Abstract
Earth’s volatile element abundances (e.g., sulfur, zinc, indium and lead) provide constraints on fundamental processes such as planetary accretion, differentiation, and the delivery of volatile species, like water, which contributed to Earth becoming a habitable planet. The composition of the silicate Earth suggests chemical affinity but isotopic disparity to carbonaceous chondrites, meteorites that record the earliest element fractionations in the protoplanetary disk. However, the volatile element depletion pattern of the silicate Earth is obscured by core formation. Another key problem is the overabundance of indium, which could not be reconciled with any known chondrite group. Here we complement recently published volatile element abundances for carbonaceous chondrites with high precision sulfur, selenium, and tellurium data. We show that both Earth and carbonaceous chondrites exhibit a unique hockey stick volatile element depletion pattern where volatile elements with low condensation temperatures (750 - 500 K) are unfractionated from each other. This abundance plateau accounts for the apparent overabundance of indium in the silicate Earth without the need of exotic building materials or vaporization from precursors or during the Moon-forming impact and suggests the accretion of 10-15 % CI-like material before core formation ceased. Finally, more accurate estimates of volatile element abundances in the core and bulk Earth can now be provided.
The chemical composition of the terrestrial planets is controlled by the composition of their planetary building blocks. All known inner solar system bodies, including Earth, Moon, Mars and the different meteorite parent bodies, are depleted in volatile elements (e.g., Cd, In, S, Cl) relative to the bulk solar system composition as represented by CI chondrites1,2. The volatile element depletion pattern constrains the nature of Earth’s building blocks and may serve as a tracer for the delivery of water during Earth’s growth3–5. Although Earth’s isotope composition cannot be reproduced by mixing known meteorites1,6–9, comparisons between Earth’s chemical composition and those of undifferentiated meteorites (chondrites), in particular for volatile elements, suggest that Earth’s building blocks experienced similar nebular fractionation processes as carbonaceous chondrites but different to those observed in ordinary and enstatite chondrites1,2,10. For example, ratios between lithophile volatile and lithophile refractory elements (e.g., K/U) for the bulk silicate Earth (BSE) always plot at the volatile-depleted end of a trend defined by carbonaceous chondrites2. Furthermore, Zn and Rb stable isotope compositions of the BSE follow the carbonaceous chondrite trend of increasingly light isotope compositions with increasing degree of volatile element depletion10,11.
Earth’s primary volatile element depletion pattern is obscured by the additional depletion of siderophile and chalcophile volatile elements in the BSE that is generally attributed to their partitioning into Earth’s core1,12. However, numerous metal-silicate partitioning experiments have thus far been unable to consistently reproduce the siderophile and chalcophile element depletion pattern observed in the BSE13–16. This is especially true regarding the abundance of the volatile element In in the BSE, which appears to be overabundant relative to the carbonaceous chondrite-like volatile element depletion pattern14,17,18. Therefore, it was concluded that Earth’s volatile element abundances were modified by post-nebular processes such as vaporization on precursor bodies and possibly during the giant Moon-forming impact, late accretion, or collisional erosion3,17,19. Alternatively, it was proposed that volatile element abundances evolved differently in the inner solar system, where the vast majority of Earth’s building materials originate14,18. There are several arguments against secondary volatile element loss related to Earth’s accretion, e.g.: (i) variations in 53Cr induced by the decay of the now extinct 53Mn (T1/2 = 3.7 Myr) suggest that Mn/Cr fractionation and therefore volatile element depletion in the precursor materials of Earth, Mars and the different meteorite parent bodies occurred early in the protoplanetary disk within about 2 Myr of CAI formation20, rather than during planetary growth, (ii) the chondritic Mn/Na of Earth argues against post nebular volatile-loss as opposed to the high Mn/Na of other differentiated rocky bodies (e.g. Mars, Moon, achondrites)19,21 and (iii) evaporative loss of volatile elements should lead to stable isotope fractionation resulting in an enrichment of heavy isotopes in the residue, which is not observed on Earth10,11,22–24. Since post-nebular volatile element loss was seemingly restricted on Earth, volatile element abundances must mirror the composition of Earth’s building blocks. Here we reassess Earth’s volatile element depletion pattern based on recently available volatile element data for carbonaceous chondrites25,26 complemented with new high precision S, Se and Te data.
Terrestrial hockey stick volatile element depletion pattern
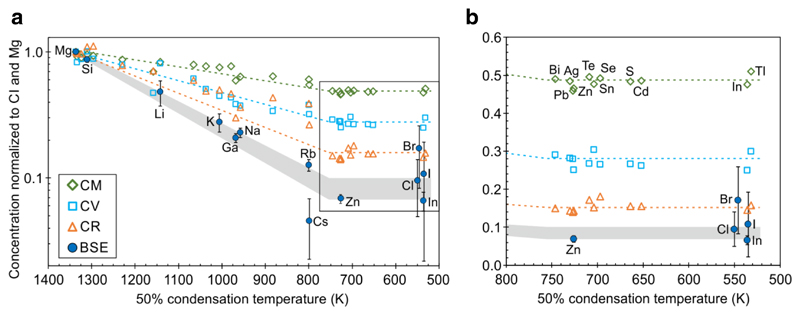
It is generally assumed that volatile element abundances in carbonaceous and ordinary chondrites decrease gradually with increasing volatility of the elements (as commonly defined by 50% condensation temperatures (T50%))27,28. However, recent high precision data25 confirm earlier suggestions29,30 that volatile elements with T50% <750 K are depleted by a constant factor in carbonaceous chondrites relative to CI chondrites. Thus, CI-normalized volatile element abundances plotted versus T50% define a hockey stick depletion pattern for all investigated carbonaceous chondrite groups (Fig. 1), where slope volatile elements with T50% between 1250 - 750 K follow the previously suggested gradual depletion trend, whereas volatile elements with T50% between 750 - 500 K are essentially unfractionated from each other and are now defined as plateau volatile elements25. Sulfur, Se and Te belong to the plateau volatile elements and our new isotope dilution data confirm their CI-chondritic relative abundances in all investigated carbonaceous chondrite groups (Supplementary Fig. 1). In contrast, ordinary and enstatite chondrite samples display no clear plateau, but more complex volatile element abundance pattern31,32 that result at least partly from additional high temperature processes as evident by their strongly fractionated Cd and Zn stable isotope compositions in comparison to carbonaceous chondrites33–35.
Figure 1. Volatile element depletion pattern for different carbonaceous chondrite groups and the bulk silicate Earth (BSE).
(a) CI chondrite and Mg normalized volatile element abundances in carbonaceous chondrites and lithophile volatile element abundances in the BSE define a hockey stick depletion pattern in a semilog plot. (b) Zoom-in on plateau region of the hockey stick. Error bars represent the propagated uncertainties of CI chondrite and CM, CV, CR or BSE concentrations, respectively. For data sources see Supplementary Table 1.
The volatile element depletion pattern for Earth can only be deduced from predominantly lithophile volatile element abundances in the BSE. Among the plateau volatile elements, these are In, Zn, Cl, Br and I, the abundances of which have been recently revised in both, the BSE and chondrites14,25,26. Importantly, all five elements are unfractionated from each other (CI-like) in the BSE within analytical uncertainty14,18,26 (Fig. 1) and (2). When combined with the abundances of lithophile slope volatile elements in the BSE, this reveals that Earth also displays hockey stick volatile element depletion pattern (Fig. 1). Recognition of this plateau in Earth’s volatile element depletion pattern means that In is no longer overabundant in the BSE, obviating the need for terrestrial building blocks with unusual In enrichments14,18 or processes, such as melting and volatile loss from precursor bodies or during the Moon-forming impact17 to explain Earth’s volatile element depletion pattern.
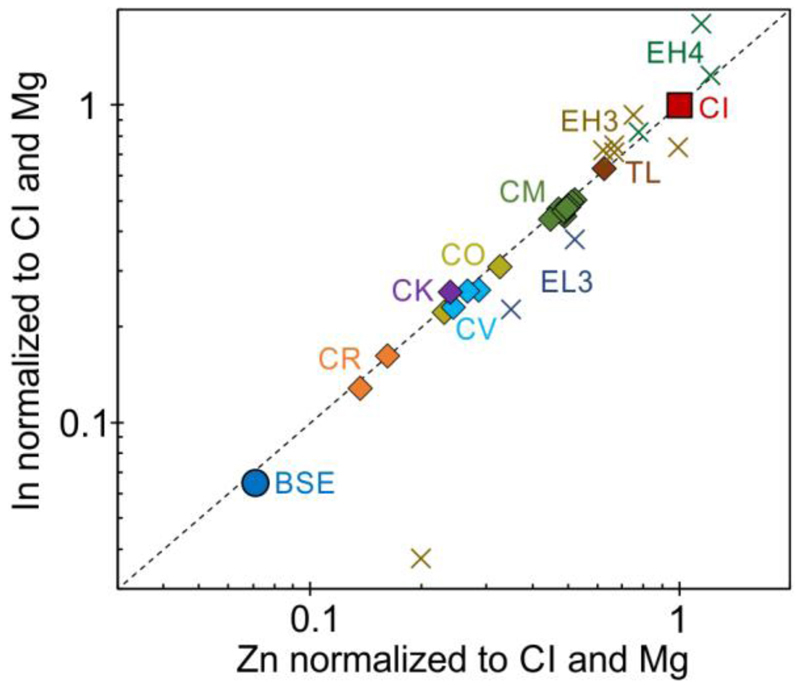
Figure 2. Indium and Zn abundances normalized to CI chondrites and Mg in chondrites and the BSE.
Carbonaceous chondrites25 and the BSE14 have a CI-chondritic In/Zn ratio as marked by the dashed line, whereas unequilibrated enstatite chondrites31 scatter around the CI-chondritic ratio. Because of the large scatter of In abundances in ordinary chondrites, representative In/Zn cannot be inferred14,18. Since the In/Zn ratio of the BSE plots on the CI-chondritic ratio, it is concluded that In and Zn both behaved predominantly lithophile during core formation.
Plateau volatile element abundances in bulk Earth and core
Recognition of the hockey stick volatile element depletion pattern allows to improve estimates of plateau volatile element abundances in bulk Earth and its core (Table 1). Bulk Earth abundances are derived from the plateau level in the BSE as defined by the lithophile elements Zn and In, whereas the core abundances are calculated from the inferred bulk Earth abundances and the depletions of siderophile and chalcophile elements relative to the BSE plateau level (Fig. 3).
Table 1. Bulk Earth and core abundances for plateau volatile elements.
| Bulk Earth [μg/g] | Core [μg/g] | Core/BSE | ||||||
|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Ref. 12 | Case 1 | Case 2 | Ref. 12 | Case 1 | Case 2 | |
| Bi | 0.012 | 0.017 | 0.010 | 0.029 | 0.046 | 0.030 | 9.7 | 15 |
| Ag | 0.023 | 0.034 | 0.050 | 0.052 | 0.086 | 0.15 | 5.8 | 9.5 |
| Pb | 0.27 | 0.40 | 0.23 | 0.46 | 0.86 | 0.40 | 2.5 | 4.6 |
| Zn | 35 | 52 | 40 | 0 | 50 | 0 | - | 0.91 |
| Te | 0.24 | 0.35 | 0.30 | 0.71 | 1.06 | 0.85 | 65 | 97 |
| Sn | 0.16 | 0.24 | 0.25 | 0.21 | 0.44 | 0.50 | 1.5 | 3.2 |
| Se | 2.2 | 3.2 | 2.7 | 6.5 | 9.6 | 8.0 | 81 | 120 |
| S | 5300 | 7900 | 6400 | 16000 | 24000 | 19000 | 76 | 113 |
| Cd | 0.073 | 0.11 | 0.10 | 0.16 | 0.27 | 0.15 | 5.1 | 8.6 |
| In | 0.0083 | 0.012 | 0.007 | 0 | 0.013 | 0 | - | 1.08 |
| Tl | 0.014 | 0.021 | 0.014 | 0.035 | 0.056 | 0.030 | 8.6 | 14 |
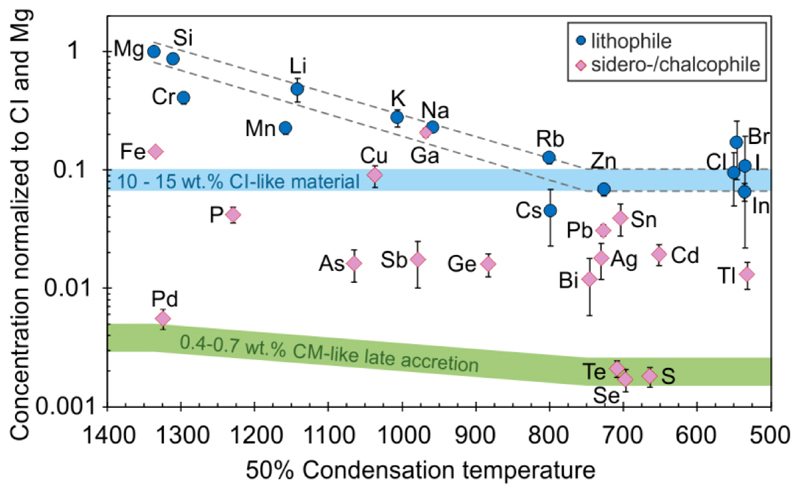
Figure 3. Magnesium normalized volatile element abundances in the BSE relative to CI as a function of their 50% condensation temperature26,41,46.
Lithophile plateau volatile element abundances in the BSE (TC < 750 K) can be explained by the addition of 10 - 15 wt.% CI-like material within Earth’s building blocks before core formation ceased. Sulfur, Se and Te were likely completely stripped of the mantle during this event before late accretion of 0.4 - 0.7 wt.% CM-like material3. Error bars represent the propagated uncertainties of CI chondrite and BSE concentrations. For data sources see Supplementary Table 1.
Our estimates of bulk Earth and core abundances hinge on the lithophile behaviour of In and Zn during core formation, but metal-silicate partition experiments revealed siderophile tendencies for both elements14,16,36. Therefore, a substantial fraction of In and Zn could have been sequestered into the core, which would drive estimates of their abundances in bulk Earth higher. Since In is considered more siderophile than Zn14,16, partitioning of Zn and In into the core, while maintaining CI-chondritic In/Zn ratios in the BSE appears possible only in extreme cases with equilibrium pressures exceeding 30 - 60 GPa during core formation16. Experiments also suggest that In is more siderophile than Cd14, which is depleted by core formation (Fig. 3). If true, In must have become even more depleted during core formation and Earths building materials must be characterized by suprachondritic In/Cd to accommodate the observed In/Cd in the BSE14. However, experiments conducted at high sulfur fugacities reveal that Cd is more chalcophile than In15,37,38. Furthermore, dissolved Si in the metal reduces the In partition coefficient more than that of Cd39 and Zn40. Consequently, a S- and Si-bearing core could explain the relative abundances of Zn, In and Cd observed in the BSE. Moreover, the lithophile behaviour of In and Zn during core formation is supported by the Cs abundance in the BSE (Fig. 1). The slope volatile element Cs plots close to the plateau level or slightly above in different carbonaceous chondrites (Fig. 1). In the BSE the lithophile element Cs plots within uncertainty at the same level as Zn and In (Fig. 1, 3) and not above. This supports the notion that both Zn and In remained largely in the silicate Earth during core formation and that both elements serve as a robust baseline for the calculation of plateau volatile element abundances in the BSE.
Based on the new hockey stick volatile element depletion pattern, we can re-evaluate plateau volatile element partitioning into the core. To this end, we calculated core and bulk Earth compositions (Table 1) for two end-member cases: 1) Zn and In did not partition into the core; and 2) there are 50 μg/g Zn in the core, which is the lower estimate of core formation models based on recent experimental data36. The latter results in significantly higher concentrations for siderophile plateau volatile elements in bulk Earth and the core. Differences to previous estimates on bulk Earth and core volatile element abundances12 result from (i) the newly recognized plateau region where volatile element depletion is the same and thus independent from uncertainties in T50%, (ii) the observation that Ag belongs to the plateau region25,41 and (iii) updated chemical compositions for CI chondrites and the BSE (Supplementary Table 1). Our new data confirm that the S abundance in the core cannot contribute substantially to the outer core density deficit12,42 and reveal the extent to which Pb and other moderately siderophile and chalcophile plateau volatile elements were sequestered into the core (Table 1; Fig. 3).
Terrestrial building materials and volatile element delivery
In carbonaceous chondrites the unfractionated plateau volatile element pattern can be explained by the presence of material with a CI-like composition in their matrices25,43. Accordingly, the unfractionated plateau volatile element abundances in Earth most likely reflect the presence of CI-like material within some of Earth’s building blocks. The abundances of the dominantly lithophile plateau volatile elements Zn, Cl, Br, I and In in the BSE allow an estimate on the maximum amount of CI-like material in Earth, which is 10-15 wt.% (Fig. 3). The depletion of siderophile and chalcophile volatile elements relative to the plateau implies that the CI-like material was accreted before core formation and sulfide segregation44,45 ceased.
In contrast, the abundances and chondritic ratios of highly siderophile elements (HSE) and the chalcogens S, Se and Te are best explained by late accretion of ~0.5 wt.% CM-like material3 (equivalent to ~0.25 wt.% CI-like material25) to a mantle that was virtually completely stripped of HSE and chalcogens during core formation (Fig. 3). Plateau volatile elements other than the chalcogens are less chalcophile and siderophile and were therefore affected to a lesser degree by metal or sulfide segregation. The depletion of Pb and Sn, however, is much smaller than predicted by equilibrium partitioning which indicates that the accretion of the CI-like material was biased towards the end of Earth’s main accretion phase13, when the composition of Earth’s building blocks shifted from reduced refractory matter to more oxidized and volatile-rich material47,48. Likewise, the overall stronger volatile element depletion of Earth compared to most carbonaceous chondrites is best explained by a mix of early refractory building materials with late volatile-rich materials47. Independent of the exact timing, the recognition that the CM-like material delivered during late accretion is also characterized by a hockey stick volatile element depletion pattern (Fig. 3), indicates that some of Earth’s building materials were chemically similar to carbonaceous chondrites during Earth’s main and late accretion phases.
There is an ongoing debate on whether Earth is made of carbonaceous chondrite-like1,2 or enstatite chondrite-like material7,49. Mass-independent isotope compositions for oxygen and several refractory elements in the BSE are indistinguishable or close to those of enstatite chondrites and clearly different from carbonaceous chondrites6–9. Consequently, it was proposed that the vast majority of Earth’s building blocks formed in the same region of the solar nebula as enstatite chondrites and cannot comprise significant amounts of carbonaceous chondrites7. In this case, however, a substantial amount of these terrestrial building blocks must be characterized by a hockey stick volatile element depletion pattern, which is so far not observed in enstatite chondrites. Alternatively, the similar mass-independent isotope composition of Earth and enstatite chondrites results from admixing carbonaceous chondrite material to non-carbonaceous material50. In this model, Earth’s average building material could comprise up to 32 wt.% of carbonaceous chondrite material while still keeping non-carbonaceous isotope compositions for oxygen and refractory elements50. Earth’s endmember composition for Mo, Ru and Nd isotopes6,8,9, would then require the presence of building materials with isotope compositions that are not present in our meteorite collections. The delivery of volatiles such as water and N2 by 2-4% carbonaceous chondrite material4,5 suggests that at least a fraction of volatile elements was added by carbonaceous chondrites.
In summary, carbonaceous chondrites and Earth display a unique, hockey stick-like volatile element depletion pattern where the most volatile elements are depleted to the same extent. This is in stark contrast to the long-standing paradigm of ever increasing depletion with elemental volatility. As a consequence, abundances of the plateau volatile elements in bulk Earth and in the core have been re-evaluated, providing a new framework for studies constraining terrestrial core formation conditions and planetary differentiation in general.
Methods
Isotope dilution analysis of S, Se and Te
Selenium and Te abundances (Extended Data Table 2) were determined by isotope dilution multiple collector-inductively coupled plasma-mass spectrometry (MC-ICP-MS). Sulfur abundances were determined using an Element XR™ sector field-inductively coupled plasma-mass spectrometry (SF-ICP-MS)51–53. Three isotope tracer solutions (spikes) enriched in 34S, 77Se and 125Te, containing 1195 ± 26 μg/g S, 420.1 ± 2.7 ng/g Se and 56.97 ± 0.83 ng/g Te (2 SD intermediate precision) were used. The intermediate precision given for the spike concentrations refers to repeated calibrations against two commercial Merck and Alfa Aesar element solutions. The isotope tracer solutions were added to ~50 mg chondrite powders. A mixture of two ml of 14 M HNO3 and 24 M HF was added for sample digestion and capped beakers were placed onto a hot plate at 120 °C for 48 h. For the determination of S contents an aliquot containing about 15 μg of S was dried down, dissolved in 0.14 M HNO3 and diluted to final S concentrations of about 1 μg/g.
For Se and Te analyses, the sample solution was dried down, taken up in 2 ml 6 M HCl and refluxed for 24 h at 80 °C to ensure complete conversion of Se (VI) and Te (VI) to the 4+ oxidation state54. Solutions were dried down again, taken up in 6 ml 6 M HCl and refluxed for another 24 h at 80 °C. After cooling, the samples were diluted with ultrapure water (18.2 MΩ*cm) to achieve 36 ml of sample solutions in 1 M HCl. Thiol cotton fibre (TCF) was employed for the separation of Se and Te from sample matrices. Preparation of TCF followed published protocols55–58. Diluted sample solutions were then loaded onto 5 ml polypropylene columns55. While all major elements pass through, Se (IV) and Te (IV) have strong affinities for the functional groups on the thiol cotton fibres56. The remaining matrix elements were then eluted with 2 ml 6 M HCl and 4 ml H2O, respectively. Subsequently, the TCF was transferred to 10 ml test tubes and attacked with 0.5 ml 7 M HNO3 in order to elute Se and Te. Test tubes were capped and placed into a boiling water bath for 20 min. Subsequently, samples were diluted by the addition of 4.6 ml ultrapure water. For Se and Te analyses, the solution was split and 0.25 ml 10 M HCl was added to both aliquots to ensure that Se and Te remain in the 4+ state.
For Se and Te isotope ratio measurements a Neptune MC-ICP-MS equipped with an ESI SC-2 autosampler and a homemade hydride generator was used52,59. A 1 μg/g solution yields about 20 mV for 82Se and 400 mV for 128Te with 1011 Ω resistors. The cup configuration includes 76Se, 77Se, 78Se and 82Se and 124Te, 125Te, 126Te, 128Te and 130Te. Each analysis comprised 20 cycles with an integration time of 4.2 s. Because Se and especially Te can be rather sticky in the sample introduction system, rinsing with 0.56 M HNO3 takes up to 20 min. The background was determined right before every standard and sample analysis. Measured background intensities were subtracted offline from sample signal intensities. Instrumental mass bias was monitored externally by repeated analyses of standard solutions and corrected using the natural 77Se/82Se of 0.87447 and 125Te/126Te of 0.37668860.
The intermediate precision of measurement is given here as two relative standard deviations (RSD) for repeated measurements of the Murchison CM2 chondrite and a slightly weathered piece of the Allende CV3 chondrite (n = number of measurements; d = number of digestions). Repeated analyses of S yielded ±1.5% (n=11; d=4) for Murchison CM2 and ±4.0% (n=20; d=8) for Allende CV3. For Se, the intermediate precision was ±1.5% for Murchison CM2 (n=5; d=5) and ±2.9% for the weathered Allende CV3 sample (n=16; d=14). The intermediate precision for Te was ±2.4% for Murchison CM2 (n=5; d=5) and ±4.7% for Allende CV3 (n=16; d=14). For S, Se and Te the precision of the measurement results from the same aliquot measured in different sessions is generally better than the intermediate precision based on repeated digestions. Thus the intermediate precision including repeated digestions is very likely compromised by sample heterogeneity between different powder aliquots. We decided to use Murchison CM2 for the estimate of the intermediate precisions for samples given in Supplementary Table 2, since the Allende CV3 test sample yielded low S (6160 μg/g) and Se (4.5 μg/g) abundances and may suffer from heterogeneities due to weathering. Procedural blanks for S, Se and Te were variable and always <90 ng for S, <0.6 ng for Se and <0.4 ng for Te. The maximum blank does not contribute significantly to S, up to 0.3% to Se and up to 1.8% to Te for the most depleted CR chondrite sample.
Calculation of core and bulk Earth plateau volatile element abundances
Core and bulk Earth plateau volatile element abundances reported in Table 1 are calculated based on their abundance in the BSE and the uniform depletion of plateau volatile elements relative to CI chondrites (Fig. 3). The extent of plateau volatile element depletion (VEDBE) in the bulk Earth is derived from the Mg and CI normalized abundances of the dominantly lithophile plateau volatile elements In and Zn in the BSE, which yields 0.067 for Case 1 and 0.100 for Case 2 (Table 1). All other plateau volatile elements are depleted relative to this plateau level in a Mg and CI normalized plot (Fig. 3), suggesting that the missing complement of these elements resides in the core. The respective bulk Earth abundances for plateau elements El (in μg/g) are calculated as ElBE = VEDBE x MgBSE x (ElCI/MgCI) x 0.675, where 0.675 refers to the silicate Earth mass fraction43. The core element abundances in μg/g are then calculated by mass balance as ElC = (ElBE - 0.675 x ElBSE)/0.325.
Supplementary Material
Acknowledgements
This research was supported by the European Commission through ERC grant No. 669666 “Infant Earth” and through DFG grant WO1594 within the priority program SPP1385 “The First 10 Million Years of the Solar System - a Planetary Materials Approach”). We thank Alexander Heuser for technical support during the ICP-MS measurements and Raúl Fonseca, Alessandro Bragagni, and Maria Kirchenbaur for fruitful discussions. We are grateful for the supply of meteorite samples by several people and institutions listed in Supplementary Table 2. This is contribution No. 57 from the DFG funded ICP-MS facility of the Steinmann-Institut für Geologie, Mineralogie und Paläontologie, University of Bonn.
Footnotes
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
Author Contributions
FW and CM designed the project. CF carried out the ICP-MS analyses for Se, Te and S. NB compiled the data, did the calculations and wrote the manuscript with substantial contributions from FW and CM. All authors contributed to the discussion of the results and editing of the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Palme H, O’Neill HSC. In: The Mantle and Core. Carlson RW, editor. Vol. 3. Treatise on Geochemistry, Elsevier Ltd; 2014. pp. 1–39. [DOI] [Google Scholar]
- 2.Allègre C, Manhès G, Lewin É. Chemical composition of the Earth and the volatility control on planetary genetics. Earth Planet Sci Lett. 2001;185:49–69. [Google Scholar]
- 3.Wang Z, Becker H. Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature. 2013;499:328–331. doi: 10.1038/nature12285. [DOI] [PubMed] [Google Scholar]
- 4.Marty B. The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet Sci Lett. 2012;313–314:56–66. [Google Scholar]
- 5.Alexander CMO, et al. The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science. 2012;337:721–723. doi: 10.1126/science.1223474. [DOI] [PubMed] [Google Scholar]
- 6.Fischer-Gödde M, Kleine T. Ruthenium isotopic evidence for an inner Solar System origin of the late veneer. Nature. 2017;541:525–527. doi: 10.1038/nature21045. [DOI] [PubMed] [Google Scholar]
- 7.Dauphas N. The isotopic nature of the Earth’s accreting material through time. Nature. 2017;541:521–524. doi: 10.1038/nature20830. [DOI] [PubMed] [Google Scholar]
- 8.Render J, Fischer-Gödde M, Burkhardt C, Kleine T. The cosmic molybdenum-neodymium isotope correlation and the building material of the Earth. Geochemical Perspect Lett. 2017;170:178. doi: 10.7185/geochemlet.1720. [DOI] [Google Scholar]
- 9.Burkhardt C, et al. Molybdenum isotope anomalies in meteorites: Constraints on solar nebula evolution and origin of the Earth. Earth Planet Sci Lett. 2011;312:390–400. [Google Scholar]
- 10.Sossi PA, Nebel O, O’Neill HSC, Moynier F. Zinc isotope composition of the Earth and its behaviour during planetary accretion. Chem Geol. 2018;477:73–84. [Google Scholar]
- 11.Pringle EA, Moynier F. Rubidium isotopic composition of the Earth, meteorites, and the Moon: Evidence for the origin of volatile loss during planetary accretion. Earth Planet Sci Lett. 2017;473:62–70. [Google Scholar]
- 12.McDonough WF. In: The Mantle and Core. Carlson RW, editor. Vol. 2. Treatise on Geochemistry, Elsevier Ltd; 2003. pp. 547–568. [Google Scholar]
- 13.Ballhaus C, et al. The great sulfur depletion of Earth’s mantle is not a signature of mantle-core equilibration. Contrib to Mineral Petrol. 2017;172:68. [Google Scholar]
- 14.Wang Z, Laurenz V, Petitgirard S, Becker H. Earth’s moderately volatile element composition may not be chondritic: Evidence from In, Cd and Zn. Earth Planet Sci Lett. 2016;435:136–146. [Google Scholar]
- 15.Wood BJ, Kiseeva ES, Mirolo FJ. Accretion and core formation : The effects of sulfur on metal – silicate partition coefficients. Geochim Cosmochim Acta. 2014;145:248–267. [Google Scholar]
- 16.Mann U, Frost DJ, Rubie DC. Evidence for high-pressure core-mantle differentiation from the metal–silicate partitioning of lithophile and weakly-siderophile elements. Geochim Cosmochim Acta. 2009;73:7360–7386. [Google Scholar]
- 17.Norris CA, Wood BJ. Earth’s volatile contents established by melting and vaporization. Nature. 2017;549:507–510. doi: 10.1038/nature23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witt-Eickschen G, Palme H, O’Neill HSC, Allen CM. The geochemistry of the volatile trace elements As, Cd, Ga, In and Sn in the Earth’s mantle: New evidence from in situ analyses of mantle xenoliths. Geochim Cosmochim Acta. 2009;73:1755–1778. [Google Scholar]
- 19.O’Neill HSC, Palme H. Collisional erosion and the non-chondritic composition of the terrestrial planets. Philos Trans A Math Phys Eng Sci. 2008;366:4205–4238. doi: 10.1098/rsta.2008.0111. [DOI] [PubMed] [Google Scholar]
- 20.Trinquier A, Birck JL, Allègre CJ, Göpel C, Ulfbeck D. 53Mn-53Cr systematics of the early Solar System revisited. Geochim Cosmochim Acta. 2008;72:5146–5163. [Google Scholar]
- 21.Siebert J, et al. Chondritic Mn/Na ratio and limited post-nebular volatile loss of the Earth. Earth Planet Sci Lett. 2018;485:130–139. [Google Scholar]
- 22.Humayun M, Clayton RN. Potassium isotope cosmochemistry: Genetic implications of volatile element depletion. Geochim Cosmochim Acta. 1995;59:2131–2148. [Google Scholar]
- 23.Wombacher F, Rehkämper M, Mezger K, Bischoff A, Münker C. Cadmium stable isotope cosmochemistry. Geochim Cosmochim Acta. 2008;72:646–667. [Google Scholar]
- 24.Nebel O, Mezger K, van Westrenen W. Rubidium isotopes in primitive chondrites: Constraints on Earth’s volatile element depletion and lead isotope evolution. Earth Planet Sci Lett. 2011;305:309–316. [Google Scholar]
- 25.Braukmüller N, Wombacher F, Hezel DC, Escoube R, Münker C. The chemical composition of carbonaceous chondrites: implications for volatile element depletion, complementarity and alteration. Geochim Cosmochim Acta. 2018;239:17–48. [Google Scholar]
- 26.Clay PL, et al. Halogens in chondritic meteorites and terrestrial accretion. Nature. 2017;551:614–618. doi: 10.1038/nature24625. [DOI] [PubMed] [Google Scholar]
- 27.Palme H, Lodders K, Jones A. In: Planets, Asteroids, Comets and The Solar System. Davis AM, editor. Vol. 2. Treatise on Geochemistry, Elsevier Ltd; 2014. pp. 15–36. [DOI] [Google Scholar]
- 28.Wasson JT, Chou C-L. Fractionation of moderately volatile elements in ordinary chondrites. Meteoritics. 1974;9:69–85. [Google Scholar]
- 29.Krähenbühl U, Morgan JW, Ganapathy R, Anders E. Abundance of 17 trace elements in carbonaceous chondrites. Geochim Cosmochim Acta. 1973;37:1353–1370. [Google Scholar]
- 30.Takahashi H, Janssens MJ, Morgan JW, Anders E. Further studies of trace elements in C3 chondrites. Geochim Cosmochim Acta. 1978;42:97–106. [Google Scholar]
- 31.Wang M-S, Lipschutz ME. Thermal Metamorphism of Primitive Meteorites - XII. The Enstatite Chondrites Revisited. Environ Chem. 2005;2:215–226. [Google Scholar]
- 32.Wang M-S, Lipschutz ME. Trace elements in primitive meteorites—VII Antarctic unequilibrated ordinary chondrites. Geochim Cosmochim Acta. 2007;71:1062–1073. [Google Scholar]
- 33.Luck JM, Othman D Ben, Albarède F. Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes. Geochim Cosmochim Acta. 2005;69:5351–5363. [Google Scholar]
- 34.Moynier F, et al. Nature of volatile depletion and genetic relationships in enstatite chondrites and aubrites inferred from Zn isotopes. Geochim Cosmochim Acta. 2011;75:297–307. [Google Scholar]
- 35.Palk C, et al. Variable Tl, Pb, and Cd concentrations and isotope compositions of enstatite and ordinary chondrites—Evidence for volatile element mobilization and decay of extinct205Pb. Meteorit Planet Sci. 2018;53:167–186. [Google Scholar]
- 36.Mahan B, Siebert J, Blanchard I, Borensztajn S, Badro J. Constraining compositional proxies for Earth’s accretion and core formation through high pressure and high temperature Zn and S metal–silicate partitioning. Geochim Cosmochim Acta. 2018;235:21–40. [Google Scholar]
- 37.Kiseeva ES, Wood BJ. A simple model for chalcophile element partitioning between sulphide and silicate liquids with geochemical applications. Earth Planet Sci Lett. 2013;383:68–81. [Google Scholar]
- 38.Righter K, et al. Volatile element signatures in the mantles of Earth, Moon, and Mars: Core formation fingerprints from Bi, Cd, In, and Sn. Meteorit Planet Sci. 2017;22:1–22. [Google Scholar]
- 39.Righter K, et al. Distribution of Sb, As, Ge, and In between metal and silicate during accretion and core formation in the Earth. Geochim Cosmochim Acta. 2017;198:1–16. [Google Scholar]
- 40.Righter K, et al. Effect of silicon on activity coefficients of siderophile elements (Au, Pd, Pt, P, Ga, Cu, Zn, and Pb) in liquid Fe: Roles of core formation, late sulfide matte, and late veneer in shaping terrestrial mantle geochemistry. Geochim Cosmochim Acta. 2018;232:101–123. [Google Scholar]
- 41.Kiseeva ES, Wood BJ. The effects of composition and temperature on chalcophile and lithophile element partitioning into magmatic sulphides. Earth Planet Sci Lett. 2015;424:280–294. [Google Scholar]
- 42.Dreibus G, Palme H. Cosmochemical constraints on the sulfur content in the Earth’s core. Geochim Cosmochim Acta. 1996;60:1125–1130. [Google Scholar]
- 43.Jacquet E, et al. Northwest Africa 5958: A weakly altered CM-related ungrouped chondrite, not a CI3. Meteorit Planet Sci. 2016;51:851–869. [Google Scholar]
- 44.O’Neill HSC. The origin of the Moon and the early history of the Earth - A chemical model. Part 1: The Moon. Geochim Cosmochim Acta. 1991;55:1135–1157. [Google Scholar]
- 45.Rubie DC, et al. Highly siderophile elements were stripped from Earth’s mantle by iron sulfide segregation. Science. 2016;353:1141–1144. doi: 10.1126/science.aaf6919. [DOI] [PubMed] [Google Scholar]
- 46.Lodders K. Solar system abundances and condensation temperatures of the elements. Astrophys J. 2003;591:1220–1247. [Google Scholar]
- 47.Schönbächler M, Carlson RW, Horan MF, Mock TD, Hauri EH. Heterogeneous accretion and the moderately volatile element budget of Earth. Science. 2010;328:884–887. doi: 10.1126/science.1186239. [DOI] [PubMed] [Google Scholar]
- 48.Rubie DC, et al. Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus. 2015;248:89–108. [Google Scholar]
- 49.Javoy M, et al. The chemical composition of the Earth: Enstatite chondrite models. Earth Planet Sci Lett. 2010;293:259–268. [Google Scholar]
- 50.Warren PH. Stable-isotopic anomalies and the accretionary assemblage of the Earth and Mars: A subordinate role for carbonaceous chondrites. Earth Planet Sci Lett. 2011;311:93–100. [Google Scholar]
- 51.Makishima A, Nakamura E. Determination of total sulfur at microgram per gram levels in geological materials by oxidation of sulfur into sulfate with in situ generation of bromine using isotope dilution high-resolution ICPMS. Anal Chem. 2001;73:2547–2553. doi: 10.1021/ac001550i. [DOI] [PubMed] [Google Scholar]
- 52.König S, Luguet A, Lorand J-P, Wombacher F, Lissner M. Selenium and tellurium systematics of the Earth’s mantle from high precision analyses of ultra-depleted orogenic peridotites. Geochim Cosmochim Acta. 2012;86:354–366. [Google Scholar]
- 53.Wang Z, Becker H, Wombacher F. Mass fractions of S, Cu, Se, Mo, Ag, Cd, In, Te, Ba, Sm, W, Tl and Bi in geological reference materials and selected carbonaceous chondrites determined by isotope dilution ICP-MS. Geostand Geoanalytical Res. 2015;39:185–208. [Google Scholar]
- 54.Marin L, Lhomme J, Carignan J. Determination of selenium concentration in sixty five reference materials for geochemical analysis by GFAAS after separation with thiol cotton. Geostand Newsl. 2001;25:317–324. [Google Scholar]
- 55.Rouxel O, Ludden J, Carignan J, Marin L, Fouquet Y. Natural variations of Se isotopic composition determined by hydride generation multiple collector inductively coupled plasma mass spectrometry. Geochim Cosmochim Acta. 2002;66:3191–3199. [Google Scholar]
- 56.Yu M, et al. Systematic studies on adsorption of trace elements Pt, Pd, Au, Se, Te, As, Hg, Sb on thiol cotton fiber. Anal Chim Acta. 2002;456:147–155. [Google Scholar]
- 57.Elwaer N, Hintelmann H. Selective separation of selenium (IV) by thiol cellulose powder and subsequent selenium isotope ratio determination using multicollector inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2008;23:733–743. [Google Scholar]
- 58.Mitchell K, et al. Selenium as paleo-oceanographic proxy: A first assessment. Geochim Cosmochim Acta. 2012;89:302–317. [Google Scholar]
- 59.Wombacher F, Ziegler A, Becker H. Geochimica et Cosmochimica Acta Supplement. Vol. 73. Pergamon Press; 2009. Selenium and tellurium abundances in mafic and ultramafic rock reference samples by ID-ICP-MS; p. 1450. [Google Scholar]
- 60.Berglund M, Wieser ME. Isotopic compositions of the elements 2009 (IUPAC Technical Report) Pure Appl Chem. 2011;83 [Google Scholar]
- 61.Lodders K, Fegley B. The planetary scientist’s companion. Oxford University Press; New York Oxford: 1998. [Google Scholar]
- 62.Wang Z, Becker H. Abundances of Ag and Cu in mantle peridotites and the implications for the behavior of chalcophile elements in the mantle. Geochim Cosmochim Acta. 2015;160:209–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.