Abstract
The lung is the primary respiratory organ of the human body and has a complicated and precise tissue structure. It comprises conductive airways formed by the trachea, bronchi and bronchioles, and many alveoli, the smallest functional units where gas-exchange occurs via the unique gas-liquid exchange interface known as the respiratory membrane. In vitro bionic simulation of the lung or its microenvironment, therefore, presents a great challenge, which requires the joint efforts of anatomy, physics, material science, cell biology, tissue engineering, and other disciplines. With the development of micromachining and miniaturization technology, the concept of a microfluidics-based organ-on-a-chip has received great attention. An organ-on-a-chip is a small cell-culture device that can accurately simulate tissue and organ functions in vitro and has the potential to replace animal models in evaluations of drug toxicity and efficacy. A lung-on-a-chip, as one of the first proposed and developed organs-on-a-chip, provides new strategies for designing a bionic lung cell microenvironment and for in vitro construction of lung disease models, and it is expected to promote the development of basic research and translational medicine in drug evaluation, toxicological detection, and disease model-building for the lung. This review summarizes current lungs-on-a-chip models based on the lung-related cellular microenvironment, including the latest advances described in studies of lung injury, inflammation, lung cancer, and pulmonary fibrosis. This model should see effective use in clinical medicine to promote the development of precision medicine and individualized diagnosis and treatment.
I. INTRODUCTION
The cell is the basic structural and functional unit of living organisms and an important basis for living organisms to possess a specific morphology and maintain specific biological functions. The cellular microenvironment refers to the sum of the peripheral environment in which cells live; it is a complex system with variable space-time composed of multiple physical and chemical factors.1 Studies have shown that changes in any minor factor in the cellular microenvironment may result in a significant change in cellular morphology and function, which are closely related to physiologic and pathologic processes such as embryonic development, repair of tissue damage, tumorigenesis, and tumor development.2 Therefore, examinations of the interaction between cells and the cellular microenvironment are of great importance in tissue engineering and biomedicine, and it is important for researchers to study the in vitro bionic construction of the cellular microenvironment.3,4
It is necessary to understand the composition of the cellular microenvironment to explore its interaction with cells,5–7 especially the response of cells to microenvironmental changes, to construct a more realistic in vitro cellular microenvironment.8,9 At present, the cellular microenvironment can be simply summarized as biochemical factors represented by soluble small molecules, proteins, and cells and biophysical factors represented by cellular mechanics (such as fluid shear stress, stretch stress, and compression stress), the physical properties of the matrix, the morphological characteristics of the matrix surface, light, and heat.10 In recent years, the development and perfection of analytic and experimental methods and detection technology have promoted great progress in the in vitro construction and study of the cellular microenvironment, integrated multiple biochemical factors, cell coculture, three-dimensional culture, and biomechanics, which enables the in vitro construction of a multidimensional and multifactorial cellular microenvironment.11,12 Moreover, with the development of micromachining technology, especially the rapid advances in soft lithography technology,13 the concept of an organ-on-a-chip based on microfluidic technology has received considerable attention.14–18
An organ-on-a-chip is a small cell-culture device that is potentially superior to animal models for the evaluation of drug toxicity and efficacy. It comprises a cell culture microchamber and fluid microchannel and can simulate the cellular microenvironment and the blood circulation system (or other vessels).19 The flexible combination and functional integration of microfluidics on the chip enable precise regulation and real-time observation of the microenvironment, including of biochemical factors such as concentration gradients and other interactions between cells, and of biophysical factors such as fluid shear stress and stretch stress. In addition, an organ-on-a-chip is a bionic system that can accurately construct in vitro tissue and organoid functions. It thus has potential as an effective tool for in vitro reconstruction of the real cellular microenvironment.20,21 In addition to lung-on-a-chip,22 other organs-on-a-chip have been constructed on an exploratory basis, including liver-on-a-chip,23 gut-on-a-chip,24 and brain-on-a-chip,25 and progress has been made in the simulation of pathologic conditions in a physiologic cellular microenvironment, including pulmonary edema, pulmonary embolism, and renal fibrosis, and in the simulation of intestinal flora and construction of the blood-brain barrier.26 In addition to the development of single-organ chips, the body-on-a-chip (BOC) concept has also been proposed. Body-on-a-chip comprises multiorgan systems contained on a microchip, to mimic physiological relations, enable recapitulation of organ-organ interactions and potentially whole-body responses to drugs, as well as serve as a model of diseases. Ultimately, it is hoped that an advanced body-on-a-chip will fully simulate the human circulatory system, providing an invaluable tool for drug discovery and screening. The establishment of kinetics and pharmacodynamics parameters provides a new platform to further consolidate this development.27,28
The lungs, as primary respiratory organs, have very complicated and precise tissue structures, composed of a variety of biochemical and biophysical factors, and possess the only gas-liquid exchange interface in humans. This interface is known as the respiratory membrane and is only several microns thick; it thus enables gas-exchange while also functioning as an air-blood barrier. Understandably, such a system poses great challenges to the in vitro bionic modeling of the lungs and respiratory membrane, that is, the simulation of the lung-related cellular microenvironment.29,30 As a consequence of environmental pollution, smoking, pathogenic bacteria, and other factors, the prevalence of lung diseases, especially lung cancer, has rapidly increased. For example, according to the annual Chinese cancer survey, published since 2015, lung cancer has become the cancer with the highest mortality and morbidity in China due to its high recurrence, easy metastasis, and drug resistance. Due to the complex etiology of other pulmonary diseases, and the variety of complications that ensue (cough, chest tightness, asthma, hemoptysis, etc.), drug therapies and compatibilities are complex, and drug resistance is common. Therefore, individualized treatment schemes are urgently needed to treat specific disease situations and patients. In vitro simulation of the pulmonary microenvironment and establishment of a pulmonary disease model in line with patients' own conditions can be used for disease research and efficacy evaluation to make clinical treatment more effective. Organs-on-a-chip are expected to achieve this goal. Among the organs-on-a-chip that have been constructed, a breathing lung-on-a-chip developed by Huh et al.31 was the first and most well known. Since then, the development of lung-on-a-chip technology has attracted research attention. On the basis of the lung-related cellular microenvironment, the study summarizes established lung-on-a-chip models and reviews the latest lung-on-a-chip advances in studies of lung injury, inflammation, lung cancer, and pulmonary fibrosis.
II. CONSTRUCTION AND BIOFUNCTION OF CURRENT LUNGS-ON-A-CHIP
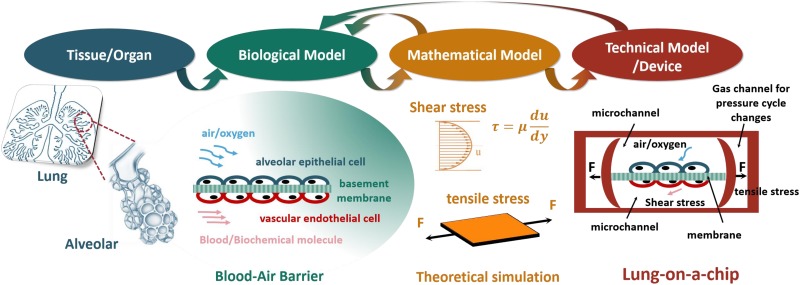
Generally, an in vitro bionic system should be based on the tissue or organ structure of living organisms, from which a biological model is abstracted. A mathematical model should then be constructed by analyzing a variety of factors in the biological model, and a technical model or device is established using existing technology.32 In this process, the mathematical model should be optimized by qualifying parameters to inform the construction of the technical model, so that the technical model accurately mimics the biological model, i.e., matches the structural features of the tissue or organ. This matching of structural features is performed because it is believed that they are necessary to represent the physiologic function of the tissue or organ. Overall, the principle of in vitro bionic system construction guides the construction of a lung-on-a-chip.
A. Lung microenvironment and model construction
When building a lung-on-a-chip, the structural characteristics of the lung tissue should be analyzed first. Anatomy indicates that the basic unit of the lungs is the pulmonary alveolus, which is a protuberant hemispheric sac that forms at the ends of the bronchioles, and the respiratory membrane of the alveolus is the main place for gas-exchange.33,34 That is, after oxygen enters the alveoli through the bronchiole, it passes through the respiratory membrane and enters the pulmonary capillaries.35,36 The biological model of alveoli can be simplified as an “air-blood barrier,” as shown in Fig. 1. The main factors of the pulmonary cellular microenvironment presented by the simplified biological model include biochemical factors,37,38 such as alveolar epithelial cells, vascular endothelial cells, and a composite model for basement membrane, air/oxygen, and blood; and biophysical factors,26,39 such as the gas pressure or flow rate, the strength and frequency of stretch stress, the fluid shear stress or flow rate of the bloodstream, and the culture environment of the three-dimensional cell. These factors are important for the construction of a lung-on-a-chip.
FIG. 1.
The schematic of the construction of lung-on-a-chip.
Current lung-on-a-chip models based on microfluidic chip technology usually simulate the trachea and blood vessels with a microfluidic channel and simulate the flow rate of the airstream and fluid shear stress of the bloodstream by controlling the flow rate of air or fluid.40 The respiratory membrane is simulated by placing “membrane materials” between the microchannel of the “trachea” and that of the “blood vessels,” and the “membrane materials” can be used for the basement of a supporting culture of cells, and used to realize stretch stress and simulate respiratory movement.29 Moreover, a series of mathematical or mechanical models, such as fluid mechanics and stretch stress, are applied during model construction. These models instruct the required parameter settings of a lung-on-a-chip, such as the flow rate of fluid, the stretch stress frequency, and size, to match the real physiologic structure.35,41
The biological model of a lung-on-a-chip is a simplified lung air-blood barrier, also known as the respiratory membrane, and the related cellular microenvironment includes biochemical and biophysical factors. The biochemical factors mainly include the cell or cellular constituent, protein (including growth factor and collagen), polysaccharide, and gas. In an in vitro cellular microenvironment that includes only biochemical factors constructed to study the interaction between lung-related cells, many studies have been performed with pore plate, transwell chamber, and various bioreactors in traditional biology.42,43 Moreover, the biophysical factors mainly include fluid shear stress, stretch stress, and three-dimensional cell culture.44 Although studies of these factors are not as extensive as those of biochemical factors, these factors have always been studied based on a parallel-plate flow chamber, drawing force model, and three-dimensional cell culture, which have provided a number of pioneering studies of in vitro construction of the lung cellular microenvironment and resulted in significant achievements.41,45 However, models based on a single factor or a few factors still have weaknesses and cannot simulate a cellular microenvironment with complicated biochemical and biophysical factors, present the complex biophysical or pathologic environment of human tissues and organs, or allow the study of tissues or organs, nor can they replace animal experiments, which have many problems, such as long cycles, high costs, bioethics issues, and species differences, to allow new drug development and clinical individualized diagnosis.39
B. Current models of lung-on-a-chip
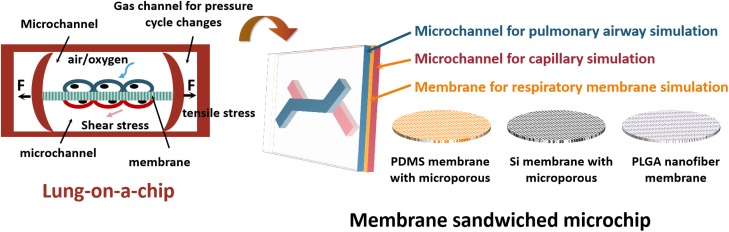
Lung-on-a-chip can to some extent integrate studies of biochemical and biological factors, enable the construction of a more complex system via micromachining technology, contain all factors in more models, even biological models, and enable fabrication of in vitro bionic lungs.46 In vitro bionic lung-on-a-chip based on microfluidic chip technology has been reported and is shown in Table I. According to the basic structure of the air-blood barrier, the lung-on-a-chip based on microfluidic chip technology is mainly a sandwiched microchip, as shown in Fig. 2. The first chip mentioned multiple times in the literature was developed by Huh et al.,36 which was constructed with polydimethylsiloxane (PDMS) and can simulate the human respiration process. The chip is divided into three layers: the upper layer is the gas channel, and the lower layer is the fluid channel, and they are separated by a flexible, porous PDMS membrane. Alveolar epithelial cells are cultured on the upper side of the membrane and placed in the gas-liquid interface, and the lower side contains vascular endothelial cells infiltrated in a dynamic fluid environment to simulate the human alveolar-capillary interface. In addition, two side channels connect to vacuums on the left and right sides of the chip's channel. Regular changes in gas pressure (0.25 Hz) deform the PDMS membrane to simulate the enlargement and contraction of the alveolar walls which occurs during breathing, which is difficult to realize in traditional in vitro models. This chip not only provides new technical thought for studies of pulmonary inflammation, edema, and embolism, but it also provides the technology for in vitro construction of other organs-on-chips. Another group developed a gut-on-a-chip using similar principles, to simulate peristalsis of the small intestine, absorption, and intestinal flora.24 In addition, an in vitro blood-brain barrier was developed to study drug transmission processes. Inspired by these studies, other membrane materials were also used to build lung chips, such as Si membrane with microporous, PLGA nanofiber membrane, etc.
TABLE I.
List for in vitro bionic lung-on-a-chip based on microfluidic chip technology.
| Diseases mimicking | Cell types | Factors investigated | Features | Ref. | |
|---|---|---|---|---|---|
| Lung injury | Mechanical cellular injury; ventilator-induced lung injury; acute respiratory distress syndrome; neonatal respiratory distress syndrome | Primary human small airway epithelial cells | Deleterious fluid mechanical stresses; air–liquid interface culture | Porous membrane sandwiched microchip; physiologic or pathologic liquid plug flows | Huh et al. (Ref. 41) |
| A549; primary murine alveolar epithelial cells | Combined effects of fluid mechanical and solid mechanical stress | Porous membrane sandwiched microchip; surface tension forces | Douville et al. (Ref. 44) | ||
| A549 | Fluid mechanical stresses of liquid plug propagation in airways; Survanta surfactant | Porous membrane sandwiched microchip; the protective role of surfactants in liquid plugs | Tavana et al. (Ref. 48) | ||
| Lung inflammation | Asthmatic; chronic obstructive pulmonary disease (COPD); pulmonary edema; emphysema; pulmonary diseases caused by particle transport and deposition | Beas-2B; primary human fibrocytes | Eosinophil cationic protein (ECP); medium flow | Porous silicon membrane sandwiched microchip; fibrocyte extravasation | Punde et al. (Ref. 30) |
| Human alveolar epithelial cells; endothelium cells, neutrophils | Mechanical stretching; air–liquid interface culture; cytokines (ICAM-1,TNF-a); bacterial, nanoparticles and interleukin-2 | Porous membrane sandwiched microchip; alveolar-capillary barrier | Huh et al. (Ref. 36) | ||
| Human alveolar epithelial cells; HUVECs | Nanoparticles (TiO2 and ZnO); cell coculture; blood vessel-tissue interface | Multichannel microfluidic chip; perfusion culture | Zhang et al. (Ref. 46) | ||
| Calu-3; human bronchial smooth muscle cells | Rat-tail type I collagen and Matrigel; cell coculture | Multilayer PMMA chip | Humayun et al. (Ref. 47) | ||
| A549 | Particles; airflow and medium flow | PMMA chip; computational fluid dynamics simulation | Moghadas et al. (2018) (Ref. 49) | ||
| Primary human airway epithelial cells; neutrophils | Interleukin-13; inflammatory cytokines; air–liquid interface culture | Porous membrane sandwiched microchip; secretion of the inflammatory cytokines | Benam et al. (2016) (Ref. 50) | ||
| Primary human airway epithelial cells obtained from healthy donors or COPD patients | Cigarette smoke extract (CSE); cigarette smoke condensate (CSC) | Porous membrane sandwiched microchip; smoke-induced pathological microenvironment | Benam et al. (Ref. 51) | ||
| Pulmonary fibrosis | Pulmonary fibrosis | Primary human small airway epithelial cells | Antifibrotic drugs pirfenidone and nintedanib; TGF-β1; mechanical stretching and traction | Simulating the membranous morphology of the alveolar wall; PDMS micropillars | Asmani et al. (Ref. 55) |
| Lung cancer | Nonsmall cell lung cancer | A549; HFL1; HUVEC | Antitumor drug Gefitinib; IGF; cell coculture; 3D microenvironment | PLGA nanofiber membrane sandwiched microchip | Yang et al. (Ref. 57) |
FIG. 2.
The schematic of the membrane sandwiched microchip.
In addition, a lung-on-a-chip made of other materials was recently reported: Humayun et al.47 developed a lung/airway-on-a-chip made of poly(methyl methacrylate) thermoplastic plastics to simulate the lung bronchia microenvironment. This microdevice comprised three vertically stacked microfluidic chambers: smooth muscle cells cultured on the base, a thin hydrogel in the middle, and a microchamber above to realize the culture of the gas-fluid interface of epithelial cells. This device was used to study the interactions between airway epithelial cells and airway smooth muscle cells, which revealed that various ratios of type I rat-tail collagen and Matrigel influence the growth of both kinds of cells; optimal cell coculture effects were achieved with high ratios of Matrigel. Moreover, Humayun et al. also detected the marker of gas-fluid interface culture of airway epithelial cells—goblet cell marker (MUC5AC)—which indicates that the model successfully simulated the growing property of pulmonary trachea cells at the gas-fluid interface. The lungs-on-chips described above incorporate human lung cells, a multicellular structure, and microchannels that allow continuous perfusion of fluid or air. These devices can be used to simulate the lung biochemical and biophysical cellular or tissue microenvironments from various perspectives, build lung disease models, and evaluate drug efficacy, thus demonstrating the great potential of the lung-on-a-chip for enhancing pulmonary research and drug development.
III. THE LATEST ADVANCES OF LUNG-ON-A-CHIP MODELS
Studies of organs-on-chips aim to better simulate the evaluation of drug toxicity and efficacy using a cell/tissue microenvironment in physiologic and/or pathologic circumstances and provide technical support for drug screening and individualized diagnosis and treatment. In addition, these studies are intended to build disease models with organs-on-a-chip. The lung-on-a-chip, as an organ-on-a-chip, not only simulates the lung cell/tissue microenvironment in physiologic and/or pathologic circumstances, it can also be used to study lung diseases, such as lung injury, inflammation, lung cancer, and pulmonary fibrosis.
A. Lung injury
According to the composition of the lung cell microenvironment, the lung is a dynamic mechanical organ, and the alveolar epithelial cells that form the alveoli are constantly stimulated by various mechanical forces. Nevertheless, external forces always cause mechanical injury of cells. The damage models for pulmonary epithelial cells developed on the basis of a microfluidic platform include a shear force model, a mechanical stretch model, and a model that includes both forces.44 In terms of the shear force model, researchers have explored the mechanical damage to small airway epithelial cells caused by a liquid plug of limited length formed in the microchannel of a microfluidic chip. The generator of the microfluidic plug transports 1 pl of blocking fluid to the microchannels of the epithelial cells cultured on the gas-liquid interface. The liquid flows out when the plug is diffused, leading to rupture of the liquid plug that causes a crackle that can be heard on an auscultator in the clinic. In addition, repeated rupture of the plug results in cell death. It has been demonstrated that reopening of the trachea may mechanically aggravate lung injury, especially when combined with surfactant dysfunction of the lungs. Pulmonary surfactant played a crucial role in mitigating the detrimental effect of reopening stresses. Airways of the peripheral lung are prone to closure at low lung volumes. Deficiency or dysfunction of pulmonary surfactant during various lung diseases compounds this event by destabilizing the liquid lining of small airways and giving rise to occluding liquid plugs in airways.41 The study facilitates our understanding of the response of cells to complex mechanical forces of the lung's arteries and may contribute to the design of a strategy to treat and prevent fluid mechanical lung injury. Based on the model above, Tavana et al.48 successfully designed a microfluidic lung-on-a-chip and simulated the damage of a liquid plug on pulmonary epithelial cells caused by the deposition of airway surface liquid. Their results indicated that a number of damaged cells are formed when the liquid plug is flowing and that surfactant can effectively prevent cell death. Therefore, surfactant can be regarded as a potential treatment for many lung diseases, including acute respiratory distress syndrome. In contrast, in a study of the mechanical stretch model, Douville et al.44 designed a new microfluidic device to reconstruct the unique mechanical stretch on the human alveolar air-blood barrier. The microfluidic device includes three parts. The upper layer simulates the alveolar cavity and can culture alveolar epithelial cells and provide a living microenvironment for cell culture. The lower channel is a driven chamber that connects easily to a programmable injection pump sold on the market to control the degree and rate of bending of the PDMS membrane. The thin PDMS membrane separates the alveolar cavity from the driven chamber, causes the cell culture room to deform under external force, and simulates the deformation process of pulmonary alveoli. In addition, researchers have simulated physiologic inflation and pathologic edema by changing the liquid load on the alveoli. The microfluidic chip has been used to successfully combine the environmental factors of solid and fluid mechanics, simulate pathologic and physiologic conditions, including lung injuries caused by a respirator, and verify that the gas-liquid interface exerts a harmful influence on cell activity after32 “respirations” in austere conditions.18
B. Lung inflammation
Pulmonary inflammation is a common lung disease that is usually caused by infection with bacteria, virus, mycoplasma, fungus, and/or other pathogens (such as parasites) or by noninfectious pneumonia, such as radiation pneumonia, allergic pneumonia, and chemical pneumonia (i.e., inhalation of irritating liquid or gas and drugs).30–49 These inflammatory factors can lead to a series of inflammatory responses, such as the expansion of capillaries and the aggregation and exudation of white blood cells. A lung-on-a-chip has been reported to simulate inflammatory responses. For example, with the double-membrane pulmonary respiration chip mentioned above,35,36 proinflammatory cytokines (tumor necrosis factor-α) and bacteria (Escherichia coli) were introduced into the alveolar microchambers of the upper layer of the alveolus-capillary interface (air-blood barrier). This led to the expression of intercellular adhesion molecule-1 by the vascular endothelial cells on the other side of the membrane, which promoted the formation of capillaries to simulate the adhesion of neutrophils in the microchannel on the surface of vascular endothelial cells to recruit neutrophils. Neutrophils were then observed to penetrate the vascular endothelial cells and the membrane and enter the alveolar microchambers, resulting in directional migration and phagocytosis of bacteria. Because the double-membrane pulmonary respiration chip has a transparent PDMS pore membrane, the entire complex physiologic reaction process can be directly observed and analyzed, and the recruitment and migration of inflammatory cells can be displayed during the occurrence of inflammation in vitro, which fully shows the advances of the lung-on-a-chip.
In addition to studies of pulmonary inflammation, models of some chronic pulmonary inflammatory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma, have been also realized with the lung-on-a-chip. Benam et al.50 designed a lung-on-a-chip to simulate the bronchia of the human lungs. Specifically, primary human airway epithelial cells were cultured on the upper layer of the porous membrane, and primary human pulmonary microvascular endothelial cells were cultured on the lower layer. A perfusion culture promotes the differentiation of human airway epithelial cells, so the cells form a real cytomembrane or tissue interface. In the lung-on-a-chip, the airway epithelial cells extracted and constructed from primary cells include mucus-producing goblet cells, club cells, and basal cells, with a ratio similar to that in normal human lung tissue, which reproduces the cell tissue microenvironment of the pulmonary bronchia in vitro. Accordingly, researchers have constructed a model of COPD to reconstruct important features of the disease in vitro with the cells of patients with COPD, including selective cytokine hypersecretion, increased neutrophil recruitment, and clinical exacerbation by exposure to viral and bacterial infections. Benam et al.51 further studied the influence of smoking on health in patients with COPD by combining two organs-on-a-chip with human bronchiolar epithelial cells with a smoking device and a micro-respirator to simulate human respiration. In addition, the influence of smoke from conventional cigarettes and electronic cigarettes was detected in cells. While the gene expression profile of COPD samples' exposure to smoke was examined, 276 genes were found with differential expression from control samples without exposure to smoke, including 147 genes for which an association with the onset of COPD has been proved. The study shows the great potential of a lung-on-a-chip to simulate lung diseases and realize individualized diagnosis and treatment, and it is predicted that these efforts will be conducive to the discovery of new drugs and will help to pave the way for more treatment directions.
Bronchial asthma is another chronic airway inflammatory condition that includes multiple cells and cell components, with symptoms such as dyspnea, wheezing, and cough mostly caused by inhalation of allergens.52,53 Furthermore, infiltration of polymorphonuclear cells (mostly eosinophilic granulocytes), mononuclear cells (mostly lymphocytes), and mast cells is the early event that leads to bronchitis. Animal models have indicated that eosinophilic granulocytes contribute to chronic bronchial asthma.54 Punde et al.30 established a bionic microsystem to simulate the lung microenvironment to monitor the effects of eosinophil cationic protein (ECP) on pulmonary inflammation. The bronchia are simulated by placing a micropore array silicon slice between two PDMS channels. The upper microchannel is used to simulate the blood circulation system. The micropores are enveloped by fibronectin to enhance the adhesion of Beas-2B bronchial epithelial cells to simulate the bronchial microenvironment together with the lower microchannel. Studies of the function of ECP in bronchial simulation with a microfluidic device found that ECP can stimulate Beas-2B cells to express CXCL12 and attract the migration of fibrocytes that express CXCR4 to epithelial cells. This study revealed the role of the CXCL12–CXCR4 axis in the mediation of ECP-induced fibrocyte extravasation in lung inflammation. The result was similar to the quantitative result of fibrocyte migration revealed in conventional experiments using a transwell chamber, which proves the effectiveness of the bronchia chip. The model provided a new method to study the quantitative detection of cell migration and concurrent cell-cell interaction.
C. Other diseases: Pulmonary fibrosis and lung cancer
In addition to studies of injury and inflammation in lungs-on-chips, a few studies have reported a bionic microenvironment of lung tumor and pulmonary fibrosis. For example, Asmani et al.55 recently developed a new class of fibrotic microtissue arrays to simulate key biomechanical events during pulmonary fibrosis, including progressive sclerosis and contraction of alveolus tissue and bronchiectasis due to lung tissue compliance decrease and tractive force. They also evaluated the antifibrosis drugs pirfenidone and nintedanib. Although the bionic system differs from common lung-on-a-chip models, a new dynamics system that simulates bronchial expansion on the basis of a combination of micropillar arrays has been developed with micromachining technology with theoretical simulation to provide more ideas for the future development of lungs-on-a-chip.
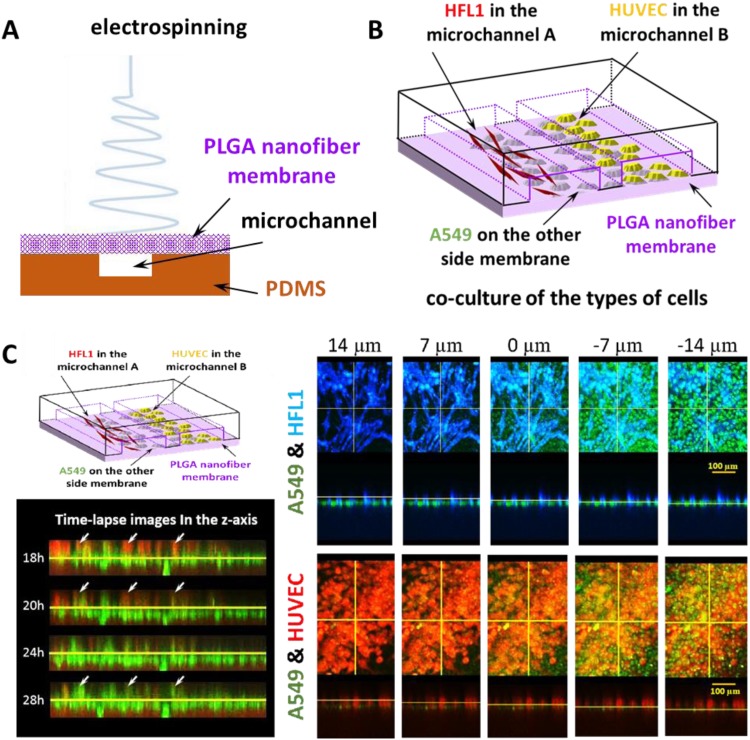
It is well known that lung cancer has the highest prevalence and mortality rate among cancers in mainland China due to its high rate of recurrence, metastasis, and drug resistance. Moreover, there are several types of lung cancer, such as small-cell carcinoma and nonsmall-cell carcinoma; the latter can be classified into adenocarcinoma, squamous carcinoma, and large-cell carcinoma, which has led to the use of numerous chemotherapeutic drugs, diverse medication regimens, and strong drug resistance. Therefore, individualized diagnosis and treatment are urgently needed as the basis for symptomatic medication. In addition, tumor development is a progressive process. Refractory cancers such as lung cancer can be better treated and controlled with comprehensive investigation including long-term culture, metastasis monitoring, and drug sensitivity assessment of tumor cells.56 The lung-on-a-chip provides a new technical method for individualized treatment of lung cancer. Although the epithelium-derived lung tumor cell line is usually used to build a lung-on-a-chip or a trachea-on-a-chip, including the replacement of pulmonary epithelial cells with A549, lung tumors are seldom studied. In a study at the Bio-microfluidics Laboratory of Shanghai University Materials Genome Project Institute, a lung-on-a-chip built with poly(lactic-co-glycolic acid) nanometer electrospinning as the backing material was used to realize three-dimensional coculture of lung tumor cells, lung fibroblasts, and vascular endothelial cells. It was also used to evaluate gefitinib, an antitumor drug that targets epidermal growth factor receptor, and lung cell A549 was observed to break through the barrier of blood vessels, leading to endosmosis. A very thin membrane can be prepared with nanometer electrospinning technology to realize cell coculture on both sides of the membrane to simulate the respiratory membrane, as shown in Fig. 3. However, biological forces (fluid shear stress and stretch stress) not stated in the study are associated with insufficient strength of poly(lactic-co-glycolic acid) materials. The team will continue to study and develop lungs-on-a-chip or lung tumor organs-on-a-chip to search for new solutions.57
FIG. 3.
A lung-on-a-chip built with poly(lactic-co-glycolic acid) nanometer electrospinning as the supporting material was used to realize three-dimensional coculture of lung tumor cells. (a) Schematic diagram of microfluidic chip with PLGA nanofiber membrane as the substrate prepared by electrospinning. (b) Schematic diagram of the coculture of A549 cells, HFL1 cells, and HUVECs. (c) Schematic diagram of the coculture of three kinds of cells on the microchip. Fluorescence image of A549, HFL1, and HUVEC cells in the direction of the Z axis layer by layer. Time-lapse images of A549 cells and HUVECs in the Z direction at the same location at different times. Reproduced with permission from Yang et al., Lab Chip 18(3), 486–495 (2018). Copyright 2018 Royal Society of Chemistry.
IV. CONCLUSION AND PROSPECTS
The lung-on-a-chip, as the first proposed and developed organ-on-a-chip, has always been a hot research topic and has flourished with advances in micromachining technology and miniaturization. The complexity of the lung cell microenvironment impedes the development of an in vitro microsystem, but the strategy of new organs-on-chips based on microfluidic chips that integrate anatomy, physics, materials science, and cell biology provides new opportunities for exploration of the lung cell microenvironment.58–61 Moreover, the strengths of organs-on-chips in drug evaluation, toxicity detection, and disease model construction may greatly promote the development of fundamental research and translational medicine. In terms of fundamental research, studies of the pathogenic factors, pathogenesis, and progression of lung diseases may benefit from exploration with organs-on-chips or in vitro bionic microphysiologic systems, especially in China, a developing country with factors that lead to severe lung diseases, including environmental pollution (PM2.5, heavy metal particles) and large smoking population. In contrast, the development of translational medicine is expected by doctors and patients to open the door to precision medicine, to provide individualized treatment regimens, and to effectively improve cure rates or survival rates. Furthermore, it is worthy of note that clinical medication or treatment may be directly instructed by models of common lung diseases, such as lung injury, pulmonary inflammation, fibrosis, and tumor, especially the establishment of individualized in vitro disease models based on the extraction of patients' cells or living tissues. Recent advances in lungs-on-a-chip have reported disease studies based on the direct extraction of patients' cells, in vitro culture and amplification, and construction of lungs-on-chips. As a result, it is anticipated that lungs-on-a-chip or similar in vitro bionic microphysiologic systems will successfully create a bionic lung microenvironment that will be acknowledged as the “gold standard” and effectively used in clinical medicine. This review aims to discuss the lung-on-a-chip as a disease model and promote further research work.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 31800848), the State Key Laboratory of Transducer Technology of China (Grant No. SKT1806), Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and Materials Genome Institute, Shanghai University.
REFERENCES
- 1.Zhang H., Dai S., Bi J., and Liu K. K., Interface Focus 1(5), 792–803 (2011). 10.1098/rsfs.2011.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom A. B. and Zaman M. H., Physiol. Genomics 46, 309–314 (2014). 10.1152/physiolgenomics.00170.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D. and Weinberg R. A., Cell 144(5), 646–674 (2011). 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Colotta F., Allavena P., Sica A., Garlanda C., and Mantovani A., Carcinogenesis 30(7), 1073–1081 (2009). 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- 5.Villanueva J. and Herlyn M., Current Oncol. Rep. 10, 439–446 (2008). 10.1007/s11912-008-0067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiao S. L., Chu G. C., and Chung L. W., Cancer Lett. 380(1), 340–348 (2016). 10.1016/j.canlet.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Liu M., Li Y. et al. , Eur. Polym. J. 72, 590–601 (2015). 10.1016/j.eurpolymj.2015.03.019 [DOI] [Google Scholar]
- 8.Ye J., Wu D., Wu P., Chen Z., and Huang J., Tumour Biol. 35(5), 3945–3951 (2014). 10.1007/s13277-013-1561-x [DOI] [PubMed] [Google Scholar]
- 9.Lorusso G. and Ruegg C., Histochem. Cell Biol. 130(6), 1091–1103 (2008). 10.1007/s00418-008-0530-8 [DOI] [PubMed] [Google Scholar]
- 10.Shieh A. C., Ann. Biomed. Eng. 39(5), 1379–1389 (2011). 10.1007/s10439-011-0252-2 [DOI] [PubMed] [Google Scholar]
- 11.Huh D., Hamilton G. A., and Ingber D. E., Trends Cell Biol. 21(12), 745–754 (2011). 10.1016/j.tcb.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker B. M. and Chen C. S., J. Cell Sci. 125(Pt. 13), 3015–3024 (2012). 10.1242/jcs.079509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcdonald J. C., Anderson J. R., Chiu D. T. et al. , Electrophoresis 21, 27–40 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Whitesides G. M., Nature 442(7101), 368–373 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- 15.Squires T. M. and Quake S. R., Rev. Mod. Phys. 77(3), 977–1026 (2005). 10.1103/RevModPhys.77.977 [DOI] [Google Scholar]
- 16.Teh S. Y., Lin R., Hung L. H., and Lee A. P., Lab Chip 8(2), 198–220 (2008). 10.1039/b715524g [DOI] [PubMed] [Google Scholar]
- 17.Bhatia S. N. and Ingber D. E., Nat. Biotechnol. 32(8), 760–772 (2014). 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 18.Moraes C., Mehta G., Lesher-Perez S. C., and Takayama S., Ann. Biomed. Eng. 40(6), 1211–1227 (2012). 10.1007/s10439-011-0455-6 [DOI] [PubMed] [Google Scholar]
- 19.Bhise N. S., Ribas J., Manoharan V. et al. , J. Controlled Release 190, 82–93 (2014). 10.1016/j.jconrel.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caplin J. D., Granados N. G., James M. R., Montazami R., and Hashemi N., Adv. Healthcare Mater. 4(10), 1426–1450 (2015). 10.1002/adhm.201500040 [DOI] [PubMed] [Google Scholar]
- 21.Zheng F., Fu F., Cheng Y., Wang C., Zhao Y., and Gu Z., Small 12(17), 2253–2282 (2016). 10.1002/smll.201503208 [DOI] [PubMed] [Google Scholar]
- 22.Pollard B. S. and Pollard H. B., Pediatr. Pulmonol. 53(S3), S12–S29 (2018). 10.1002/ppul.24118 [DOI] [PubMed] [Google Scholar]
- 23.Lee S. A., No D. Y., Kang E., Ju J., Kim D. S., and Lee S. H., Lab Chip 13(18), 3529–3537 (2013). 10.1039/c3lc50197c [DOI] [PubMed] [Google Scholar]
- 24.Kim H. J., Huh D., Hamilton G., and Ingber D. E., Lab Chip 12(12), 2165–2174 (2012). 10.1039/c2lc40074j [DOI] [PubMed] [Google Scholar]
- 25.Park J., Lee B. K., Jeong G. S., Hyun J. K., Lee C. J., and Lee S. H., Lab Chip 15(1), 141–150 (2015). 10.1039/C4LC00962B [DOI] [PubMed] [Google Scholar]
- 26.Mertens T. C. J., Karmouty-Quintana H., Taube C., and Hiemstra P. S., Pulm. Pharmacol. Ther. 45, 101–113 (2017). 10.1016/j.pupt.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 27.Sung J. H., Wang Y. I., Kim J. H., Lee J. M., and Shuler M. L., AIChE J. 64(12), 4351–4360 (2018). 10.1002/aic.16448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palaninathan V., Kumar V., Maekawa T. et al. , MRS Commun. 8(03), 652–667 (2018). 10.1557/mrc.2018.148 [DOI] [Google Scholar]
- 29.Doryab A., Amoabediny G., and Salehi-Najafabadi A., Biotechnol. Adv. 34(5), 588–596 (2016). 10.1016/j.biotechadv.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 30.Punde T. H., Wu W. H., Lien P. C. et al. , Integr. Biol. 7(2), 162–169 (2015). 10.1039/c4ib00239c [DOI] [PubMed] [Google Scholar]
- 31.Huh D. D., Ann. Am. Thoracic Soc. 12(Suppl. 1), S42–44 (2015). 10.1513/AnnalsATS.201410-442MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu T., Guo Z., Fan H. et al. , Oncotarget 7(18), 25593–25603 (2016). 10.18632/oncotarget.8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haefeli-Bleuer B. and Weibel E. R., Anat. Rec. 220, 401–414 (1988). 10.1002/ar.1092200410 [DOI] [PubMed] [Google Scholar]
- 34.Ochs M., Nyengaard J. R., Jung A. et al. , Am. J. Respir. Crit. Care Med. 169(1), 120–124 (2004). 10.1164/rccm.200308-1107OC [DOI] [PubMed] [Google Scholar]
- 35.Huh D., Leslie D. C., Matthews B. D. et al. , Sci. Transl. Med. 4(159), 159ra147 (2012). 10.1126/scitranslmed.3004249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huh D., Matthews B. D., Mammoto A. et al. , Science 328(5986), 1662–1668 (2010). 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schilders K. A., Eenjes E., van Riet S. et al. , Respir. Res. 17, 44 (2016). 10.1186/s12931-016-0358-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson A., Singh S., Fernekorn U., and Schober A., Lab Chip 13(18), 3471–3480 (2013). 10.1039/c3lc50237f [DOI] [PubMed] [Google Scholar]
- 39.Konar D., Devarasetty M., Yildiz D. V., Atala A., and Murphy S. V., Biomed. Eng. Comput. Biol. 7(Suppl. 1), 17–27 (2016). 10.4137/BECB.S34252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajaj P., Harris J. F., Huang J.-H., Nath P., and Iyer R., ACS Biomater. Sci. Eng. 2(4), 473–488 (2016). 10.1021/acsbiomaterials.5b00480 [DOI] [PubMed] [Google Scholar]
- 41.Huh D., Fujioka H., Tung Y. C. et al. , Proc. Natl. Acad. Sci. U.S.A. 104(48), 18886–18891 (2007). 10.1073/pnas.0610868104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji X., Ji J., Shan F., Zhang Y., Chen Y., and Lu X., Int. J. Clin. Exp. Med. 8(5), 7002–7008 (2015), available at http://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC4509182&blobtype=pdf. [PMC free article] [PubMed] [Google Scholar]
- 43.Chu Y., Yang P., Yang S. et al. , Am. J. Respir. Cell Mol. Biol. 17, 353–360 (1997). 10.1165/ajrcmb.17.3.2837 [DOI] [PubMed] [Google Scholar]
- 44.Douville N. J., Zamankhan P., Tung Y. C. et al. , Lab Chip 11(4), 609–619 (2011). 10.1039/C0LC00251H [DOI] [PubMed] [Google Scholar]
- 45.Kniazeva T., Hsiao J. C., Charest J. L., and Borenstein J. T., Biomed. Microdevices 13(2), 315–323 (2011). 10.1007/s10544-010-9495-1 [DOI] [PubMed] [Google Scholar]
- 46.Zhang M., Xu C., Jiang L., and Qin J., Toxicol. Res. 7(6), 1048–1060 (2018). 10.1039/C8TX00156A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humayun M., Chow C. W., and Young E. W. K., Lab Chip 18(9), 1298–1309 (2018). 10.1039/C7LC01357D [DOI] [PubMed] [Google Scholar]
- 48.Tavana H., Zamankhan P., Christensen P. J., Grotberg J. B., and Takayama S., Biomed. Microdevices 13(4), 731–742 (2011). 10.1007/s10544-011-9543-5 [DOI] [PubMed] [Google Scholar]
- 49.Moghadas H., Saidi M. S., Kashaninejad N., and Nguyen N. T., Drug Delivery Transl. Res. 8(3), 830–842 (2018). 10.1007/s13346-017-0467-3 [DOI] [PubMed] [Google Scholar]
- 50.Benam K. H., Villenave R., Lucchesi C. et al. , Nat. Methods 13(2), 151–157 (2016). 10.1038/nmeth.3697 [DOI] [PubMed] [Google Scholar]
- 51.Benam K. H., Novak R., Nawroth J. et al. , Cell Syst. 3(5), 456–466 e454 (2016). 10.1016/j.cels.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 52.Corrigan C., Medicine 40(5), 223–227 (2012). 10.1016/j.mpmed.2012.02.007 [DOI] [Google Scholar]
- 53.Wilson J. W. and Bamford T. L., Pulm. Pharmacol. Ther. 14(3), 229–247 (2001). 10.1006/pupt.2001.0294 [DOI] [PubMed] [Google Scholar]
- 54.Humbles A. A., Lloyd C. M., McMillan S. J. et al. , Science 305(5691), 1776–1779 (2004). 10.1126/science.1100283 [DOI] [PubMed] [Google Scholar]
- 55.Asmani M., Velumani S., Li Y. et al. , Nat. Commun. 9(1), 2066 (2018). 10.1038/s41467-018-04336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Z., Gao Y., Hao Y. et al. , Biomaterials 34(16), 4109–4117 (2013). 10.1016/j.biomaterials.2013.02.045 [DOI] [PubMed] [Google Scholar]
- 57.Yang X., Li K., Zhang X. et al. , Lab Chip 18(3), 486–495 (2018). 10.1039/C7LC01224A [DOI] [PubMed] [Google Scholar]
- 58.Zhang C., Zhao Z., Abdul Rahim N. A., van Noort D., and Yu H., Lab Chip 9(22), 3185–3192 (2009). 10.1039/b915147h [DOI] [PubMed] [Google Scholar]
- 59.Luni C., Serena E., and Elvassore N., Curr. Opin. Biotechnol. 25, 45–50 (2014). 10.1016/j.copbio.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 60.Sei Y., Justus K., LeDuc P., and Kim Y., Microfluidics Nanofluidics 16(5), 907–920 (2014). 10.1007/s10404-014-1341-y [DOI] [Google Scholar]
- 61.Li K., Yang X., and Gao X., Biomicrofluidics 13, 014102 (2019). 10.1063/1.5064838 [DOI] [PMC free article] [PubMed] [Google Scholar]