Abstract
Background
Component-resolved diagnosis might improve the prediction of future allergy in young children.
Objective
We sought to investigate the association between IgE reactivity to the pathogenesis-related class 10 (PR-10) protein family and allergic rhinitis to birch pollen (ARbp) from early childhood up to age 16 years.
Method
Questionnaire data and sera obtained at 4, 8, and 16 years of age from the Barn/Children Allergi/Allergy Milieu Stockholm Epidemiologic (BAMSE) study birth cohort were used. Sera from 764 children were analyzed for IgE reactivity to 9 PR-10 allergen proteins at the 3 time points by using an allergen chip based on ISAC technology. ARbp was defined as upper airway symptoms during birch pollen exposure.
Results
IgE reactivity to Bet v 1 was found in 12%, 17%, and 25% of children at 4, 8, and 16 years of age. IgE reactivity of PR-10 proteins showed a hierarchic intrarelationship: Bet v 1 > Mal d 1 > Cor a 1.04 > Ara h 8 > Pru p 1 > Aln g 1 > Api g 1 > Act d 8 > Gly m 4. There was an increased risk of incidence and persistence of ARbp up to age 16 years with increasing levels of Bet v 1–specific IgE or increasing numbers of IgE-reactive PR-10 proteins at 4 years. Children with severe ARbp at age 16 years had higher levels of Bet v 1–specific IgE at age 4 years compared with children with mild symptoms.
Conclusion
ARbp at age 16 years can be predicted by analysis of IgE reactivity to PR-10 proteins in early childhood. (J Allergy Clin Immunol 2015;135:1199-206.)
Keywords: Allergen components, allergic rhinitis, oral allergy syndrome, BAMSE, birch pollen, cohort, cross-reactivity, IgE, MeDALL, microarray
Allergic rhinitis (AR), the most common chronic disease in childhood, has a substantial effect on quality of life.1 One of the major challenges that remain to be addressed is the prediction of onset, persistence, and severity of allergic diseases across the life cycle. Long-term birth cohort studies are essential to understanding the life course and childhood predictors of allergy and the complex interplay between genes and the environment.2 Respiratory allergy to birch and other Fagales pollens, such as hazel and alder, is frequent in the Northern Hemisphere.3 Allergen molecules from Fagales pollen (ie, Bet v 1 from birch pollen, Cor a 1.01 from hazel pollen, and Aln g 1 from alder), as well as some proteins in fruits, vegetables, and nuts, such as apple (Mal d 1), peach (Pru p 1), kiwi (Act d 8), celery (Api g 1), soy (Gly m 4), hazelnut (Cor a 1.04), and peanut (Ara h 8), all belong to the pathogenesis-related class 10 (PR-10) protein family and share common epitopes.4–6 Therefore IgE antibodies to such allergen molecules might cross-react.7
The introduction of component-resolved diagnosis based on molecular allergens has increased the accuracy of allergy diagnosis and prognosis, particularly for peanut and hazelnut allergy.8,9 Furthermore, component-resolved IgE testing has also improved our knowledge regarding the progression of sensitization and development of symptoms10 and selection of immunotherapy.11
Molecular multiplex platforms, such as ISAC,12 are promising tools for obtaining an overview of the sensitization profile to a number of allergen sources, enabling discrimination between genuine versus cross-reactive sensitization.
PR-10 component-resolved diagnosis has been used in various populations of allergic patients,13,14 but no study has thus far assessed the predictive value of IgE testing to PR-10 proteins in a population-based child cohort.
The aim of this study was to investigate whether IgE reactivity to allergenic molecules of the PR-10 protein family in childhood was associated with the occurrence, incidence, and persistence of AR to birch pollen up to 16 years of age. Secondary aims were to assess the association between IgE reactivity to PR-10 proteins and the severity of AR to birch pollen and occurrence of oral allergy syndrome (OAS) at age 16 years.
Methods
Barn/Children Allergi/Allergy Milieu Stockholm Epidemiologic study cohort
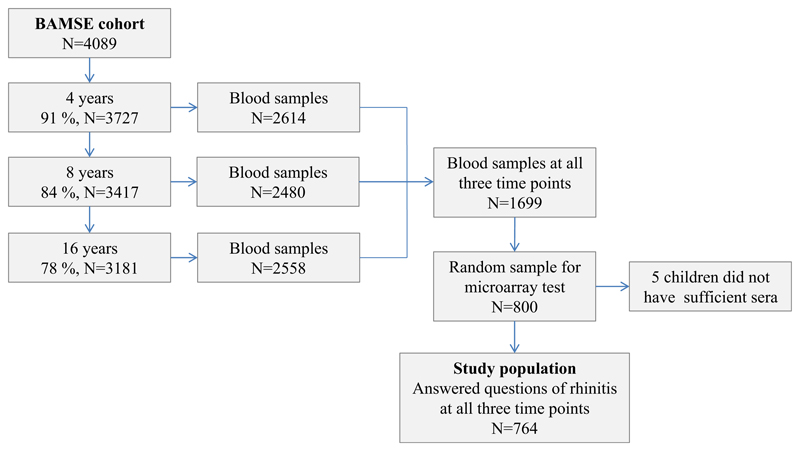
Within the framework of Mechanisms for the Development of Allergies (MeDALL), a European Union–funded project (http://medall-fp7.eu/),15 we analyzed data from the population-based birth cohort Barn/Children Allergi/Allergy Milieu Stockholm Epidemiologic (BAMSE) study of 4089 children born in the mid-1990s in Sweden. The cohort has been described in detail elsewhere.16 In brief, baseline data were obtained shortly after birth and repeatedly thereafter up to age 16 years by using parental questionnaires on symptoms of allergy-related disorders (see Fig E1 in this article’s Online Repository at www.jacionline.org). At age 16 years, the children answered questionnaires as well. The response rate at the latest follow-up at age 16 years was 78% from baseline. Families who completed the questionnaire for their children at 4, 8, and 16 years of age were invited for blood sampling. The number of children with blood samples obtained at all 3 time points was 1699. In a previous study background characteristics between the 1699 children and the BAMSE cohort were assessed, and no significant differences were found.17 Permission for the study was obtained from the Regional Ethical Review board at Karolinska Institutet, Stockholm, Sweden, throughout the study years, and parents provided informed consent for each follow-up.
Study population
Eight hundred of the 1699 children with available sera from all 3 time points were randomly selected for microarray testing. Children with results from the microarray testing and complete information from the parental questionnaires on AR symptoms when exposed to birch pollen at 4, 8, and 16 years of age were included in the study (n = 764, see Fig E1).
Definition of symptoms
Allergic rhinitis to birch pollen (ARbp) was defined as sneezing; runny, itchy, or blocked nose; and itchy eyes when exposed to birch pollen.18,19 Birch pollen–related asthma was defined as respiratory symptoms (difficult breathing, tightness of chest, and wheezy or raspy breathing) or bothersome cough when exposed to birch pollen.
Incident ARbp at 8 and 16 years of age refers to children with ARbp at the respective time points but with no previously reported ARbp. Children who reported ARbp at an earlier age were excluded from the analysis. Persistent ARbp among 4-year-olds was defined as ARbp at 4, 8, and 16 years of age, and that among 8-year-olds was defined as ARbp at 8 and 16 years of age but no ARbp at age 4 years. Severity of ARbp at age 16 years was classified according to Allergic Rhinitis and its Impact on Asthma into mild (no effect on daily activities or sleep) and moderate/severe (effect on daily activities, sleep, or both).20
OAS was defined as itch in the mouth, throat, or ears and/or swollen feeling in the mouth or throat after consumption of PR-10 allergen–containing plant food.21
Information on outcome (ARbp and OAS) was obtained from the parental questionnaires, except for severity of disease, which was based on children’s answers.
Specific IgE reactivity
The serum samples were analyzed for IgE reactivity to microarrayed allergen molecules by using the MeDALL chip, which is based on the ISAC microarray platform (Phadia Multiplexing; Thermo Fisher Scientific, Uppsala, Sweden) but differs from the commercially available ISAC regarding outlay and the number of allergens. The technical details and features of the MeDALL chip together with the cutoff of 0.3 ISAC standardized units for IgE detection (ISU-E) or greater are described in detail by Lupinek et al.22 IgE reactivity profiles and levels were measured for PR-10 proteins (Bet v 1, Mal d 1, Aln g 1, Cor a 1.04, Ara h 8, Pru p 1, Api g 1, Act d 8, and Gly m 4). A level of 0.3 ISU-E or greater was considered positive. Briefly, aliquots of 35 μL of serum were incubated on the microarray, and after 120 minutes of incubation at room temperature, slides were washed, and fluorescence-labeled anti-IgE antibodies (Thermo Fisher) were added and incubated for 30 minutes. Chips were then washed, dried, and analyzed with a Laser Scan Confocal microarray reader (LuxScan 10K/A; Capital-Bio, Beijing, China). The results were evaluated by using Phadia Microarray Image Analysis (MIA) software and are reported in ISU-E.22
Statistical analyses
The distributions of selected baseline characteristics for the study population and the original cohort were compared by using the t test with finite population correction. For significant results, a sensitivity analysis was performed. IgE reactivity levels are presented as box plots with median levels and 25th and 75th percentiles. Comparison of specific IgE levels between time points or between those with mild or moderate/severe symptoms was performed with quantile regression. Correlations between levels of Bet v 1–specific IgE and numbers of IgE-reactive components were assessed with the Spearman correlation test. Fitted predicted probability curves were plotted based on a logistic regression model to assess the probability of ARbp in relation to Bet v 1–specific IgE levels. Fitted predicted probability curves were also performed for comparison between Bet v 1–specific IgE levels (ISAC) and birch-specific IgE levels (ImmunoCAP) and between the study population and the original cohort. Similarly, probability curves for OAS from apple, hazelnut, peanut, kiwi, and soy in relation to specific IgE levels of the corresponding PR-10 proteins were plotted. Children with IgE reactivity to other allergen components (eg, Ara h 2 and Cor a 9) known to produce severe reactions were excluded from the analyses in this context.
The number of recognized PR-10 proteins was categorized into 3 groups at 4 and 8 years of age (1, Bet v 1 only; 2, Bet v 1 and up to the median number of other recognized PR-10 proteins; and 3, Bet v 1 and greater than the median number of other PR-10 proteins). The association of IgE reactivity according to these categories in relation to ARbp up to age 16 years was calculated with generalized estimating equations. As a complement, absolute risks of ARbp at 8 or 16 years of age was calculated.
P values of less than .05 were considered as statistically significant. A detailed description of the statistical analyses is presented in the Methods section in this article’s Online Repository at www.jacionline.org. All analyses were conducted with STATA Statistical Software, version 13.1 (StataCorp, College Station, Tex). For drawing of proportional Venn diagrams, eulerAPE was used (http://www.eulerdiagrams.org/eulerAPE).
Results
Study population versus study base
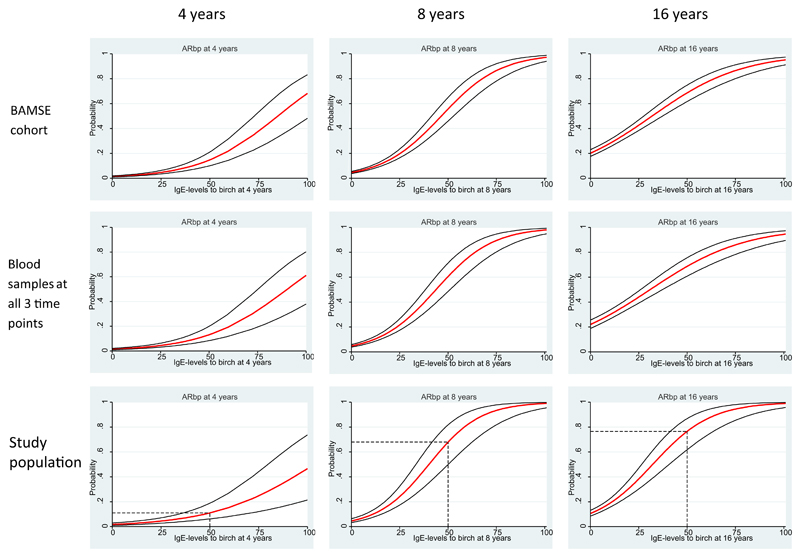
When comparing baseline characteristics at birth between the study population and the BAMSE cohort, no significant differences were found, except for low socioeconomic status and birth month (see Table E1 in this article’s Online Repository at www.jacionline.org). However, these differences were small and shown by means of sensitivity analysis not to influence the results (data not shown). Moreover, the probability of ARbp at 4, 8, or 16 years of age in relation to IgE levels to birch did not differ between the study population and the original cohort (see Fig E2 in this article’s Online Repository at www.jacionline.org).
IgE reactivity to PR-10 proteins
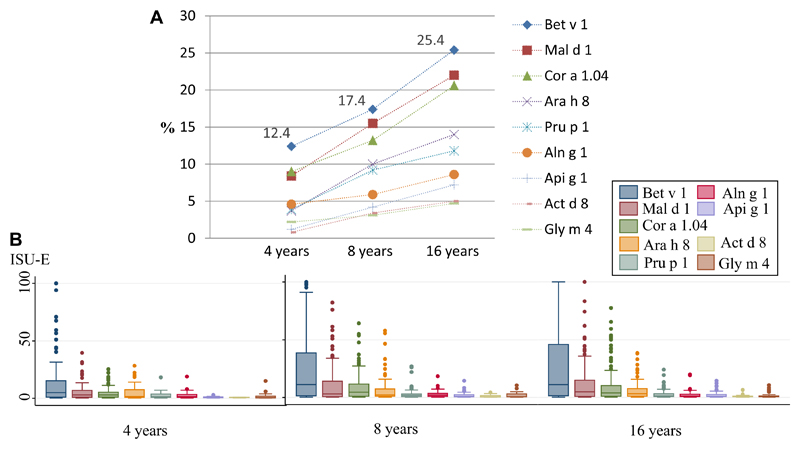
At 4, 8, and 16 years of age, 12.4%, 17.4%, and 25.4% of the children had IgE reactivity to Bet v 1, respectively. The presence of IgE reactivity to the other PR-10 proteins was seen in the following order at 8 and 16 years of age: Bet v 1 > Mal d 1 > Cor a 1.04 > Ara h 8 > Pru p 1 > Aln g 1 > Api g 1 > Act d 8 > Gly m 4 (Fig 1). Among children with IgE reactivity to Bet v 1, the proportion of IgE reactivity to any of the other 8 remaining PR-10 proteins was 75.8%, 82.0%, and 83.0% at 4, 8, and 16 years of age, respectively. IgE levels at the 3 time points were highest for Bet v 1 compared with the other PR-10 proteins (Fig 1). Between 4 and 8 years of age, the median IgE level for Bet v 1 doubled (5.1 ISU to 11.3 ISU-E, P = .055), but from 8 to 16 years of age, the corresponding median level remained unchanged (11.1 ISU-E, 4 vs 16 years; P = .080).
Fig 1.
A, Prevalence of IgE reactivity to PR-10 proteins in the population (≥0.3 ISU-E). B, Specific IgE levels to the different PR-10 proteins among sensitized children (≥0.3 ISU-E), where boxes show the median levels and 25th and 75th percentiles at 4, 8, and 16 years of age, respectively.
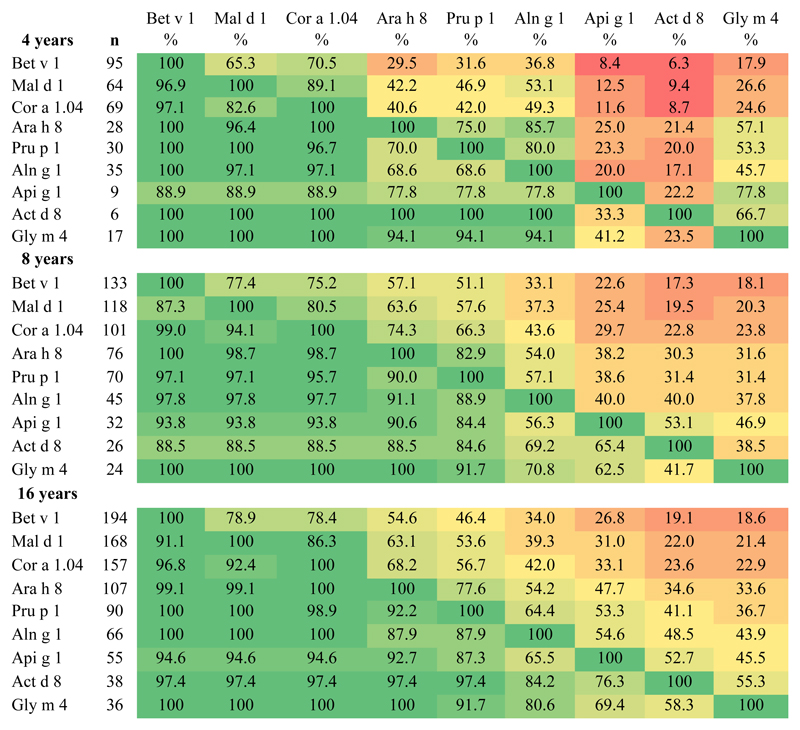
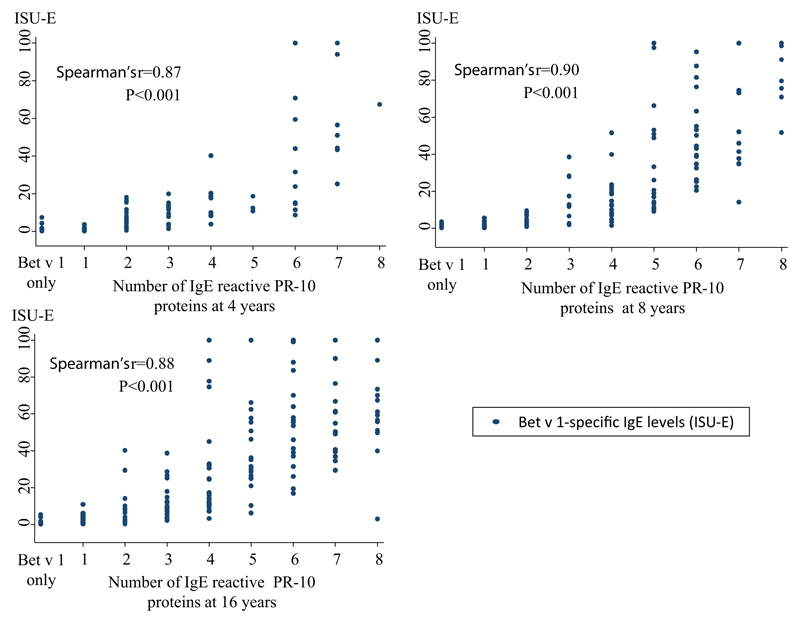
The hierarchy of IgE reactivity to the different PR-10 proteins was further assessed (Fig 2). Among the 4-, 8-, and 16-year-olds with IgE reactivity to Bet v 1, between 65% and 79% also exhibited IgE reactivity to Mal d 1, but only 18% to 19% exhibited IgE reactivity to Gly m 4. On the other hand, among children with IgE reactivity to Gly m 4, 100% had IgE reactivity to Bet v 1, Mal d 1, and Cor a 1.04. A close correlation (Rho = 0.87-0.90) between IgE levels to Bet v 1 and the number of other IgE-reactive PR-10 proteins was noted (see Fig E3 in this article’s Online Repository at www.jacionline.org).
Fig 2.
Number of children with IgE-reactivity to the different PR-10 proteins (left column) and percentages with additional IgE-reactivity to other PR-10 proteins. Green, High degree of IgE cross reactivity; Red, low degree of IgE cross reactivity.
IgE reactivity to PR-10 proteins in relation to symptoms
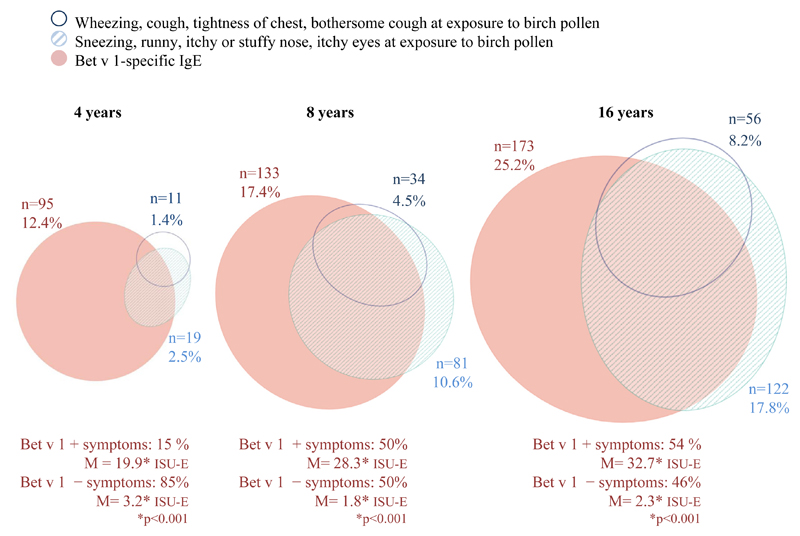
The prevalence of birch-related airway symptoms in Bet v 1–sensitized subjects is shown in Fig 3. At 4, 8, and 16 years of age, 2.5%, 10.6%, and 17.8% of the subjects had ARbp. At age 4 years, only 15% (n = 14) of Bet v 1–specific IgE–positive children had ARbp (Fig 3). At 8 and 16 years of age, the corresponding proportions were 50% (n = 66) and 54% (n = 104). The median ISU-E levels in asymptomatic children were significantly lower in all age groups compared with those in symptomatic children (3.2 vs 19.9 at age 4 years, 1.8 vs 28.3 at age 8 years, and 2.3 vs 32.7 at age 16 years; Fig 3).
Fig 3.
Proportional Venn diagram of numbers of children who reported symptoms after exposure to birch pollen from the upper and lower airways, respectively, and IgE reactivity to Bet v 1 at 4 (n = 764), 8 (n = 763), and 16 (n = 686) years of age.
The probability of reporting ARbp in relation to Bet v 1–specific IgE levels at the different time points was assessed cross-sectionally. At 4, 8, and 16 years of age, the cross-sectional probability to report ARbp increased with increasing levels of Bet v 1–specific IgE (see Fig E4 in this article’s Online Repository at www.jacionline.org) and with the number of IgE-reactive PR-10 proteins (see Table E2 in this article’s Online Repository at www.jacionline.org). As a comparison, probability curves for ARbp in relation to IgE to birch was performed. The probability of ARbp was higher at a Bet v 1–specific IgE level of 50 ISU-E compared with a birch-specific IgE level of 50 kU/L at 4 and 8 years of age (see Fig E2).
Trajectories of PR-10 protein IgE, sensitization, and symptoms
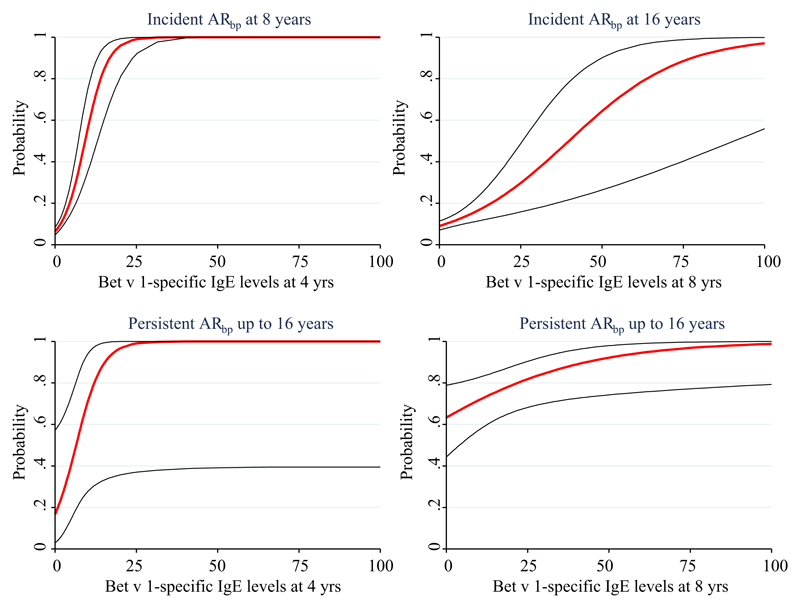
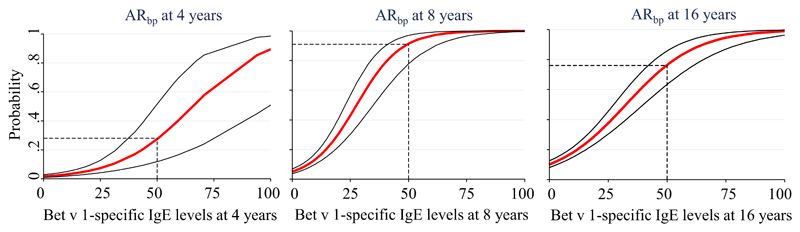
Onset, persistence, and severity of ARbp were studied longitudinally. The probability to report incident symptoms of ARbp at age 8 years increased with increasing levels of Bet v 1–specific IgE at 4 years, as did the probability to report incident symptoms at age 16 years with increasing Bet v 1–specific IgE levels at age 8 years (Fig 4).
Fig 4.
Probabilities for incident or persistent ARbp from 4 to 16 years of age in relation to Bet v 1–specific IgE levels (ISU-E) at 4 and 8 years of age, respectively.
The odds ratios (ORs) for incident ARbp in relation to the number of IgE-reactive PR-10 proteins is displayed in Table I, and absolute risks are shown in Table E3 in this article’s Online Repository at www.jacionline.org. Among asymptomatic children with IgE reactivity to Bet v 1 only at age 4 years, the overall OR for having ARbp at 8 or 16 years of age was 7.1 (95% CI, 3.3-15.3). The OR increased if 1 to 2 (26.2; 95% CI, 13.1-52.3) or 3 or more (45.1; 95% CI, 21.3-95.5) IgE reactivities beside Bet v 1 were present (Table I). Between 8 and 16 years of age, this pattern was less clear (Table I and see Table E3).
Table I. Proportions and ORs (generalized estimating equations) for onset of ARbp at 8 and 16 years of age in relation to the number of IgE-reactive PR-10 proteins at 4 and 8 years of age, respectively.
| ARbp at age 8 y | ARbp at age 16 y | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percent | OR | 95% CI | No. | Percent | OR | 95% CI | OR | 95% CI | ||
| No. of PR-10 at age 4 y (n = 740)† | |||||||||||
| 0 | 659 | 23 | 3.5 | Reference | Reference | 51 | 7.7 | Reference | Reference | Reference | Reference |
| Bet v 1 only | 23 | 4 | 17.4 | 5.8 | 1.8-18.5 | 8 | 34.8 | 8.2 | 3.5-19.4 | 7.1§ | 3.3-15.3 |
| Bet v 1 and 1-2* | 29 | 19 | 65.5 | 52.5 | 22.0-125.6 | 3 | 10.3 | 12.7 | 5.8-27.8 | 26.2§ | 13.1-52.3 |
| Bet v 1 and ≥3 | 29 | 19 | 65.5 | 52.5 | 22.0-125.6 | 5 | 17.2 | 43.2 | 15.9-116.8 | 45.1§ | 21.3-95.5 |
| No. of PR-10 at age 8 y (n = 656)‡ | No. | ||||||||||
| 0 | 590 | NA | NA | NA | NA | 36 | 6.1 | Reference | Reference | NA | NA |
| Bet v 1 only | 21 | 9 | 42.9 | 11.5 | 4.6-29.2 | ||||||
| Bet v 1 and 1-2* | 23 | 9 | 39.1 | 9.9 | 4.0-24.4 | ||||||
| Bet v 1 and ≥3 | 22 | 11 | 50.0 | 15.4 | 6.2-37.9 | ||||||
Median number of components or less.
No symptoms of ARbp reported at age 4 years.
No symptoms of ARbp reported at age 4 or 8 years.
P for trend < .001.
No child with ARbp at age 4 years had IgE reactivity to Bet v 1 alone (see Table E4 in this article’s Online Repository at www.jacionline.org). The absolute risk of persistence of ARbp was 67% in subjects with IgE reactivity to Bet v 1 in combination with 1 to 3 other PR-10 proteins (see Table E4). If IgE reactivity was present to 4 or more PR-10 proteins at age 4 years, the corresponding risk for persistence of ARbp reached 100%. At 8 years of age, only 3 children with ARbp had IgE reactivity to Bet v 1 alone, and all of them had persistent symptoms up to age 16 years (see Table E4). Analysis of the other PR-10 proteins for persistence of symptoms from 8 to 16 years of age did not provide much added value (see Table E5 in this article’s Online Repository at www.jacionline.org).
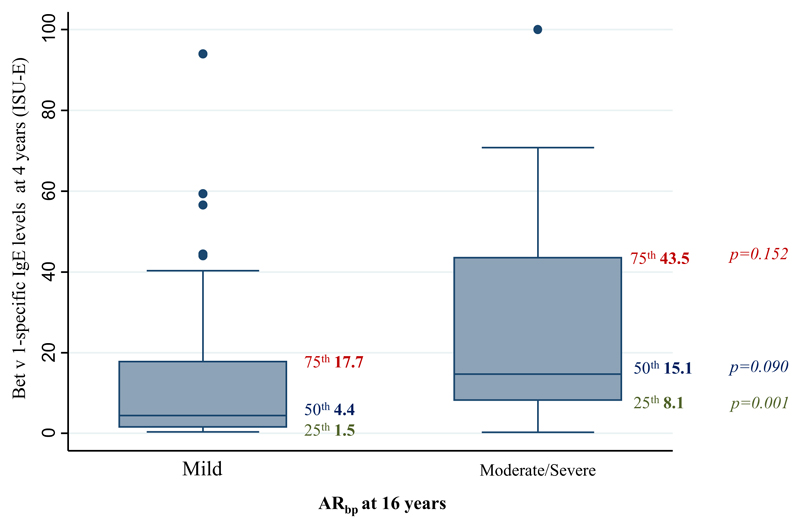
Among the 30% of 16-year-old children who were classified as having moderate/severe ARbp, Bet v 1–specific IgE levels at age 4 years were higher than those in children with mild ARbp (25th percentile: 1.5 vs 8.1 ISU-E, P = .001; median: 4.4 vs 15.1 ISU-E, P = .090; Fig 5).
Fig 5.
Bet v 1–specific IgE levels (ISU-E) at age 4 years among children with mild ARbp compared with those with moderate/severe ARbp at 16 years of age, with box plots showing median levels and 25th and 75th percentiles.
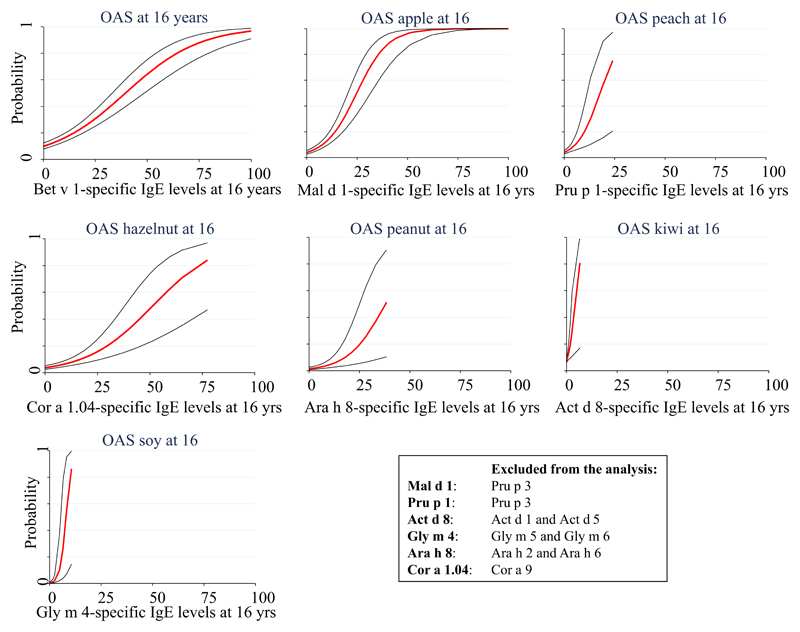
Among children with ARbp and IgE reactivity to Bet v 1 at age 16 years, 63% reported symptoms of OAS to any of the food items apple, hazelnut, peanut, peach, kiwi, or soy. Apple was the most frequently reported food item (47%), followed by peach (29%) and hazelnut (27%). The probability of reporting symptoms of OAS after ingestion of any of the food items apple, hazelnut, peanut, peach, kiwi, or soy increased with increasing Bet v 1–specific IgE levels (see Fig E5 in this article’s Online Repository at www.jacionline.org). A similar or even higher probability was seen for OAS after ingestion of a specific food item in relation to the IgE level of the corresponding PR-10 component (see Fig E5).
Discussion
To our knowledge, this is the first study to investigate IgE reactivity to PR-10 allergen components as a possible predictor for ARbp during childhood to adolescence. We found that the risk of later onset or persistence of symptoms of ARbp increased with increasing levels of Bet v 1–specific IgE or increasing numbers of recognized PR-10 proteins at 4 years. High levels of IgE to Bet v 1 at age 4 years were associated with severe ARbp at age 16 years. Furthermore, the likelihood of reported OAS increased by increasing ISU-E levels to the corresponding PR-10 protein.
Bet v 1 was the most prevalent sensitizing PR-10 protein at 4, 8, and 16 years of age, and median levels of Bet v 1–specific IgE were higher than for the other PR-10 proteins. This was consistent with results from earlier studies indicating that in a birch-endemic region, Bet v 1 is the driving allergen in sensitization to the other PR-10 proteins.23,24
The IgE reactivity to PR-10 proteins showed a hierarchic intrarelationship as follows: Bet v 1 > Mal d 1 > Cor a 1.04 > Ara h 8 > Pru p 1 > Aln g 1 > Api g 1 > Act d 8 > Gly m 4, where IgE reactivity to the more prevalent components was almost always present when IgE reactivity to the less common components were seen. The result might reflect routes and amounts of allergen exposure, different allergenicity, and/or the degree of homology between Bet v 1 and the other PR-10 protein molecules.25 This pattern might look different in a region with less dominant Bet v 1 exposure.26
A large proportion of children with IgE reactivity to Bet v 1 did not report symptoms. The presence of IgE sensitization without clinical symptoms is well known.10,19,27 IgE levels were higher among children with symptoms than among asymptomatic children, a finding in accordance with previous studies.27 There were also children who reported symptoms but did not have IgE reactivity. This has also been reported and might be due to nonallergic rhinitis or infectious rhinitis misinterpreted as allergic symptoms18,28 or local AR.29
At 4 or 8 years of age, the possibility to predict onset of ARbp up to 16 years of age increased with increasing Bet v 1–specific IgE levels. Several studies have shown that sensitization often precedes rhinitis symptoms.10,19,30 In our study we saw that levels of Bet v 1–specific IgE correlated very well with numbers of other IgE-reactive PR-10 proteins at all 3 time points. Consequently and in line with our hypothesis, the more PR-10 proteins recognized by IgE, the higher the probability to report symptoms to birch pollen. When analyzing the data longitudinally, we saw a tendency of higher risk for incident symptoms from 4 to 8 or 16 years of age, as well as persistence of symptoms from age 4 up to 16 years with increasing numbers of PR-10 proteins, although interpretation should be carried out with caution because of low numbers of children in these analyses. Yet one has to bear in mind that PR-10 cross-reactivity will increase with Bet v 1–specific IgE levels and that levels and affinities of Bet v 1–specific IgE are the driving forces in this process.
The probability of having persistence of symptoms from 8 to 16 years of age was high already at low IgE levels to Bet v 1 at the age of 8 years. Analysis of Bet v 1–specific IgE levels and IgE reactivity to other PR-10 proteins did not seem to provide much added value in this context. Thus it appears as if the 8-year-old children, who were sensitized already at age 4 years, were no longer in their early phase of disease. In addition, those who were sensitized and had symptoms at this age had a persistence of disease,10 which is important information when intervention treatment, such as allergen-specific immunotherapy, is considered. In fact, it has been reported that allergen-specific immunotherapy prevents the progression of AR to asthma,31 and based on our data, it is quite tempting to consider specific immunotherapy as a preventive intervention in the early phase of allergic sensitization when there is still plasticity of the IgE response.
At 16 years of age, the probability of reporting symptoms of OAS after exposure to apple, peach, hazelnut, peanut, and soy also increased with increasing IgE reactivity to the corresponding PR-10 protein. This is consistent with what is seen for other types of food allergies.32,33 These results most likely reflect the probability of reporting OAS symptoms for the corresponding PR-10 protein because we excluded children with IgE to allergen components known to cause severe reactions, such as storage and lipid transfer proteins in the analysis. Among children with ARbp and IgE reactivity to Bet v 1 at 16 years of age, the most commonly reported food item was apple, which is consistent with studies mostly among adults.34,35 However, the proportion of children reporting OAS symptoms from apple (47%), as well as from hazelnut, peanut, peach, kiwi, and soy, was lower than previously reported. These studies were conducted among adults and at allergy clinics, which differs from the current study, which was conducted among adolescents in a population-based setting.
The strengths of this study are the large sample size of children with results from IgE testing in a population-based design and the high follow-up rate (ie, 78% from baseline at the 16-year-follow up). The use of the MeDALL chip has allowed us to perform a comprehensive analysis of IgE reactivities to multiple PR-10 proteins, requiring only a small serum volume, which would have been impossible with traditional diagnostic tests. Therefore the MeDALL chip is well suited for the analysis of sera in birth cohorts and children, where only small volumes of serum are available.
However, there are some limitations. The study population was a sample of the original cohort, but a comparison of baseline characteristics, as well as cross-sectional probability curves for ARbp in relation to levels of specific IgE to birch, between the study population and the original cohort showed no major differences.
The definition of AR is based on questionnaires only, and reporting of symptoms might not be as accurate as in a clinical study. However, in a validation study from Finland among 290 students aged 18 to 25 years, the question of symptoms after exposure to an allergen had a positive predictive value of 75% in relation to a doctor’s diagnosis of symptoms in combination with a positive skin prick test response.18 In our study a similar proportion (74% to 81%) of children who reported symptoms of AR at exposure to birch pollen had IgE reactivity to Bet v 1. The questionnaires were answered without parents or children knowing about the IgE reactivity, and thus any misclassification would be nondifferential.
In conclusion, in this birth cohort of well-characterized children, we show that the risk of onset and persistence of ARbp up to 16 years of age increased with Bet v 1–specific IgE levels, as well as the number of IgE-reactive PR-10 proteins in early childhood. Thus analysis of early IgE reactivity to PR-10 allergen components might be a useful tool in predicting the course of ARbp up to 16 years of age.
Methods
Statistical analyses
To analyze whether there were any differences in important background characteristics between the study population (the 764 children included in this study) and the study base (the original BAMSE cohort), we used the t test with finite population correction. When using finite population correction, one is able to account for the fact that the sample (n = 764) is not picked from an infinite population (n = 4089), which means that the variance gets smaller, and consequently, the CIs get smaller.
In Fig 1 the prevalence of IgE reactivity to PR-10 proteins at 4, 8, and 16 years of age is expressed as a percentage of the total number of available observations. Levels of the different PR-10 proteins are presented as box plots with medians and 25th and 75th percentiles. Quantile regression was used to compare the median IgE levels of the different PR-10 proteins. This method was also used to compare the median IgE levels of Bet v 1 between time points. Because the prevalence and IgE levels of the PR-10 proteins seemed to follow a certain order, we wanted to further analyze the hierarchy within the PR-10 protein group, which is presented in Fig 2. Among children with IgE reactivity to a certain PR-10 protein, we calculated the proportion of children with IgE reactivity to each of the other PR-10 proteins. Because the highest prevalence and IgE level was seen for Bet v 1 and there seemed to be a certain hierarchy within the PR-10 protein group, we wanted to assess whether IgE levels of Bet v 1 were correlated with the number of IgE reactivities to other PR-10 proteins. For the analysis of correlation, we used Spearman rho.
The next step was to analyze IgE reactivity in relation to symptoms of ARbp. A Venn diagram was performed to see the proportions of symptoms to birch pollen from the upper and lower airways, respectively, and IgE reactivity to Bet v 1 in relation to each other. Levels of specific IgE to Bet v 1 among asymptomatic children compared with those in children with symptoms were again analyzed with quantile regression. The cross-sectional probability to report symptoms of ARbp in relation to Bet v 1–specific IgE levels was assessed with fitted predicted probability curves, which are based on a logistic regression model. The same method was used to analyze the cross-sectional probability to report OAS from apple, hazelnut, peanut, kiwi, and soy in relation to specific IgE levels of the corresponding PR-10 protein. Children with IgE reactivity to other than PR-10 allergen components known to produce severe reactions were excluded from these analyses. For Mal d 1– and Pru p 1–specific IgE, children with concomitant IgE to Pru p 3 were excluded. For Act d 8–specific IgE, children with IgE to Act d 1/Act d 5 were excluded. For Ara h 8–specific IgE, children with IgE to Ara h 2/Ara h 6 were excluded. For Gly m 4–specific IgE, children with IgE to Gly m 5/Gly m 6 were excluded. For Cor a 1.04–specific IgE, children with IgE to Cor a 9 were excluded. To test whether we had to perform a logarithmic transformation before analysis of specific IgE levels, we tested the log-linear relationship. The different IgE levels showed a log-linear relationship to the respective outcome and were thus not logarithmically transformed.
Finally, we wanted to analyze whether the onset, persistence, or severity of ARbp could be predicted by either levels of specific IgE to Bet v 1 or numbers of IgE reactivites to other PR-10 proteins. For analysis of probability of onset or persistence of symptoms in relation to levels of specific IgE to Bet v 1, the same method of fitted predicted probability curves as used for the cross-sectional analysis was performed. For probability of onset of ARbp, the analysis was performed among children without symptoms of ARbp at baseline, and for persistence of symptoms, the analysis was performed among children with ARbp at baseline.
For assessing the possible association between onset or persistence of ARbp in relation to the number of recognized PR-10 proteins, we divided the number of recognized PR-10 proteins into 3 categories; Bet v 1 only, Bet v 1 and up to the median number of other recognized PR-10 proteins, and Bet v 1 and a number of other recognized PR-10 proteins above the median. The association between these categories of numbers of recognized PR-10 proteins at baseline and onset of ARbp at ages 8 and 16 years was calculated with generalized estimating equations. As a complement, absolute risks of ARbp at 8 or 16 years of age were calculated. The absolute risk was calculated as the number of children with the exposure (specific IgE ≥0.3 ISU-E) and the outcome (ARbp) at 8 or 16 years of age, respectively, as divided by the total number of children with the exposure at baseline. Ninety-five percent CIs were calculated with the binomial test of statistical significance. P values of less than .05 were considered statistically significant.
The severity of ARbp at 16 years of age in relation to Bet v 1–specific IgE levels at age 4 years are presented as box plots with median levels and 25th and 75th percentiles. Specific IgE levels at the 50th percentile (median), as well as the 25th and 75th percentiles, were compared between patients with mild and those with moderate/severe ARbp by using quantile regression.
Extended Data
Fig E1.
Flow chart of the study.
Fig E2.
Cross-sectional probabilities to report ARbp at 4, 8, and 16 years of age in relation to levels of specific IgE to birch (measured with ImmunoCAP) among all children in the BAMSE cohort, the 1699 children with blood samples from all 3 time points, and the study population.
Fig E3.
Correlation between Bet v 1–specific IgE levels (ISU-E) and numbers of other IgE-reactive PR-10 proteins.
Fig E4.
Cross-sectional probabilities to report ARbp in relation to Bet v 1–specific IgE levels (ISU-E) at 4, 8, and 16 years of age.
Fig E5.
Cross-sectional probabilities to report symptoms of OAS to any food item in relation to Bet v 1–specific IgE levels or specific food items in relation to levels of IgE specific for the corresponding PR-10 protein at 16 years of age.
Table E1. Background characteristics of participants in the BAMSE birth cohort, Stockholm, Sweden.
| Study population (n = 764) |
BAMSE cohort (n = 4089) |
P value | |||||
|---|---|---|---|---|---|---|---|
| No. | Percent | 95% CI | No. | Percent | 95% CI | ||
| Sex | |||||||
| Male | 383 | 50.1 | 46.6-52.8 | 2065 | 50.5 | 49.0-52.0 | .821 |
| Heredity for AR | |||||||
| Yes | 286 | 37.6 | 34.1-41.0 | 1397 | 34.5 | 33.1-36.0 | .055 |
| Low socioeconomic status | |||||||
| Yes | 109 | 14.3 | 12.0-17.0 | 695 | 17.1 | 15.9-18.2 | .016 |
| Birth month | |||||||
| December-February | 139 | 18.2 | 15.6-21.1 | 722 | 17.7 | 16.5-18.8 | .672 |
| March-May | 240 | 31.4 | 28.2-34.8 | 1201 | 29.4 | 28.0-30.8 | .177 |
| June-August | 223 | 29.2 | 26.1-32.5 | 1190 | 29.1 | 27.7-30.5 | .952 |
| September-November | 162 | 21.2 | 18.4-24.3 | 976 | 23.9 | 22.6-25.2 | .046 |
| Mother’s age | |||||||
| <26 y | 57 | 7.5 | 5.6-9.3 | 319 | 7.8 | 7.0-8.6 | .692 |
| Parent born outside Scandinavia | |||||||
| Yes | 142 | 18.6 | 15.8-21.4 | 707 | 20.8 | 19.4-22.2 | .076 |
| Older siblings | |||||||
| Yes | 382 | 50.0 | 45.4-53.6 | 1980 | 48.4 | 46.9-50.0 | .336 |
| Breast-feeding exclusively ≥4 mo | |||||||
| Yes | 595 | 78.9 | 76.0-81.8 | 3116 | 79.5 | 78.2-80.8 | .655 |
| Furred animals at home | |||||||
| Yes | 120 | 15.7 | 13.1-18.3 | 629 | 15.4 | 14.3-16.5 | .783 |
| Mother smoking | |||||||
| Yes | 95 | 12.4 | 10.1-14.8 | 563 | 13.8 | 12.7-14.8 | .212 |
| Smell of mildew in home | |||||||
| Yes | 55 | 7.2 | 5.4-8.8 | 324 | 7.9 | 7.1-8.8 | .393 |
| Moisture damage in home | |||||||
| Yes | 150 | 19.6 | 16.8-22.5 | 812 | 19.9 | 18.6-21.1 | .861 |
Boldface indicates statistical significance.
Table E2. Cross-sectional proportions of ARbp among children with IgE reactivity to Bet v 1 only or in combination with the other PR-10 proteins, as well as for certain numbers of other PR-10 proteins, at 4, 8, and 16 years of age (n = 764).
| AR at age 4 y |
AR at age 8 y |
AR at age 16 y |
||||
|---|---|---|---|---|---|---|
| No./† | Percent | No./† | Percent | No./† | Percent | |
| Bet v 1 only | 0/23 | 0 | 3/24 | 12.5 | 5/33 | 15.2 |
| Bet v 1 + any PR-10 | 14/72 | 19.4 | 63/109 | 57.8 | 99/161 | 61.5 |
| Bet v 1 + Mal d 1 | 14/62 | 22.6 | 61/103 | 59.2 | 95/153 | 62.1 |
| Bet v 1 + Cor a 1.04 | 13/67 | 19.4 | 62/100 | 62.0 | 94/152 | 61.8 |
| Bet v 1 + Ara h 8 | 6/28 | 21.4 | 55/76 | 72.4 | 76/106 | 71.7 |
| Bet v 1 + Pru p 1 | 11/30 | 36.7 | 50/68 | 73.5 | 72/90 | 80.0 |
| Bet v 1 + Aln g 1 | 9/35 | 25.7 | 32/44 | 72.7 | 50/66 | 75.8 |
| Bet v 1 + Api g 1 | 2/8 | 25.0 | 25/30 | 83.3 | 40/52 | 76.9 |
| Bet v 1 + Act d 8 | 4/6 | 66.7 | 21/23 | 91.3 | 29/37 | 78.4 |
| Bet v 1 + Gly m 4 | 3/17 | 17.7 | 19/24 | 79.2 | 29/36 | 80.6 |
| No. of PR-10 proteins | ||||||
| 0 | 5/664 | 0.8 | 15/608 | 2.5 | 27/546 | 5.0 |
| Bet v 1 only | 0/23 | 0 | 3/24 | 12.5 | 5/33 | 15.2 |
| Bet v 1 and median* | 6/42 | 14.3 | 22/56 | 39.3 | 42/90 | 46.7 |
| Bet v 1 and above median | 8/30 | 26.7 | 41/53 | 77.4 | 57/71 | 80.3 |
Median number of components: age 4 years, ≤3; 8 and 16 years of age, ≤4.
Number of children with IgE reactivity to the particular PR-10 protein analyzed.
Table E3. Absolute risks for onset of ARbp at 8 or 16 years of age in relation to IgE reactivity to PR-10 proteins among asymptomatic children at 4 and 8 years of age, respectively.
| Incident ARbp at age 8 or 16 y | No onset of symptoms at age 8 or 16 y | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| No. | Percent | 95% CI | No. | Percent | |||
| 4 y (n = 740)* | No. | n = 86 | n = 608 | ||||
| No IgE reactivity | 659 | 74 | 11.2 | 585 | 88.8 | ||
| Bet v 1 only | 23 | 12 | 52.2 | 30.6-73.2 | 11 | 47.8 | |
| Bet v 1 + any PR-10 protein | 58 | 46 | 79.3 | 66.6-88.8 | 12 | 20.7 | |
| Bet v 1 + Mal d 1 | 48 | 40 | 83.3 | 69.8-92.5 | 8 | 16.7 | |
| Bet v 1 + Cor a 1.04 | 54 | 42 | 77.8 | 64.4-88.0 | 12 | 22.2 | |
| Bet v 1 + Ara h 8 | 22 | 19 | 86.4 | 65.1-97.1 | 3 | 13.6 | |
| Bet v 1 + Pru p 1 | 19 | 16 | 84.2 | 60.4-96.6 | 3 | 15.8 | |
| Bet v 1 + Aln g 1 | 26 | 22 | 84.6 | 65.1-95.6 | 4 | 15.4 | |
| Bet v 1 + Api g 1 | 6 | 6 | 100 | 54.1-100 | 0 | 0 | |
| Bet v 1 + Act d 8 | 2 | 2 | 100 | 15.8-100 | 0 | 0 | |
| Bet v 1 + Gly m 4 | 14 | 13 | 92.9 | 66.1-99.8 | 1 | 7.1 | |
| Incident ARbp at age 16 y | |||||||
| 8 y (n = 656)† | No. | n = 65 | n = 591 | ||||
| No IgE reactivity | 590 | 36 | 6.1 | 4.3-8.3 | 554 | 93.9 | |
| Bet v 1 only | 21 | 9 | 42.9 | 21.8-66.0 | 12 | 57.1 | |
| Bet v 1 + any PR-10 protein | 45 | 20 | 44.4 | 29.6-60.0 | 25 | 55.6 | |
| Bet v 1 + Mal d 1 | 41 | 18 | 43.9 | 28.4-60.3 | 23 | 56.1 | |
| Bet v 1 + Cor a 1.04 | 37 | 18 | 48.6 | 31.9-65.6 | 19 | 51.4 | |
| Bet v 1 + Ara h 8 | 20 | 10 | 50.0 | 27.2-72.8 | 10 | 50.0 | |
| Bet v 1 + Pru p 1 | 17 | 9 | 52.9 | 27.8-77.0 | 8 | 47.1 | |
| Bet v 1 + Aln g 1 | 12 | 7 | 58.3 | 27.7-84.8 | 5 | 41.7 | |
| Bet v 1 + Api g 1 | 5 | 3 | 60.0 | 14.7-94.7 | 2 | 40.0 | |
| Bet v 1 + Act d 8 | 2 | 2 | 100 | 15.8-100 | 0 | 0 | |
| Bet v 1 + Gly m 4 | 5 | 2 | 40.0 | 5.3-85.3 | 3 | 60.0 | |
No symptoms of ARbp reported at age 4 years.
No symptoms of ARbp reported at age 4 or 8 years.
Table E4. Absolute risks for persistence of ARbp up to age 16 years in relation to the number of IgE-reactive PR-10 proteins at 4 and 8 years of age, respectively.
| Transient | Persistent | |||||
|---|---|---|---|---|---|---|
| No. | No. | Percent | No. | Percent | 95% CI | |
| No. of PR-10 proteins at age 4 y (n = 19)† | ||||||
| 0 | 5 | 5 | 100 | 0 | 0 | 0-52.2 |
| Bet v 1 only | 0 | — | — | — | — | |
| Bet v 1 and 1-3* | 6 | 2 | 33.3 | 4 | 66.7 | 22.3-95.7 |
| Bet v 1 and ≥4 | 8 | 0 | 0 | 8 | 100 | 63.1-100 |
| No. of PR-10 proteins at age 8 y (n = 66)‡ | ||||||
| 0 | 13 | 7 | 53.9 | 6 | 46.2 | 19.2-74.9 |
| Bet v 1 only | 3 | 0 | 0 | 3 | 100 | 29.2-100 |
| Bet v 1 and 1-4* | 17 | 4 | 23.5 | 13 | 76.5 | 50.1-93.2 |
| Bet v 1 and ≥5 | 33 | 3 | 9.1 | 30 | 90.9 | 75.7-98.1 |
Median number of IgE-reactive proteins or less.
ARbp at age 4 years.
ARbp at age 8 years but not age 4 years.
Table E5. Absolute risks for persistence of ARbp up to age 16 years in relation to IgE reactivity to PR-10 proteins at 4 and 8 years of age, respectively.
| 4 y (n = 19)† | No. |
Transient (n = 7 [36.8%]) |
Persistent* (n = 12 [63.2%]) |
||
| No. | Percent | No. | Percent | ||
| No IgE reactivity | 5 | 5 | 100 | 0 | 0 |
| Bet v 1 only | 0 | — | — | — | — |
| Bet v 1 + any PR-10 protein | 14 | 2 | 14.3 | 12 | 85.7 |
| Bet v 1 + Mal d 1 | 14 | 2 | 14.3 | 12 | 85.7 |
| Bet v 1 + Cor a 1.04 | 13 | 2 | 15.4 | 11 | 84.6 |
| Bet v 1 + Ara h 8 | 6 | 0 | 0 | 6 | 100 |
| Bet v 1 + Pru p 1 | 11 | 1 | 9.1 | 10 | 90.9 |
| Bet v 1 + Aln g 1 | 9 | 0 | 0 | 9 | 100 |
| Bet v 1 + Api g 1 | 2 | 0 | 0 | 2 | 100 |
| Bet v 1 + Act d 8 | 4 | 0 | 0 | 4 | 100 |
| Bet v 1 + Gly m 4 | 3 | 0 | 0 | 3 | 100 |
| 8 y (n = 66)‡ | No. |
Transient (n = 14 [21.2%]) |
Persistent (n = 52 [78.8%]) |
||
| No. | Percent | No. | Percent | ||
| No IgE reactivity | 13 | 7 | 53.9 | 6 | 46.2 |
| Bet v 1 only | 3 | 0 | 0 | 3 | 100 |
| Bet v 1 + any PR-10 protein | 50 | 7 | 14.0 | 43 | 86.0 |
| Bet v 1 + Mal d 1 | 48 | 5 | 10.4 | 43 | 89.6 |
| Bet v 1 + Cor a 1.04 | 49 | 6 | 12.2 | 43 | 87.8 |
| Bet v 1 + Ara h 8 | 44 | 5 | 11.4 | 39 | 88.6 |
| Bet v 1 + Pru p 1 | 39 | 4 | 10.3 | 35 | 89.7 |
| Bet v 1 + Aln g 1 | 28 | 3 | 10.7 | 25 | 89.3 |
| Bet v 1 + Api g 1 | 18 | 1 | 5.6 | 17 | 94.4 |
| Bet v 1 + Act d 8 | 14 | 1 | 7.1 | 13 | 92.9 |
| Bet v 1 + Gly m 4 | 15 | 1 | 6.7 | 14 | 93.3 |
Reported symptoms to birch pollen at both 8 and 16 years of age. Only 1 child reported symptoms at age 8 years but not at age 16 years and 1 child at age 16 years but not at age 8 years.
tARbp at age 4 years.
ARbp at age 8 years but not at age 4 years.
Clinical implications.
Analysis of IgE reactivity to PR-10 allergen molecules in childhood might be a useful tool for predicting the onset and persistence of ARbp up to 16 years of age.
Acknowledgments
We thank all the families who participated and the staff working with the BAMSE project. We also thank Renata Kiss, Department of Pathophysiology and Allergy Research, Medical University of Vienna, Austria, for technical assistance regarding chip measurements.
Supported by the Swedish Asthma and Allergy Research Foundation; the Frimurare Barnhuset Foundation; the Acta Oto-Laryngologica Foundation; Stockholm County Council; the Swedish Research Council of Health, Working Life and Welfare; the Swedish Research Council, Swedish Heart-Lung Foundation; the Swedish Cancer and Allergy Foundation; the European Commission’s Seventh Framework 29 Program MeDALL under grant agreement no. 261357, and in part by grant F4605 of the Austrian Science Fund (FWF).
Abbreviations used
- AR
Allergic rhinitis
- ARbp
Allergic rhinitis to birch pollen
- BAMSE
Barn/Children Allergi/Allergy Milieu Stockholm Epidemiologic
- ISU-E
ISAC standardized units for IgE detection
- MeDALL
Mechanisms for the Development of Allergies
- OAS
Oral allergy syndrome
- OR
Odds ratio
- PR-10
Pathogenesis-related class 10
Footnotes
Disclosure of potential conflict of interest: C. Lupinek has received payment for delivering lectures from Thermo Fisher. J. Bousquet has received consultancy fees from Actelion, Almirall, Meda, Merck, MSD, Novartis, Sanofi-Aventis, Takeda, Teva, Uriach, AstraZeneca, Chiesi, GlaxoSmithKline, OM Pharma, Schering Plough, and Stallergènes and received support for travel to meetings for this study or other purposes from Actelion, Almirall, Meda, Merck, MSD, Novartis, Sanofi-Aventis, Takeda, Teva, and Uriach. P. Stjärne’s institution has received funding, and has received or has grants pending from the ALF. K. C. Lødrup Carlsen’s institution has received funding from the EU-MeDALL (grant no. 261357). K.-H. Carlsen has received compensation for board membership from Meda and Boehringer Ingelheim, as well as consultancy fees from Novartis, and payment for delivering lectures from Takeda, Novartis, and Boehringer Ingelheim, and he has received compensation for travel and other meeting-related expenses from Sandoz. J. M. Antó’s institution has received funding from the European Commission (grant no. 261357). R. Valenta’s institution has received funding from the EU (grant no. 261357), he has received consultancy fees, and he has received or has grants pending from Biomay AG, Thermo Fisher Scientific, and Fresenius Medical Care. M. van Hage has received consultancy fees from Hycor Biomedical, as well as payment for delivering lectures from Thermo Fisher Scientific, Novartis, and ALK-Abelló. M. Wickman’s institution has received funding from the European Union (grant no. 261357); he has received consultancy fees, payment for delivering lectures, and has received or has grants pending from Thermo Fisher Scientific; and he has also received consultancy fees from Mictrotest DX. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(suppl):S43–70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Gern JE, Martinez FD, Anto JM, Johnson CC, Holt PG, et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop. J Allergy Clin Immunol. 2014;133:1535–46. doi: 10.1016/j.jaci.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–90. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen MB, Hall S, Dragsted LO. Identification of European allergy patterns to the allergen families PR-10, LTP, and profilin from Rosaceae fruits. Clin Rev Allergy Immunol. 2011;41:4–19. doi: 10.1007/s12016-009-8177-3. [DOI] [PubMed] [Google Scholar]
- 5.Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106:27–36. doi: 10.1067/mai.2000.106929. [DOI] [PubMed] [Google Scholar]
- 6.Breiteneder H, Pettenburger K, Bito A, Valenta R, Kraft D, Rumpold H, et al. The gene coding for the major birch pollen allergen Betv1, is highly homologous to a pea disease resistance response gene. EMBO J. 1989;8:1935–8. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebner C, Hirschwehr R, Bauer L, Breiteneder H, Valenta R, Ebner H, et al. Identification of allergens in fruits and vegetables: IgE cross-reactivities with the important birch pollen allergens Bet v 1 and Bet v 2 (birch profilin) J Allergy Clin Immunol. 1995;95:962–9. doi: 10.1016/s0091-6749(95)70096-x. [DOI] [PubMed] [Google Scholar]
- 8.Asarnoj A, Moverare R, Ostblom E, Poorafshar M, Lilja G, Hedlin G, et al. IgE to peanut allergen components: relation to peanut symptoms and pollen sensitization in 8-year-olds. Allergy. 2010;65:1189–95. doi: 10.1111/j.1398-9995.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 9.Masthoff LJ, Mattsson L, Zuidmeer-Jongejan L, Lidholm J, Andersson K, Akker-daas JH, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013;132:393–9. doi: 10.1016/j.jaci.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Hatzler L, Panetta V, Lau S, Wagner P, Bergmann RL, Illi S, et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J Allergy Clin Immunol. 2012;130:894–901.e5. doi: 10.1016/j.jaci.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 11.Stringari G, Tripodi S, Caffarelli C, Dondi A, Asero R, Di Rienzo Businco A, et al. The effect of component-resolved diagnosis on specific immunotherapy prescription in children with hay fever. J Allergy Clin Immunol. 2014;134:75–81. doi: 10.1016/j.jaci.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO-ARIA-GA(2)LEN consensus document on molecular-based allergy diagnostics. World Allergy Org J. 2013;6:17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panzner P, Vachova M, Vitovcova P, Brodska P, Vlas T. A comprehensive analysis of middle-European molecular sensitization profiles to pollen allergens. Int Arch Allergy Immunol. 2014;164:74–82. doi: 10.1159/000362760. [DOI] [PubMed] [Google Scholar]
- 14.Vieira T, Cunha L, Neves E, Falcao H. Diagnostic usefulness of component-resolved diagnosis by skin prick tests and specific IgE to single allergen components in children with allergy to fruits and vegetables. Allergol Immunopathol (Madr) 2014;42:127–35. doi: 10.1016/j.aller.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bousquet J, Anto J, Auffray C, Akdis M, Cambon-Thomsen A, Keil T, et al. MeD-ALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy. 2011;66:596–604. doi: 10.1111/j.1398-9995.2010.02534.x. [DOI] [PubMed] [Google Scholar]
- 16.Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol. 2002;13(suppl 15):11–3. doi: 10.1034/j.1399-3038.13.s.15.10.x. [DOI] [PubMed] [Google Scholar]
- 17.Wickman M, Asarnoj A, Tillander H, Andersson N, Bergstrom A, Kull I, et al. Childhood-to-adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J Allergy Clin Immunol. 2014;133:580–2. doi: 10.1016/j.jaci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Kilpelainen M, Terho EO, Helenius H, Koskenvuo M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy. 2001;56:377–84. doi: 10.1034/j.1398-9995.2001.056005377.x. [DOI] [PubMed] [Google Scholar]
- 19.Westman M, Stjarne P, Asarnoj A, Kull I, van Hage M, Wickman M, et al. Natural course and comorbidities of allergic and nonallergic rhinitis in children. J Allergy Clin Immunol. 2012;192:403–8. doi: 10.1016/j.jaci.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 21.Ortolani C, Ispano M, Pastorello E, Bigi A, Ansaloni R. The oral allergy syndrome. Ann Allergy. 1988;61:47–52. [PubMed] [Google Scholar]
- 22.Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazemi-Shirazi L, Pauli G, Purohit A, Spitzauer S, Froschl R, Hoffmann-Sommer-gruber K, et al. Quantitative IgE inhibition experiments with purified recombinant allergens indicate pollen-derived allergens as the sensitizing agents responsible for many forms of plant food allergy. J Allergy Clin Immunol. 2000;105:116–25. doi: 10.1016/s0091-6749(00)90186-6. [DOI] [PubMed] [Google Scholar]
- 24.Valenta R, Kraft D. Type 1 allergic reactions to plant-derived food: a consequence of primary sensitization to pollen allergens. J Allergy Clin Immunol. 1996;97:893–5. doi: 10.1016/s0091-6749(96)80062-5. [DOI] [PubMed] [Google Scholar]
- 25.Valenta R, Steinberger P, Duchene M, Kraft D. Immunological and structural similarities among allergens: prerequisite for a specific and component-based therapy of allergy. Immunol Cell Biol. 1996;74:187–94. doi: 10.1038/icb.1996.26. [DOI] [PubMed] [Google Scholar]
- 26.Scala E, Alessandri C, Bernardi ML, Ferrara R, Palazzo P, Pomponi D, et al. Cross-sectional survey on immunoglobulin E reactivity in 23,077 subjects using an allergenic molecule-based microarray detection system. Clin Exp Allergy. 2010;40:911–21. doi: 10.1111/j.1365-2222.2010.03470.x. [DOI] [PubMed] [Google Scholar]
- 27.Bousquet J, Anto JM, Bachert C, Bousquet PJ, Colombo P, Crameri R, et al. Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitization to allergens. A GA2LEN project. Allergy. 2006;61:671–80. doi: 10.1111/j.1398-9995.2006.01048.x. [DOI] [PubMed] [Google Scholar]
- 28.Bousquet J, Fokkens W, Burney P, Durham SR, Bachert C, Akdis CA, et al. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy. 2008;63:842–53. doi: 10.1111/j.1398-9995.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 29.Rondon C, Canto G, Blanca M. Local allergic rhinitis: a new entity, characterization and further studies. Curr Opin Allergy Clin Immunol. 2010;10:1–7. doi: 10.1097/ACI.0b013e328334f5fb. [DOI] [PubMed] [Google Scholar]
- 30.Kellberger J, Dressel H, Vogelberg C, Leupold W, Windstetter D, Weinmayr G, et al. Prediction of the incidence and persistence of allergic rhinitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2012;129:397–402,e1-3. doi: 10.1016/j.jaci.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 32.Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008;122:145–51. doi: 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Yunginger JW, Ahlstedt S, Eggleston PA, Homburger HA, Nelson HS, Ownby DR, et al. Quantitative IgE antibody assays in allergic diseases. J Allergy Clin Immunol. 2000;105:1077–84. doi: 10.1067/mai.2000.107041. [DOI] [PubMed] [Google Scholar]
- 34.Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, Greiderer A, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011;127:616–22.e1. doi: 10.1016/j.jaci.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Rashid RS, Smith KA, Nambiar KZ, Frew AJ, Tarzi MD. Pollen-food syndrome is related to Bet v 1/PR-10 protein sensitisation, but not all patients have spring rhinitis. Allergy. 2011;66:1391–2. doi: 10.1111/j.1398-9995.2011.02618.x. [DOI] [PubMed] [Google Scholar]