for 27 patients (F), and OS versus normalized T1 for 31 patients (G). n_rTBV_c, normalized corrected relative tumor blood volume; n_rTBF, normalized relative tumor blood flow; n_ADC, normalized apparent diffusion coefficient; n_FA, normalized fractional anisotropy; n_ FLAIR, normalized fluid attenuated inversion recovery; n_T1, normalized T1.
Figure 4 depicts the ROC curves with the best cutoff values for all 36 patients. The highest AUC values were observed for n_rTBV_c (0.92) and n_rTBF (0.89). For diffusion parameters, including n_ADC or n_FA, AUC was 0.79 and 0.77, respectively; for anatomical parameters of n_T1 or n_FLAIR, AUC was 0.79 and 0.81, respectively; for age, AUC was 0.78.
Figure 4.
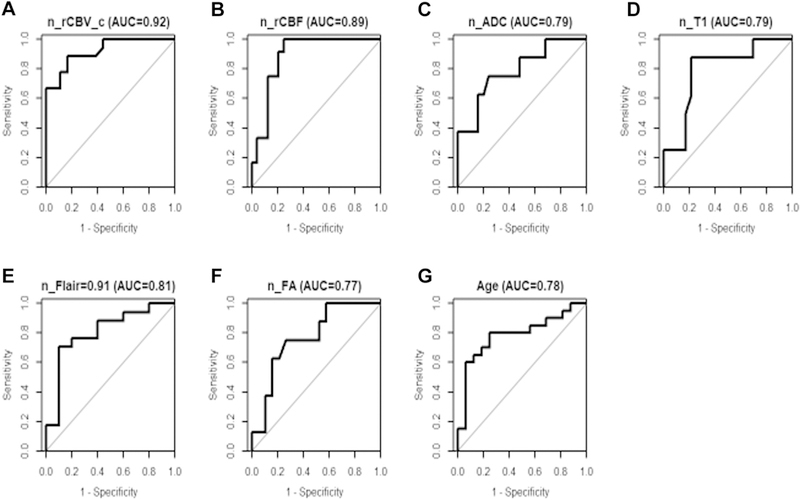
Receiver operating characteristic curves for all examined variables with the best cutoff values for all 36 patients. AUC, area under the curve; n_rCBV_c, normalized corrected relative cerebral blood volume; _rCBF, normalized relative cerebral blood flow; n_ADC, normalized apparent diffusion coefficient; n_T1, normalized T1; n_ FLAIR, normalized fluid attenuated inversion recovery; n_FA, normalized fractional anisotropy.
A multivariate Cox model on OS was used to assess the effect of age and each of the imaging parameters simultaneously (Table 4). The perfusion imaging parameters (n_rTBV and n_rTBF) were most significant in predicting OS compared with age and the other MRI parameters. In particular, the hazard ratio for n_rTBV was 5.97 (P = 0.0001) compared with 1.63 (P = 0.19) for age. Additional plots (available on request) reveal that with increasing values of n_rTBV_c and n_rTBF, the difference between OS of the 2 age groups decreases, suggesting that there is no interaction between age and total blood volume or total blood flow data.
Table 4.
Multivariable Cox Model Showing Interactions Between Age ≥65 Years and Each Imaging Parameter Using All 36 Patients with Glioblastomoa.
| Cox Models | HR | Lower 95% CI | Upper 95% CI | P Value |
|---|---|---|---|---|
| n_rTBV_c >2.5 | 5.97 | 2.43 | 14.71 | 0.0001 |
| Age ≥65 | 1.63 | 0.78 | 3.39 | 0.19 |
| n_rTBF >2.79 | 8.76 | 3.00 | 25.57 | 0.0001 |
| Age ≥65 | 2.13 | 1.04 | 4.37 | 0.04 |
| n_ADC >1.54 | 0.34 | 0.12 | 0.94 | 0.0374 |
| Age ≥65 | 1.83 | 0.87 | 3.83 | 0.11 |
| n_T1 >1.4 | 3.50 | 1.40 | 8.79 | 0.0075 |
| Age ≥65 | 2.63 | 1.22 | 5.68 | 0.01 |
| n_FLAIR >0.91 | 3.66 | 1.32 | 10.19 | 0.0128 |
| Age ≥65 | 0.66 | 0.24 | 1.83 | 0.42 |
| n_FA >1.4 | 0.56 | 0.19 | 1.64 | 0.29 |
| Age ≥65 | 1.40 | 0.54 | 3.64 | 0.49 |
HR, hazard ratio; CI, confidence interval; n_rTBV_c, normalized corrected relative tumor blood volume; n_rTBF, normalized relative tumor blood flow; n_ADC, normalized apparent diffusion coefficient; n_T1, normalized T1; n_FLAIR, normalized fluid attenuated inversion recovery; n_FA, normalized fractional anisotropy.
DISCUSSION
Perfusion MRI may play several roles in the management of brain tumors. It may assist in differential diagnosis, tumor grading, treatment monitoring, and prognosis, and it has been shown to have highly repeatable results.21 Genomic and cellular expression patterns of proangiogenic factors, including vascular endothelial growth factor, have been shown to influence perfusion MRI metrics.22 Glioblastomas with aggressive genetic alterations (e.g., MGMT methylation—negative, no PTEN loss, and/or higher Ki-67 index) have been demonstrated to have higher normalized relative tumor blood volume.23 Additional studies have demonstrated a role of perfusion imaging in identifying patients with EGFR gene amplification and endothelial growth factor receptor variant III mutation status in glioblastoma, helping to stratify survival and potentially provide targets for individualized treatment protocols.24,25
In addition to providing insight into the glioblastoma subtype, perfusion imaging may predict outcomes. OS of a patient with glioblastoma is influenced by many prognostic factors, such as age, rTBV, rTBF, and ADC mean value.1–6 Age is linked to overall life expectancy; rTBV and rTBF are correlated to angiogenesis; and mean ADC value is linked to tumor cellularity and mitotic activity. Studies have demonstrated that DSC perfusion could identify patients with glioblastoma with decreased OS via elevation of tumor cerebral blood volume, which has consistently demonstrated predictive value with regard to OS regardless of confounding factors, such as extent of surgical resection or patient age.6,14,17,26 DCE MRI parameters, elevated volume transfer constants, and kinetic texture analyses obtained from perfusion maps have also been associated with OS in patients with glioblastoma.27,28 In another study assessing a cohort patients with recurrent glioblastoma treated with bevacizumab, increased survival was demonstrated among patients with a larger decrease in relative blood volume, theorized to be indicative of the efficacy of the antiangiogenic bevacizumab therapy.29 Perfusion imaging has also been demonstrated to be a tool to differentiate pseudoprogression from progression or recurrent glioblastoma.30–32 Finally, by combining diffusion tensor imaging with perfusion and spectroscopy, the limits of glioblastoma invasion—which may precede the development of contrast enhancement—may become more evident; this may aid in achieving a gross total resection and improving survival.33,34 Thus, the use of perfusion imaging may predict genetic and biologic alterations and, in effect, prognosis.
This study retrospectively assessed MRI data of 36 patients with primary glioblastoma and analyzed the perfusion and diffusion data with respect to OS. In this study, corrected rCBV (i.e., rTBV_c for a tumor region) was used for evaluating the impact of this parameter on OS, as there is vessel leakage in glioblastoma. This correction was done in Olea Sphere software by including the terms of T1 and T2 shortening effects from the leakage of the contrast agent on the recovering DSC signal baseline. This correction was necessary to avoid underestimation of rTBV owing to T1 leakage effect and potentially overestimation of rTBV owing to residual susceptibility and/or dipolar T2 leakage effect.
When comparing parameters between the tumor and nontumor hemispheres, there were significant differences for rTBV_c, rTBV, rTBF, tMIP, mean transit time, mean ADC, minimum ADC, fractional anisotropy, and FLAIR for all 36 patients (P < 0.05). There were no significant differences between the 2 hemispheres when assessing K2, T1, and time to peak. After separating the patients into age groups ≥65 years or <65 years, only rTBV_c, rTBV, rTBF, and tMIP were different between the 2 brain hemispheres for the older group. For the younger group, rTBV_c, rTBV, rTBF, tMIP, mean ADC, minimum ADC, and FA were significantly different. The only parameters that were different for the 2 brain hemispheres for all 3 groups (i.e., all the patients, the older patients, and the younger patients) were perfusion parameters rTBV_c and rTBF.
The Pearson R values and the corresponding P values demonstrated that there were strong negative correlations for OS with n_rTBV_c and n_rTBF in all 36 patients and in both age subgroups. There was no correlation of OS time with n_ADC, n_FA (an index of dead cell or necrosis density), and n_T1. There was a weakly negative correlation for n_FLAIR.
Regression tree analyses were used to obtain the optimal cutoff value of each imaging biomarker used in the Kaplan-Meier survival analysis. The P value for the 2 groups separated by the best cutoff value suggested that only n_FA was not a factor for OS. The smallest P values for the best cutoff values corresponded to n_rTBV_c and n_rTBF, which meant the 2 perfusion bioimaging parameters could be the best indices for predicting OS. The ROC curves with the best cutoff values again exposed that the highest AUC values in the 6 bioimaging parameters were n_rTBV_c (0.92) and n_rTBF (0.89). Finally, a multivariate Cox model including age and each imaging parameter determined that n_rTBV and n_rTBF were most significant in predicting OS with a hazard ratio of 5.97 (P = 0.0001) for n_rTBV (compared with 1.63 for age, P = 0.19) and 8.76 (P = 0.0001) for n_rTBF (compared with 2.13 for age, P = 0.04). As an internal control, χ2 testing confirmed that there were no differences in MGMT status, extent of resection, and Stupp protocol adherence between older and younger (<65 years old) patients and between patients with high and low (n_rTBV_C <2.5) tumor blood volume, differences that could have biased the survival results.
For a certain value of n_rTBV or n_rTBF, the younger patients had better OS compared with the older patients. However, with increasing values ofn_rTBV_c and n_rTBF, the difference between the OS of the 2 age groups decreased. If the n_rTBV_c of a patient was smaller than 2.5, 7 total patients (of 36 total patients), 3 older patients (of 20 older patients), or 6 younger patients (of 16 younger patients) lived >480 days (16 months). If n_TBV was ≥2.5, the 16-month survival rate was zero among all 3 groups. The Kaplan-Meier survival analysis for all patients with glioblastoma also demonstrated that the 16-month OS was 20% and the median OS was 7.5 months. Taken together, these data suggest that perfusion MRI and certain parameters, particularly tumor blood volume and blood flow, are the best indices for predicting survival in patients with glioblastoma, and as such should be accounted for when comparing cohorts with this diagnosis.
Limitations and Future Directions
This is a retrospective study from a single institution with a limited number of patients. The relatively small sample sizes (20 patients in the older subgroup and 16 patients in the younger subgroup) influenced the statistical power of the results. Although there were no significant differences in extent of surgical resection and Stupp protocol adherence when the patients were dichotomized (n_rTBV_C <2.5 or ≥2.5; age <65 or ≥65), it is possible the study was not statistically powered enough to identify the differences given the number of patients with insufficient data. The second uncertainty results from the sizes of the ROIs, which also influences the values listed in Table 3. To counteract this limitation, we used the normalized values—obtained by dividing the mean value in the tumor ROI by the mean value in the contralateral mirror area—instead of the direct values calculated from the ROIs for all the bioimaging parametersin the Pearson correlation, Kaplan-Meier survival analyses, ROC curves, and Cox multivariableanalyses. Additionally, the study did not address other factors that may affect survival, such as extent of resection, tumor volume, functional status of patient, or genetic makeup of tumor. The tumor molecular makeup from patients whose surgeries were done several years ago remain unknown. Patients were not stratified by known prognostic factors (e.g., isocitrate dehydrogenase-1 or MGMT status) except for age to see if perfusion was an independent predictor. Age was assessed to determine if there was a correlation between age and perfusion and if age was a better predictor of survival. Patient characteristics were not significantly different when comparing high perfusion versus low perfusion in the present study.
Future directions include assessing how surgery and/or chemotherapy affects these MRI parameters and OS. Additional studies may also assess whether the n_rTBV_c cutoff value of 2.5 could further characterize patients who will have the highest chances of tumor recurrence. Lastly, it is possible that combining parameters may have increased predictive capabilities with regard to OS.
CONCLUSIONS
Perfusion imaging may be used as a surrogate to predict glioblastoma aggressiveness and survival. OS for a patient with glioblastoma after surgery is mainly determined by preoperative n_rTBV_c and/or n_rTBF. If n_rTBV_c is <2.5 (the best cutoff value), OS rate at 16 months is 20% and median OS is 7.5 months; if n_ rTBV_c is ≥2.5, OS rate at 16 months is zero. Calculated from the preoperative MRI scan, these results were independent of subsequent treatment and likely reflect an aggressive class of glioblastoma. Critical analysis of glioblastoma imaging may help predict tumor aggressiveness and survival.
Abbreviations and Acronyms
- ADC
Apparent diffusion coefficient
- CI
Confidence interval
- DCE
Dynamic contrast enhancement
- DSC
Dynamic susceptibility contrast
- FLAIR
Fluid attenuated inversion recovery
- MGMT
Methylguanine-DNA methyltransferase
- MRI
Magnetic resonance imaging
- n_ADC
Normalized apparent diffusion coefficient
- n_FA
Normalized fractional anisotropy
- n_FLAIR
Normalized fluid attenuated inversion recovery
- n_rTBF
Normalized relative tumor blood flow
- n_rTBV_c
Normalized corrected relative tumor blood volume
- n_T1
Normalized T1
- OS
Overall survival
- rCBF
Relative cerebral blood flow
- rCBV
Relative cerebral blood volume
- ROC
Receiver operating characteristic
- ROI
Region of interest
- rTBF
Relative tumor blood flow
- rTBV
Relative tumor blood volume
- rTBV_c
Corrected relative tumor blood volume
- tMIP
temporal maximum intensity projection
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Chaudhry NS, Shah AH, Ferraro N, et al. Predictors of long-term survival in patients with glioblastoma multiforme: advancements from the last quarter century. Cancer Invest. 2013)31:287–308. [DOI] [PubMed] [Google Scholar]
- 2.Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope WB, Qiao XJ, Kim HJ, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- 5.Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang- Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379:1984–1996. [DOI] [PubMed] [Google Scholar]
- 6.Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehkordi ANV, Kamali-Asl A, Wen N, Mikkelsen T, Chetty IJ, Bagher-Ebadian H. DCE-MRI prediction of survival time for patients with glioblastoma multiforme: using an adaptive neuro-fuzzy-based model and nested model selection technique. NMR Biomed. 2017;30. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill AF, Qin L, Wen PY, de Groot JF, Van den Abbeele AD, Yap JT. Demonstration of DCE-MRI as an early pharmacodynamic biomarker of response to VEGF trap in glioblastoma. J Neurooncol. 2016;130:495–503. [DOI] [PubMed] [Google Scholar]
- 9.Chang W, Pope WB, Harris RJ, et al. Diffusion MRI characteristics following concurrent radiochemotherapy predicts progression-free and overall survival in newly diagnosed glioblastoma. Tomography. 2015;1:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalpathy-Cramer J, Gerstner ER, Emblem KE, Andronesi O, Rosen B. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res. 2014;74:4622–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas DL, Lythgoe MF, Pell GS, Calamante F, Ordidge RJ. The measurement of diffusion and perfusion in biological systems using magnetic resonance imaging. Phys Med Biol. 2000;45: R97–R138. [DOI] [PubMed] [Google Scholar]
- 12.Parker GJ, Tofts PS. Pharmacokinetic analysis of neoplasms using contrast-enhanced dynamic magnetic resonance imaging. Top Magn Reson Imaging. 1999;10:130–142. [DOI] [PubMed] [Google Scholar]
- 13.Axel L Cerebral blood flow determination by rapid-sequence computed tomography: theoretical analysis. Radiology. 1980;137:679–686. [DOI] [PubMed] [Google Scholar]
- 14.Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25:746–755. [PMC free article] [PubMed] [Google Scholar]
- 15.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17:357–367. [DOI] [PubMed] [Google Scholar]
- 16.Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am. 2009;19:527–557. [DOI] [PubMed] [Google Scholar]
- 17.Hirai T, Murakami R, Nakamura H, et al. Prognostic value of perfusion MR imaging of highgrade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol. 2008;29:1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224:177–183. [DOI] [PubMed] [Google Scholar]
- 19.Sinha S, Bastin ME, Whittle IR, Wardlaw JM. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol. 2002;23: 520–527. [PMC free article] [PubMed] [Google Scholar]
- 20.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. [DOI] [PubMed] [Google Scholar]
- 21.Jafari-Khouzani K, Emblem KE, Kalpathy-Cramer J, et al. Repeatability of cerebral perfusion using dynamic susceptibility contrast MRI in glioblastoma patients. Transl Oncol. 2015;8:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barajas RF, Phillips JJ, Vandenberg SR, et al. Pro-angiogenic cellular and genomic expression patterns within glioblastoma influences dynamic susceptibility weighted perfusion MRI. Clin Radiol. 2015;70:1087–1095. [DOI] [PubMed] [Google Scholar]
- 23.Ryoo I, Choi SH, Kim J-H, et al. Cerebral blood volume calculated by dynamic susceptibility contrast-enhanced perfusion MR imaging: preliminary correlation study with glioblastoma genetic profiles. PLoS One. 2013;8:e71704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao XJ, Ellingson BM, Kim HJ, et al. Arterial spin-labeling perfusion MRI stratifies progression-free survival and correlates with epidermal growth factor receptor status in glioblastoma. AJNR Am J Neuroradiol. 2015;36:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Young RJ, Shah AD, et al. Pretreatment dynamic susceptibility contrast MRI perfusion in glioblastoma: prediction of EGFR gene amplification. Clin Neuroradiol. 2015;25:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain R, Poisson L, Narang J, et al. Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology. 2013;267:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonekamp D, Deike K, Wiestler B, et al. Association of overall survival in patients with newly diagnosed glioblastoma with contrast-enhanced perfusion MRI: Comparison of intraindividually matched T1-and T2-based bolus techniques. J Magn Reson Imaging. 2015;42:87–96. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Jain R, Khalil K, et al. Texture feature ratios from relative CBV maps of perfusion MRI are associated with patient survival in glioblastoma. AJNR Am J Neuroradiol. 2016;37:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RJ, Cloughesy TF, Hardy AJ, et al. MRI perfusion measurements calculated using advanced deconvolution techniques predict survival in recurrent glioblastoma treated with bev-acizumab. J Neurooncol. 2015;122:497–505. [DOI] [PubMed] [Google Scholar]
- 30.Khalifa J, Tensaouti F, Chaltiel L, et al. Identification of a candidate biomarker from perfusion MRI to anticipate glioblastoma progression after chemoradiation. Eur Radiol. 2016;26:4194–4203. [DOI] [PubMed] [Google Scholar]
- 31.Thomas AA, Arevalo-Perez J, Kaley T, et al. Dynamic contrast enhanced T1 MRI perfusion differentiates pseudoprogression from recurrent glioblastoma. J Neurooncol. 2015;125:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young RJ, Gupta A, Shah AD, et al. MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin Imaging. 2013;37:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price SJ, Young AMH, Scotton WJ, et al. Multimodal MRI can identify perfusion and metabolic changes in the invasive margin of glioblastomas. J Magn Reson Imaging. 2016;43:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou Z, Cai X, Li H, et al. Quantitative assessment of invasion of high-grade gliomas using diffusion tensor magnetic resonance imaging. World Neuro-surg. 2018;113:e561–e567. [DOI] [PubMed] [Google Scholar]


