Abstract
Background
Allergic sensitization is frequently present in asthma and rhinitis, but the role of specific immunoglobulin E (s-IgE) is not always clear. Multiple s-IgE analyses may provide insight into this relationship, thus a microarray chip was developed within the EU-funded MeDALL project. The main objective was to evaluate the performance of the MeDALL-chip compared to ImmunoCAP and skin prick test (SPT) in detecting allergic sensitization in children and secondarily to investigate the association to asthma and allergic rhinitis.
Methods
From the ‘Environment and Childhood Asthma Study’, 265 children were investigated at 10 and 16 yr of age with clinical examination, interview, SPT, ImmunoCAP, and the MeDALL-chip including 152 allergen components in the analysis.
Results
Allergic sensitization at 10 yr was more frequently detected using the MeDALL-chip (38.1%) compared to the ImmunoCAP (32.8%) (p = 0.034) and SPT (25.5%) (p < 0.001), but no significant difference was seen at 16 yr (MeDALL-chip 49.8%, ImmunoCAP 48.6%, SPT 45.8%). The MeDALL-chip did not differ significantly from the ImmunoCAP or SPT in terms of detecting allergic sensitization in subjects with rhinitis or asthma at 10 or 16 yr.
Conclusion
The prevalence of allergic sensitization increased by all three diagnostic tests from 10 to 16 yr was similar by SPT and ImmunoCAP and significantly higher with the MeDALL-chip at 10 yr. All three tests were comparable for identification of allergic sensitization among children with current rhinitis or asthma.
Keywords: allergy, allergen components, allergy diagnosis, asthma, childhood allergy, microarray, rhinitis
The classical way of diagnosing allergic sensitization and determining the presence of specific immunoglobulin E (s-IgE) has been by the use of skin prick test (SPT) (1–3) and serological tests like ImmunoCAP (4). Analyses of s-IgE with ELISA, chemiluminescence, or ImmunoCAP methods usually require about 40 μl of serum per allergen, limiting the availability of testing for a multitude of allergens or allergen components in children, particularly in the very young.
The microarray technique differs from other serologic tests by measuring specific IgE to a large number of allergens and allergen components necessitating small amounts of serum only (5). The multiplex microarray chip technique measures IgE binding in low amounts of allergens whereas ImmunoCAP technology measures IgE under conditions of excess allergen. Several studies have analyzed the reproducibility and compared the microarray chip, ISAC-chip, to other methods of measuring s-IgE including singleplex platforms for component analysis (6–10). Overall, the results are acceptable, but the sensitivity of the ISAC is often found to be lower than ImmunoCAP (6–8, 10).
As part of defining phenotypes and characterizing allergic disease in childhood, the Mechanisms of the Development of Allergy (MeDALL) (11) cooperation developed a multiplex allergen chip, the MeDALL-chip, to determine specific IgE to multiple allergen components for early diagnosis of allergic sensitization. The MeDALL-chip has the advantage of simultaneously analyzing a large number of allergen components with very small amounts of serum (9) and contains more allergen components than the ISAC-chip test (9). However, the clinical usefulness of assessment of s-IgE to multiple allergen components remains to be determined (6).
The main objective of this study was to compare the MeDALL-chip with ImmunoCAP and SPT including common allergens for detecting allergic sensitization in children and to investigate the association to asthma and allergic rhinitis.
Subjects and methods
Study design
The MeDALL-chip was analyzed in subjects representative of the general cohort population from the ‘Environment and Childhood Asthma’ (ECA) birth cohort study in Oslo (12) who had blood samples available at 10 and 16 yr of age. Lung function measurements, structured interviews, skin prick tests, and blood samplings were performed at 10 and 16 yr (see details in the Data S1).
Written informed consent was obtained from parents of all subjects. The study was approved by the regional medical ethics committee and the Norwegian Data Directorate and reported to the National Biobank Register. The analysis of de-identified serum samples was performed with permission of the Ethics committee of the Medical University of Vienna.
Subjects
The 265 children from the ECA study, 53.6% boys, were representative at birth of the entire birth cohort (n = 3754) (Table 1) and were selected on the basis of having lung function measured at birth, completed questionnaires at 2 yr of age, and attending the 10 and 16 yr investigations.
Table 1.
Demography at birth for the subjects included in the study compared to the remaining birth cohort (total included at birth n = 3754). Values are given as numbers (n), percent (%), and mean with standard deviation (SD). Family income is given in five categories: from 1 < 100,000 NOK to 5 > 500,000 NOK. Parental education is given in six categories from 1, maximum 9 yr elementary school, to 6, university degree
| Included (n = 265) | Non-included (n = 3489) | p-Value | |
|---|---|---|---|
| Gender (boys %, n) | 53.6 (142) | 51.6 (1801) | 0.54 |
| Cat at home (%, n) | 5.7 (15) | 7.7 (267) | 0.24 |
| Dog at home (%, n) | 9.1 (24) | 9.2 (320) | 0.95 |
| Maternal smoking during pregnancy (%, n) | 21.2 (56) | 24.7 (859/3482) | 0.20 |
| Parental asthma (%, n) | 11.3 (30) | 12.2 (426) | 0.67 |
| Maternal asthma (%, n) | 5.7 (15) | 6.9 (241) | 0.44 |
| Paternal asthma (%, n) | 5.7 (15) | 5.7 (199) | 0.98 |
| Parental eczema (%, n) | 34.0 (90) | 28.1 (980) | 0.04 |
| Maternal eczema (%, n) | 21.9 (58) | 17.9 (624) | 0.10 |
| Paternal asthma (%, n) | 15.8 (42) | 13.2 (459) | 0.21 |
| Parental hayfever (%, n) | 30.6 (81) | 27.4 (955) | 0.26 |
| Maternal hayfever (%, n) | 16.6 (44) | 14.2 (497) | 0.29 |
| Paternal hayfever (%, n) | 17.4 (46) | 16.6 (580) | 0.76 |
| Older siblings (%, n) | 47.9 (124) | 44.6 (1479) | 0.31 |
| Number of previous maternal births (mean (SD)) | 0.67 (0.79) | 0.64 (0.81) | 0.53 |
| Number of older siblings (mean (SD)) | 0.58 (0.70) | 0.56 (0.72 | 0.58 |
| Maternal education (mean category (SD)) | 4.54 (1.25) | 4.41 (1.34) | 0.11 |
| Paternal education (mean category (SD)) | 4.70 (1.18) | 4.55 (1.40) | 0.09 |
| Household income (mean category (SD)) | 3.92 (0.80) | 3.72 (1.00) | 0.003 |
| Birthweight (mean g (SD)) | 3598 (500) | 3552 (510) | 0.16 |
| Birth length (mean cm (SD)) | 50.4 (2.8) | 49.7 (3.5) | 0.002 |
| Maternal year of birth (mean (min, max) | 1961 (1948, 1972) | 1962 (1946, 1976) | |
| Paternal year of birth (mean (min, max) | 1958 (1937, 1971) | 1959 (1935, 1976) |
Methods
Parental structured interview including central questions from the International study of asthma and allergies in childhood (ISAAC) (validated in Norwegian language (13)) related to airways symptoms of the child, in addition to detailed questions regarding environmental exposure, lifestyle, and diseases (index child and family).
SPTs to allergen extracts from 10 common inhalant and four food allergen sources were performed with Soluprick® allergens (ALK, Albello, Denmark). S-IgE was measured with a solid phase fluorescence immunoassay (ImmunoCAP® system, Thermo Fisher Scientific/Phadia, Uppsala, Sweden) to allergen extracts from nine inhalant and four food allergen sources. Details of the methods and allergens are described in the Data S1.
Microarray allergen component testing was performed with the MeDALL-chip as previously described (9). The original MeDALL-chip comprises of 176 allergen components. The components analyzed in this study are listed in the Data S1, along with a description of why certain allergen components were not included in the analysis. A s-IgE level of at least 0.3 ISU/l was considered positive (9).
For all three methods (ImmunoCAP, SPT, and MeDALL-chip), the allergen extracts from inhalant allergen sources or components are included in ImmunoCAP Inhalation, SPT Inhalation, and MeDALL-chip Inhalation as described in the Data S1.
Clinical outcomes
Rhinitis
At least one of the following symptoms in the absence of having a cold: runny nose, blocked nose, or sneezing.
Current rhinitis
Rhinitis and the presence of symptoms (as in the rhinitis definition) during the last 12 months.
Asthma
Defined in subjects with a positive response to at least two of the following: doctor’s diagnosis of asthma, asthma symptoms, and the use of anti-asthmatic medication.
Current asthma
Fulfilling the asthma definition and reporting symptoms and/or the use of asthma medication last 12 months (14, 15).
Statistical analysis
Continuous variables are presented as mean with standard deviation SD (demographic data) or 95% confidence intervals (CI) and categorical data as counts and percentages. Comparisons between the three methods were carried out by Mc Nemar’s test. Relative risk (RR) and absolute risk (AR) were calculated according to Fleiss (16). The statistical significance level was set to 5%. Statistical analyses were carried out with Statistical Package for Social Science (SPSS) version 21 (SPSS, Chicago, IL, USA) and Statistical Analysis System (SAS 9.4, SAS Institute, Chapel Hill, NC, USA). To create Venn diagrams, eulerAPE® (University of Kent, UK, www.eulerdiagrams.org/eulerAPE) was used.
Results
Allergic sensitization increased from 10 to 16 yr
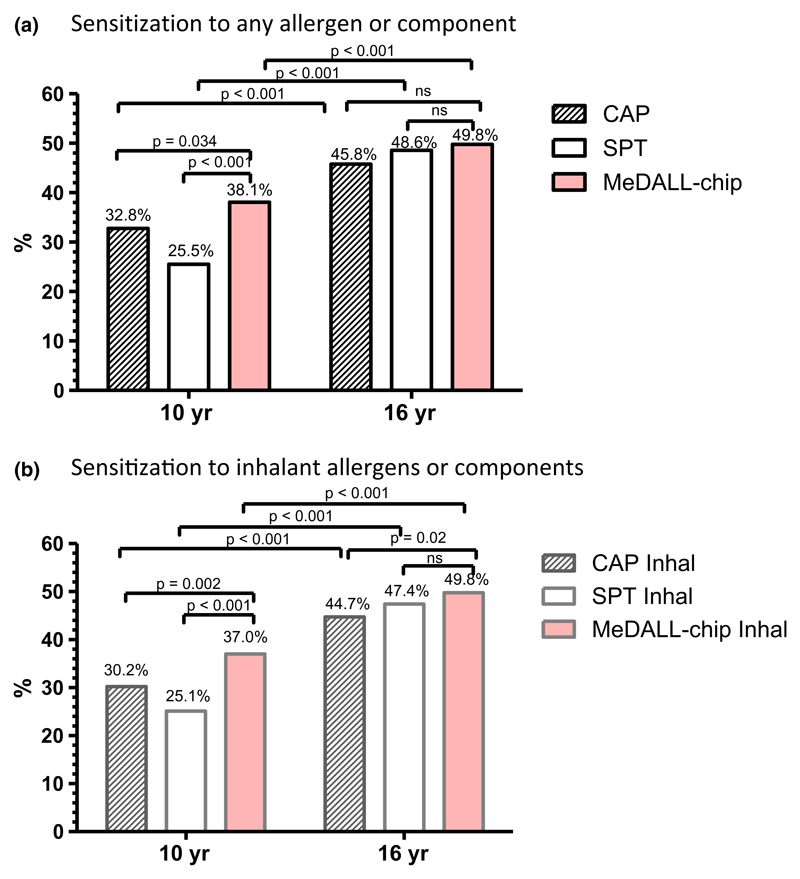
Allergic sensitization increased significantly from 10 to 16 yr measured by all three methods, p < 0.001 for any allergen or component as well as for inhalant allergens or components only (Fig. 1).
Figure 1.
Allergic sensitization to any (a) or inhalant (b) allergens, respectively, is given as measured by ImmunoCAP (CAP) or ImmunoCAP Inhalation allergens (CAP Inhal), skin prick test (SPT) or SPT Inhalation allergens (SPT Inhal) and MeDALL-chip or MeDALL-chip Inhalation allergens (MeDALL Inhal) at 10 and 16 yr, respectively. Stripes: CAP. Blank: SPT. Red: MeDALL-chip.
At 10 yr of age, the allergic sensitization rate was significantly higher when measured with the MeDALL-chip (38.1%) compared to ImmunoCAP (32.8%) (p = 0.034) or SPT (25.5%) (p < 0.001). The allergic sensitization rates were similar for all three methods (49.8, 45.8, and 48.6% respectively) at 16 yr (MeDALL-chip and ImmunoCAP: p = 0.087, MeDALL-chip and SPT: p = 1.0) (Fig. 1a). Similarly, allergic sensitization rates to extracts from inhalant allergen sources or components were significantly higher by the MeDALL-chip Inhalation compared to SPT Inhalation (p < 0.001) and ImmunoCAP Inhalation (p = 0.002) at 10 yr. At 16 yr, a significant difference between the MeDALL-chip Inhalation and ImmunoCAP Inhalation was seen (p = 0.02) (Fig. 1b).
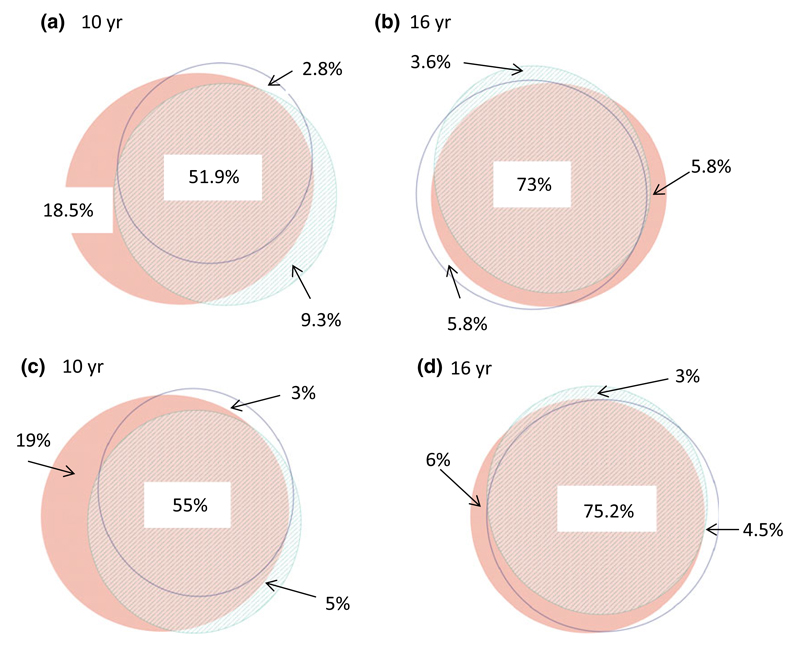
All three methods had a high degree of overlap at both ages for being sensitized to at least one allergen (Fig. 2). A more complete overlap was observed at 16 compared to 10 yr of age for allergic sensitization (73% vs. 51.9%, p < 0.001) (Fig. 2a,b) and for sensitization to extracts from inhalant allergen sources or components only (75.2% vs. 55%, p < 0.001) (Fig. 2c,d). However, comparing allergen by allergen the three tests were largely similar, but varied within some allergens, as outlined in the Fig. S1.
Figure 2.
Venn diagrams showing allergic sensitization to any allergens or components at 10 yr (a) and 16 yr (b), inhalant allergens or components at 10 yr (c), and 16 yr (d). Stripes: CAP. Blank: SPT. Red: MeDALL-chip.
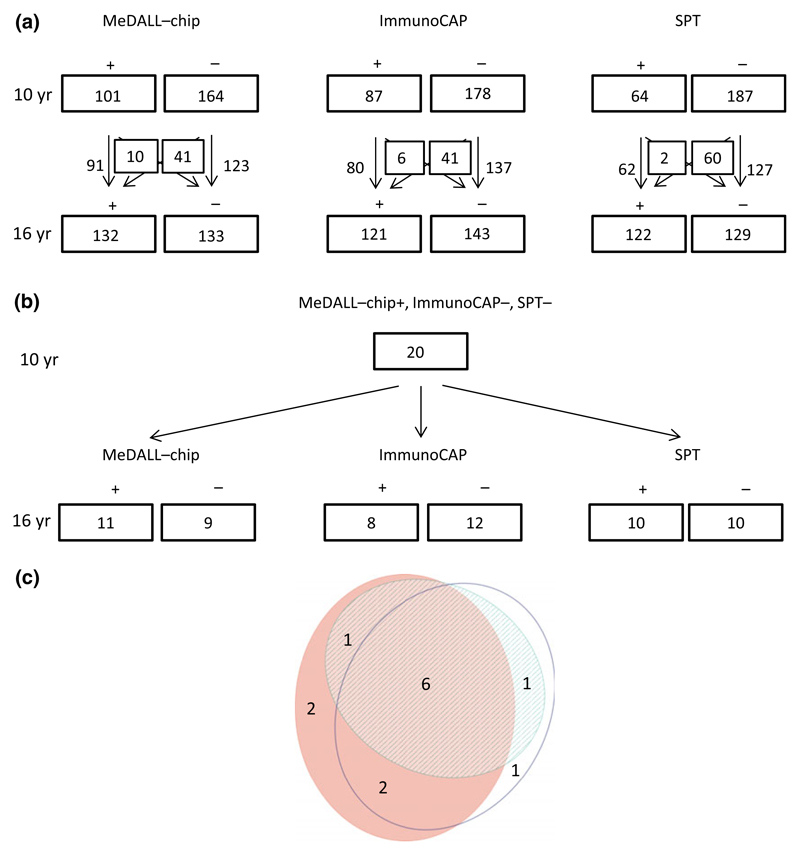
More children had a positive MeDALL-chip with corresponding negative SPT and ImmunoCAP (18.5% vs. 5.8%; p = 0.023) at 10 compared to 16 yr, with similar results for the MeDALL-chip Inhalation, SPT Inhalation, and ImmunoCAP Inhalation (19.0% vs. 6.0%; p = 0.035), respectively (Fig. 2a-d). The numbers of children detected with the MeDALL-chip, ImmunoCAP, and SPT at 10 and 16 yr are shown in Fig. 3a, as well as the numbers of children who change their sensitization status (Fig. 3a). Twenty children at 10 yr of age had a positive MeDALL-chip only with negative SPT and ImmunoCAP. In all but one of these subjects, the specific IgE levels measured by the MeDALL-chip value were low (<2 ISU/l). The components measured were not only ‘new’ components not covered by ImmunoCAP or SPT, but a mixture of components which should be present in the allergen extracts covered by Immuno-CAP and SPT and ‘new’ components which eventually are not present in the tested allergen extracts (data not shown). At 16 yr, 11 of 20 children remained sensitized by the MeDALL-chip (Fig. 3b), whereas eight had developed a positive ImmunoCAP and 10 a positive SPT. In six of the 20 children, all three tests had become positive at 16 yr of age, and in two children, the MeDALL-chip was still positive, while ImmunoCAP and SPT were negative (Fig. 3b,c).
Figure 3.
Allergic sensitization to any allergens or allergen components at 10 and 16 yr, and the numbers of children who gain or lose sensitization (a). Allergic sensitization only detected by the MeDALL-chip at 10 yr and sensitization by MeDALL-chip, ImmunoCAP, and SPT at 16 yr (b). Venn diagram showing sensitization to any allergens or allergen components at 16 yr (n: 13) within the children with only positive MeDALL-chip at 10 yr (n: 20). Stripes: CAP. Blank: SPT. Red: MeDALL-chip (c).
Airway disease and allergic sensitization
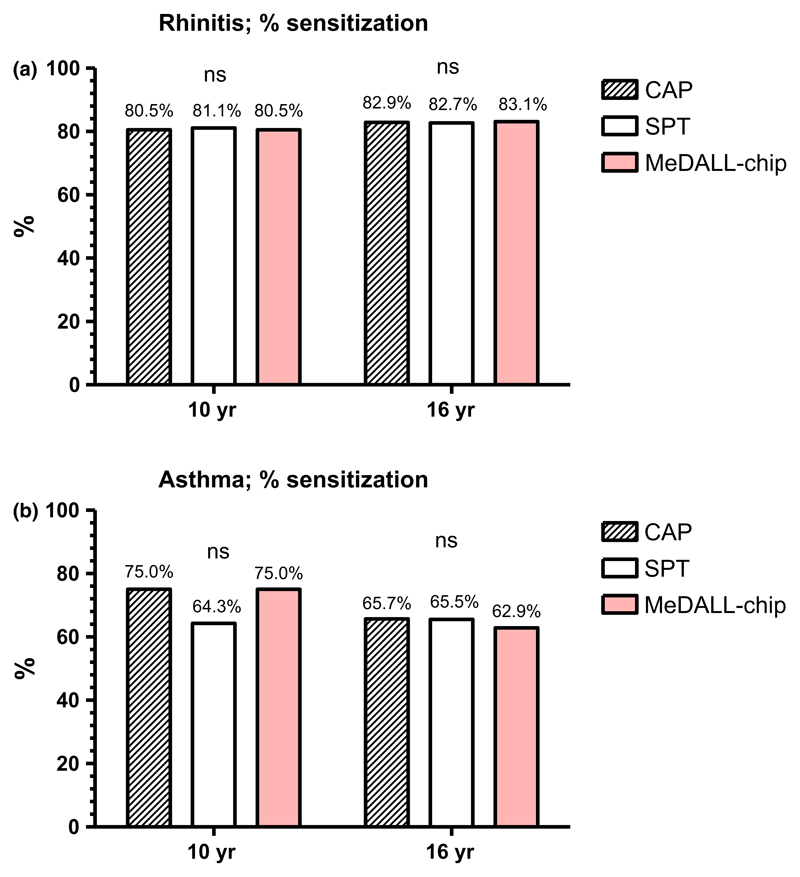
The number of children with rhinitis increased from 41 (15.5%) at 10 yr to 83 (31.3%) at 16 yr, with the corresponding results for asthma of 16 (6.1%) and 35 (13.2%), respectively. In subjects with current rhinitis, allergic sensitization to any allergen extract or allergen component was 80.5–81.1% at 10 yr and 82.7–83.1% at 16 yr. The sensitization rates were similar as assessed by each of the three methods (Fig. 4a). The corresponding results for current asthma tended to be lower when assessed by SPT (64.3%) compared to the 75% observed with the ImmunoCAP and MeDALL-chip at 10 yr, but were slightly higher with SPT (65.5%) and ImmunoCAP (65.7%) compared to 62.9% (MeDALL-chip) at 16 yr. None of these differences were, however, statistically significant (Fig. 4b). The corresponding sensitization rates for inhalant allergens were almost identical to those of sensitization to any allergen or component at both 10 and 16 yr (Fig. S1).
Figure 4.
The percentage of subjects with (a) rhinitis and (b) asthma sensitized to at least one allergen or allergen component as measured by ImmunoCAP (CAP), SPT, and the MeDALL-chip at 10 and 16 yr, respectively. Stripes: CAP. Blank: SPT. Red: MeDALL-chip.
Allergic sensitization to food allergens was observed in 1.1% of the children at 10 yr by the MeDALL-chip, 2.6% by ImmunoCAP, and 0.4% by SPT. The corresponding results at 16 yr were 0%, 1.2%, and 1.1% (data not shown). The associations between allergic sensitization as measured by the three different methods and both current asthma and rhinitis at 10 and 16 yr were almost identical with very similar relative risks (RR) and largely overlapping interval confidence limits, as also confirmed by absolute risk (AR) (Table S2).
Discussion
Allergic sensitization to at least one allergen or allergen component, as well as for inhalant allergens or allergen components, was in the present study significantly higher using the MeDALL-chip when analyzing 152 allergen components compared to SPT or ImmunoCAP analyses of selected common allergen extracts at 10 yr of age. At 16 yr of age, there was a significant difference between the MeDALL-chip and the ImmunoCAP for inhalant allergens only. The rates of allergic sensitization as identified by each of the three methods were almost identical in subjects with rhinitis at both ages and similar in subjects with asthma.
The high and increasing rate of allergic sensitization from 10 to 16 yr of age in the present study (38.1% to 49.8%) is consistent with findings from other European studies (17–19). Similar rates were also found in an American population based study reported for 1- to 6-yr-old children (overall sensitization rate of 36.2%) increasing to 44.6% in subjects older than 6 yr of age (20), as well as in a study from Latin America with an overall allergic sensitization rate in children of 20% (21). In the Swedish BAMSE study, overall sensitization measured with a screening test for specific IgE to respiratory allergen extracts (the Phadiatop ®) increased from 8 yr of approximately 25% to approximately 45% at 16 yr of age.
The MeDALL-chip identified significantly more subjects classified as sensitized to at least one allergen or inhalant allergen compared to the ImmunoCAP and the SPT at 10, but at 16 yr, there is only a significant difference between the MeDALL-chip and ImmunoCAP for inhalant allergens only. The increase in sensitization observed by the three methods appears largest for SPT (difference 23.1% points) and smallest for the microarray analyses (difference 10.7% points). Thus, in young school age, the microarray technique detected allergic sensitization more often than did the ImmunoCAP and SPT, which include common, carefully chosen allergen extracts believed to be clinically relevant for the Norwegian population. The allergic sensitization measured with the MeDALL-chip was not lower than found by ImmunoCAP or SPT at any age, in contrast to what has previously been observed in multiplex microarray chip techniques (6). The sensitivity of the Me-DALL-chip thus appears as good as the two other methods, and the results of the present study suggest that the MeDALL-chip may identify allergic sensitization at an earlier stage in the allergic sensitization process than the other two methods. However, with the lack of agreement on a ‘gold standard’ for clinically relevant allergic sensitization in epidemiological studies (22), we compared the analytical features of the three different methods. For many of the allergens, the three tests appeared similarly sensitive, with some variations in sensitivity as outlined in the (Fig. S1).
Allergic sensitization to inhalant allergens among subjects with airway disease were almost identical as assessed by the ImmunoCAP, SPT, and the MeDALL-chip, whereas very few subjects were sensitized to food allergens only at both ages. This is in line with findings from the Swedish BAMSE birth cohort study demonstrating that co-occurrence of s-IgE to inhalant and food allergens increased over time (18). The similarity between the present three methods in identifying sensitized subjects appears to be in contrast to other studies. The microarray-based serology has been reported to miss detection of some subjects with confirmed food allergy by SPT (23), whereas low agreement was found for indication and use of allergens with respect to immunotherapy based upon SPT and ImmunoCAP (ISAC) molecular analyses (24). These differences may be explained by the higher sensitivity of the MeDALL-chip and/or the presence of a larger panel of allergen components as compared to the commercially available allergen chip (10).
The identical rate of allergic sensitization observed at 16 yr by the MeDALL-chip and MeDALL-chip Inhalation, and the similar identification at 10 yr (difference of 1.1%) suggest that the MeDALL-chip identified sensitization to inhalant allergens in all the sensitized children. This was almost but not entirely so for ImmunoCAP and SPT. The overlap between the three methods for measuring allergic sensitization, carrying differences in choice of allergens, was greater at 16 (73%) than at 10 (51.9%) years of age. The conversion to allergic sensitization detected also by SPT or ImmunoCAP by 16 yr of age in 11 of 20 subjects with a positive MeDALL-chip only at 10 yr of age indicates that the chip appeared more sensitive than the two other methods for identifying subjects who were developing allergic sensitization by 16 yr of age. On the other hand, nine of these 20 children were no longer sensitized to any allergen measured by the MeDALL-chip at 16 yr, possibly explained by the very low level of s-IgE antibody characterizing the positive MeDALL-chip test results (<2 ISU in all but one subject) at 10 yr of age. It is thus possible that the s-IgE levels dropped below the cutoff limit at 16 yr in these children.
The MeDALL-chip is a further development of the ImmunoCAP ISAC-chip to improve the diagnosis of sensitization and allergy (9). A few components from the MeDALL-chip were not included in the analysis for different reasons. Some components that were unlikely to be of clinical relevance for respiratory allergy (i.e., venom and Anisakis allergens) or where the quality of the components was inadequate (Api g 1, bacterial toxins) were not included in the analysis.
A major difference between the MeDALL-chip and ImmunoCAP is that the MeDALL-chip measures s-IgE binding in low amounts of allergens, while ImmunoCAP measures s-IgE under conditions of allergen excess (9). SPT detects IgE bound to mast cells in the skin with a bell-shaped response curve depending on the tested allergen concentration (5–10). Hence, the magnitudes/IgE levels measured by the different tests cannot be compared directly. In the present study, we therefore compared only qualitative (i.e., positive and negative) test results.
In the present study, rhinitis is defined based on patient’s history. However, the interview included central questions from the International study of asthma and allergies in childhood (ISAAC) (validated in Norwegian language (13)), and the definitions used are similar to those used by other birth cohorts (25–28). Our asthma definition has been shown to correlate well with objective measures such as spirometry, exhaled nitric oxide, and bronchial hyper-responsiveness (14, 29).
Another possible limitation of the study is that we compared results from IgE measurements performed with the MeDALL-chip with ImmunoCAP measurements using only 0.35 kUA/l as cutoff value for ImmunoCAP. However, 0.35 kUA/l is the cutoff recommended by the manufacturer. A lowering of the cutoff limit might have resulted in false-positive results. Furthermore, we did not have results below 0.35 kUA/l for the 10-yr samples.
A major advantage of the MeDALL-chip is the small amount of serum required (approximately 35 μl serum), compared to the more conventional ImmunoCAP that usually requires about 40 μl serum per allergen for analysis and the SPT which is time consuming and inconvenient for the child. In birth cohort studies where the sample number is high and serum samples are limited, the MeDALL-chip could be advantageous.
Costs and time used for diagnosis are important issues in clinical practice. For individual ImmunoCAPs, the costs are usually low, but often repeated CAP testing is necessary to obtain a complete serological analysis. For SPT, the costs of the allergen extracts are reasonably low, but the test requires extended time in the clinic by patients and health personnel. At present the MeDALL-chip is available as a research tool only and costs for clinical use cannot be calculated. It may, however, be cost-effective in clinical practice due to the large number of components that can be analyzed in tiny amounts of serum.
Conclusion
Allergic sensitization in subjects from a general population increased from 10 to 16 yr was similar, at 16 yr as assessed by SPT, ImmunoCAP, and the MeDALL-Chip, but significantly higher for the MeDALL-chip than ImmunoCAP when measuring the inhalant allergens only. At 10 yr of age, allergic sensitization was similar when assessed by SPT and Immuno-CAP, but significantly higher by the MeDALL-chip for both all the components and for inhalant components only. All three tests were comparable for identification of allergic sensitization among children with current rhinitis or current asthma at both ages. The clinical usefulness and predictive value of the MeDALL-chip needs further investigations.
Supplementary Material
Acknowledgments
The authors thank the children and parents participating in the Environment and Childhood Asthma study. We are especially grateful to Solveig Knutsen, Runa Kaldestad, Christine Sachs Olsen, Sveinung Berntsen Stølevik, Tale Torjussen, and Geir Håland for contributing to the data collection. We also want to thank the members of the ORAACLE (the Oslo Research group of Asthma and Allergy in Childhood; the Lung and Environment) for helpful discussion and Thermo Fisher/Phadia for performing IgE analyses.
Funding
The MeDALL study was supported by funding from the European Union under the Health Cooperation Work Programme of the 7th Framework Programme (grant agreement No. 261357). The ECA study was further supported through the 16 yr by the following public funding bodies; The Norwegian Research Council, The Regional Health Board South East, Health and Rehabilitation, the European Union (Ga2len and MeDALL projects), by unrestricted grants from the Norwegian Association of Asthma and Allergy, Rimi as well as by financing PhD students by the Kloster foundation and Astra-Zeneca, and by providing IgE analyses by Thermo Fisher, Uppsala, Sweden in collaboration with Fürst Medical Laboratory, Oslo, Norway. The study was also supported in part by project F4605 of the Austrian Science Fund (FWF) and the Christian Doppler research Association (Austria).
The study was performed within the Oslo Research Group of Asthma and Allergy in Childhood; the Lung and Environment (ORAACLE), as part of Mechanisms of the Development of ALLergy (MeDALL) a collaborative project conducted within the European Union under the Health Cooperation Work Programme of the 7th Framework Programme (grant agreement No. 261357).
References
- 1.Dreborg SF. A. Position paper: allergen standardization and skin tests. Allergy. 1993;47(Suppl. 14):48s–82s. [PubMed] [Google Scholar]
- 2.van Kampen V, de Blay F, Folletti I, et al. EAACI position paper: skin prick testing in the diagnosis of occupational type I allergies. Allergy. 2013;68:580–4. doi: 10.1111/all.12120. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Heinzerling L, Bachert C, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 4.Glovsky MM. Measuring allergen-specific IgE: where have we been and where are we going? Methods Mol Biol. 2007;378:205–19. doi: 10.1007/978-1-59745-323-3_15. [DOI] [PubMed] [Google Scholar]
- 5.Hiller R, Laffer S, Harwanegg C, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–6. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 6.Canonica GW, Ansotegui IJ, Pawankar R, et al. A WAO - ARIA - GA(2)LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lizaso MT, Garcia BE, Tabar AI, et al. Comparison of conventional and component-resolved diagnostics by two different methods (Advia-Centaur/Microarray-ISAC) in pollen allergy. Ann Allergy Asthma Immunol. 2011;107:35–41. doi: 10.1016/j.anai.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Melioli G, Bonifazi F, Bonini S, et al. The ImmunoCAP ISAC molecular allergology approach in adult multi-sensitized Italian patients with respiratory symptoms. Clin Biochem. 2011;44:1005–11. doi: 10.1016/j.clinbiochem.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Lupinek C, Wollmann E, Baar A, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohrl S, Vigl K, Zehetmayer S, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006;61:633–9. doi: 10.1111/j.1398-9995.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 11.Bousquet J, Anto J, Auffray C, et al. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy. 2011;66:596–604. doi: 10.1111/j.1398-9995.2010.02534.x. [DOI] [PubMed] [Google Scholar]
- 12.Lodrup Carlsen KC. The environment and childhood asthma (ECA) study in Oslo: ECA-1 and ECA-2. Pediatr Allergy Immunol. 2002;13(Suppl 15):29–31. doi: 10.1034/j.1399-3038.13.s.15.2.x. [DOI] [PubMed] [Google Scholar]
- 13.Selnes A, Bolle R, Holt J, Lund E. Cumulative incidence of asthma and allergy in north-Norwegian schoolchildren in 1985 and 1995. Pediatr Allergy Immunol. 2002;13:58–63. doi: 10.1034/j.1399-3038.2002.01009.x. [DOI] [PubMed] [Google Scholar]
- 14.Hovland V, Riiser A, Mowinckel P, Carlsen KH, Carlsen KC. Asthma with allergic comorbidities in adolescence is associated with bronchial responsiveness and airways inflammation. Pediatr Allergy Immunol. 2014;25:351–9. doi: 10.1111/pai.12241. [DOI] [PubMed] [Google Scholar]
- 15.Pinart M, Benet M, Annesi-Maesano I, et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med. 2014;2:131–40. doi: 10.1016/S2213-2600(13)70277-7. [DOI] [PubMed] [Google Scholar]
- 16.Statistical methods for Rates and Proportions. Second Edition. New York: John Wiley & Sons; 1981. [Google Scholar]
- 17.Scott M, Raza A, Karmaus W, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65:258–62. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wickman M, Asarnoj A, Tillander H, et al. Childhood-to-adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J Allergy Clin Immunol. 2014;133:580–2. doi: 10.1016/j.jaci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Roberts G, Zhang H, Karmaus W, et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clin Exp Allergy. 2012;42:1501–9. doi: 10.1111/j.1365-2222.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 20.Salo PM, Arbes SJ, Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol. 2014;134:350–9. doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper PJ, Chico ME, Rodrigues LC, et al. Risk factors for atopy among school children in a rural area of Latin America. Clin Exp Allergy. 2004;34(6):845–52. doi: 10.1111/j.1365-2222.2004.01958.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S284–96. doi: 10.1016/j.jaci.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 23.Onell A, Hjalle L, Borres MP. Exploring the temporal development of childhood IgE profiles to allergen components. Clin Transl Allergy. 2012;2:24. doi: 10.1186/2045-7022-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sastre J, Landivar ME, Ruiz-Garcia M, Andregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67:709–11. doi: 10.1111/j.1398-9995.2012.02808.x. [DOI] [PubMed] [Google Scholar]
- 25.Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68:1102–16. doi: 10.1111/all.12235. [DOI] [PubMed] [Google Scholar]
- 26.Bousquet J, Gern JE, Martinez FD, et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop. J Allergy Clin Immunol. 2014;133:1535–46. doi: 10.1016/j.jaci.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballardini N, Kull I, Lind T, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy. 2012;67:537–44. doi: 10.1111/j.1398-9995.2012.02786.x. [DOI] [PubMed] [Google Scholar]
- 28.Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy. 2011;41:851–9. doi: 10.1111/j.1365-2222.2011.03765.x. [DOI] [PubMed] [Google Scholar]
- 29.Riiser A, Hovland V, Carlsen KH, Mowinckel P, Lodrup Carlsen KC. Does bronchial hyperresponsiveness in childhood predict active asthma in adolescence? Am J Respir Crit Care Med. 2012;186:493–500. doi: 10.1164/rccm.201112-2235OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.