Abstract
Exosomes are nano-sized membrane vesicles (50–120 nm), which are released from a wide variety of cells. Depending on their cellular origin, they can induce immune stimulatory-, inhibitory-, or tolerance-inducing effects. However, it is still unclear what role exosomes play during human inflammatory diseases. It has not been studied whether exosomes derived from human dendritic cells (DCs), the first cells to encounter allergens in the mucosa, can carry aeroallergens and contribute to allergic immune responses. We therefore explored whether DC-derived exosomes can present the major cat allergen Fel d 1 and whether they thereby contribute to the pathogenesis of allergic disease. Our results demonstrate that exosomes are able to present aeroallergens and thereby induce T-cell T(H)2-like cytokine production in allergic donors. Thus, these exosomes may be important immune-stimulatory factors in allergic immune responses and important targets or engineered tools in immunotherapy.
Keywords: aeroallergens, allergy, dendritic cells, exosomes, Fel d 1
Exosomes are nano-sized membrane vesicles (50–120 nm) derived from the endosomal compartment, released from a wide variety of cells, and they can be found in several body fluids such as plasma, breastmilk, and bronchoalveolar lavage (BAL) (1, 2). They have been demonstrated to have immune stimulatory-, inhibitory-, or tolerance-inducing effects, depending on their cellular origin (3). In particular, exosomes from antigen-presenting cells (APCs) have gained interest, as they carry immunorelevant molecules such as MHC classes I and II and costimulatory molecules, and thus are investigated for use in vaccine and immune therapeutic strategies. Previous studies have demonstrated that exosomes are able to transfer functional MHC I/II/peptide complexes to bystander APCs (2). It has also been demonstrated that exosomes isolated from B cells can present T-cell-activating peptides from the major birch allergen Bet v 1 and thereby induce T-cell proliferation and T(H)2-like cytokine production (4). However, it has not been studied whether exosomes derived from human dendritic cells (DCs), the first cells to encounter allergens in the mucosa, can carry aeroallergens and contribute to allergic inflammation. We therefore explored whether DC-derived exosomes can present aeroallergens, such as the major cat allergen Fel d 1, and whether they thereby contribute to the allergic immune response.
Material and methods
Detailed experimental methods are given in Supporting information.
Results and discussion
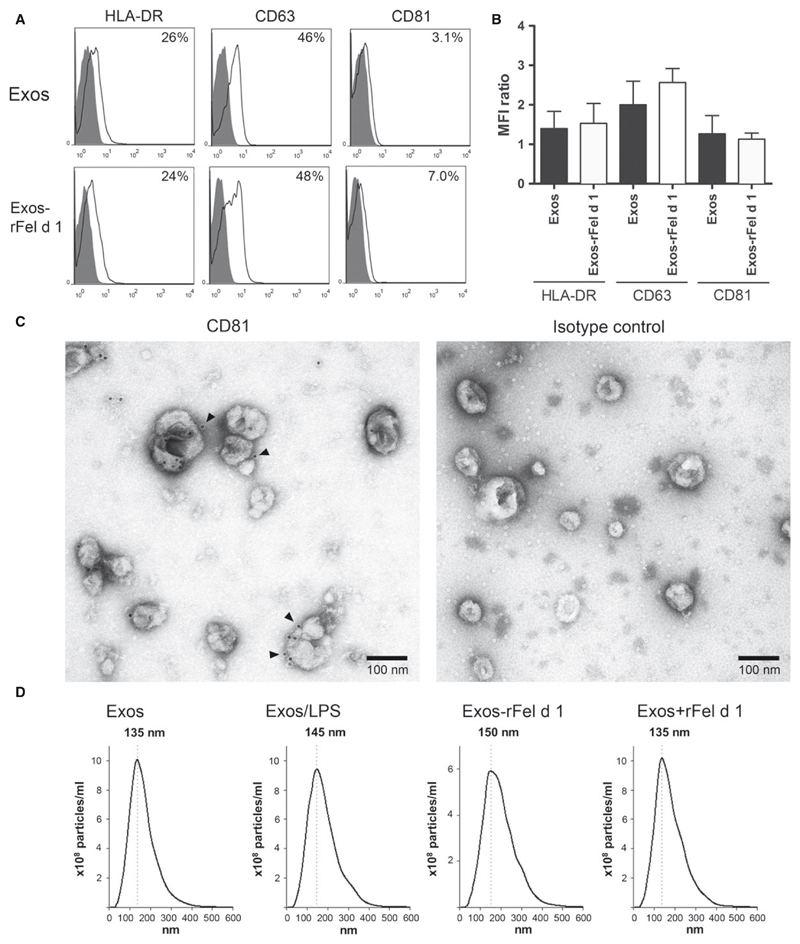
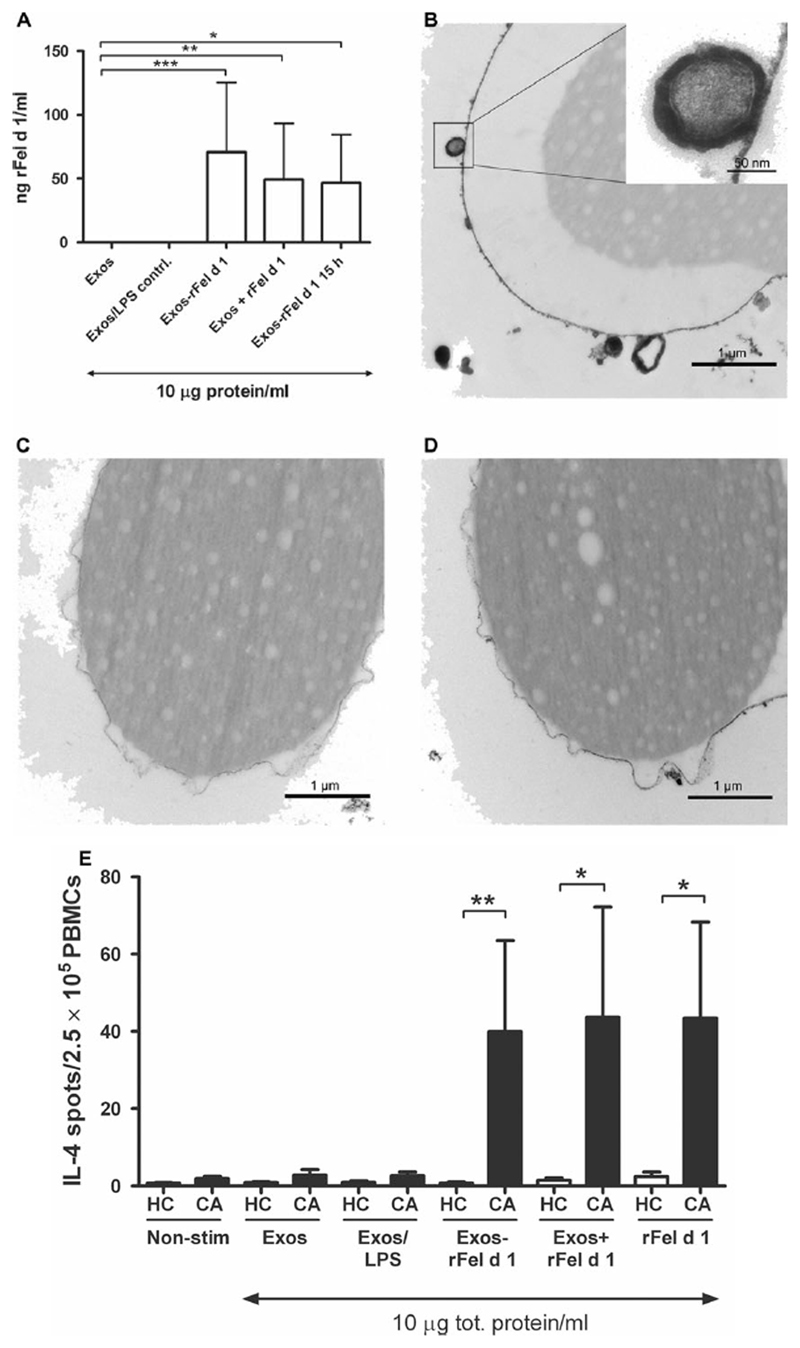
Peripheral blood mononuclear cells (PBMCs) were isolated from cat-allergic or healthy blood donors, whose plasma had been analyzed for IgE and IgG4 to Fel d 1 (Table S1). The concentration of IgE was in the range of 0.49–37 kAU/l to Fel d 1 in the allergic donors and below <0.10 kAU/l in each of the healthy blood donors, and the IgG4 levels were above 0.05 mg/l in only two of the cat-allergic donors (Table S1). We started to investigate exosomes isolated from healthy blood donors. The exosomes were either generated from monocyte-derived dendritic cells (MDDCs) cultured alone (Exos), from MDDCs cocultured with rFel d 1 (10 μg/ml; Exos-rFel d 1), or from MDDCs cultured with similar amount of LPS (0.08 ng/ml; Sigma-Aldrich, Steinheim, Germany) found in the rFel d 1 preparation (Exos/LPS) for 48 h. To prove that Exos-rFel d 1 carries Fel d 1 which first has been taken up by MDDCs, another control was added where MDDCs were first cultured in the presence of rFel d 1 for 15 h; thereafter, the MDDCs were washed to remove any rFel d 1 not endocytosed by the MDDCs. After additional culture for 48 h in fresh media without rFel d 1, the supernatant was collected for isolation of exosomes (Exos-rFel d 1 15 h). To address whether Fel d 1 is able to bind directly to exosomes without the involvement of cells, rFel d 1 (10 μg/ml) was added for 30 min to one part of the supernatant from MDDCs cultured alone (Exos + rFel d 1). The phenotype of MDDCs was not affected by the LPS amount found in rFel d 1 or by rFel d 1 itself as seen by flow cytometry (Fig. S1). The exosomes from the various supernatants were isolated by ultracentrifugation. Flow cytometry showed that both Exos and Exos-rFel d 1 had a similar surface expression of HLA-DR, CD63, and CD81 (Fig. 1A,B), which corresponds to a typical phenotype of MDDC-derived exosomes (3). Immuno-negative electron microscopy staining (iEM) showed the characteristic morphology of exosomes and the presence of the exosome marker CD81 on the surface of Exos-rFel d 1 (Fig. 1C) (3), which was not seen when using the isotype control (Fig. 1C). Nanoparticle tracking analysis indicated that Exos, Exos/LPS, Exos – rFel d 1, and Exos + rFel d 1 exhibited relatively similar sizes with a diameter ranging from 135 to 150 nm (Fig. 1D). To investigate the presence of rFel d 1 on exosomes, ELISA was performed, indicating that Exos-rFel d 1, Exos + rFel d 1, and Exos-rFel d 1 15 h are able to carry rFel d 1 (Fig. 2A). Similar amounts of rFel d 1 were found on the different exosomes, within the range of 3–390 ng rFel d 1/ml (n = 15) in 10 μg exosome preparations (Fig. 2A). The wide range reflects the individual variations between the different blood donors. As rFel d 1 is detected on all types of rFel d 1-exosomes, Fel d 1 seems to be able to be endocytosed by MDDCs, loaded onto exosomes and released on exosomes (Exos + rFel d 1 15 h), and also to bind directly to exosomes isolated from MDDCs cultured alone (Exos + rFel d 1). The presence of rFel d 1 on rFel d 1-exosomes was further supported by TEM, which visualizes how rFel d 1-exosomes (Fig. 2B), but not Exos (Fig. 2C), bind to latex beads covered with anti-Fel d 1 Abs, representing the presence of rFel d 1 on the surface of exosomes. Beads covered with the isotype control showed no binding to Exos-rFel d 1 (Fig. 2D).
Figure 1.
Characterization of Exos-rFel d1. Expression of the surface markers HLA-DR, CD63, and CD81 on Exos and Exos-rFel d 1 was measured by flow cytometry and is shown as (A) histograms and % positive cells from one representative experiment of three independent experiments using exosomes from different healthy blood donors (isotype control in black), or as (B) the ratio of the mean fluorescence intensity (MFI). Results represent the mean ± SEM from three independent experiments. (C) The morphology of Exos-rFel d 1 was visualized by performing iEM on pools of Exos-rFel d 1 from five healthy blood donors using a mAb against CD81 (detected with a 10 nm gold particle-conjugated anti-rabbit Ab; indicated by arrows) and corresponding isotype control. (D) The mean particle size distribution from five different exosome batches for Exos, Exos/LPS, Exos-rFel d 1 and Exos + rFel d 1 analyzed by nanoparticle tracking analysis (NanoSight).
Figure 2.
Exosomes generated from MDDCs carry rFel d 1 and induce IL-4 production in PBMCs from cat-allergic donors. (A) The presence of rFel d 1 on Exos-rFel d 1 (n = 7), Exos + rFel d 1 (n = 5), and Exos-Fel d 1 15 h (n = 3) generated from MDDCs from healthy nonallergic blood donors was analyzed using ELISA and a mouse mAb against Fel d 1. Exos and Exos/LPS were used as controls (n = 7). (B) Exos-rFel d 1, but not (C) Exos, binds to latex beads coupled with anti-Fel d 1 rabbit Abs, as visualized using TEM on a pool of Exos-rFel d 1 from 2 healthy blood donors. (D) No binding is seen with latex beads coupled with an isotype control Ab. (E) IL-4 production in PBMCs from cat-allergic (CA) donors and healthy controls (HC) measured by ELISpot when cultured alone (nonstimulated; n = 7), with Exos (n = 7), Exos/LPS (n = 7), Exos-rFel d 1 (n = 7), Exos + rFel d 1 (n = 5), or only rFel d 1 (10 μg; n = 7) for 48 h. Data represent mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, Mann–Whitney U-test.
To investigate whether rFel d 1 on the exosomes has immunogenic effects on PBMCs, cultures of PBMCs from healthy or cat-allergic donors were set up pairwise and cocultured with either Exos, Exos/LPS control, Exos-rFel d 1, Exos + rFel d 1, Exos-rFel d 1 15 h, or only rFel d 1. Cytokine analysis was performed using ELISpot. Results showed that Exos-rFel d 1 and Exos + rFel d 1 did not induce IL-4 production in PBMCs from healthy donors, whereas in the cat-allergic donors, Exos-rFel d 1 and Exos + rFel d 1 were able to induce IL-4 responses (Fig. 2E). IL-4 responses were also detected in control experiments with Exos-rFel d 1 15 h, where we had a response of 10 and 47 IL-4 spots/2.5 9 105 PBMCs, respectively, from two cat-allergic donors, whereas no spots were detected by PBMCs from two healthy donors. Despite the low amount of rFel d 1 found on the different rFel d 1-exosomes (Fig. 2A), they are still able to induce similar IL-4 responses as 10 μg/ml pure rFel d 1 (Fig. 2E and Fig. S2). Thus, these data suggest that exosomes are able to enhance the IL-4 stimulatory capacity of rFel d 1 in cat-allergic individuals.
Additional cytokine analysis (IL-6, IL-10, IL-17A, IFN-γ, and TNF) was performed with CBA, but no significant differences were observed between PBMCs from cat-allergic and healthy donors cocultured with the different exosome preparations (data not shown). Similar results for IL-10 and IFNγ have been previously observed for PBMCs treated with only Fel d 1 (5).
Our results demonstrate a novel route for distribution of aeroallergens via exosomes derived from DCs. These exosomes are able to present allergens and thereby induce T-cell T(H)2-like cytokine production in allergic donors, and may therefore be important immune-stimulatory factors in allergic immune responses. Thus, exosomes carrying allergens may be important targets in immunotherapy. Our novel finding also shows the ability to engineer exosomes by adding allergens, which may be useful for future exosome-based vaccines.
Supplementary Material
Acknowledgments
We thank Zekiye Cansu for skillful technical assistance with preparation of MDDCs and exosomes, Jonas Binnmyr for technical support with ELISA, Catharina Johansson and nurse Agneta Sollén Nilsson for help with recruitment of blood donors and patient handling, and our collaborators in the EuroNanoMed project NANO Allergen Specific Immuno Therapy (NANOASIT) for fruitful discussions. This study was supported by the Swedish Research Council, the Swedish Cancer and Allergy Fund, the ERA-NET EuroNanoMed project NANOASIT, and in part by Grant F4605 of the Austrian Science Fund (FWF).
Footnotes
Author contributions
Annika Scheynius and Helen Vallhov conceived and designed the study; Helen Vallhov, Cindy Gutzeit, and Kjell Hultenby performed experiments and analysis. Rudolf Valenta and Hans Grönlund contributed with reagents. All authors participated in interpretation of data. Helen Vallhov and Annika Scheynius drafted the manuscript, and all co-authors approved the final version.
Conflicts of interest
Rudolf Valenta has received research grants from Biomay AG, Vienna, Austria, and Thermo Fisher, Uppsala, Sweden, and serves as a consultant for these companies.
References
- 1.Admyre C, Telemo E, Almqvist N, Lötvall J, Lahesmaa R, Scheynius A, et al. Exosomes – nanovesicles with possible roles in allergic inflammation. Allergy. 2008;63:404–408. doi: 10.1111/j.1398-9995.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- 2.Théery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 3.Chaput N, Théery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 4.Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Cirkovic Velickovic T, Thunberg S, Polovic N, Neimert-Andersson T, Grönlund H, van Hage M, et al. Low levels of endotoxin enhance allergen-stimulated proliferation and reduce the threshold for activation in human peripheral blood cells. Int Arch Allergy Immunol. 2008;146:1–10. doi: 10.1159/000112497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.