Abstract
BACKGROUND:
Activity-dependent release of brain-derived neurotrophic factor (BDNF) in the medial prefrontal cortex (mPFC) is essential for the rapid and sustained antidepressant actions of ketamine, and a recent study shows a similar requirement for vascular endothelial growth factor (VEGF). Since BDNF is reported to stimulate VEGF expression/release in neuroblastoma cells, the present study tested the hypothesis that the actions of BDNF are mediated by VEGF.
METHODS:
The role of VEGF in the antidepressant behavioral actions of BDNF was tested by intra-mPFC co-infusion of a VEGF neutralizing antibody and by neuron-specific deletion of VEGF. The influence of BDNF on the release of VEGF and the role of VEGF in the neurotrophic actions of BDNF were determined in rat primary cortical neurons. The role of BDNF in the behavioral and neurotrophic actions of VEGF were also determined.
RESULTS:
The results show that the rapid and sustained antidepressant-like actions of intra-mPFC BDNF are blocked by co-infusion of a VEGF neutralizing antibody, and that neuron-specific mPFC deletion of VEGF blocks the antidepressant-like actions of BDNF. Studies in primary cortical neurons demonstrate that BDNF stimulates the release of VEGF, and that BDNF-induction of dendrite complexity is blocked by a selective VEGF-Flk-1 antagonist. Surprisingly, the results also show reciprocal interactions, indicating that the behavioral and neurotrophic actions of VEGF are dependent on BDNF.
CONCLUSIONS:
These findings indicate that the antidepressant-like and neurotrophic actions of BDNF require VEGF signaling, but also demonstrate reciprocal interdependence for BDNF in the actions of VEGF.
Keywords: BDNF, Depression, Medial prefrontal cortex, Mood disorder, Rapid antidepressants, VEGF-A
Introduction
Major depressive disorder (MDD) is a widespread debilitating illness, affecting approximately 17% of the population in the United States, causing enormous personal and socioeconomic burden (1, 2). Conventional antidepressants, notably monoamine reuptake inhibitors, take weeks to months to produce a therapeutic response, and have limited efficacy, as approximately one-third of depressed patients fail to respond to typical antidepressants and are considered treatment-resistant (3). Recent studies demonstrate that a single subanesthetic dose of ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, produces rapid (within hours) and sustained (up to a week) antidepressant actions even in patients suffering from treatment-resistant depression (4, 5); similar rapid and long-lasting effects are observed in rodent models (6, 7).
Although the mechanisms underlying the pathophysiology of MDD and the therapeutic actions of ketamine remain unclear, growing evidence supports a neurotrophic hypothesis of depression and antidepressant response (8–10). This hypothesis is based on evidence that reduced neurotrophic factor levels, notably brain-derived neurotrophic factor (BDNF) and/or vascular endothelial growth factor (VEGF), are tightly linked with neuronal atrophy in brain regions implicated in MDD, including the prefrontal cortex (PFC) and hippocampus (8–10). BDNF and VEGF are two completely different pleiotrophic growth factors that bind to and activate different tyrosine kinase receptors, TrkB and Flk-1 (VEGF receptor 2), respectively, that have unique as well as overlapping signaling pathways (11–13). In support of this hypothesis, neuroimaging studies have consistently reported decreased volume of the PFC and hippocampus in depressed patients (14, 15), where neuronal atrophy and glial loss have been reported in postmortem studies of depression and rodent chronic stress models (10, 16). Studies of postmortem depressed subjects and rodent chronic stress report decreased levels of BDNF and VEGF, as well as their receptors, TrkB and Flk-1, respectively, in the PFC and hippocampus (9, 17–22); VEGF is also decreased in cerebrospinal fluid of suicide attempters (23).
Conversely, preclinical studies reveal that ketamine and other rapid acting antidepressants act at least in part by producing the opposite effects, increasing BDNF and/or VEGF release and signaling in the PFC and hippocampus (10, 24–27). Ketamine blockade of NMDA receptors located on GABAergic interneurons leads to disinhibition and a rapid and transient glutamate burst that activates postsynaptic AMPA receptors, resulting in stimulation of Ca2+ influx through voltage-dependent calcium channels that activates BDNF release (10); this increases and reverses the synaptic deficts in PFC caused by chronic stress (6, 7), and it required for the the antidepressant-like behavioral actions of ketamine (24, 28). The actions of conventional antidepressants are also linked to BDNF and VEGF, although these monoaminergic agents only increase trophic factor levels after chronic treatment and only increase expression, but not release of BDNF and VEGF (9, 29–35).
We have recently reported that neuronal VEGF-Flk-1 signaling in the mPFC is also required for the neurotrophic and antidepressant-like behavioral actions of ketamine (25). Since BDNF is reported to stimulate VEGF expression and release in neuroblastoma cells (36), we hypothesized that VEGF signaling acts downstream of BDNF to produce the neurotrophic and antidepressant-like actions. The current study addresses this hypothesis as well as the interdependence between BDNF and VEGF signaling.
Methods and Materials
Animals
Male C57BL/6J (Jackson Laboratories, Bar Harbor, ME), Camk2a-cre;Vegfaflox/flox (hereafter, VegfNEURON−/−) (25), and Vegfaflox/flox mice (25, 37) were used. Pregnant female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used to harvest embryonic rats for primary cortical neuronal cultures. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committee.
Reagents
Recombinant mouse VEGF164 (the predominant VEGF isoform), recombinant rat VEGF164, recombinant BDNF, goat anti-mouse VEGF neutralizing antibody (nAb), normal goat IgG, and normal sheep IgG were obtained from R&D Systems (Minneapolis, MN), and reconstituted according to the manufacturer’s instruction. Sheep anti-BDNF nAb (Millipore, Billerica, MA) was also reconstituted according to the manufacturer’s instruction. Selective TrkB (ANA-12, Millipore) and selective Flk-1 (ZM323881, Selleck, Houston, TX) inhibitors were dissolved in dimethyl sulfoxide (DMSO).
Surgery and drug treatments
Intra-mPFC infusions (1.8 mm rostral, ±0.4 mm lateral, 2.8 mm ventral to bregma) (38) were performed as previously described (25). Mice were bilaterally infused with VEGF (5 ng/side), BDNF (100 ng/side), or vehicle (0.1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS)) in a volume of 0.2 μL/side. For co-infusion of VEGF and BDNF nAb, a mixture of VEGF with either sheep anti-BDNF nAb (200 ng/side) or normal sheep IgG (200 ng/side) was delivered into the mPFC. For co-infusion of BDNF and VEGF nAb, a mixture of BDNF with either goat anti-VEGF nAb (80 ng/side) or normal goat IgG (80 ng/side) was delivered into the mPFC. These doses of BDNF, VEGF and the neutralizing antibodies were determined based on our previous works (24, 25, 39).
Behavioral testing
The forced swim test (FST), female urine sniffing test (FUST), novelty-suppressed feeding (NSF) test, and locomotor activity (LMA) test were performed as previously described (25). The FST was conducted twice, 1 and 2 days after treatments. The FUST is based on the attraction of male rodents to pleasurable pheromones in female urine and serves as a measure of reward-seeking behavior (40). Further details are provided in the Supplemental Information.
Histology
After behavioral tests, histological analyses were performed. Coronal sections (30 μm) were prepared on a cryostat and stained with cresyl violet. Infusion sites were examined under a bright field microscope (Zeiss, Oberkochen, Germany). Animals with incorrect infusion placements were excluded from analyses.
Primary Cortical Neuronal Cultures
Cortical neurons were dissected from E18 rat embryos and maintained as previously described (24, 25, 35, 41). Further details are provided in the Supplemental Information.
Measurements of BDNF and VEGF
On DIV 10, the medium was changed to fresh medium without B27 supplement (Thermo Fisher Scientific, Waltham, MA) 4 h prior to addition of growth factors. Neurons were treated with either 0.1% DMSO, ANA-12 (5 μM), or ZM323881 (10 nM). After 30 min, neurons were treated with either vehicle (0.0001% BSA/PBS), BDNF (50 ng/mL), or rat VEGF (50 ng/mL). These doses of BDNF, VEGF and the inhibitors were determined based on our pilot experiments and previous reports (25, 42, 43). The media were collected 1 and 3 h after VEGF and BDNF treatments, respectively, for ELISA analysis. The duration of incubation was determined based on our pilot experiments in which 1-h incubation of BDNF did not increase VEGF release (Figure S1). Measurement of BDNF was performed as previously described (24, 35). On DIV 10, the medium was changed to B27-free medium containing an anti-BDNF antibody (2 μg/mL; Santa Cruz, Dallas, TX) 4 h before growth factor incubation; the secreted BDNF captured by the antibody was immunoprecipitated using Protein G-Sepharose beads (GE Healthcare, Little Chalfont, UK), and BDNF was detected via ELISA assays (BDNF Emax Immunoassay system; Promega, Madison, WI). VEGF was measured directly in the media by VEGF Immunoassay (Quantikine ELISA; R&D systems) according to the manufacturer’s instructions.
Sholl analysis
Dendritic complexity in primary cortical neurons was analyzed as previously described (25, 35, 41). Further details are provided in the Supplemental Information.
Statistical Analyses
Data are presented as mean ± SEM. Data were analyzed by one-way ANOVA or two-way ANOVA followed by the Tukey’s or Dunnett’s post hoc test using GraphPad Prism 6 (GraphPad Software, San Diego, CA). Differences with p < .05 were considered significant.
Results
Rapid and sustained antidepressant-like effects of BDNF require VEGF release
In a previous study, we showed that intra-mPFC infusion of BDNF (100 ng/side) produces an antidepressant-like effect in the FST 1 day after the infusion (39). We have also reported that a single intra-mPFC infusion of VEGF (5 ng/side) produces rapid and sustained antidepressant-like effects in three different behavioral paradigms: the FST (behavioral despair), FUST (motivation/reward) and NSF (anxiety) (25). Here, we replicate and extend these findings on the antidepressant-like actions of BDNF (100 ng infusions per side of mPFC) and compare with VEGF (5 ng/side), or vehicle (0.1% BSA/PBS); these doses of BDNF and VEGF produce ketamine-like rapid antidepressant responses (25, 39). The reason for the difference in dose is unclear but could be related to receptor affinities or presence of truncated TrkB receptors that bind BDNF, but lack the intracellular signaling tyrosinse kinase domain (44–46). Behavioral testing was conducted on days 1–5 (Figure S2A); FST was conducted twice, 1 and 2 days after dosing. A single intra-mPFC infusion of BDNF, or VEGF, significantly decreased immobility in both FST1 and FST2, but did not influence locomotor activity (Figure S2B–D). Infusion of BDNF or VEGF also increased time spent sniffing female urine in the FUST (Figure S2E). A subset of mice was examined in the NSF test 5 days after the infusion, and both BDNF and VEGF decreased latency to feed at this later time point (Figure S2F); there were no effects on home cage feeding (HCF; Figure S2G). These results indicate that intra-mPFC infusions of BDNF or VEGF produce rapid and sustained antidepressant-like actions in three different behavioral paradigms.
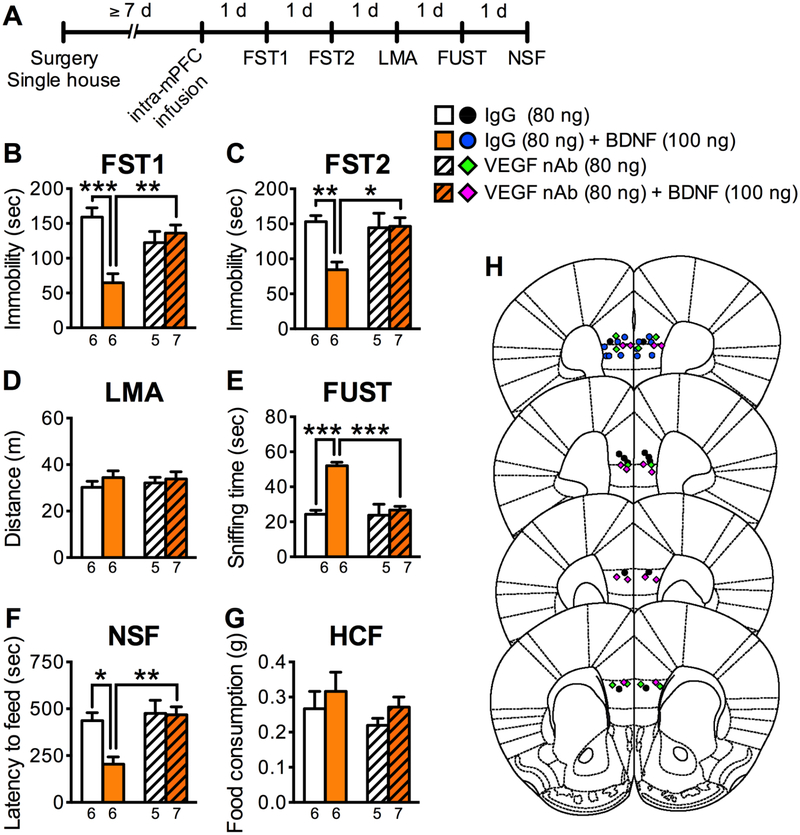
To test the role of VEGF in the antidepressant-like actions of BDNF, a VEGF nAb or control IgG (80 ng/side) was co-infused with BDNF (100 ng/side) into the mPFC, and subjected to behavioral testing (Figure 1A). Immunoblot studies demonstrate that the VEGF nAb reacts with VEGF as expected, but not recombinant BDNF (Figure S3). Intra-mPFC BDNF infusion in control IgG mice significantly decreased immobility in FST1 and FST2, increased sniffing female urine in the FUST, and decreased latency to feed in the NSF test (Figure 1B, C, E, F). However, in mice co-infused with the VEGF nAb, the antidepressant-like actions of BDNF were completely blocked (Figure 1B, C, E, F). These treatments had no effect on LMA or HCF (Figure 1D, G), and the VEGF nAb alone, in the absence of BDNF had no significant effects on any of the behaviors tested (Figure 1B–G). Since the VEGF nAb would bind and sequester VEGF in the extracellular space, the results suggest that VEGF release is required for the rapid and sustained antidepressant-like actions of intra-mPFC infusion of BDNF.
Figure 1.
The antidepressant-like effects of intra-mPFC BDNF infusion are blocked by co-infusion of VEGF nAb. (A) Experimental timeline for behavioral testing after intra-mPFC infusion of control IgG (80 ng/side), VEGF nAb (80 ng/side), IgG + BDNF (100 ng/side), or VEGF nAb + BDNF. (B) Immobility time in the FST1 1 day after intra-mPFC infusion (interaction, F1,20 = 15.9, p =.0007, n = 5–7). (C) Immobility time in the FST2 2 days after intra-mPFC infusion (interaction, F1,20 = 7.13, p = .0147, n = 5–7). (D) LMA 3 days after intra-mPFC infusion (interaction, F1,20 = .199, p = .660; VEGF nAb, F1,20 = .0531, p = .820; BDNF, F1,20 = 1.01, p = .327, n = 5–7). (E) Time spent sniffing female urine in the FUST 4 days after intra-mPFC infusion (interaction, F1,20 = 14.4, p = .0011, n = 5–7). (F) Latency to feed in the NSF 5 days after intra-mPFC infusion (interaction, F1,20 = 5.45, p = .0301, n = 5–7). (G) HCF just after the NSF (interaction, F1,20 = .000296, p = .986; VEGF nAb, F1,20 = 1.23, p = .282; BDNF, F1,20 = 1.49, p = .236, n = 5–7). (H) Schematic representation of mPFC infusion sites. Data are expressed as means ± SEM. *p < .05, **p < .01, ***p < .001.
Neuronal VEGF signaling mediates the antidepressant-like effects of intra-mPFC BDNF infusion
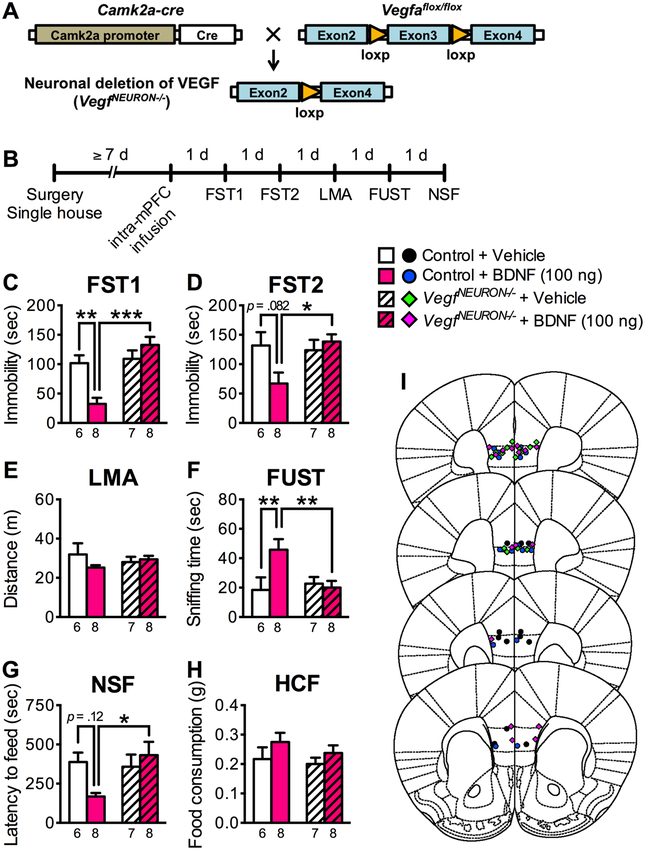
VEGF is expressed by different cell types in brain, including neurons, astrocytes, and endothelial cells (34). We have recently developed a neuron specific VEGF deletion mutant (25) (Figure 2A) and here used these mice to determine if the VEGF required for the effects of BDNF is derived from neurons. We examined the effects of intra-mPFC infusion of BDNF (100 ng/side) in mice with neuronal specific deletion of VEGF, referred to as VegfNEURON−/− in which Cre recombinase is driven by the Camk2a promoter; littermate controls were Vegfaflox/flox, but Camk2a-cre negative. Mice were tested in the FST, FUST and NSF (Figure 2B). In littermate control mice, intra-mPFC BDNF infusion significantly decreased immobility in the FST1, increased time spent sniffing female urine in the FUST, with strong tendancies for decreased immobility in FST2 and latency in the NSF (Figure 2C, D, F, G). However, the antidepressant-like actions of BDNF were completely blocked in VegfNEURON−/− mice in all three behavioral paradigms. There were no differences in LMA or HCF in the VegfNEURON−/− mutant mice (Figure 2E, H). These results indicate that VEGF in pyramidal neurons is required for the antidepressant-like actions of intra-mPFC infusion of BDNF.
Figure 2.
The antidepressant-like effects of intra-mPFC BDNF infusion are blocked in VegfNEURON−/− mice. (A) Schematic representation of neuronal deletion of VEGF. (B) Experimental timeline for behavioral testing after intra-mPFC infusion of either vehicle or BDNF (100 ng/side) in control or VegfNEURON−/− mice. (C) Immobility time in the FST1 1 day after intra-mPFC infusion (interaction, F1,25 = 12.6, p = .0015, n = 6–8). (D) Immobility time in the FST2 2 days after intra-mPFC infusion (interaction, F1,25 = 4.94, p = .0355, n = 6–8). (E) LMA 3 days after intra-mPFC infusion (interaction, F1,25 = 1.93, p = .177; genotype, F1,25 = .00238, p = .962; BDNF, F1,25 = .824, p = .373, n = 6–8). (F) Time spent sniffing female urine in the FUST 4 days after intra-mPFC infusion (interaction, F1,25 = 5.62, p = .0257, n = 6–8). (G) Latency to feed in the NSF 5 days after intra-mPFC infusion (interaction, F1,25 = 4.93, p = .0358, n = 6–8). (H) HCF just after the NSF (interaction, F1,25 = .120, p = .732; genotype, F1,25 = .809, p = 0.377; BDNF, F1,25 = 2.53, p = .124, n = 6–8). (I) Schematic representation of mPFC infusion sites. Data are expressed as means ± SEM. *p < .05, **p < .01, ***p < .001.
Neurotrophic effects of BDNF require VEGF release in primary cortical neurons
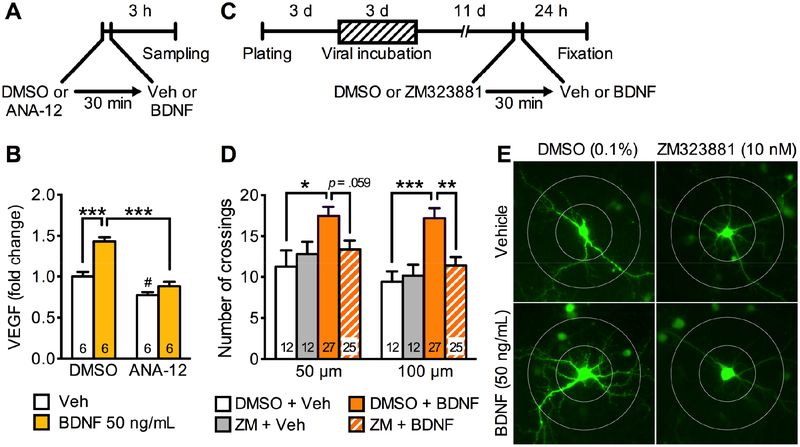
Since the antidepressant-like actions of intra-mPFC infusion of BDNF require VEGF derived from pyramidal neurons (Figures 1, 2), we tested whether BDNF stimulates VEGF release from cultured primary cortical neurons (Figure 3A, B). We measured VEGF levels in media after 3-h incubation of recombinant BDNF (50 ng/mL) or vehicle; we also tested the influence of a selective TrkB inhibitor ANA-12 (5 μM) or vehicle (0.1% DMSO) (30 min pre-incubation prior to BDNF) (Figure 3A). BDNF incubation significantly increased VEGF levels in the media, and co-incubation with the TrkB antagonist ANA-12 completely blocked this effect (Figure 3B). ANA-12 alone also significantly reduced VEGF levels in vehicle-treated neurons (Figure 3B). These results indicate that BDNF stimulates VEGF release from cultured primary cortical neurons, and that BDNF-TrkB signaling regulates VEGF release under unstimulated conditions.
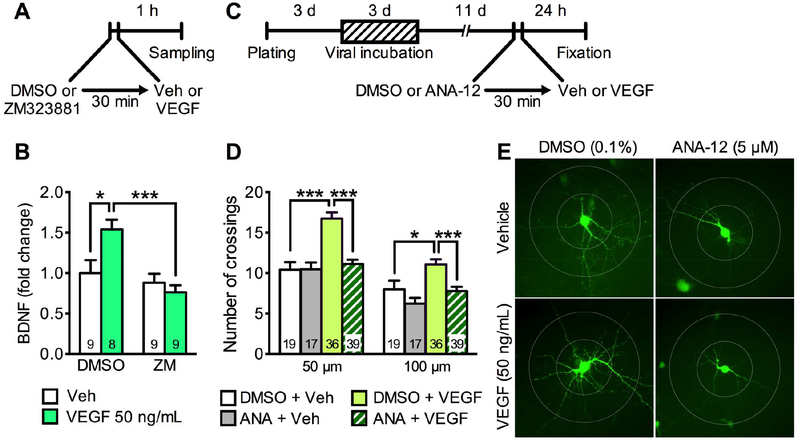
Figure 3.
BDNF stimulates VEGF release and produces neurotrophic effects via VEGF-Flk-1 signaling in rat primary cultured cortical neurons. (A) Experimental timeline for BDNF-stimulated VEGF release. (B) VEGF levels in media after 3-h incubation of vehicle (0.0001% BSA/PBS) or BDNF (50 ng/mL) with 30-min pre-incubation of 0.1% DMSO with or without the TrkB inhibitor ANA-12 (5 μM; interaction, F1,20 = 10.2, p = .0046, n = 6). (C) Experimental timeline for dendritic morphology in rat primary cultured cortical neurons. (D) The number of dendritic crossings at 50 and 100 μm distances from the soma after 24-h vehicle (0.0001% BSA/PBS) or BDNF (50 ng/mL) treatment with 30-min pre-incubation of 0.1% DMSO with or without ZM323881 (10 nM; 50 μm, interaction, F1,72 = 4.00, p = .0493; 100 μm, interaction, F1,72 = 5.78, p = .0188, n = 12–27). (E) Representative images of EGFP-expressing rat primary cortical neurons from each group with concentric circles (100- and 200-μm diameter). Data are expressed as means ± SEM. *p < .05, **p < .01, ***p < .001; #p < .05 relative to DMSO + vehicle.
Our recent study reveals that Flk-1 is expressed in CaMKII-positive neurons and the selective Flk-1 inhibitor ZM323881 suppresses the neurotrophic actions of both ketamine and VEGF in primary cultured cortical neurons (25). Since BDNF stimulates VEGF release, we next tested whether neurotrophic responses to BDNF require VEGF-Flk-1 signaling (Figure 3C–E). For these studies, primary cortical neurons were infected with AAV2 encoding Egfp to visualize neuronal processes and 2 weeks later the influence of BDNF incubation (50 ng/mL, 24-h) on dendrite branching was determined (Figure 3C). BDNF significantly increased the number of dendritic branch crossings compared with vehicle control at both 50- and 100-μm distances from the soma (Figure 3D, E). Moreover, BDNF-stimulation of dendrite complexity was blocked by pre-incubation with ZM323881 (10 nM in 0.1% DMSO, 30-min pre-incubation) (Figure 3D, E). These results indicate that the neurotrophic actons of BDNF require VEGF-Flk-1 signaling.
Role of BDNF in the antidepressant-like and neurotrophic effects of VEGF
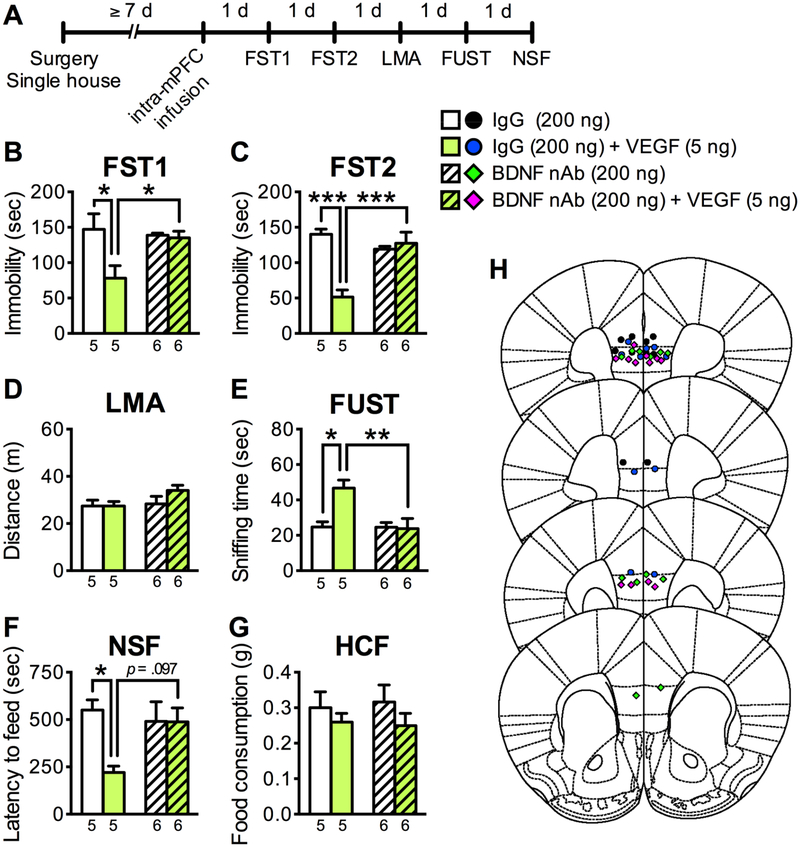
To extend these studies we also examined the reciprocal interdependence for BDNF in the antidepressant-like behavioral and neurotrophic effects of VEGF. To test the role of BDNF in the antidepressant-like actions of intra-mPFC VEGF infusion, a BDNF nAb (200 ng/side) or control IgG (200 ng/side) were co-infused with VEGF (5 ng/side) into the mPFC, and subjected to behavioral testing (Figure 4A). Immunoblot studies demonstrate that the BDNF nAb recognizes BDNF, but did not react with VEGF (Figure S3). As observed in Figure S1, control IgG mice with intra-mPFC VEGF infusion displayed significant antidepressant-like behaviors in FST1 and FST2 (decreased immobility), FUST (increased time spent sniffing female urine), and NSF test (decreased latency to feed) (Figure 4B, C, E, F). Importantly, these effects were completely blocked by co-infusion of BDNF nAb (Figure 4B, C, E, F). These treatments had no effect on LMA or HCF (Figure 4D, G), and the BDNF nAb alone had no significant effects on any of the behaviors tested (Figure 4B–G). These data indicate that BDNF is required for the antidepressant-like actions of intra-mPFC infusions of VEGF.
Figure 4.
The antidepressant-like effects of intra-mPFC VEGF infusion are blocked by co-infusion of BDNF nAb. (A) Experimental timeline for behavioral testing after intra-mPFC infusion of control IgG (200 ng/side), BDNF nAb (200 ng/side), IgG + VENF (5 ng/side), or BDNF nAb + VEGF. (B) Immobility time in the FST1 1 day after intra-mPFC infusion (interaction, F1,18 = 5.58, p = .00296, n = 5–6). (C) Immobility time in the FST2 2 days after intra-mPFC infusion (interaction, F1,18 = 21.4, p = .0002, n = 5–6). (D) LMA 3 days after intra-mPFC infusion (interaction, F1,18 = 1.24, p = .280; BDNF nAb, F1,18 = 2.09, p = .166; VEGF, F1,18 = 1.20, p = .288, n = 5–6). (E) Time spent sniffing female urine in the FUST 4 days after intra-mPFC infusion (interaction, F1,18 = 7.36, p = .0142, n = 5–6). (F) Latency to feed in the NSF 5 days after intra-mPFC infusion (interaction, F1,18 = 4.61, p = .0456, n = 5–6). (G) HCF just after the NSF (interaction, F1,18 = .112, p = .741; BDNF nAb, F1,18 = .00702, p = .934; VEGF, F1,18 = 1.80, p = .197, n = 5–6). (H) Schematic representation of mPFC infusion sites. Data are expressed as means ± SEM. *p < .05, **p < .01, ***p < .001.
Next we tested the influence of VEGF on BDNF release and neurotrophic responses in cultured primary cortical neurons. We measured BDNF levels in the media after 1-h incubation of VEGF (50 ng/mL); we also tested the influence of ZM323881 (10 nM, 30-min pre-incubation) or vehicle (0.1% DMSO) (Figure 5A). VEGF incubation significantly increased BDNF levels in the media, and this effect was completely blocked by ZM323881 (Figure 5B); ZM323881 alone had no effect on BDNF levels.
Figure 5.
VEGF stimulates BDNF release and produces neurotrophic effects via BDNF-TrkB signaling in primary cultured cortical neurons. (A) Experimental timeline for VEGF-stimulated BDNF release. (B) BDNF levels in media after 1-h incubation of vehicle (0.0001% BSA/PBS) or VEGF (50 ng/mL) with 30-min pre-incubation of 0.1% DMSO with or without the Flk-1 inhibitor ZM323881 (10 nM; interaction, F1,31 = 7.09, p = .0122, n = 8–9). (C) Experimental timeline for dendritic morphology in rat primary cultured cortical neurons. (D) The number of dendritic crossings at 50 and 100 μm distances from the soma after 24-h vehicle (0.0001% BSA/PBS) or VEGF (50 ng/mL) treatment with 30-min pre-incubation of 0.1% DMSO with or without ANA-12 (5 μM; 50 μm, interaction, F1,107 = 13.0, p = .0005; 100 μm, interaction, F1,107 = 1.05, p = .307; ZM323881, F1,107 = 11.6, p = .0009; BDNF, F1,107 = 9.78, p = .0023, n = 17–39). (E) Representative images of EGFP-expressing rat primary cortical neurons from each group with concentric circles (100- and 200-μm diameter). *p < .05, **p < .01, ***p < .001.
For studies of the neurotrophic responses, we tested whether BDNF-TrkB signaling is required for VEGF-induction of dendrite complexity. Primary cortical neurons were labeled with Egfp and incubated with VEGF (50 ng/mL, 24 hr), with or without ANA-12 (5 μM in 0.1% DMSO, 30-min pre-incubation; Figure 5C–E). VEGF significantly increased the number of dendritic branch crossings at both 50- and 100-μm distances from the soma, and this effect was blocked by ANA-12 (Figure 5D, E). Together, these results indicate that the neurotrophic actions of VEGF on dendrite complexity require BDNF-TrkB signaling.
Discussion
The current results demonstrate several important points. First, a single intra-mPFC infusion of BDNF produces rapid and sustained antidepressant-like actions similar to those of ketamine and intra-mPFC VEGF infusion, consistent with our recent findings (25, 39). Second, the antidepressant-like actions of BDNF in three different behavioral paradigms require neuronal-derived extracellular VEGF. Third, BDNF-TrkB signaling stimulates VEGF release in primary cortical neurons, consistent with a previous report showing BDNF-induced VEGF release in a neuroblastoma cell line (36). Fourth, BDNF-induced neurotrophic actions on dendrite complexity require VEGF-Flk-1 signaling in primary cortical neurons. Fifth, the results also demonstrate a reciprocal interdependence, showing that the antidepressant-like and neurotrophic actions of VEGF require BDNF, and that VEGF stimulates BDNF release in primary cortical neurons. These results provide the first evidence of a crucial role for interplay between BDNF and VEGF signaling in the neurotrophic and rapid/sustained antidepressant-like responses of these factors. Because these studies were conducted in males, further studies are needed to determine if similar effects are observed in females.
Previous studies demonstrate that the rapid antidepressant-like actions of ketamine are blocked by infusion of a BDNF nAb into the mPFC (24) and blocked in mice with a knockin of the BDNF Val66Met polymorphism, which blocks activity-dependent BDNF release (28). The behavioral actions of two other rapid-acting antidepressants, rapastinel (an NMDA receptor modulator) and scopolamine (a nonselective muscarinic acetylcholine receptor antagonist) are also blocked by intra-mPFC infusion of a BDNF nAb and in Val66Met knockin mice (39, 47). These effects differ from typical monoaminergic antidepressants, which increase the expression, but not the release of BDNF, and indicate that BDNF release accounts for the rapid actions of ketamine and other rapid acting agents (9, 10). In addition, we have recently demonstrated that VEGF release in the mPFC is also required for the rapid antidepressant-like actions of ketamine (25).
The results of the current study demonstrate that infusion of a VEGF nAb into the mPFC is sufficient to block the antidepressant-like effects of BDNF. The dependence on VEGF was observed in three different antidepressant behavioral paradigms, including a model of behavioral despair (FST), motivation and reward (FUST), and anxiety (NSF). While future studies will be needed to test this BDNF-VEGF interaction in chronic stress models, such as chronic unpredictable stress or social defeat (7, 48), the current results indicate a broad effect across these different behavioral paradigms. Together, the results demonstrate that the antidepressant-like behavioral actions of BDNF are dependent on release of VEGF from excitatory neurons in the mPFC. Immunoblot analysis demonstrates that the VEGF nAb does not cross-react with recombinant BDNF, but further studies are needed to demonstrate the lack of cross-reactivity under physiological conditions. In any case the results of the nAb approach were confirmed with an independent approach, neuronal deletion of VEGF (Camk2a-cre recombinase line crossed with a Vegfaflox/flox) (25). Here we show that the antidepressant-like behavioral actions of BDNF in all three behavioral paradigms are also blocked in the neuronal VEGF deletion mutants. Since VEGF is expressed by multiple cell types, including neurons, astrocytes, and endothelial cells (34), we cannot rule out the possibility that VEGF derived from one or more of the other cell types contributes to the BDNF response. However, the results indicate an essential role for VEGF derived from neurons.
Analysis of VEGF release in vivo is technically difficult, so we utilized a primary cortical neuron cell culture system that we have developed to demonstrate that ketamine stimulates BDNF release (24, 35). Here we show that incubation with BDNF increases the release of VEGF in primary cortical neurons, and that co-incubation with a selective TrkB inhibitor blocks both BDNF-induced as well as basal VEGF release. The mechanisms underlying BDNF-TrkB-stimulated VEGF release are unclear, but could involve effects on neuronal activity or signaling pathways linked with neurotrophic factor release. Evidence for an activity-dependent mechanism is provided by previous studies reporting that infusion of BDNF into the mPFC (49) or hippocampus induces c-Fos expression (50). Induction of c-Fos is coupled with neuronal activity, although stimulation of intracellular signaling pathways independent of neuronal activity can also increase this immediate early gene. More direct evidence is provided by electrophysiological studies demonstrating that BDNF potentiates glutamatergic transmission by increasing the probability of presynaptic release in hippocampal primary neurons or slices (51–54) and in visual cortex slices (55). BDNF stimulates the release of Ca2+ from intracellular stores via activation of phospholipase Cγ (PLCγ) (13), which could stimulate VEGF release (Figure 6). These findings are consistent with the possibility that BDNF-enhancement of glutamatergic transmission stimulates activity-dependent VEGF release. There is also evidence that BDNF stimulates VEGF expression and release via the mechanistic target of rapamycin complex 1 pathway and induction of hypoxia-inducible factor-1α in a neuroblastoma cell line (36). These reports raise the possibility that activity-dependent, as well as intracellular signaling could be involved in VEGF release, and further studies are needed to determine the exact pathways.
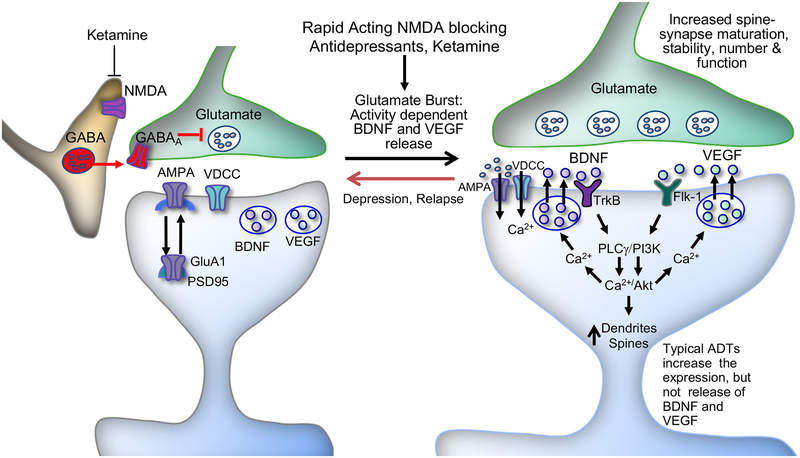
Figure 6.
Model for the cellular mechanisms underlying the rapid antidepressant-like actions of ketamine. Ketamine induces a rapid glutamate burst via blockade of NMDA receptors located on GABAergic interneurons and the resulting disinhibition. This stimulates postsynaptic AMPA receptors, which causes depolarization and activation of L-type voltage-dependent Ca2+ channels (VDCCs), leading to stimulation of BDNF and VEGF release. BDNF and VEGF stimulate TrkB and Flk-1, respectively, and the activation of TrkB and Flk-1 stimulates not only the phosphoinositide 3-kinase (PI3K)/Akt/mechanistic target of rapamycin complex 1 (mTORC1) pathway, but also release of Ca2+ from intracellular stores via activation of phospholipase Cγ (PLCγ), which stimulates hetelorogous release of the other factor. The PI3k/Akt/mTORC1 pathway controls dendritic arborization and the translation and synthesis of synaptic proteins, including GluA1 and postsynaptic density protein 95 (PSD95), which are required for increases in synaptogenesis and spine maturation. These cellular events are associated with the rapid and sustained antidepressant-like actions of ketamine. Previous studies demonstrate that BDNF and VEGF also induce further glutamate release via presynaptic TrkB and Flk-1, respectively, resulting in further activity-dependent release of these trophic factors (see discussion). ADT, antidepressant; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GABAA, γ-aminobutyric acid receptor A; GluA1, subtype of AMPA receptor; NMDA, N-methyl-D-aspartate receptor.
Ketamine rapidly increases the number and function of spine synapses on layer V pyramidal neurons in the mPFC, and these synaptic effects require activity-dependent BDNF and VEGF release (6, 10, 25, 28). Similar to ketamine, rapastinel and scopolamine also increase the number and function of spine synapses in the mPFC (39, 47, 56, 57). All of these rapid-acting antidepressants produce neurotrophic actions in primary cortical neurons, including increased BDNF release and increased dendrite complexity (24, 25, 35, 47). We have also reported that ketamine, as well as VEGF induction of dendrite complexity is completely blocked by a selective Flk-1 inhibitor (25). The current study demonstrates that BDNF increases dendrite complexity in primary neurons, and that these neurotrophic effects are completely blocked by incubation with a selective Flk-1 inhibitor. These findings provide further evidence that VEGF is required for the neurotrophic actions of BDNF on dendrite complexity.
The results clearly demonstrate a requirement for VEGF in the antidepressant-like and neurotrophic actions of BDNF, but we also examined reciprocal interactions between these two factors. Somewhat surprising, we found that the antidepressant-like and neurotrophic effects of VEGF required BDNF release and TrkB signaling. Using similar experimental approaches, the results show that co-infusion of a BDNF nAb into the mPFC blocks the antidepressant-like behavioral responses of VEGF in the three behavioral paradigms tested. The neutralizing antibody used for these studies was specific to BDNF as there was no cross-reactivity with VEGF examined by immunoblot analysis. We also found that incubation of primary cortical neurons with VEGF stimulates the release of BDNF into the culture media, and that this effect is blocked by a selective Flk-1 anagonist. In addition, the results show that VEGF-stimulation of dendrite complexity is blocked by incubation with a selective TrkB receptor antagonist. The mechanisms underlying VEGF stimulation of BDNF release are unclear, but could also involve activity-dependent effects. VEGF increases presynaptic glutamate release probability, leading to enhanced glutamatergic transmission in primary hippocampal slices (58) and also increases excitatory transmission via postsynaptic NMDA receptors (59). VEGF also stimulates the release of Ca2+ from intracellular stores via activation of PLCγ (11), which could stimulate BDNF release (Figure 6).
In conclusion, the current results in combination with our recent findings (24, 25, 28, 35, 39, 47), demonstrate a key interdependence between BDNF and VEGF signaling in the mPFC, and suggest that this reciprocal dependence plays a crucial role in the neurotrophic and antidepressant-like effects of rapid-acting antidepressants. This is particularly clear for the antidepressant-like actions of ketamine, which are blocked by inhibition of either BDNF or VEGF (24, 25, 28). Although the requirement for VEGF in the actions of other agents, notably rapastinel and scopolamine have not been tested and the role of VEGF in depressed patients remains unclear, the prediction is that there is also a requirement for VEGF. These findings raise several interesting possibilities regarding the consequences of this interdependence. For example, previous studies demonstrate that deletion of either BDNF or VEGF in mice is insufficient to produce depressive behaviors, possibly due to the antidepressant-like and neurotrophic actions of the remaining factor (25, 28, 29), and it would be interesting to determine if dual deletion mutants display depressive-like behaviors. A related consequence is whether a functional polymorphism of one factor would increase vulnerability but is insufficient alone to produce depression, which appears to be the case for the BDNF Val66Met polymorphism (28, 60, 61). In contrast, the antidepressant actions of ketamine and other rapid acting agents could be attenuated by a functional polymorphism of one factor, as reported for the ketamine response in carriers of the BDNF Met allele (28, 39, 47, 62), although this effect also appears to be race specific (63). The present results provide new insights on the complex interdependence of these two critical neurotrophic factors that could have important consequences for understanding the pathophysiology and treatment of depression.
Supplementary Material
Acknowledgements and Disclosures
This study was supported by National Institute of Mental Health Grants MH045481 (R.S.D.), MH093897 (R.S.D.), and the State of Connecticut.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005): Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC (2015): The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76: 155–162. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. (2006): Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163: 28–40. [DOI] [PubMed] [Google Scholar]
- 4.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. (2000): Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 5.Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. (2006): A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. (2010): mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. (2011): Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duman RS, Heninger GR, Nestler EJ (1997): A molecular and cellular theory of depression. Arch Gen Psychiatry 54: 597–606. [DOI] [PubMed] [Google Scholar]
- 9.Duman RS, Monteggia LM (2006): A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016): Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO, et al. (2008): Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal 20: 714–725. [DOI] [PubMed] [Google Scholar]
- 12.Nowacka MM, Obuchowicz E (2012): Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 46: 1–10. [DOI] [PubMed] [Google Scholar]
- 13.Sasi M, Vignoli B, Canossa M, Blum R (2017): Neurobiology of local and intercellular BDNF signaling. Pflugers Arch 469: 593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drevets WC, Price JL, Simpson JR Jr., Todd RD, Reich T, Vannier M, et al. (1997): Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386: 824–827. [DOI] [PubMed] [Google Scholar]
- 15.Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. (2017): Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry 22: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. (1999): Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45: 1085–1098. [DOI] [PubMed] [Google Scholar]
- 17.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R (2005): Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 136: 29–37. [DOI] [PubMed] [Google Scholar]
- 18.Qi XR, Zhao J, Liu J, Fang H, Swaab DF, Zhou JN (2015): Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb Cortex 25: 75–83. [DOI] [PubMed] [Google Scholar]
- 19.Elfving B, Plougmann PH, Wegener G (2010): Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci Lett 474: 13–16. [DOI] [PubMed] [Google Scholar]
- 20.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ (2005): Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 21: 1304–1314. [DOI] [PubMed] [Google Scholar]
- 21.Howell KR, Kutiyanawalla A, Pillai A (2011): Long-term continuous corticosterone treatment decreases VEGF receptor-2 expression in frontal cortex. PLoS One 6: e20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi XR, Zhao J, Liu J, Fang H, Swaab DF, Zhou JN (2015): Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb Cortex 25: 75–83. [DOI] [PubMed] [Google Scholar]
- 23.Isung J, Aeinehband S, Mobarrez F, Martensson B, Nordstrom P, Asberg M, et al. (2012): Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry 2: e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS (2014): BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deyama S, Bang E, Wohleb ES, Li XY, Kato T, Gerhard DM, et al. (2018): Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. (2011): NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi M, Lee Sh, Chang HL, Son H (2016): Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochem Biophys Acta 1862: 1247–1254. [DOI] [PubMed] [Google Scholar]
- 28.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK (2012): Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, et al. (2004): Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A 101: 10827–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nibuya M, Morinobu S, Duman RS (1995): Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15: 7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nibuya M, Nestler EJ, Duman RS (1996): Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16: 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. (2003): Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warner-Schmidt JL, Duman RS (2007): VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A 104: 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS (2009): Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology 34: 2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS (2016): Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 111: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ (2006): Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res 66: 4249–4255. [DOI] [PubMed] [Google Scholar]
- 37.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, et al. (1999): VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159. [DOI] [PubMed] [Google Scholar]
- 38.Franklin KBJ, Paxinos G (2007): The mouse brain in stereotaxic coordinates, 3rd ed. Burlington, MA: Elsevier. [Google Scholar]
- 39.Kato T, Fogaca MV, Deyama S, Li XY, Fukumoto K, Duman RS (2018): BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry 23: 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, et al. (2010): The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry 67: 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dwyer JM, Maldonado-Aviles JG, Lepack AE, DiLeone RJ, Duman RS (2015): Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. Proc Natl Acad Sci U S A 112: 6188–6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong H, Futamura T, Jourdi H, Zhou H, Takei N, Diverse-Pierluissi M et al. (2002): Neurotrophins induce BDNF expression through the glutamate receptor pathway in neocortical neurons. Neuropharmacology 42: 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenstein JM, Mani N, Khaibullina A, Krum, JM (2003): Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci 23: 11036–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS (1994): Neurotrophic factors: from molecule to man. Trends Neurosci 17: 182–190. [DOI] [PubMed] [Google Scholar]
- 45.Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF (1996): Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci 16: 3123–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenner BM (2012): Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev 23: 15–24. [DOI] [PubMed] [Google Scholar]
- 47.Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, et al. (2018): Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry 83: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–868. [DOI] [PubMed] [Google Scholar]
- 49.Yue L, Ma LY, Cui S, Liu FY, Yi M, Wan Y (2017): Brain-derived neurotrophic factor in the infralimbic cortex alleviates inflammatory pain. Neurosci Lett 655: 7–13. [DOI] [PubMed] [Google Scholar]
- 50.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002): Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang H, Schuman EM (1995): Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267: 1658–1662. [DOI] [PubMed] [Google Scholar]
- 52.Carmignoto G, Pizzorusso T, Tia S, Vicini S (1997): Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol 498: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lessmann V, Heumann R (1998): Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience 86: 399–413. [DOI] [PubMed] [Google Scholar]
- 54.Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N (1998): Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci 18: 10231–10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng Q, Song SH, Augustine GJ (2017): Calcium-dependent and synapsin-dependent pathways for the presynaptic actions of BDNF. Front Cell Neurosci 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. (2017): GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 42: 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. (2013): Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 74: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, Yang C, Liu C, Zhang T, Yang Z (2016): Paradoxical effects of VEGF on synaptic activity partially involved in Notch1 signaling in the mouse hippocampus. Hippocampus 26: 589–600. [DOI] [PubMed] [Google Scholar]
- 59.De Rossi P, Harde E, Dupuis JP, Martin L, Chounlamountri N, Bardin M, et al. (2016): A critical role for VEGF and VEGFR2 in NMDA receptor synaptic function and fear-related behavior. Mol Psychiatry 21: 1768–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. (2006): Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314: 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY (2012): Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 32: 4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. (2012): Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 72: e27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su TP, Chen MH, Li CT, Lin WC, Hong CJ, Gueorguieva R, et al. (2017): Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology 42: 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.