Abstract
Background
Sensitization to individual cat and dog allergen molecules can contribute differently to development of allergy to these animals.
Objective
We sought to investigate the association between sensitization patterns to cat and dog allergen molecules during childhood and symptoms to these furry animals up to age 16 years.
Methods
Data from 779 randomly collected children from the Barn/Children Allergy/Asthma Milieu Stockholm Epidemiologic birth cohort at 4, 8, and 16 years were used. IgE levels to cat and dog were determined by using ImmunoCAP, and levels to allergen molecules were determined by using an allergen chip based on ISAC technology (Mechanisms for the Development of Allergy chip). Allergy was defined as reported rhinitis, conjunctivitis, or asthma at exposure to cat or dog.
Results
Cross-sectionally, IgE to Fel d 1 and cat extract had similar positive predictive values for cat allergy. IgE to Can f 1 showed a higher positive predictive value for dog allergy than dog extract IgE. Sensitizations to Fel d 1 and Can f 1 in childhood were significantly associated with symptoms to cat or dog at age 16 years. Polysensitization to 3 or more allergen molecules from cat or dog was a better longitudinal predictor of cat or dog symptoms than results of IgE tests with cat or dog allergen extract, respectively. Cross-sectionally, cat/dog-polysensitized children had higher IgE levels and more frequent symptoms to cat and dog than monosensitized children.
Conclusions
Sensitization to Fel d 1 and Can f 1 in childhood and polysensitization to either cat or dog allergen molecules predict cat and dog allergy cross-sectionally and longitudinally significantly better than IgE to cat or dog extract.
Keywords: Allergy, allergen, Barn/Children Allergy/Asthma Milieu Stockholm Epidemiologic, birth cohort, cat, children, dog, IgE, ISAC technology, microarray, pet, sensitization, Can f 1, Can f 5, Fel d 1, prediction
Allergy to cat and dog is common in the Western world,1,2 and the prevalence of sensitization to furry animals has increased both in Europe1,3 and the United States.4 It is well known that increased IgE levels to furry animal allergens are associated with allergy-related symptoms.5–7 Using ImmunoCAP, we recently reported an increase in sensitization to cat (from 6.4% to 19.0%) and dog (from 4.8% to 22.6%) in children followed from 4 to 16 years of age in the population-based Barn/Children Allergy/Asthma Milieu Stockholm Epidemiologic (BAMSE) birth cohort.8
A number of allergen molecules have been described in cats (Felis domesticus) and dogs (Canis familiaris). The major cat allergen is the uteroglobulin Fel d 1,9 which accounts for 60% to 90% of all IgE reactivity to cat dander.10,11 Among the minor cat allergens are the serum albumin Fel d 2, the lipocalins Fel d 4 and Fel d 7, and the latherin-like Fel d 8.12,13 The major dog (Canis familiaris) allergen Can f 1, together with Can f 2, Can f 4, and Can f 6, belongs to the lipocalin family. Can f 3, a serum albumin, and Can f 4 have been reported to be allergens of less importance.14,15 Can f 5, a prostatic kallikrein in male dog urine, has recently been reported to be recognized by up to 70% of dog-sensitized patients.14,16
More knowledge is needed in the area of pet allergy, where the clinical history often is inconclusive and many patients are polysensitized to several furry animals, such as cat, dog, and horse. Furthermore, some allergen molecules might be poorly represented in crude allergen extracts,17 leading to uncertain results. However, molecular allergy diagnostics allow for an increased accuracy in allergy diagnosis and prognosis and are able to reveal the specific pet allergen molecules responsible for sensitization and symptoms.18
The aims of the present study, which is part of the European Union–funded project Mechanisms for the Development of Allergy (MeDALL; http://medall-fp7.eu/)19 program, were (1) to investigate IgE reactivity to individual cat and dog allergen molecules in childhood through adolescence for the first time by using the BAMSE birth cohort, (2) compare the results with cat and dog extract IgE levels, (3) identify risk markers in preschool- and school-aged children for allergic symptoms to cat and dog up to the age of 16 years, and (4) assess the phenotypes of monosensitized and polysensitized subjects to cat and dog allergens.
Methods
Study cohort
The BAMSE study is an unselected population-based birth cohort study of 4089 children.20,21 For this study, data from baseline and the 4-, 8-, and 16-year follow-ups, the time points when blood was drawn, were used in conjunction with serologic allergy testing. At the respective follow-ups, sera were available for 64%, 60%, and 62% of the population. Background data were retrieved from the baseline questionnaire. Reported respiratory symptoms at pet exposure were obtained from the questionnaires at 4, 8, and 16 years of age. Symptoms to cat and dog were defined as reported symptoms from the upper airways, lower airways, or both at exposure to cat/dog (for definitions of symptoms, see the Methods section in this article’s Online Repository at www.jacionline.org).
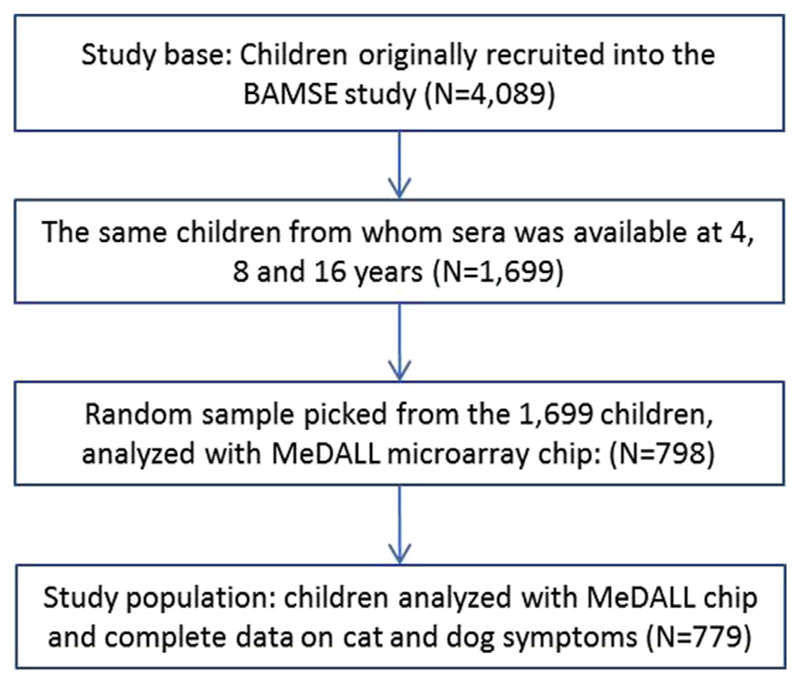
For 1699 (42%) children, blood samples were obtained from the same children at each of the 3 time points of clinical follow-up (4, 8, and 16 years). A subset of 798 children was randomly picked by using the Stata software randomization function (StataCorp, College Station, Tex), providing 2394 (3 × 798) serum samples for analysis. Complete data on reported airway symptoms to cat and dog were available in 779 of these children, which constitute our study population. Permission for the study was obtained from the Regional Ethical Review board at Karolinska Institutet at each follow-up, and parents of participating children/children themselves (when applicable) provided informed consent.
Allergen-specific IgE measurement
Serum samples were initially tested with ImmunoCAP (Thermo Fisher AB, Uppsala, Sweden) for allergen-specific IgE antibodies to cat (e1) and dog (e5) extract. The results were expressed in kilounits of allergen per liter, and a positive test result was defined as 0.35 kUA/L or greater. An IgE antibody level of greater than 100 kUA/L was given the value of 101 kUA/L in statistical evaluations.
IgE reactivity to the cat allergen molecules Fel d 1, 2, and 4, as well as the dog allergen molecules Can f 1, 2, 3, 5, and 6, were analyzed with an allergen chip based on ISAC technology (Thermo Fisher) developed in the MeDALL FP7-funded research program.22 In brief, serum aliquots of 35 μL were incubated on the microarray for 120 minutes at room temperature, and slides were washed and incubated with fluorescence-labeled anti-IgE antibodies (Thermo Fisher) for 30 minutes. Chips were then washed, dried, and analyzed with a Laser Scan Confocal microarray reader (LuxScan 10K/A; Capital-Bio, Beijing, China). The results were evaluated with Phadia Microarray Image Analysis software and are reported in ISAC standardized units. The cutoff was set at 0.3 ISAC standardized units for IgE detection (ISU-E).
Statistical analysis
Results are expressed as numbers and proportions (percentages). Group IgE levels are expressed as medians and ranges. Median ISU-E values were calculated on values of greater than the cutoff. Two-tailed t tests were used for 2-sample tests of proportions, as well as on the logarithmic scale for group comparisons of IgE levels. Positive predictive values (PPVs), which were defined as the number of true-positive results (ie, cat/dog-symptomatic and specific allergen-sensitized subjects) divided by the number of true-positive results plus the number of false-positive results (ie, all subjects sensitized to the specific allergen), respectively, and binomial exact 95% CIs for the PPVs, were calculated. Negative predictive values (NPVs), which were defined as the number of true-negative results (ie, cat/dog-asymptomatic subjects without sensitization to the specific allergen) divided by the number of true-negative results plus the number of false-negative results (ie, all subjects not sensitized to the specific allergen), respectively, and binomial exact 95% CIs for the PPVs, were calculated. Odds ratios (ORs) for symptoms to cat or dog, respectively, in relation to sensitization were estimated by using logistic regression models and 95% CIs. Fitted predicted probability estimates were plotted according to the number of IgE-reactive (≥0.3 ISU-E) cat or dog allergen molecules, respectively, per subject by using the results from the logistic regression. Logistic regression with clustered SEs was also used to investigate cat/dog symptoms in relation to sensitization to different allergen molecules using both crude ORs and ORs adjusted for concomitant sensitization to the other allergen molecules. P values of less than .05 and 95% CIs not including 1 were considered significant. All statistical analyses were performed with Stata statistical software (release 14.0, StataCorp).
Results
Demographic characteristics of the study population, including reported symptoms and sensitization to cat and dog extracts
Baseline characteristics were similar between the children included in the study and children in the original cohort (see Table E1 in this article’s Online Repository at www.jacionline.org). No significant differences were seen between the 2 groups except for a higher prevalence of allergic heredity in the study group. However, this difference in prevalence did not affect the results (data not shown). The prevalences of IgE reactivities to cat and dog extract (ImmunoCAP) at the 3 different time points were 6.8%, 13.9%, and 19.8% for cat and 5.1%, 11.6%, and 22.9% for dog, respectively. At 4, 8, and 16 years, reported upper and/or lower respiratory symptoms were 4.8%, 7.1%, and 11.2% to cat and 3.1%, 3.1%, and 5.5% to dog (see Table E2 in this article’s Online Repository at www.jacionline.org). Conjunctival challenges with cat or dog extract were performed in 34 cat- or dog-sensitized children without reported cat or dog symptoms, respectively (see the Methods section and Table E3 in this article’s Online Repository at www.jacionline.org).
IgE reactivity to microarrayed pet allergen molecules
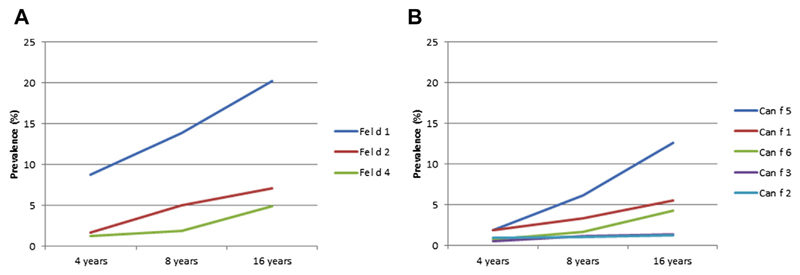
IgE reactivity to any of the 3 cat allergen molecules tested increased from 9.2% at 4 years to 14.5% at 8 and 21.8% at 16 years. Fel d 1 was the dominant sensitizing cat allergen during the entire childhood (8.7% to 20.3%; Fig 1, A) and in line with the rates of sensitization to cat extract. Fel d 1 also induced the highest IgE levels at all 3 time points (Fig 2). Only 4.4% to 7.1% of the subjects sensitized to any cat allergen molecule had IgE to Fel d 2, Fel d 4, or both but not to Fel d 1.
Fig 1.
Prevalence (percentage) of IgE reactivity (≥0.3 ISU-E) to cat (A) and dog (B) allergen components at 4, 8, and 16 years of age (n = 779).
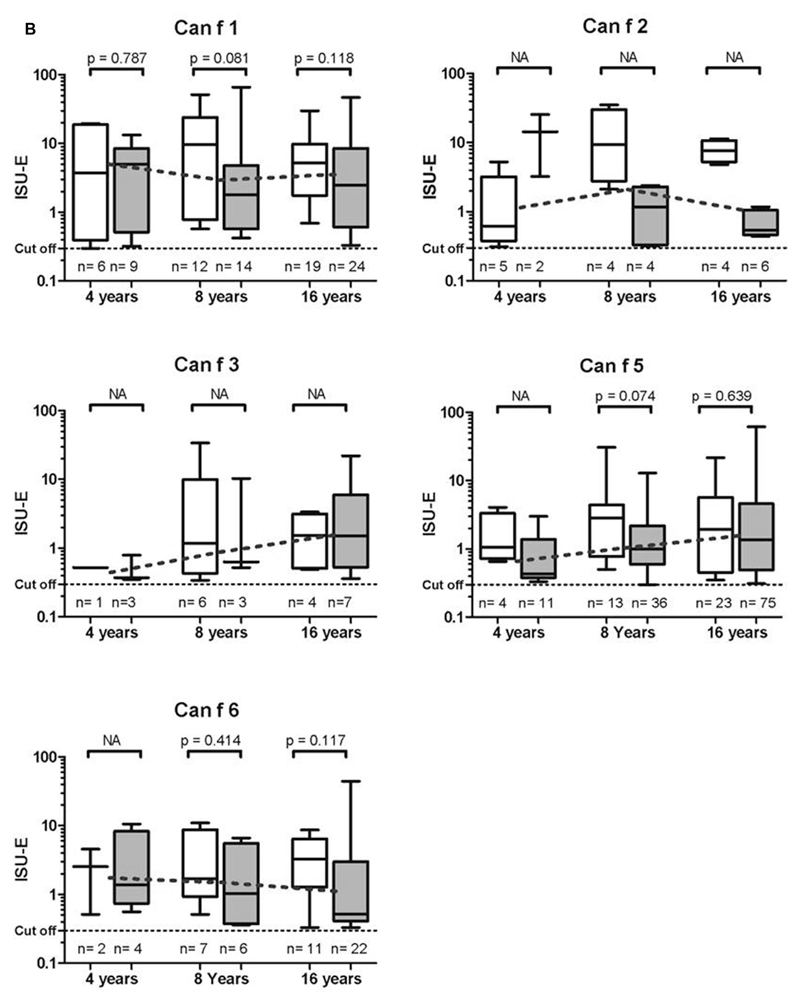
Fig 2.
Allergen-specific IgE levels (≥0.3 ISU-E) to cat (A) and dog (B) allergens in children with (white box plots) or without (gray box plots) symptoms to cat, dog, or both at 4, 8, and 16 years of age. Median IgE levels per age group (connected dotted lines) and P values are indicated. n, Number of children with positive results (N = 779); NA, not applicable (subgroup number <5 observations).
The proportion of children with IgE reactivity to any of the 5 dog allergen molecules increased from 3.6% through 8.2% to 14.8% at the corresponding time points, which was lower in comparison with dog extract ImmunoCAP results (5.1%, 11.6%, and 22.9% at ages 4, 8, and 16 years, respectively). The most frequently recognized dog allergen was Can f 5 (1.9% to 12.6%; Fig 1, B), but the highest IgE levels were directed toward Can f 1 (Fig 2).
Cross-sectional IgE reactivity to pet allergen molecules in relation to symptoms
IgE levels to Fel d 1 were significantly higher at each of the analyzed ages among children with symptoms to cat compared with nonsymptomatic children (Fig 2, A). No such associations could be seen for the dog allergen molecules in relation to dog symptoms. However, there were too few observations of IgE reactivity to the minor dog allergens Can f 2 and Can f 3 to allow for any statistical calculations. Even though a large proportion of children sensitized to a single dog allergen molecule at 16 years of age were sensitized to Can f 5 (81%), only about one tenth of them reported symptoms to dog (data not shown).
Cross-sectional PPVs were calculated for symptoms among subjects with IgE reactivity to cat or dog allergens extract or to the different pet allergen molecules (Table I). PPVs were significantly higher for IgE reactivity to Can f 1 compared with dog extract at 8 (53.8% vs 20.0%) and 16 (44.2% vs 18.5%) years of age. No significant differences in PPVs were seen for IgE to Can f 5 or Fel d 1 compared with dog or cat allergen extracts with ImmunoCAP, respectively. NPVs were quite similar for the different tested allergens and varied between 91.0% (Fel d 4 at 16 years) and 99.3% (dog extract at 8 years, data not shown). For corresponding sensitivity and specificity values, see Table E4 in this article’s Online Repository at www.jacionline.org.
Table I. Cross-sectional PPVs: proportion of cat/dog-symptomatic children among subjects with IgE reactivity to cat/dog extract (ImmunoCAP) versus cat/dog allergen molecules (microarray; binomial exact 95% CIs; n = 779).
| Cat | ||||||
| Age | Cat extract | Fel d 1 | Fel d 2 | Fel d 4 | ||
| 4 y | 30.2% (18.3% to 44.3%) | 25.0% (15.3% to 37.0%) | 61.5% (31.6% to 86.1%) | 30.0% (6.7% to 65.2%) | ||
| 8 y | 41.7% (32.2% to 51.5%) | 42.6% (33.1% to 52.5%) | 53.8% (37.2% to 69.9%) | 60.0% (32.3% to 83.7%) | ||
| 16 y | 45.5% (37.4% to 53.7%) | 41.8% (34.0% to 49.9%) | 47.3% (33.7% to 61.2%) | 52.6% (35.8% to 69.0%) | ||
| Dog | ||||||
| Age | Dog extract | Can f 1 | Can f 2 | Can f 3 | Can f 5 | Can f 6 |
| 4 y | 27.5% (14.6% to 43.9%) | 40.0% (16.3% to 67.7%) | 71.4% (29.0% to 96.3%) | 25% (0.6% to 80.6%) | 26.7% (7.8% to 55.1%) | 33.3% (4.3% to 77.8%) |
| 8 y | 20.0% (12.3% to 29.8%) | 53.8% (33.4% to 73.4%) | 50% (15.7% to 84.3%) | 66.7% (29.9% to 92.5%) | 25.0% (13.6% to 39.6) | 53.8% (25.1% to 80.8%) |
| 16 y | 18.5% (13.1% to 25.0%) | 44.2% (29.1% to 60.1%) | 40.0% (12.2% to 73.8%) | 36.4% (10.9% to 69.2%) | 23.5% (15.5% to 33.1%) | 33.3% (18.0% to 51.8%) |
Boldface values indicate significant differences (95% CIs not overlapping) compared with IgE to dog/cat extract (ImmunoCAP), respectively. NPVs varied between 91.0% (Fel d 4 at age 16 years) and 99.3% (dog extract at age 8 years).
The proportion of children cosensitized to cat and dog allergen molecules increased from 4 to 16 years (Fig 3). Having IgE reactivity to both cat and dog allergen molecules increased the median IgE levels to Fel d 1 and Can f 1 and increased the prevalence rates of allergic symptoms to cats and dogs compared with being sensitized to 1 furry animal only (Fig 3). Of the 197 (25.3%) children with IgE reactivity to at least 1 cat or dog allergen at 16 years of age, 80 (40.6%) reported symptoms to cat, dog, or both at the same age. In the sensitized but asymptomatic group of 177 children, 26.5% were sensitized to cat or dog allergens at all ages (4, 8, and 16 years), and 47.9% were sensitized only at age 16 years (data not shown).
Fig 3.
Overlapping IgE reactivity (≥0.3 ISU-E) to cat and dog allergen components and median IgE levels to Fel d 1 (cat), Can f 1 (dog), and Can f 5 (dog) at 4, 8, and 16 years of age. Cat/dog symptom prevalence in the subgroups is shown (n = 779). Blue circles, IgE reactivity (≥0.3 ISU-E) to any cat allergen component: Fel d 1, Fel d 2, or Fel d 4. Green circles, IgE reactivity (≥0.3 ISU-E) to any dog allergen component: Can f 1, Can f 2, Can f 3, Can f 5, or Can f 6.
Longitudinal IgE reactivity to pet allergen molecules in relation to symptoms
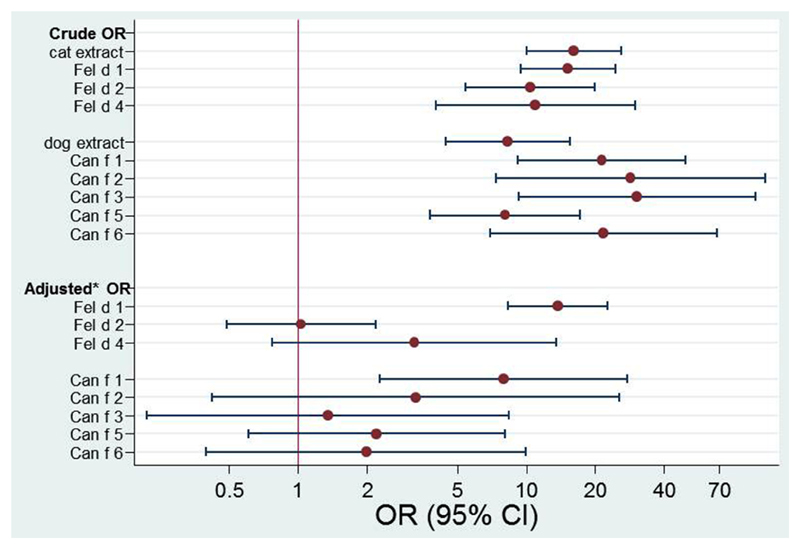
We also assessed our data longitudinally by investigating the ORs of reported symptoms to cat or dog at 16 years of age in relation to IgE reactivity to the cat and dog allergen molecules and whole extracts at 4 and 8 years of age. At ages 4 and 8 years, IgE reactivity to each of the cat or dog allergen molecules or to cat or dog extract was significantly associated with reported symptoms to cat or dog at age 16 years, respectively (Fig 4, upper part). However, when adjusting for sensitization to the other cat allergen molecules, only IgE reactivity to Fel d 1 remained significantly associated with symptoms (OR, 13.7; 95% CI, 8.3-22.7; Fig 4, lower part). Similarly, only IgE levels to Can f 1 were significantly associated with symptoms to dog at age 16 years after adjusting for sensitization to the other dog allergen molecules at 4 and 8 years of age (OR, 8.0; 95% CI, 2.3-27.7; Fig 4, lower part, and see Table E5 in this article’s Online Repository at www.jacionline.org).
Fig 4.
Longitudinal logistic regression. Crude and adjusted ORs for IgE sensitizations to cat and dog allergen (ISU-E ≥0.3) at 4 and 8 years of age in relation to reported cat/dog allergy at 16 years of age (n = 779). *Adjusted for concomitant sensitization to the other cat or dog components, respectively. For sensitization matrix, see Table E5.
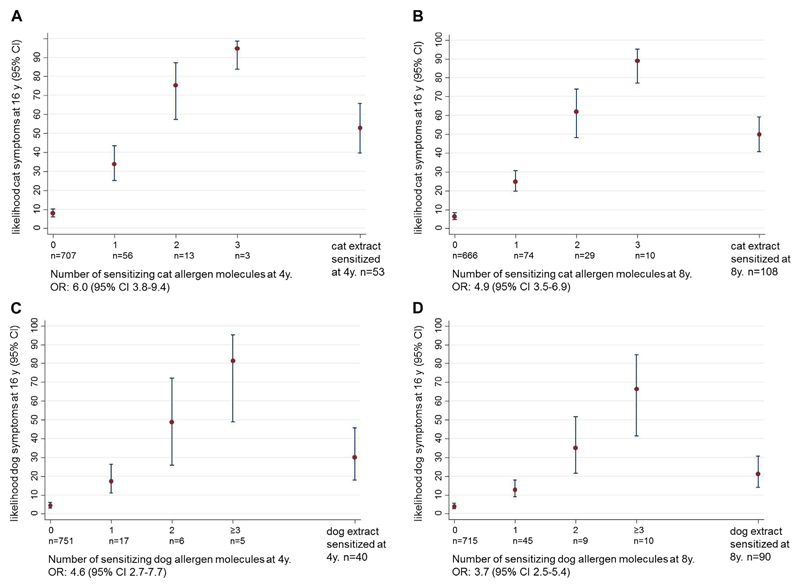
Sensitization to more than 1 cat allergen molecule at age 4 or 8 years significantly increased the likelihood of reporting cat allergy at 16 years of age (Fig 5, A and B). A 95% probability of cat symptoms at 16 years of age was present for 4-year-old children with IgE reactivity to all 3 cat allergen molecules. However, sensitization to cat extract at 4 and 8 years of age predicted only a 50% probability of cat symptoms at age 16 years. Similarly, sensitization to an increasing number of dog allergen molecules at age 4 or 8 years gave rise to a higher probability of symptoms to dog at 16 years of age (Fig 5, B and C). Sensitization to at least 3 dog allergens at 4 years of age led to an 81% likelihood for dog symptoms at 16 years of age. For comparison, the likelihood of dog symptoms at 16 years of age only reached 20% and 30% when sensitization to dog extract with ImmunoCAP at 4 or 8 years of age was used, respectively.
Fig 5.
A and B, Likelihood (y-axis, percentage) of reporting symptoms to cat at 16 years of age depending of the number of IgE-reactive cat allergens (Fel d 1, Fel d 2, and Fel d 4) and ImmunoCAP cat extract sensitization (x-axes) at 4 (Fig 5, A) and 8 (Fig 5, B) years of age. C and D, Likelihood (y-axis, percentage) of reporting symptoms to dog at 16 years of age depending of the number of IgE-reactive dog allergens (Can f 1, Can f 2, Can f 3, Can f 5, and Can f 6) and ImmunoCAP dog extract sensitization (x-axes) at 4 (Fig 5, C) and 8 (Fig 5, D) years of age. Numbers, ORs, and 95% CIs are shown (n = 779).
Discussion
For the first time, we present a cross-sectional and longitudinal population-based study showing the effect of sensitization to cat and dog allergen molecules during early childhood in relation to future clinical respiratory symptoms at cat or dog exposure. The novel findings are that polysensitization (ie, sensitization to ≥3 cat or dog allergen molecules, respectively) at 4 or 8 years of age is superior in predicting future cat or dog symptoms than sensitization to cat or dog extract, respectively. Furthermore, sensitization to both cat and dog allergen molecules is associated with more prevalent cat- and dog-related symptoms and higher IgE levels to these molecules. With respect to dog, our results indicate that sensitization to Can f 1 is the most important prognostic marker of dog allergy and superior to measurement of IgE levels to dog allergen extract (ImmunoCAP), whereas measurement of IgE levels to Can f 5 shows a weaker association to dog allergy. Moreover, we show that sensitization to Fel d 1 is a predictor of cat allergy at 16 years of age. Finally, monosensitized and polysensitized subjects appear to belong to 2 different allergy phenotypes. Thus molecular allergy diagnostics offer important advantages for the diagnosis of cat and dog allergy in early childhood and help to predict the course of disease to adolescence.
The strengths of the study are the large sample size of children from a population-based birth cohort and the high follow-up rate; nearly 80% from baseline answered the questionnaire at the 16-year follow-up, and there are blood samples from 42% at all 3 clinical follow-ups.
One limitation of the small sample sizes in some subgroups is illustrated by large CIs in the corresponding analyses. Therefore the associated results need to be confirmed by other studies.
Another limitation could be the geographic relevance of the findings given the fact that the study is based on a Swedish cohort. However, we do not believe that exposure to furry animals or reactions on exposure to cat or dog allergens differ substantially among children in our cohort compared with those in children in the Western world as a whole. Breeds of cats and dogs are usually the same in major parts of the Western world.
Yet another limitation might be that symptom data are based on questionnaires and that avoidance of animals might be difficulty to assess. What might introduce a misclassification is limited contact with animals and not precisely knowing about current allergy to cats or dogs. To eliminate this bias, we performed conjunctival challenges with cat or dog extracts that actually confirmed the questionnaire data (see the Methods section and Table E3 in the online repository).23
We found that sensitization to Fel d 1 and Can f 1 at 4 and 8 years of age are risk markers of cat and dog allergy at 16 years of age, as is cosensitization to several cat or dog allergen molecules. The dominant role of IgE to Fel d 1 compared with Fel d 2 and Fel d 4 in cat-sensitized patients was observed in each of the tested age groups. We also noted that the prevalence of IgE to Fel d 1 almost tripled from 4 to 16 years of age and reached 20% in the oldest age group. Fel d 1 was the only cat allergen molecule independently predictive for cat symptoms and also induced the highest IgE levels among the analyzed cat allergen molecules, which reflects that Fel d 1 accounts for the major allergenic activity in cat dander.7 Compared with other mammalian allergens, Fel d 1 stands out because of its very dominant role in cat allergy.9 Thus testing with cat allergen molecules provides additional strength in predicting current or future cat allergy.
Regarding sensitization to dog allergen molecules, we found that the prevalence more than tripled from childhood to adolescence, but the prevalence rates were lower compared with those to cat allergen. Interestingly, Can f 5, a kallikrein protein that is suggested to be found only in male dogs,14 was the most prevalent dog allergen molecules recognized by IgE in all age groups. A large proportion of children who were sensitized to a single dog allergen molecule had IgE to Can f 5, but only about one of 10 of them reported symptoms to dog. This allergen has been reported as a major allergen among European subjects with dog allergy; among these subjects, 26 (70%) of 37 were sensitized, 14 of whom lacked IgE reactivity to Can f 1, Can f 2, or Can f 3.14 Sensitization to Can f 5 was also recently highlighted among children with severe asthma who, compared with children with controlled asthma, had an IgE response to more than 3 animal-derived allergen molecules, of which Can f 5 was one.24,25 However, adjustment for cosensitization to other allergen molecules was not performed. Furthermore, these studies were all investigating small samples of allergic patients. In our large population-based study Can f 1 was more related to dog symptoms than Can f 5, and Can f 1 also induced the highest IgE levels. Furthermore, IgE reactivity to Can f 1 was the only parameter that independently could predict future symptoms to dog. Thus having IgE to Can f 1 seems to be the strongest marker related to development of dog allergy, which is of importance when allergen-specific immunotherapy treatment is to be considered. In contrast, according to current literature, having IgE to Can f 5 appears, at least according to our data, to be more prominent for allergic airway disease in general than for dog allergy in particular. One of the novelties of our study is that the associations described are observed in a population-based longitudinal study and are not due to selection bias or lack of adjustment. Thus the findings are of public health relevance.
For the diagnosis of cat allergy in our population, we also noted that IgE testing with just 1 cat allergen molecule, Fel d 1, is as good as testing for IgE to cat allergen extract (ImmunoCAP), which is line with previous observations.7 Importantly, for diagnosis of dog allergy, measuring IgE levels to Can f 1 seems to have advantages over measuring IgE levels to dog allergen extract (ImmunoCAP). In fact, IgE reactivity to Can f 1 in childhood predicted the development of dog allergy in adolescence significantly better than IgE reactivity to dog allergen extract. Actually, dog allergen extracts have shown considerable heterogeneity in their allergen composition, which will have a negative influence on the accuracy of diagnosis of dog allergy.17
Interestingly, we also observed that cosensitization to cat and dog allergen molecules became more common with increasing age. Being cosensitized to both cat and dog allergen molecules was associated with higher IgE levels to cat and dog allergen molecules and a higher proportion of subjects having symptoms compared with being monosensitized to either cat or dog allergen molecules. Notably, few children with IgE to only 1 dog allergen molecule reported symptoms at 16 years of age. This can be explained by IgE to Can f 5, which was the dominant sensitizing molecule in this group of children.
At age 4 or 8 years, the possibility to predict symptoms at age 16 years increased with the number of IgE-reactive cat or dog allergens. A high number of IgE-reactive allergens (3 cat molecules and ≥3 dog molecules) at a young age (4 years) was associated with a very high probability (95% and 81%, respectively) of having symptoms to cat or dog at exposure in adolescence. The results are in line with previous findings in which multiple sensitizations were shown to be a risk marker for asthma in children.24,26,27 However, other studies were either based on a small sample of allergic patients or did not focus specifically on sensitization to cat or dog allergen molecules in relation to cat or dog symptoms. If the present study is confirmed, immunotherapy in young children with polysensitization to cat and dog allergen molecules might be a treatment of choice because of the risk of persistence of symptoms. In such children molecular-based allergy diagnostics with individual allergen molecules will be of considerable importance.
The common IgE tests with crude allergen extract might be sufficient for cat allergy but less likely for dog allergy. In addition, young children with high IgE levels to Fel d 1 or Can f 1 or children who are polysensitized to cat or dog allergen molecules, as well as children sensitized to both cat and dog allergen components, are more likely to have present and future allergy and might be more important to treat (eg, with more frequent doctor’s office visits or early immunotherapy to cat or dog).
In conclusion, this is the first study to elucidate the benefit of using cat and dog allergen molecules longitudinally and in a population-based sample as predictors of cat and dog allergy development from childhood to adolescence. Molecular-based allergy diagnostics offer new opportunities for improving the diagnosis of furry animal allergy and in particular dog allergy. Our data suggest that different allergy phenotypes exist and that both clinical management and research should be considered differently in patients with monosensitization versus polysensitization to cat, dog, or both.
Methods
Definition of symptoms and background characteristics (questionnaire answers)
Cat allergy
Asthma at age 4 years: Has your child had trouble with wheezy breathing or cough after contact with the following after the age of 2 years? (Yes and cat indicated)
Rhinitis/conjunctivitis at age 4 years: Has your child after the age of 2 years ever had trouble with sneezing, runny nose, stuffy nose, or red itchy eyes after contact with the following? (Yes and cat indicated)
Asthma at age 8 years: Has your child had wheezing, raspy breathing, or disruptive cough in conjunction with any of the following since age 4 years? (Yes and cat indicated)
Rhinitis/conjunctivitis at age 8 years: Has your child been afflicted with sneezing, runny nose, stuffy nose, or red irritated eyes in conjunction with any of the following since age 4 years? (Yes and cat indicated)
Asthma at age 16 years: Has your child had respiratory symptoms or a troublesome cough after contact with any of the following in the past 12 months? (Yes and cat indicated)
Rhinitis/conjunctivitis at age 16 years: Has your child had nose or eye symptoms without simultaneously having a cold after contact with any of the following in the past 12 months? (Yes and cat indicated)
Dog allergy
Asthma at age 4 years: Has your child had trouble with wheezy breathing or cough after contact with the following after the age of 2 years? (Yes and dog indicated)
Rhinitis/conjunctivitis at age 4 years: Has your child after the age of 2 years ever had trouble with sneezing, runny nose, stuffy nose, or red itchy eyes after contact with the following? (Yes and dog indicated)
Asthma at age 8 years: Has your child had wheezing, raspy breathing, or disruptive cough in conjunction with any of the following since age 4 years? (Yes and dog indicated)
Rhinitis/conjunctivitis at age 8 years: Has your child been afflicted with sneezing, runny nose, stuffy nose, or red irritated eyes in conjunction with any of the following since age 4 years? (Yes and dog indicated)
Asthma at age 16 years: Has your child had respiratory symptoms or a troublesome cough after contact with any of the following in the past 12 months? (Yes and dog indicated)
Rhinitis/conjunctivitis at age 16 years: Has your child had nose or eye symptoms without simultaneously having a cold after contact with any of the following in the past 12 months? (Yes and dog indicated)
Background characteristics
Heredity allergy: Mother, father, or both with a doctor’s diagnosis of asthma and asthma medication and/or a doctor’s diagnosis of hay fever in combination with furred pets and/or pollen allergy at the time of the baseline questionnaire.
Breast-fed ≥4 months: Exclusively breast-fed for 4 months or more.
Parental smoking: Either of the parents smoked at least 1 cigarette per day at the time of the first questionnaire.
Young mother at birth (≤25 years): Mother’s age less than 25 years at birth of the child.
White collar parent: Socioeconomic status for the household according to dominance order: “Socioeconomic division (SEI); Reports on Statistical Coordination 1982:4.”
Cat ownership: Having a cat at home at the time of baseline and 1-year questionnaires.
Dog ownership: Having a dog at home at the time of baseline and 1-year questionnaires.
Conjunctival challenge
A random subset of 34 children (median age, 19.3 years; age range, 17.9-20.1 years) who had never reported symptoms to cat or dog at exposure at any of the 3 time points but were sensitized to cat or dog underwent conjunctival challenges with cat or dog allergen extract to elucidate whether they were still asymptomatic. The challenge was performed with a single drop (about 0.03 mL) of 100,000 SQ-E of undiluted Aquagen of cat or dog allergen extract (ALK-Abelló Nordic) in the lower conjunctival sac in one eye and normal saline (NaCl 0.9%) solution in the other eye. The substance was unknown to the patients. The left eye was chosen for provocation. The patients were informed not to rub the eyes after instillation. The same investigator (A.A.) performed all challenges. Conjunctival signs of redness and chemosis were graded 10 minutes after the provocationE1 and assessed by using a 4-grade score, as follows: 0, no signs at all; 1, mild; 2, moderate; and 3, severe signs.
Extended Data
Fig E1.
Flow chart of children in the BAMSE cohort study base (n = 4089) and study population (n = 779).
Table E1. Baseline (median age, 2 months) and age 1-year characteristics of the study group (n = 779) compared with children in the original cohort (n = 4089).
| Variables | Study base cohort* (n = 4089) | Study population* (n = 779) | |||||
|---|---|---|---|---|---|---|---|
| No. | Percent | 95% CI | No. | Percent | 95% CI | P value | |
| Female sex | 2024 | 49.5 | 48.0-51.0 | 393 | 50.4 | 46.9-54.0 | .63 |
| Heredity allergy | 1200 | 29.7 | 28.3-31.1 | 258 | 33.2 | 29.9-36.7 | .05 |
| Breast-fed ≥4 mo | 3116 | 79.5 | 78.2-80.8 | 609 | 79.2 | 76.2-82.0 | .84 |
| Parental smoking | 855 | 21.0 | 19.8-22.3 | 159 | 20.5 | 17.7-23.5 | .75 |
| Young mother at birth (≤25 y) | 319 | 7.8 | 7.0-8.7 | 58 | 7.4 | 5.7-9.5 | .73 |
| White collar parent | 695 | 17.3 | 16.1-18.5 | 112 | 14.5 | 12.1-17.2 | .06 |
| Older siblings | 1980 | 48.4 | 46.9-50.0 | 389 | 49.9 | 46.4-53.5 | .44 |
| Cat ownership† | 405 | 11.1 | 10.1-12.1 | 85 | 11.8 | 9.6-14.3 | .39 |
| Dog ownership† | 221 | 6.4 | 5.7-7.2 | 38 | 5.6 | 4.1-7.4 | .54 |
Missing values between 0 and 170 observations in the study base cohort and between 0 and 7 observations in the study population.
At baseline, age 1 year, or both.
Table E2. Symptoms at exposure to cat and dog, respectively, at 4, 8, and 16 years of age (n = 779).
| Age | Any symptom (asthma and/or rhinitis), no. (%) | Asthma, no. (%) | Rhinitis, no. (%) | Asthma and rhinitis, no. (%) |
|---|---|---|---|---|
| Cat symptoms | ||||
| 4 y | 37 (4.7) | 25 (3.2) | 27 (3.5) | 15 (1.9) |
| 8 y | 55 (7.1) | 19 (2.4) | 52 (6.7) | 16 (2.0) |
| 16 y | 87 (11.2) | 36 (4.6) | 83 (10.7) | 32 (4.1) |
| Dog symptoms | ||||
| 4 y | 24 (3.1) | 15 (1.9) | 18 (2.3) | 9 (1.2) |
| 8 y | 24 (3.1) | 10 (1.3) | 22 (2.8) | 8 (1.0) |
| 16 y | 43 (5.5) | 23 (3.0) | 39 (5.0) | 19 (2.4) |
Table E3. Conjunctival challenge with cat/dog extract* in a subset of adolescents (median age, 19.2 years; age range, 17.9-20.1 years) without reported symptoms to cat/dog at exposure at 16 years of age but with IgE reactivity (≥0.3 ISU) to at least 1 cat/dog allergen component at 16 years of age, respectively (n [cat] = 18, n [dog] = 16).
| IgE reactivity to cat allergen molecules (≥0.3 ISU-E) |
IgE reactivity to dog allergen molecules (≥0.3 ISU-E) |
||
|---|---|---|---|
| Cat allergen | Challenged (n = 18) | Dog allergen | Challenged (n = 16) |
| Fel d 1, no. (%) | 14 (77.8) | Can f 1, no. (%) | 1 (6.3) |
| IgE level (ISU-E), median (range) | 0.49 (0.32-20.12) | IgE level (ISU-E) | 16.5 |
| Fel d 2, no. (%) | 3 (16.7) | Can f 2, no. (%) | 1 (6.3) |
| IgE level (ISU-E) | 0.3, 0.55, 0.62 | IgE level (ISU-E) | 1.17 |
| Fel d 4, no. (%) | 3 (16.7) | Can f 3, no. (%) | 3 (18.8) |
| IgE level (ISU-E) | 1.9, 3.22, 6.16 | IgE level (ISU-E) | 0.36, 0.53, 21.91 |
| Can f 5, no. (%) | 15 (93.8) | ||
| IgE level (ISU-E), median (range) | 1.56 (0.38-34.93) | ||
| Can f 6, no. (%) | 3 (18.8) | ||
| IgE level (ISU-E) | 0.41, 0.41, 0.47 | ||
| Reaction at provocation, no. (%) | 3 (16.7) | Reaction at provocation, no. (%) | 1 (6.3) |
| Highest IgE reactivity if reaction, cat allergen, (degree of reaction) | Fel d 1 = 20.12 (2) Fel d 1 = 9.12 (1) Fel d 1 = 0.49 (1) |
Highest IgE reactivity if reaction, dog allergen, (degree of reaction) | Can f 1 = 16.5 (1) |
Degree of reaction: 1, mild; 2, moderate; and 3, severe.
Four subjects were challenged with both cat and dog extracts (but with an at least 1-week interval).
Table E4. Diagnostic sensitivity (true-positive rate) and specificity (true-negative rate) for cat or dog symptoms, respectively, and presence or absence of IgE reactivity to cat/dog extract or allergen molecules at 4, 8, and 16 years of age (n = 779).
| Sensitivity | Specificity | |
|---|---|---|
| 4 y | ||
| Cat extract | 43.2% (27.1% to 60.5%) | 95.0% (93.2% to 96.5%) |
| Any cat component | 45.9% (29.5% to 63.1%) | 92.6% (90.5% to 94.4%) |
| Fel d 1 | 45.9% (29.5% to 63.1%) | 93.1% (91.1% to 94.8%) |
| Fel d 2 | 21.6% (9.83% to 38.2%) | 99.3% (98.4% to 99.8%) |
| Fel d 4 | 8.1% (1.7% to 21.9%) | 99.1% (98.1% to 99.6%) |
| Dog extract | 45.8% (25.6% to 67.2%) | 96.2% (94.5% to 97.4%) |
| Any dog component | 33.3% (15.6% to 55.3%) | 97.4% (95.9% to 98.4%) |
| Can f 1 | 25.0% (9.8% to 46.7%) | 98.8% (97.7% to 99.5%) |
| Can f 2 | 20.8% (7.1% to 42.2%) | 99.7% (99.0% to 100%) |
| Can f 3 | 4.2% (0.1% to 21.1%) | 99.6% (98.8% to 99.9%) |
| Can f 5 | 16.7% (4.7% to 37.4%) | 98.5% (97.4% to 99.3%) |
| Can f 6 | 8.3% (1.0% to 27.0%) | 99.5% (98.6% to 99.9%) |
| 8 y | ||
| Cat extract | 83.3% (70.7% to 92.1%) | 91.2% (89.0% to 93.2%) |
| Any cat component | 83.6% (71.2% to 92.2%) | 90.7% (88.4% to 92.8%) |
| Fel d 1 | 83.6% (71.2% to 92.2%) | 91.4% (89.2% to 93.4%) |
| Fel d 2 | 38.2% (25.4% to 52.3%) | 97.5% (96.1% to 98.5%) |
| Fel d 4 | 16.4% (7.8% to 28.8%) | 99.2% (98.2% to 99.7%) |
| Dog extract | 78.3% (56.3% to 92.5%) | 90.4% (88.1% to 92.4%) |
| Any dog component | 75.0% (53.3% to 90.2%) | 93.9% (92.0% to 95.5%) |
| Can f 1 | 58.3% (36.6% to 77.9%) | 98.4% (97.2% to 99.2%) |
| Can f 2 | 16.7% (4.7% to 37.4%) | 99.5% (98.6% to 99.9%) |
| Can f 3 | 25.0% (9.8% to 46.7%) | 99.6% (98.8% to 99.9%) |
| Can f 5 | 50.0% (29.1% to 70.9%) | 95.2% (93.5% to 96.6%) |
| Can f 6 | 29.2% (12.6% to 51.1%) | 99.2% (98.3% to 99.7%) |
| 16 y | ||
| Cat extract | 80.5% (70.6% to 88.2%) | 87.9% (85.2% to 90.2%) |
| Any cat component | 79.3% (69.3% to 87.3%) | 85.4% (82.6% to 88.0%) |
| Fel d 1 | 75.9% (65.5% to 84.4%) | 86.7% (83.9% to 89.1%) |
| Fel d 2 | 29.9% (20.5% to 40.6%) | 95.8% (94.0% to 97.2%) |
| Fel d 4 | 23.0% (14.6% to 33.2%) | 97.4% (95.9% to 98.5%) |
| Dog extract | 76.7% (61.4% to 88.2%) | 80.3% (77.2% to 83.1%) |
| Any dog component | 65.1% (49.1% to 79.0%) | 88.2% (85.6% to 90.4%) |
| Can f 1 | 44.2% (29.1% to 60.1%) | 96.7% (95.2% to 97.9%) |
| Can f 2 | 9.3% (2.6% to 22.1%) | 99.2% (98.2% to 99.7%) |
| Can f 3 | 9.3% (2.6% to 22.1%) | 99.0% (98.1% to 99.6%) |
| Can f 5 | 53.5% (37.7% to 68.8%) | 89.8% (87.4% to 91.9%) |
| Can f 6 | 25.6% (13.5% to 41.2%) | 97.0% (95.5% to 98.1%) |
Value are shown as proportions (95% CIs).
Table E5. Sensitization matrix: allergen molecules at 4, 8, and 16 years of age.
| Cat | |||||||
| No., 4 y | No., 8 y | No., 16 y | Fel d 1 | Fel d 2 | Fel d 4 | ||
| 707 | 666 | 609 | – | – | – | ||
| 4 | 3 | 5 | – | – | X | ||
| 0 | 1 | 6 | – | X | – | ||
| 0 | 1 | 1 | – | X | X | ||
| 52 | 70 | 95 | X | – | – | ||
| 3 | 1 | 15 | X | – | X | ||
| 10 | 27 | 31 | X | X | – | ||
| 3 | 10 | 17 | X | X | X | ||
| Dog | |||||||
| No., 4 y | No., 8 y | No., 16 y | Can f 1 | Can f 2 | Can f 3 | Can f 5 | Can f 6 |
| 751 | 715 | 664 | – | – | – | – | – |
| 1 | 3 | 3 | – | – | – | – | X |
| 8 | 32 | 55 | – | – | – | X | – |
| 0 | 1 | 8 | – | – | – | X | X |
| 2 | 0 | 1 | – | – | X | – | – |
| 0 | 0 | 1 | – | – | X | X | – |
| 0 | 0 | 2 | – | – | X | X | X |
| 0 | 0 | 1 | – | X | – | X | – |
| 0 | 0 | 1 | – | X | – | X | X |
| 2 | 2 | 0 | – | X | – | – | – |
| 4 | 8 | 9 | X | – | – | – | – |
| 1 | 2 | 2 | X | – | – | – | X |
| 4 | 5 | 10 | X | – | – | X | – |
| 0 | 1 | 10 | X | – | – | X | X |
| 0 | 1 | 1 | X | – | X | – | – |
| 0 | 1 | 2 | X | – | X | X | – |
| 1 | 2 | 1 | X | – | X | X | X |
| 1 | 0 | 1 | X | X | – | – | – |
| 2 | 0 | 0 | X | X | – | – | X |
| 1 | 1 | 1 | X | X | – | X | – |
| 0 | 0 | 3 | X | X | – | X | X |
| 0 | 1 | 0 | X | X | X | X | – |
| 1 | 4 | 3 | X | X | X | X | X |
X, Sensitized (≥0.3 ISU-E); –, not sensitized (<0.3 ISU-E).
Clinical implications.
IgE to Fel d 1, Can f 1, and polysensitization to cat or dog allergen molecules in preschool- or school-aged childhood are predictive risk markers for the development of allergy to cat and dog at age 16 years.
Acknowledgments
We thank all the families who have participated and the staff working with the BAMSE project. The technical help of Renata Kiss, Department of Pathophysiology and Allergy Research, Medical University of Vienna, is acknowledged.
Supported by the Swedish Asthma and Allergy Association’s Research Foundation, the Foundation for Health Care Sciences and Allergy Research, the Centre for Allergy Research (CfA), Stockholm County Council, the Swedish Research Council, the Swedish Heart-Lung Foundation, the Center for Inflammatory Diseases, Karolinska Institutet, the Swedish Cancer and Allergy Foundation, the Konsul Th C Bergs Foundation, the King Gustaf V Research Foundation, the Swedish Society of Medicine, the Magnus Bergvall Foundation and Karolinska Institutet, the Austrian Science Fund (FWF project F4605), and the European Commission’s Seventh Framework 29 Program MeDALL under grant agreement no. 261357.
Abbreviations used
- BAMSE
Barn/Children Allergy/Asthma Milieu Stockholm Epidemiologic
- ISU-E
ISAC standardized units for IgE detection
- MeDALL
Mechanisms for the Development of Allergy
- NPV
Negative predictive value
- OR
Odds ratio
- PPV
Positive predictive value
Footnotes
Disclosure of potential conflict of interest: A. Asarnoj has received research support from the Stockholm County Council, Karolinska Institutet, the Swedish Cancer and Allergy Foundation, the Konsul Th C Bergs Foundation, and the Swedish Society of Medicine; has received travel support from the Swedish Society of Medicine; and is employed by the Stockholm County Council. C. Lupinek has received lecture fees from Thermo Fisher. J. Anto has received research support from the European Commission. J. Bousquet is a board member for Stallergenes; has received consultancy fees from Actelion, Almirall, Meda, Merck, Merck Sharp Dohme, Novartis, Sanofi-Aventis, Takeda, Teva, and Uriach; and has received lecture fees and payments for development of educational presentations from Almirall, AstraZeneca, Chiesi, GlaxoSmithKline, Meda, Merck, Merck Sharp Dohme, Novartis, OM Pharma, Sanofi-Aventis, Schering-Plough, Takeda, Teva, and Uriach. R. Valenta has received research support from the European Union, the Austrian Science Fund (FWF), and Biomay AG (Vienna, Austria) and has received consultancy fees from Thermo Fisher (Uppsala, Sweden), Biomay AG, and Fresenius Medical Care (Bad Homburg, Germany). M. Wickman has received research support and lecture fees from Thermo Fischer Scientific, consultancy fees from Thermo Fischer Scientific and Microtest Dx, and payment for development of educational presentations from Stallergenes. M. van Hage has received a consultancy fee from Hycor Biomedical and has received lecture fees from Thermo Fisher Scientific, Novartis, and ALK-Abelló. The rest of the authors declare that they have no relevant conflicts of interest.
The CrossMark symbol notifies online readers when updates have been made to the article such as errata or minor corrections
References
- 1.Ronmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundback B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J Allergy Clin Immunol. 2009;124:357–63, e1-15. doi: 10.1016/j.jaci.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz R, Ellert U, Kalcklosch M, Dahm S, Thamm M. Patterns of sensitization to inhalant and food allergens—findings from the German Health Interview and Examination Survey for Children and Adolescents. Int Arch Allergy Immunol. 2013;162:263–70. doi: 10.1159/000353344. [DOI] [PubMed] [Google Scholar]
- 3.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Increasing prevalence of specific IgE to aeroallergens in an adult population: two cross-sectional surveys 8 years apart: the Copenhagen Allergy Study. J Allergy Clin Immunol. 2000;106:247–52. doi: 10.1067/mai.2000.108312. [DOI] [PubMed] [Google Scholar]
- 4.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Salo PM, Calatroni A, Gergen PJ, Hoppin JA, Sever ML, Jaramillo R, et al. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2011;127:1226–35.e7. doi: 10.1016/j.jaci.2010.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton RG, MacGlashan DW, Jr, Saini SS. IgE antibody-specific activity in human allergic disease. Immunol Res. 2010;47:273–84. doi: 10.1007/s12026-009-8160-3. [DOI] [PubMed] [Google Scholar]
- 7.Gronlund H, Adedoyin J, Reininger R, Varga EM, Zach M, Fredriksson M, et al. Higher immunoglobulin E antibody levels to recombinant Fel d 1 in cat-allergic children with asthma compared with rhinoconjunctivitis. Clin Exp Allergy. 2008;38:1275–81. doi: 10.1111/j.1365-2222.2008.03003.x. [DOI] [PubMed] [Google Scholar]
- 8.Wickman M, Asarnoj A, Tillander H, Andersson N, Bergstrom A, Kull I, et al. Childhood-to-adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J Allergy Clin Immunol. 2014;133:580–2. doi: 10.1016/j.jaci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Grönlund H, Saarne T, Gafvelin G, van Hage M. The major cat allergen, Fel d 1, in diagnosis and therapy. Int Arch Allergy Immunol. 2010;151:265–74. doi: 10.1159/000250435. [DOI] [PubMed] [Google Scholar]
- 10.van Ree R, van Leeuwen WA, Bulder I, Bond J, Aalberse RC. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J Allergy Clin Immunol. 1999;104:1223–30. doi: 10.1016/s0091-6749(99)70017-5. [DOI] [PubMed] [Google Scholar]
- 11.Kleine-Tebbe J, Kleine-Tebbe A, Jeep S, Schou C, Lowenstein H, Kunkel G. Role of the major allergen (Fel d I) in patients sensitized to cat allergens. Int Arch Allergy Immunol. 1993;100:256–62. doi: 10.1159/000236421. [DOI] [PubMed] [Google Scholar]
- 12.Hales BJ, Chai LY, Hazell L, Elliot CE, Stone S, O’Neil SE, et al. IgE and IgG binding patterns and T-cell recognition of Fel d 1 and non-Fel d 1 cat allergens. J Allergy Clin Immunol Pract. 2013;1:656–65, e1-5. doi: 10.1016/j.jaip.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Smith W, Butler AJ, Hazell LA, Chapman MD, Pomes A, Nickels DG, et al. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34:1732–8. doi: 10.1111/j.1365-2222.2004.02090.x. [DOI] [PubMed] [Google Scholar]
- 14.Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol. 2009;123:362–8. doi: 10.1016/j.jaci.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson OB, van Hage M, Gronlund H. Mammalian-derived respiratory allergens —implications for diagnosis and therapy of individuals allergic to furry animals. Methods. 2014;66:86–95. doi: 10.1016/j.ymeth.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Curin M, Swoboda I, Wollmann E, Lupinek C, Spitzauer S, van Hage M, et al. Microarrayed dog, cat, and horse allergens show weak correlation between allergen-specific IgE and IgG responses. J Allergy Clin Immunol. 2014;133:918–21.e6. doi: 10.1016/j.jaci.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–63. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- 18.Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO-ARIA-GA(2)LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bousquet J, Anto J, Auffray C, Akdis M, Cambon-Thomsen A, Keil T, et al. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy. 2011;66:596–604. doi: 10.1111/j.1398-9995.2010.02534.x. [DOI] [PubMed] [Google Scholar]
- 20.Asarnoj A, Ostblom E, Kull I, Lilja G, Pershagen G, Hedlin G, et al. Sensitization to inhalant allergens between 4 and 8 years of age is a dynamic process: results from the BAMSE birth cohort. Clin Exp Allergy. 2008;38:1507–13. doi: 10.1111/j.1365-2222.2008.03046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kull I, Almqvist C, Lilja G, Pershagen G, Wickman M. Breast-feeding reduces the risk of asthma during the first 4 years of life. J Allergy Clin Immunol. 2004;114:755–60. doi: 10.1016/j.jaci.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nivenius E, Van der Ploeg I, Gafvelin G, Van Hage M, Montan PG. Conjunctival provocation with airborne allergen in patients with atopic keratoconjunctivitis. Clin Exp Allergy. 2012;42:58–65. doi: 10.1111/j.1365-2222.2011.03858.x. [DOI] [PubMed] [Google Scholar]
- 24.Nordlund B, Konradsen JR, Kull I, Borres MP, Onell A, Hedlin G, et al. IgE antibodies to animal-derived lipocalin, kallikrein and secretoglobin are markers of bronchial inflammation in severe childhood asthma. Allergy. 2012;67:661–9. doi: 10.1111/j.1398-9995.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- 25.Konradsen JR, Nordlund B, Onell A, Borres MP, Grönlund H, Hedlin G. Severe childhood asthma and allergy to furry animals: Refined assessment using molecular-based allergy diagnostics. Pediatr Allergy Immunol. 2014;25:187–92. doi: 10.1111/pai.12198. [DOI] [PubMed] [Google Scholar]
- 26.Patelis A, Gunnbjornsdottir M, Malinovschi A, Matsson P, Onell A, Hogman M, et al. Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol. 2012;130:397–402.e2. doi: 10.1016/j.jaci.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Prosperi MC, Belgrave D, Buchan I, Simpson A, Custovic A. Challenges in interpreting allergen microarrays in relation to clinical symptoms: a machine learning approach. Pediatr Allergy Immunol. 2014;25:71–9. doi: 10.1111/pai.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Nivenius E, Van der Ploeg I, Gafvelin G, Van Hage M, Montan PG. Conjunctival provocation with airborne allergen in patients with atopic keratoconjunctivitis. Clin Exp Allergy. 2012;42:58–65. doi: 10.1111/j.1365-2222.2011.03858.x. [DOI] [PubMed] [Google Scholar]