Abstract
Background
Prevention of new IgE sensitizations has been described during allergen-specific immunotherapy. However, prospective data using a preventive approach in very young children who would benefit most are missing. We initiated a prospective pilot study investigating the safety, immunomodulatory, and sensitization-preventive effect of sublingual immunotherapy (SLIT) in mono/oligoclonally sensitized, clinically asymptomatic children 2–5 yr of age.
Methods
In this double-blinded, randomized, placebo-controlled pilot study, 31 mono-/oligosensitized children to house-dust mite or grass pollen were included. SLIT with the respective source (n = 15) or placebo (n = 16) was applied. After dose-up-phase therapy was continued for 2 yr. Parents recorded clinical events, vaccinations, and drug intake in a diary. Skin prick testing and specific IgE and IgG measurements were recorded at baseline, 12 and 24 months. At the same time, allergen-specific proliferation and IL10- and TGFβ-dependent Treg function were measured.
Results
Preventive application of SLIT in young children was safe (no relevant side effects in 21,170 single applications). After 12 and 24 months of treatment, the rate of allergen-specific sensitization (specific IgE and SPT reactivity) was comparable in the treatment and the placebo group. However, verum-treated patients displayed a significant up-regulation of allergen-specific IgG (p < 0.05). Furthermore, IL10-dependent inhibition (p < 0.05) was observed in vitro in the treatment group but not in the placebo group.
Conclusion
Preventive SLIT is safe in children 2–5 yr of age and induces regulatory mechanisms involving allergen-specific IgG and IL10. Based on this pilot study, large-scale trials will need to investigate the modulation of sensitization and clinically relevant allergy.
Keywords: allergy prevention, allergen-specific immunotherapy, IgE sensitization, skin prick test, specific IgE
Allergen-specific immunotherapy (AIT) is regarded as the most efficient therapy in established IgE-mediated allergic diseases (insect venom allergy, allergic rhinitis, and bronchial asthma). Early and long-lasting clinical improvements based on immunologic changes can be achieved (1–4). Although the clinical response to immunotherapy has been proven to be allergen-specific (5, 6), there is emerging evidence that administration of appropriate immunotherapy from one allergen source to mono/oligo-sensitized children can reduce the likelihood of patients developing additional sensitizations from other allergen sources (3, 7–10). Thus, an allergen-specific and a bystander effect of preventive AIT have been postulated. These effects have been shown in retrospective and prospective studies with subcutaneous allergen immunotherapy and sublingual immunotherapy (SLIT) using conventional treatment protocols (3, 7–11). Due to the less invasive approach, the sublingual route (SLIT) is preferred among pediatricians, in particular in young children. Immunologic changes in SLIT have been described within 1–2 months of treatment (12, 13). However, placebo-controlled trials addressing the allergy/sensitization-preventive effect have not yet been performed. This study has thus been designed to understand the effect and mechanism in such a setting. In this first randomized, placebo-controlled, allergen-specific, preventive sublingual immunotherapy (pSLIT) pilot trial mono/oligosensitized children 2–5 yr of age were treated with a conventional dosing and duration protocol for 2 yr. Children had symptoms of the upper/lower airways and the skin, but these were infection-related and not allergen exposure associated. Thus, change in clinical symptoms was not taken as outcome parameter. Instead, the main primary outcome parameter was defined as development of new (or additional) sensitizations during the treatment period and not monitoring of clinical symptoms.
Materials and methods
Study population and design
We included (randomized) 31 patients, aged 2–5 yr, with a sensitization to house-dust mite or grass pollen as defined either by skin prick test (ALK-Abelló, Hamburg, Germany) or by specific IgE antibody testing (ImmunoCAP®; Thermo Fisher Scientific-Phadia AB, Uppsala, Sweden) (Fig. 1). Children with defined allergen-(season) related symptoms and children under persistent treatment with topical steroids (for upper or lower airways or skin) were excluded.
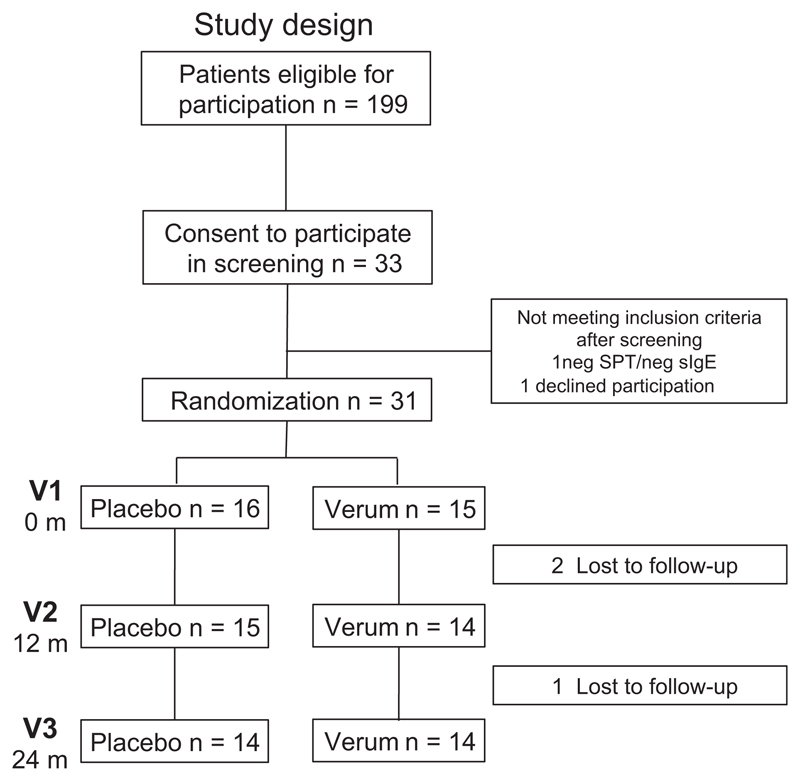
Figure 1.
Study design, enrollment, randomization, and outcomes.
Skin prick testing with a panel of standardized allergens (Dermatophagoides pteronyssinus, 6-grass–pollen mix, birch pollen, Cladosporium herbarum, Alternaria alternata, peanut, cat- and dog dander, hen’s egg, and cow’s milk; ALK-Abelló) was performed by the blinded personnel according to established guidelines before and at 12 and 24 months of immunotherapy treatment. A wheal of at least 3 mm was regarded as positive. Serum-specific IgE and IgG antibody levels were measured at the same time points with the use of the Immuno-CAP® (Thermo Fisher Scientific-Phadia AB and chip-based microarray [Thermo Fisher Scientific-Phadia AB (14)]. A positive-specific IgE level was defined as larger than 0.35 ku/L for Immuno-CAP® and as larger than 0.3 ISU (ISAC standard units) for the microarrayed chip measurements.
Heparinized peripheral blood was collected at time point of inclusion (before IT; V1) and after 12 (V2) and 24 (V3) months of immunotherapy treatment. Peripheral blood mononuclear cells (PBMC) were isolated.
The study protocol and consent forms were approved by the institutional review board of the Medical University of Vienna. Written informed consent was obtained from parents or guardians. The study protocol has been approved by the European Drug Agency (EMA; EudraCT 2006-007096-32).
Randomization and SLIT protocol
Immunotherapy extract (NovoHelisen®) and placebo were provided by Allergopharma (Reinbek, Germany). Allergen concentrations were defined as follows: der p1 9.4 μg/ml and der f1 5.7 μg/ml, group V grass pollen 13.4 μg/ml, respectively. The participants were randomly assigned by means of a block randomization 2:2 to receive either placebo or verum immunotherapy with either grass pollen or house-dust mite extract (according to the individual sensitization profile, with a total of 15 children receiving active SLIT and 16 receiving sublingual placebo). In case of oligosensitization (2–3 sensitizations), house-dust mite or grass pollen extract was chosen (there was no house-dust mite/grass pollen double sensitization in any case). The protocol for SLIT consisted of two phases: an initial dose escalation and a maintenance phase as described by the manufacturer and used for treatment purposes in grass pollen or house-dust mite allergic individuals. During the treatment period, parents were instructed to note all clinical events, vaccinations, and medical treatments on a diary record that was collected every 3 months by the study nurse.
The study was blinded until the final data lock file was sent to the Center for Medical Statistics, Informatics and Intelligent Systems, Medical University of Vienna. The randomization institution (Allergopharma) provided thereafter the unblinded patient list to the Center for Medical Statistics, Informatics and Intelligent Systems. Data analysis of the primary outcome parameter was thereafter provided to the study center. For further calculations of the immunologic parameters study, personnel was unblinded.
Antigens and antibodies
Purified nDer p 1 was kindly provided by Wayne Thomas (Telethon Institute for Child Health Research, University of Western Australia, Perth, Australia), and rDer p 2 was generously provided by Susanne Vrtala (Institute of Pathophysiology and Allergy Research, Medical University of Vienna, Austria). rPhl p 1, rPhl p 5, and rBet v 1 were purchased from Biomay (Vienna, Austria). Human TGFβ Receptor II and IL10 were blocked using mAb from R&D Systems (Abingdon, UK).
Proliferation assays
For proliferation experiments, freshly isolated PBMCs were cultured in triplicates in serum-free UltraCulture medium (Lonza, Basel, Switzerland, supplemented with 2 mm l-glutamine and 170 mg/l gentamycin sulfate, both Sigma-Aldrich, St. Louis, MO, USA) alone or in the presence of rPhl p 1, rPhl p 5, nDer p 1, rDer p 2, rBet v 1 (10 μg/ml each), and anti-CD3 (250 ng/ml). For inhibition experiments, PBMC was incubated with the above mentioned allergens in the presence of a neutralizing anti-TGFβ- (150 ng/ml), or anti-IL-10- (both 50 ng/ml) and an isotype-matched control antibody. After 6 days, proliferation was determined after 16 h by 3H-thymidine (Perkin Elmer, Waltham, MA, USA) incorporation. Results are displayed as stimulation index, calculated by mean cpm of stimulated cultures/mean cpm of unstimulated cultures. The degree of cytokine-mediated inhibition was calculated as fold increase of proliferation to the respective allergen in the presence of anti-IL10 or anti-TGF-β antibodies as compared to the respective isotype controls and the allergen (13).
Statistical analysis
Sample size calculation wase based on results from uncontrolled or open controlled studies investigating the effect of AIT in young allergic children. Considering IgE sensitization effects, a sample size of 70 in each group would have 80% power to detect a probability of 0.320 that an observation in placebo group is less than an observation in verum group using a Wilcoxon (Mann–Whitney) rank-sum test with a 0.050 two-sided significance level. A smaller sample size was used in this pilot study to evaluate at first safety and effectiveness in a vulnerable study group that has not yet been treated with such a treatment.
The main outcome parameter was the number of positive sensitizations (of the 10 performed tests: Dermatophagoides pteronyssinus, 6-grass–pollen mix, birch pollen, cladosporium herbarum, alternaria alternata, peanut, cat- and dog dander, henn’s egg, and cow’s milk) calculated for each patient.
Poisson regression models (for repeated measurements) were used to investigate the influence of group (verum vs. placebo), year, age, gender, positive family history, and number of infections on the number of sensitizations. Therefore, first, an univariate model was calculated and then univariate significant variables were further investigated in a multiple poisson regression model.
Comparisons between the time points (baseline, 12 and 24 months) of allergen-specific IgG and IgE levels and impact of anti-IL10 and anti-TGFb treatment on proliferation were performed using Wilcoxon-signed rank test (secondary outcome measure). For comparisons of baseline characteristics between the two groups, Mann–Whitney U-test or Fisher Exact test was applied. p-values were declared significant at levels <0.05. All analyses were performed using the sas 9.1 System release 2.12.
Results
Patient characteristics and side effects
Based on the inclusion criteria as defined by IgE-mono/oligosensitization to the most common allergens in this age group at an age of 2–5 yr without clear-cut clinical symptoms, 199 patients were eligible for participation in the trial. Thirty-three consented to participate and underwent a screening procedure. Thirty-one of these 33 patients were included in the pilot trial to receive either SLIT to house-dust mite or grass pollen according to their sensitization profile or the placebo (verum n = 15, placebo n = 16, Fig. 1). Three children received a grass pollen, and 12 children received a house-dust mite extract. Groups were matched according to age, number of sensitization, gender, and family history (Table 1). In both groups, patients were predominantly male (70%). The median age at inclusion was 4 yr. One and two patients, respectively, were lost upon follow-up in the verum and in the placebo group (Fig. 1).
Table 1. Baseline characteristics, according to study group.
| Placebo |
Verum |
|||||
|---|---|---|---|---|---|---|
| Visit | V1 | V2 | V3 | V1 | V2 | V3 |
| n | 16 | 15 | 14 | 15 | 14 | 14 |
| Age (median) | 3.78 | 4.78 | 5.78 | 4.08 | 5.22 | 6.20 |
| Gender (f/m) | 4/12 | 4/11 | 4/10 | 5/10 | 4/10 | 4/10 |
| Allergy family history (pos/neg) | 9/7 | 8/7 | 8/6 | 9/6 | 9/5 | 9/5 |
| Specific treatment assigned (grass pollen/house-dust mite) | 4/12 | 4/11 | 4/10 | 3/12 | 3/11 | 3/11 |
Any side effect that could potentially be linked to SLIT was documented in a diary. Preventive SLIT was reported to be safe by the patients and parents within this trial. Among 21,170 single doses applied, one adverse event was reported in the symptom diaries. One patient who received grass pollen SLIT reported self-limiting burning of the tongue after applying the medication on three consecutive days with decreasing intensity in the first week of verum treatment. No further reactions were reported.
Preventive SLIT does not decrease the rate of new sensitizations
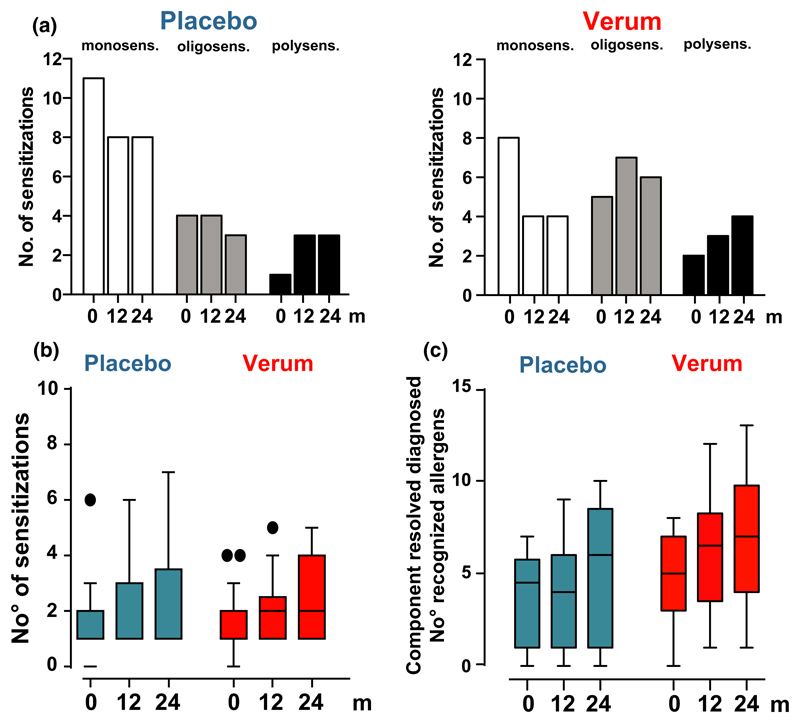
Sensitization was assessed at time point before start and at 12 and 24 months immunotherapy. The number of monosensitized patients decreased while the number of oligosensitized and polysensitized patients increased in both groups without significant differences (Fig. 2a). Neither the number of new sensitizations to extracts measured by ImmunoCAP, including also non-treated allergen sources (Fig. 2b), nor the rate of new sensitizations to allergen components (n = 58) of the 10 allergen sources measured by microarray technology were higher in the treatment group than in the placebo group (Fig. 2c). In both groups, component resolved diagnosis revealed that the rate of new sensitizations to HDM and timothy grass pollen allergens increased significantly over time (Fig. 2c).
Figure 2.
No evidence for an enhanced or reduced IgE sensitization rate in patients treated with preventive sublingual immunotherapy (SLIT). (a) The number of sensitizations to a panel of allergens (Dermatophagoides pteronyssinus, 6-grass–pollen mix, birch pollen, cladosporium herbarum, alternaria alternata, peanut, cat- and dog dander, hen’s egg, and cow’s milk) was assessed via ImmunoCAP. Each patient was assigned as monosensitized (one sensitization), oligosensitized (2–3 sensitizations), and polysensitized (>4 sensitizations). Changes over treatment period are shown. (b) Changes of IgE sensitizations to the 10 allergen sources are shown over treatment period. (c) Microarray analysis of IgE sensitization was used to assess specific IgE antibodies to 58 components of 10 allergen sources (Dermatophagoides pteronyssinus, 6-grass–pollen mix, birch pollen, cladosporium herbarum, alternaria alternata, peanut, cat- and dog dander, hen’s egg, and cow’s milk). Each dot represents one patient’s serum recognizing an individual number of allergen components of the 10 allergen sources. p < 0.05; Wilcoxon-signed rank test.
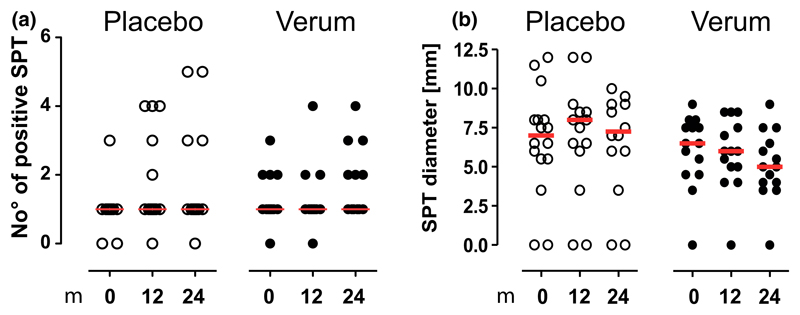
In vivo evaluation of the sensitization profiles by SPT did not show an increased rate of new sensitizations under verum treatment (Fig. 3a). However, there was a non-significant trend toward a decreased wheal size to the treated allergen observed in the verum group (Fig. 3b).
Figure 3.
Skin prick test reactivity is not altered by preventive sublingual immunotherapy (SLIT). Skin prick tests to a panel of allergens (Dermatophagoides pteronyssinus, 6-grass pollen mix, birch pollen, cladosporium herbarum, alternaria alternata, peanut, cat- and dog dander, hen’s egg, and cow’s milk) were performed and assessed during the course of treatment. The number of positive tests (a) and cumulative diameter was assessed (b). Median levels are shown in red lines. Differences are only shown if significant.
At a diary basis, subjective improvement during therapy and clinical symptoms that may be attributed to allergen exposure were assessed. Irrespective of the type of treatment improvement was reported in the majority of the patients (not shown).
Induction of pro-tolerogenic mechanisms via preventive SLIT
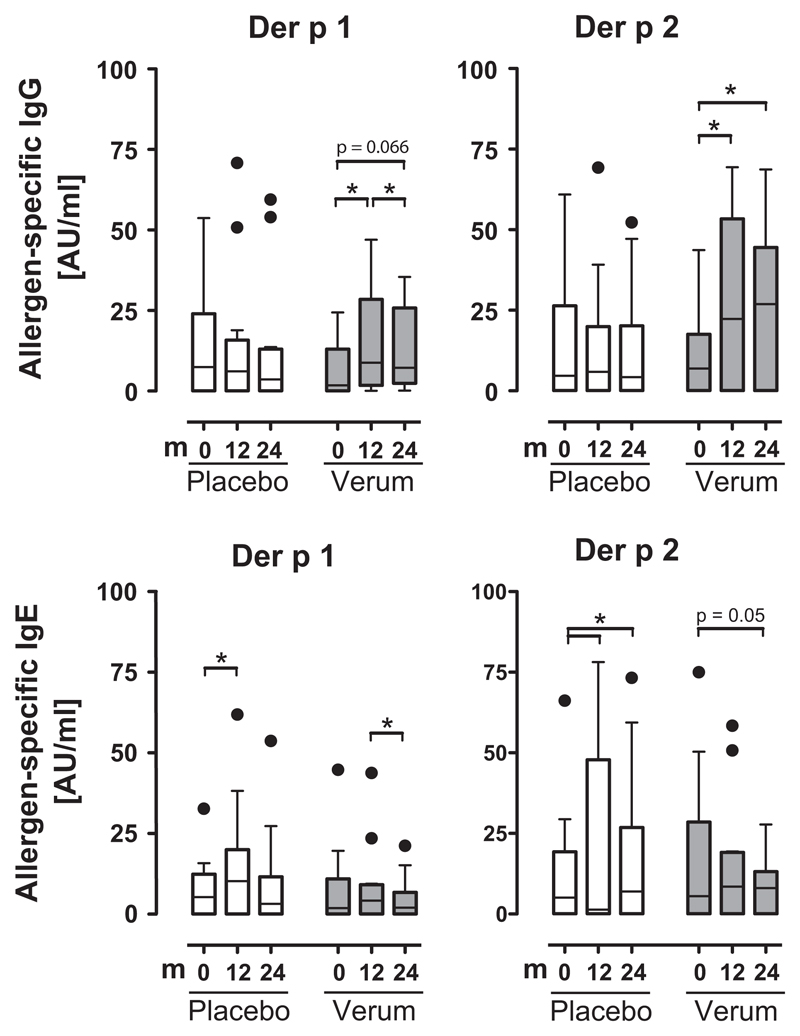
Allergen-specific IgG production to two major house-dust mite allergens Der p 1 and Der p 2 significantly increased in HDM-SLIT-treated patients at 12 and 24 month. This was not observed in sera of placebo-treated patients (Fig. 4). Allergen-specific IgE production to the respective allergens significantly increased in the placebo group and decreased in the verum group (Fig. 4).
Figure 4.
Specific IgG but not specific IgE to house-dust mite allergens significantly increases during preventive sublingual immunotherapy (SLIT). Specific IgE to Der p 1 and Der p 2 was measured by ImmunoCAP. Specific IgG was measured by microarray technology. *p < 0.05, Wilcoxon-signed rank test. Bars are shown with median and s.e.; dots represent individual outliners.
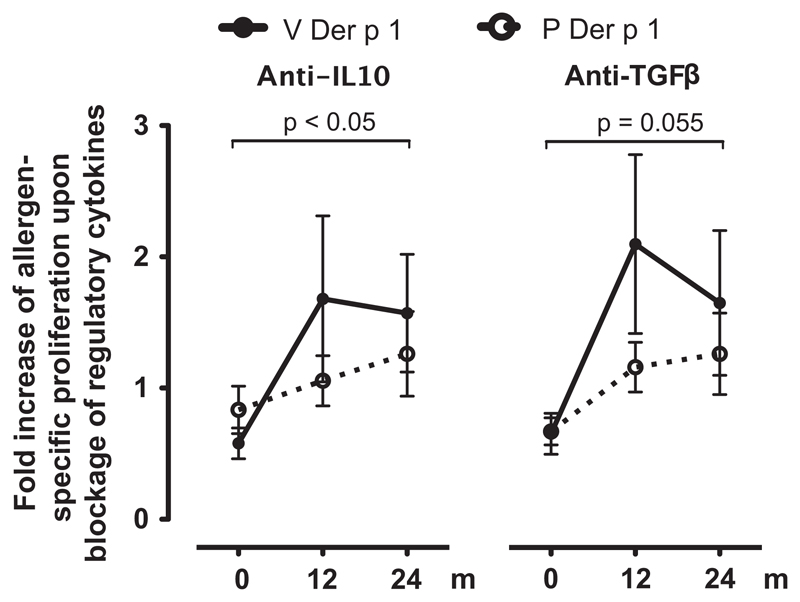
To evaluate the role of IL10 and TGF-β in the context of allergen-specific proliferation, blocking experiments were performed. In HDM-SLIT-treated individuals, inhibition of IL10 and TGF-β via addition of blocking antibodies led to increased Der p 1-specific proliferation. This inhibitory effect was not seen upon addition of anti-IL10 or anti-TGF-β antibodies in placebo-treated patients. The impact of IL10 and TGFβ on allergen-specific proliferation was statistically significant in the HDM-treated group after 24 months of SLIT but not in the placebo-treated group (Fig. 5).
Figure 5.
Induction of allergen-specific IL10- and TGFβ-dependent control in preventive sublingual immunotherapy (SLIT)-treated patients. Allergen-specific proliferation to the major house-dust mite allergen Der p 1 was assessed in the presence of anti-IL10 or anti -TGFβ antibodies relative to allergen-specific proliferation in the presence of the respective isotype control in the house-dust mite SLIT-treated group (V, n = 10) and the placebo group (P, n = 14). The degree of cytokine-mediated inhibition was calculated as fold increase of proliferation to the respective allergen in the presence of anti-IL10 or anti-TGF-β antibodies as compared to the respective isotype controls and the allergen. V, Verum; P, Placebo; p < 0.05; Wilcoxon-signed rank test.
Allergen-specific proliferation to the major HDM-allergens Der p 1 and Der p 2 decreased trend-wise in HDM and placebo-treated patients. This trend was not observed in response to the control allergen Bet v 1, to which patients were not sensitized at baseline (not shown).
Discussion
Allergen-specific immunotherapy used for allergy treatment has previously been evaluated for its ability to prevent new IgE sensitizations (7–11, 15). The current study showed safe application of preventive sublingual allergen-specific immunotherapy in small children (age 2–5 yr) and, unlike previous studies, revealed immunologic changes suggestive of sustained immunomodulation in a double-blinded, randomized, controlled study design. Previous treatment studies have consistently shown a reduction of new sensitizations in children treated for allergic diseases (allergic rhinitis and/or bronchial asthma) (10, 15). In treatment studies on monosensitized children, although non-placebo controlled, this effect was also clearly described (7–9, 11). This effect was not seen in the current study. However, the preventive nature via application of SLIT in children who are IgE-sensitized but non-allergic is novel. Indeed, placebo group and verum group showed a similar increase in new sensitizations over the 2-yr treatment period. The induction of new IgE sensitizations (not significant after correction for multiple testing) by application of preventive SLIT with a mixture of allergens was very recently observed in a pilot study including atopy prone infants/children without IgE sensitization to aeroallergens (house-dust mite, cat, and timothy grass) (16). The authors stressed the point that future study designs addressing this issue should particularly focus on pre-sensitized individuals, because the induction of antigen-specific tolerance might typically arise after an obligatory step of transient activation of primary immunity to the treatment antigen (17, 18). Importantly, SLIT treatment in our trial did not lead to enhanced rates of sensitization as compared to placebo treatment.
Allergen-specific immunotherapy in allergic individuals aims to reduce symptom scores and medication use and to increase quality of life (4, 19). All these items were not applicable as outcome parameters in the current study, as inclusion criteria limited eligible individuals to sensitized but non-(yet) allergic children. Thus, specific focus was put on the immunologic changes along the 2-yr treatment period. Two parameters were of particular interest: involvement of IL10 and TGFβ as regulatory T-cell products (20) and increase of allergen-specific IgG antibodies (21). Along successful subcutaneous and SLIT IL10 and TGFβ were shown to increase as surrogate marker of tolerance development. Low-allergen-dose studies did not reveal a sustained production of IL10 and/or TGFβ in their culture systems (22), but more recent studies applying mediumallergen doses were more successful in measuring regulatory cytokine involvement in successfully treated patients (12, 13). High-dose treatment approaches using self-dissolving sublingual tablets revealed induction of an IL10-dependent modulation (23) in some responders. Thus, it is highly remarkable in the present study that IL10- and trendwise TGFβ involvement was detectable even though immunotherapy was used in a preventive setting in non-allergic children. The functional involvement of these cytokines was reflected by re-induction of proliferation upon allergen encounter (house-dust mite) when anti-IL10 or anti-TGFβ was applied in culture. The major interpretation of these observations might reflect the strong potential of immunomodulation by immunotherapy in this particular age group coming closer to the described ‘window of opportunity’ for successful treatment/prevention of allergies in the prenatal and early postnatal phase (24–26).
Allergen-specific IgG/IgG4 antibodies are induced under allergen-specific immunotherapy (20). Under particular situations, these antibodies are called blocking antibodies (19, 27), but not all studies have successfully shown an involvement of IgG antibodies (28, 29). In the current study, allergen-specific IgG was predominantly induced in the treatment group. The functional role of the increase of allergen-specific IgG could not be evaluated in the present study.
Appearance and spreading of IgE sensitizations over infancy and young childhood followed by sequential development of particular allergic diseases represent the basis of the ‘allergic march’ (30). The overall focus of allergy preventive strategies is to interfere in the early phases of this development. The present approach clearly followed the secondary preventive strategy in sensitized but not (yet)- allergic children, based on the fact of an allergen-specific immune reactivity in which treatment by immunotherapy would induce immunomodulation. The primary outcome parameter chosen was prevention of new sensitizations. The results of our study clearly show that immunologic changes are inducible and detectable as early as by year 1 of treatment. Whether or not the treatment needs 2 yr for achieving sustained effects cannot be answered by the current approach, but the changes in immunomodulation clearly reveal a promising approach. Unlike expected, immunomodulation (IL10- and IgG-increase) was more pronounced than the effect on IgE sensitization. In fact, new IgE sensitizations developed in both groups, the number of monosensitized children decreased and the number of oligosensitized and polysensitized children increased in the whole study population irrespective of the treatment modality.
The SLIT approach in very young children has raised skepticism concerning the use of soluble allergen drops in an age group that cannot sufficiently hold sublingual allergen long enough under the tongue to deliver allergens to mucosal immune cells (16). The current study might provide evidence that preventive SLIT over a treatment period of 1–2 yr would deliver enough allergen as to mediate immunologic changes (31). Nevertheless, optimizing allergen dosing and treatment duration for the use of preventive immunotherapy (sublingual or subcutaneous) in very young children is one of the main questions to be solved. Overall, the current pilot study underlines that the approach of immunomodulation by preventive allergen-specific immunotherapy in early infancy is feasible and larger studies should delineate more details of optimal application modalities.
Acknowledgments
We thank C. Ebner from the Allergy Clinic Reumannplatz, F. Horak from the Allergy Clinic Hütteldorf, R. Jarisch from the Allergy Clinic Floridsdorf and W. Emminger from the Allergy Clinic Rennweg for support in patient recruitment.
Funding
This work was supported by the Austrian Science Fund Project F4615-B19 and F4605-B19 (SFB), and Medical Science Fund of the Mayor of the City of Vienna, project 11013.
Abbreviations
- PBMC
peripheral blood mononuclear cell
- pSLIT
preventive sublingual immunotherapy
- SCIT
subcutaneous allergen immunotherapy
- SLIT
sublingual immunotherapy
- AIT
allergen-specific immunotherapy
Footnotes
Clinical trial Registration: The study protocol has been approved by the European Drug Agency (EMA; EudraCT 2006-007096-32).
Conflict of interest
All authors have no conflicts of interest to disclose.
Author contributions
Dr Szépfalusi designed the study and drafted the manuscript; Dr Bannert participated in the data collection, supervised the data collection, and critically reviewed the manuscript; Dr Ronceray carried out the initial analyses, and critically reviewed the manuscript; Ms Mayer designed the data collection instruments, coordinated the data collection, and critically reviewed and revised the mansucript; Ms Hassler and Ms Wissmann participated in the design of the data collection instruments and carried out the initial analyses and reviewed the mansucript; Drs Dehlink and Gruber participated in and supervised the data collectionand critically reviewed and revised the manuscript; Dr Graf performed the statistical analysis and critically reviewed the manuscript; Dr. Lupinek participated in data aquisition and analyses and critically reveiwed the manuscript; Dr Valenta participated in the design of the study, supervised data aquisition, and analyses, critically reviewed and revised the manuscript; Dr Eiwegger participated in the design of the study and participated in the draft of the manuscript; Dr Urbanek participated in the design of the study and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
References
- 1.Marogna M, Bruno M, Massolo A, Falagiani P. Long-lasting effects of sublingual immunotherapy for house dust mites in allergic rhinitis with bronchial hyperreactivity: a long-term (13-year) retrospective study in real life. Int Arch Allergy Immunol. 2007;142:70–8. doi: 10.1159/000096001. [DOI] [PubMed] [Google Scholar]
- 2.Di Rienzo V, Marcucci F, Puccinelli P, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003;33:206–10. doi: 10.1046/j.1365-2222.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 3.Eng PA, Reinhold M, Gnehm HP. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy. 2002;57:306–12. doi: 10.1034/j.1398-9995.2002.1o3264.x. [DOI] [PubMed] [Google Scholar]
- 4.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 5.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–91. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 7.Purello-D’Ambrosio F, Gangemi S, Merendino RA, et al. Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not. A retrospective study. Clin Exp Allergy. 2001;31:1295–302. doi: 10.1046/j.1365-2222.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 8.Des Roches A, Paradis L, Menardo JL, Bouges S, Daures JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99:450–3. doi: 10.1016/s0091-6749(97)70069-1. [DOI] [PubMed] [Google Scholar]
- 9.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–37. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 11.Inal A, Altintas DU, Yilmaz M, Karakoc GB, Kendirli SG, Sertdemir Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J Investig Allergol Clin Immunol. 2007;17:85–91. [PubMed] [Google Scholar]
- 12.Kinaciyan T, Jahn-Schmid B, Radakovics A, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119:937–43. doi: 10.1016/j.jaci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–13. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Lupinek C, Wollmann E, Baar A, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2013;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng PA, Borer-Reinhold M, Heijnen IA, Gnehm HP. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198–201. doi: 10.1111/j.1398-9995.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 16.Holt PG, Sly PD, Sampson HA, et al. Prophylactic use of sublingual allergen immunotherapy in high-risk children: a pilot study. J Allergy Clin Immunol. 2013;132:991–3.e1. doi: 10.1016/j.jaci.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Hoyne GF, Askonas BA, Hetzel C, Thomas WR, Lamb JR. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+ T cells precedes the development of tolerance in vivo. Int Immunol. 1996;8:335–42. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- 18.Holt PG, Rowe J, Kusel M, et al. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol. 2010;125:653–9. doi: 10.1016/j.jaci.2009.12.018. 59.e1–59.e7. [DOI] [PubMed] [Google Scholar]
- 19.Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–25.e5. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 20.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. quiz 47–8. [DOI] [PubMed] [Google Scholar]
- 21.James LK, Shamji MH, Walker SM, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–16.e1–5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 22.Dehlink E, Eiwegger T, Gerstmayr M, et al. Absence of systemic immunologic changes during dose build-up phase and early maintenance period in effective specific sublingual immunotherapy in children. Clin Exp Allergy. 2006;36:32–9. doi: 10.1111/j.1365-2222.2006.02400.x. [DOI] [PubMed] [Google Scholar]
- 23.Bonvalet M, Moussu H, Wambre E, et al. Allergen-specific CD4+ T cell responses in peripheral blood do not predict the early onset of clinical efficacy during grass pollen sublingual immunotherapy. Clin Exp Allergy. 2012;42:1745–55. doi: 10.1111/cea.12015. [DOI] [PubMed] [Google Scholar]
- 24.von Mutius E. Infection: friend or foe in the development of atopy and asthma? The epidemiological evidence. Eur Respir J. 2001;18:872–81. doi: 10.1183/09031936.01.00268401. [DOI] [PubMed] [Google Scholar]
- 25.Prescott SL, Smith P, Tang M, et al. The importance of early complementary feeding in the development of oral tolerance: concerns and controversies. Pediatr Allergy Immunol. 2008;19:375–80. doi: 10.1111/j.1399-3038.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 26.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 27.Vrtala S, Akdis CA, Budak F, et al. T cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J Immunol. 2000;165:6653–9. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]
- 28.Baron-Bodo V, Horiot S, Lautrette A, et al. Heterogeneity of antibody responses among clinical responders during grass pollen sublingual immunotherapy. Clin Exp Allergy. 2013;43:1362–73. doi: 10.1111/cea.12187. [DOI] [PubMed] [Google Scholar]
- 29.Selb R, Eckl-Dorna J, Vrtala S, Valenta R, Niederberger V. An assay that may predict the development of IgG enhancing allergen-specific IgE binding during birch immunotherapy. Allergy. 2013;68:1199–202. doi: 10.1111/all.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopper JL, Bui QM, Erbas B, et al. Does eczema in infancy cause hay fever, asthma, or both in childhood? Insights from a novel regression model of sibling data. J Allergy Clin Immunol. 2012;130:1117–22.e1. doi: 10.1016/j.jaci.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–46. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]