To the Editor
Recent studies regarding the prevalence and type of respiratory and food allergies in Asian-Pacific populations have revealed not only parallels but also interesting differences concerning the occurrence of allergies in populations in these countries when compared with Westernized populations. Clinically relevant peanut and tree nut allergies occurred rarely in Asian-Pacific countries such as the Philippines, whereas fish allergy was very frequent.1,2 However, the “Allergies in Asia-Pacific Survey” indicated that allergic rhinitis, in particular seasonal allergic rhinitis, may be very common in the Asian-Pacific countries.3 Moreover, a high prevalence of IgE sensitizations to pollen extracts prepared from pollen of local tropical grasses was reported for a population from the Philippines.4
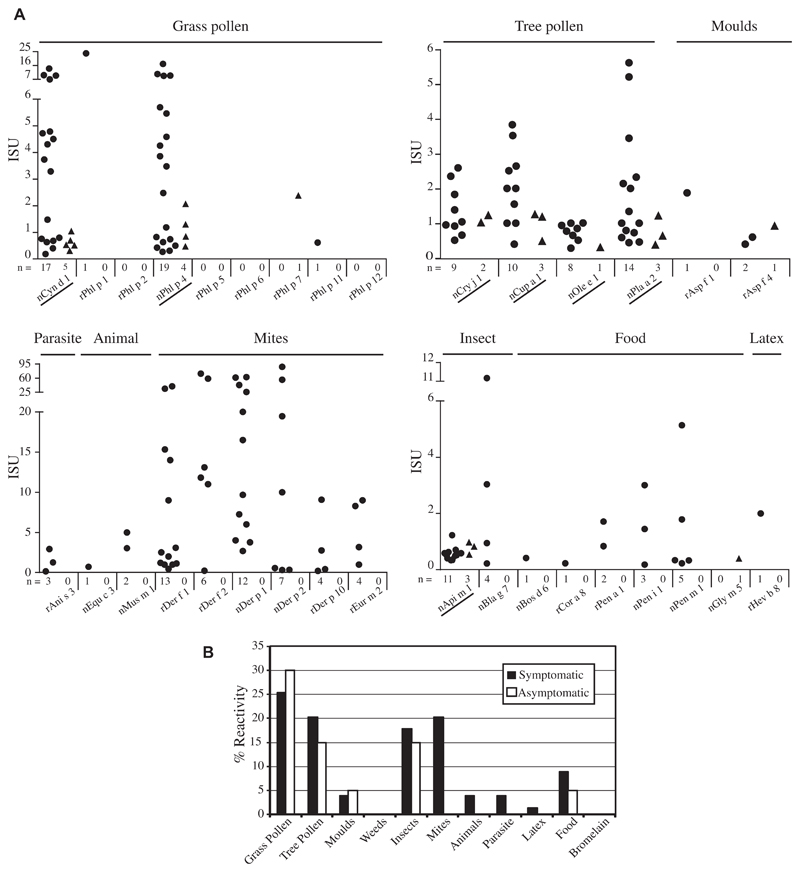
The International Study of Asthma and Allergies in Childhood Questionnaire on Allergic Screening (see the Appendix in this article’s Online Repository at www.jacionline.org) was used to recruit symptomatic subjects who reported symptoms indicative of allergy and asymptomatic subjects without such symptoms in the Philippines.4 An in-depth analysis of the molecular IgE reactivity profiles in 79 sera from symptomatic subjects and in 20 sera from asymptomatic subjects was performed by using an allergen microarray containing 103 allergen molecules (see Table E1 and Methods in the Online Repository at www.jacionline.org). IgE antibodies were found to at least 1 of the 103 allergens in 48% symptomatic subjects and, interestingly, in 40% of the asymptomatic subjects. Unlike grass pollen–allergic patients from temperate climate areas who are sensitized to the major grass pollen allergens, Phl p 1, Phl p 2, Phl p 5, and Phl p 6,5 25% of symptomatic patients showed an almost exclusive IgE reactivity to natural glycosylated grass pollen allergens nCyn d 1 or nPhl p 4 whereas nonglycosylated recombinant grass pollen allergens were almost not recognized (Fig 1, A). nCyn d 1 and nPhl p 4, and several other glycoproteins present on the microarray (Fig 1, A, underlined) contain N-linked carbohydrates, which in most cases are β(1,2)-xylose and α(1,3)-fucose motifs. These motifs occur as cross-reactive carbohydrate determinants (CCD) in various allergen sources (eg, plants, insects, and animals) and react with IgE antibodies without inducing relevant clinical symptoms.6 Only 2 sera reacted with nonglycosylated recombinant grass pollen allergens (ie, rPhl p 1, Phl p 7, and rPhl p 11) (Fig 1, A). Interestingly, asymptomatic subjects also showed IgE reactivity to nCyn d 1 and/or nPhl p 4 (Fig 1, A). Similar results were obtained for other pollen allergens where both symptomatic and asymptomatic subjects showed an almost exclusive IgE reactivity to natural glycosylated pollen allergens (eg, nCry j 1, nCup a 1, nOle e 1, and nPla a 2) but did not react with nonglycosylated pollen allergens (Fig 1, A). Rice is a frequently grown plant in the Philippines but only 1 of the sera containing IgE antibodies to nCyn d 1 reacted with the homologous recombinant major rice allergen Ory s 1 (data not shown).
Fig 1.
IgE reactivities of symptomatic and asymptomatic individuals from the Philippines to micro-arrayed allergens. A, IgE reactivities (y-axes: ISU) of symptomatic (dots) and asymptomatic individuals (triangles) to allergens on the chip (x-axes: allergens, allergen sources, number of positive sera, allergens with known glycosylation are underlined). B, Summary of the frequencies of IgE reactivities in symptomatic and asymptomatic individuals according to allergen sources.
Natural glycosylated major bee venom allergen nApi m 1 was also frequently recognized by 14% and 15% sera from symptomatic and nonsymptomatic subjects, respectively (Fig 1, A). Interestingly, none of the subjects showed IgE reactivity to Bromelain (Fig 1, B), which because of its carbohydrate chain similarities with many N-linked glycoproteins has been suggested as a marker for carbohydrate sensitization. Weed pollen, animal, plant food, latex, parasite and mould allergens were either rarely or not recognized (Fig 1, A and B; see Table E1). House dust mites (HDM) represented the second major allergen source after grass pollen. Similar to data obtained for Middle Europe, twenty percent of the symptomatic patients recognized HDM allergens, in particular group 1 and 2 allergens, but none of the asymptomatic subjects showed IgE reactivity to HDM (Fig 1, A and B), which is similar to Middle Europe.7 Animal allergens were also recognized only by symptomatic subjects (Fig 1, B). Furthermore, tropomyosin from shrimp and mites was quite frequently recognized by symptomatic subjects (Fig 1, A). Thus, symptomatic individuals were discriminated from asymptomatic subjects through IgE reactivity to non-glycosylated allergens.
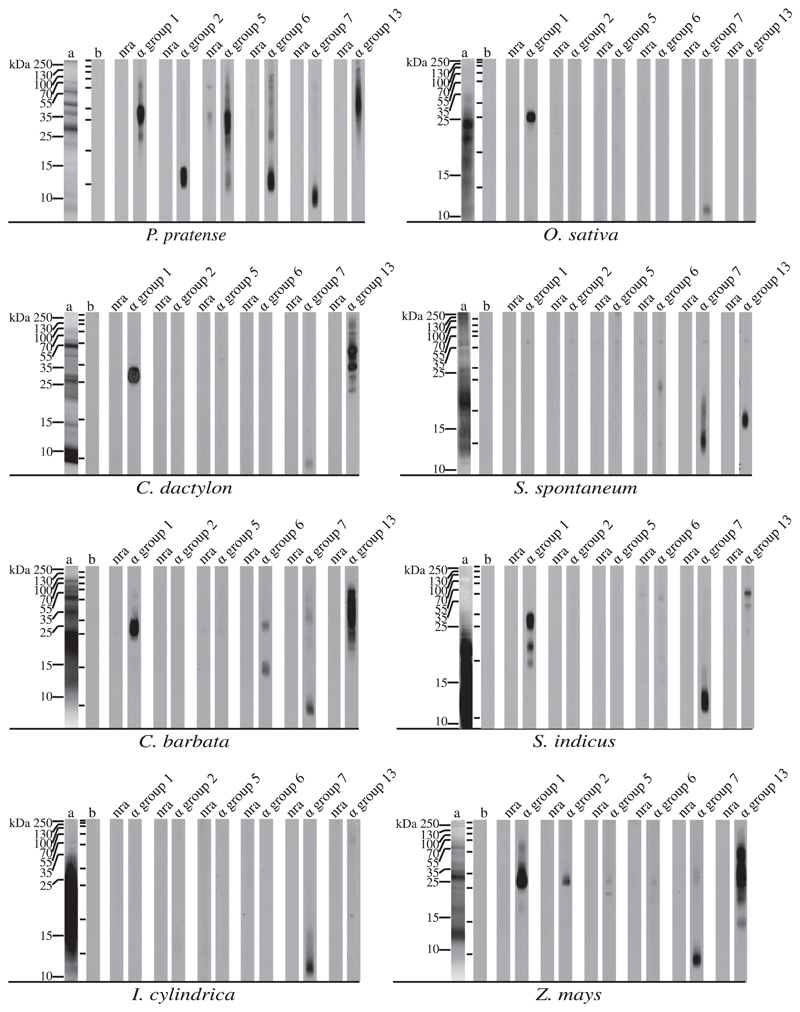
By using antibody probes specific for group 1, 2, 5, 6, 7, and 13 grass pollen allergens, we searched for related allergens in extracts from 5 tropical grasses prevalent in the Philippines (ie, Cynodon dactylon, Saccharum spontaneum, Chloris barbata, Sporobulus indicus, and Imperata cylindrica), rice (Oryza sativa), and maize (Zea mays) and used timothy grass (Phleum pratense) pollen extract for control purposes (see Fig E1 in the Online Repository at www.jacionline.org; see Methods). Each of the 6 allergen groups could be detected in timothy grass, whereas only group 7 allergens, which are highly cross-reactive calcium-binding proteins, were found in the tropical grasses, rice, and maize. The major group 1 allergens were detected in rice, C dactylon, C barbata, S indicus, and maize, and group 13 allergens were found in C dactylon, S spontaneum, C barbata, S indicus, and maize. Group 2 and group 5 allergens could not be detected in any of the tropical grasses or rice. Aweak reactivity of the anti–group 6 antibodies was observed in C barbata, with bands of approximately 15 and 23 kDa, which however did not match the molecular weight of group 6 allergens (ie, approximately 12 kDa). The band detected by group 2–specific antibodies at approximately 30 kDa in maize most likely results from cross-reactivity with group 1 allergens, which contain similar regions of sequence to group 1. Our results demonstrated that the allergen profiles of the tropical grasses resemble that of Bermuda grass in-as-much as they contained group 1, group 7, and group 13 allergens. Because the sequence and structural similarities of group 1 allergens among grasses are very high,8 the lack of sensitization to the protein backbone of group 1 allergens cannot be explained by lack of group 1–related allergens in grasses growing in the Philippines. We therefore must consider alternative explanations for the lack of clinically relevant sensitizations toward grass pollen. One possibility would be parasitic infestations inducing mainly IgE antibodies to carbohydrate epitopes as reported for other tropical populations,9 but according to the International Study of Asthma and Allergies in Childhood questionnaire used to select the individuals, worm infestations were excluded.
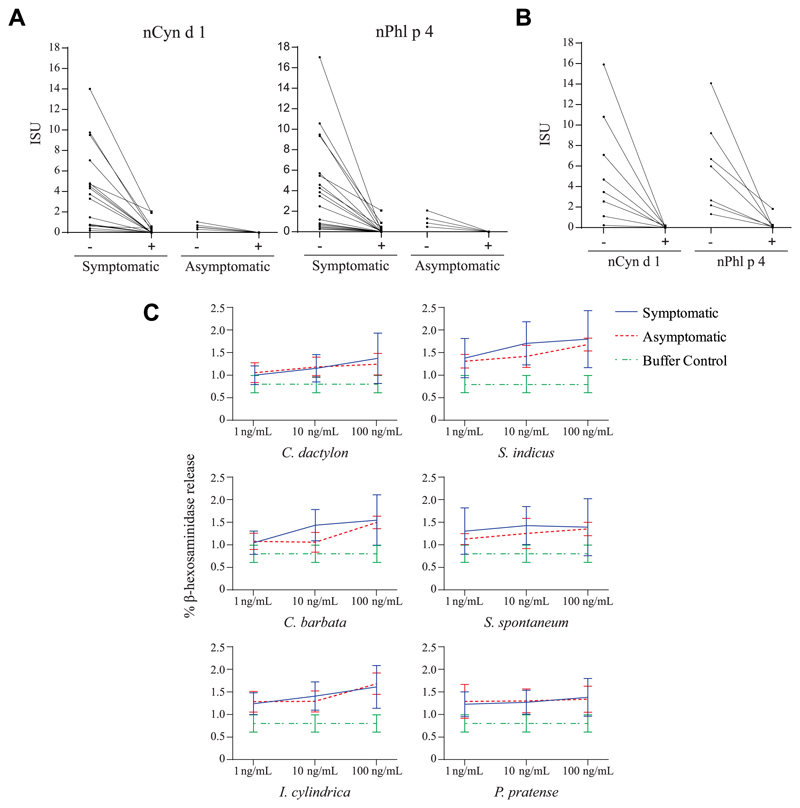
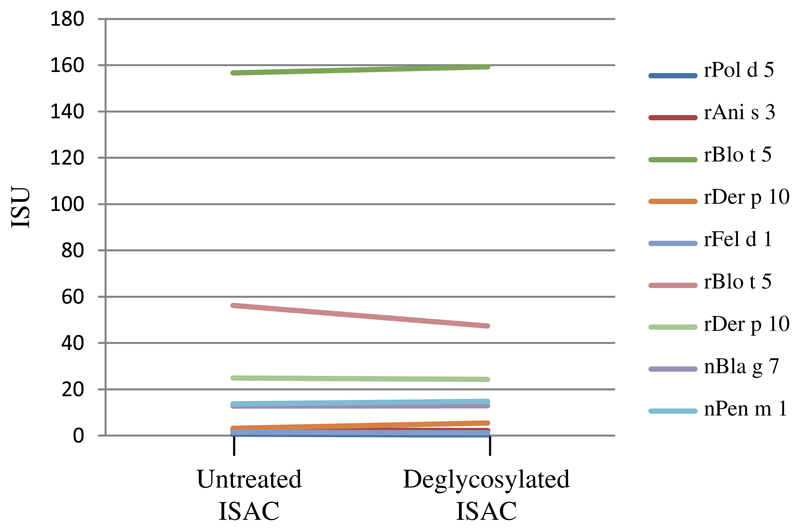
Another possibility would be that everyday contact of an individual with plant materials such as grass pollen grains may favor the development of anti-CCD antibodies.6 This could explain the detected predominance of anti-CCD IgEs in both symptomatic and asymptomatic subjects. Therefore, we performed IgE inhibition studies (see Methods) to investigate whether Phl p 4–associated carbohydrate epitopes represent major IgE-reactive structures. Preincubation of sera with nPhl p 4 caused a more than 90% inhibition of IgE binding to nCyn d 1 (Fig 2, A, left panel). Moreover, nPhl p 4 strongly inhibited IgE binding to the other glycosylated pollen allergens (ie, nCry j 1: 78.6%; nCup a 1: 82.3%; nOle e 1: 100%; and nPla a 2: 99.1%) and to the venom allergen Api m 1 (100%) but had no relevant effect on IgE binding to the nonglycosylated allergens on the chip. Autoinhibition of a similar degree of IgE binding to nPhl p 4 was obtained by preincubation of the sera with nPhl p 4 (Fig 2, A, right panel). Furthermore, the deglycosylation of the allergens on the chip by periodate treatment caused a more than 90% reduction in IgE binding to nCyn d 1 and nPhl p 4 (Fig 2, B) but had no relevant effects on IgE binding to nonglycosylated allergens (see Fig E2 in the Online Repository at www.jacionline.org).
Fig 2.
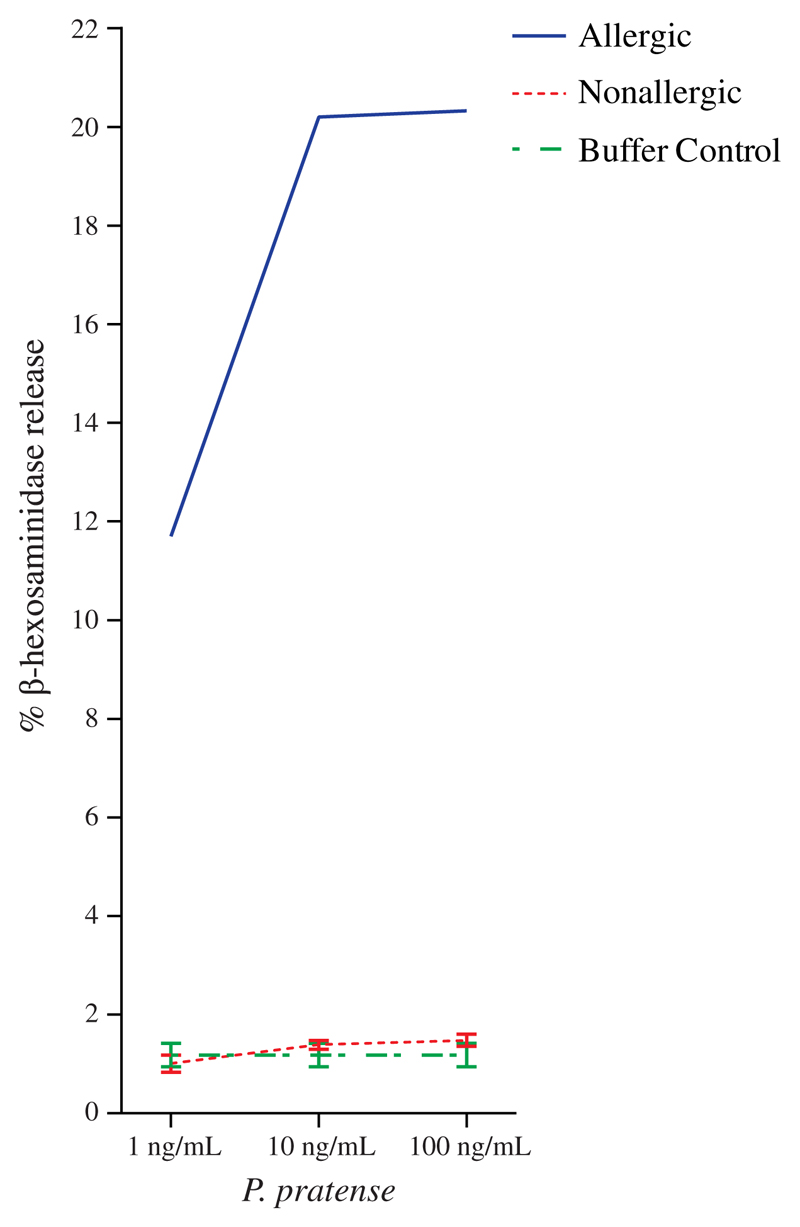
A, IgE reactivity (y-axis) of sera from subjects with grass pollen–specific IgE to nCyn d 1 (left panel) and nPhl p 4 (right panel) after preincubation with (+) or without (−) nPhl p 4. B, IgE reactivity (y-axis) of sera to untreated (−) and deglycosylated (+) nCyn d 1 and nPhl p 4. C, Mediator release induced by grass pollen allergens (x-axis) displayed as percentages of total β-hexosaminidase (y-axes) for symptomatic and asymptomatic subjects or buffer.
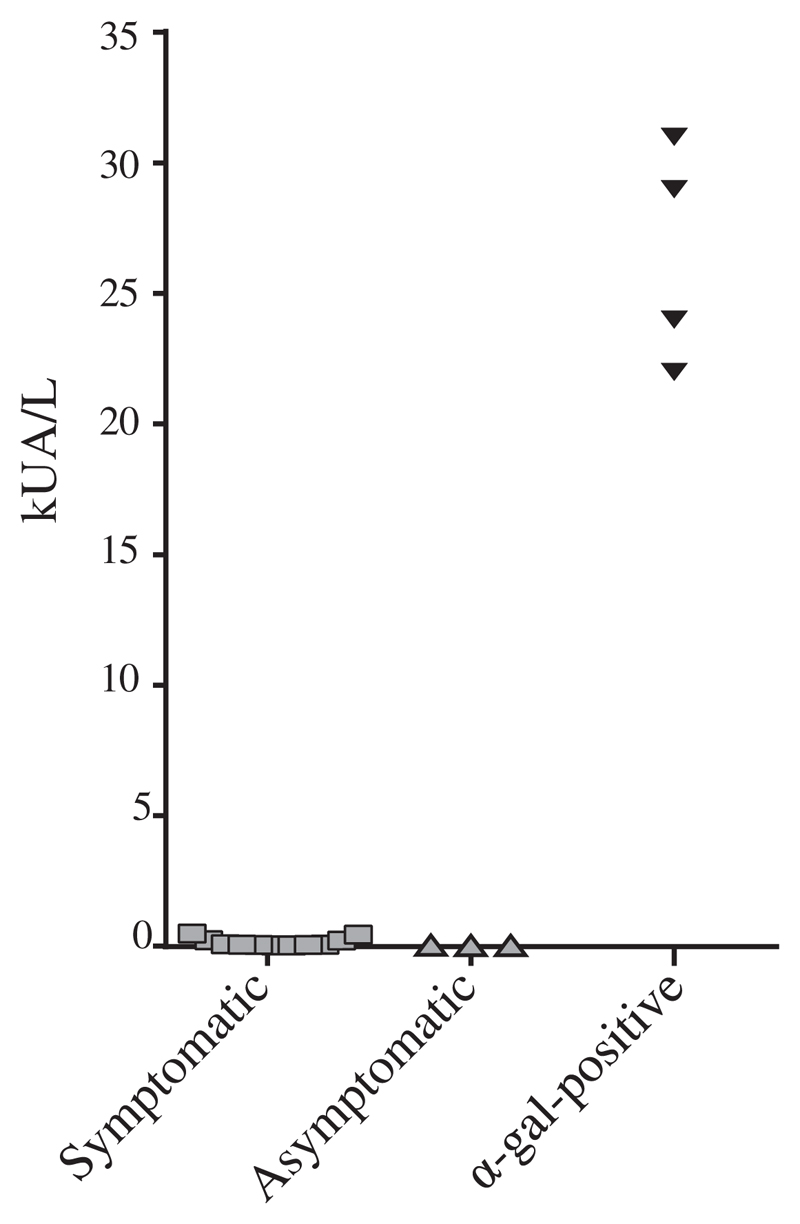
Galactose alpha-1,3-galactose (α-Gal), an animal-derived glycan, has been described as a frequent cause of clinically relevant as well as asymptomatic sensitization in allergic patients.9 However, only 2 of the 11 tested sera from symptomatic or asymptomatic patients showed weak IgE reactivity to α-Gal (ie, <0.48 kUA/L) whereas sera from α-Gal-positive patients (positive control) displayed strong IgE reactivity (>20 kUA/L) (see Fig E3 in the Online Repository at www.jacionline.org).
We therefore assumed that the tropical flora, and in particular the tropical grasses, produce highly glycosylated allergens where protein epitopes are hidden by the carbohydrates and as a result primarily induce clinically irrelevant IgE sensitizations against carbohydrate epitopes. This assumption was supported by experiments studying the possible allergenic activity of carbohydrate IgE epitopes in tropical grasses. Rat basophil leukemia-2H3 cells expressing the human high-affinity receptor for IgE were loaded with serum IgE from subjects who had shown IgE reactivity to Phl p 4 and then exposed to natural pollen extracts from the tropical grasses (Fig 2, C) and to timothy grass (P pratense) extract. Although each of the tested sera had shown IgE reactivity to pollen extracts from the tropical grasses, no basophil degranulation was observed (Fig 2, C). When serum from an Austrian timothy grass pollen allergic patient was used to load the rat basophil leukemia cells followed by incubation to timothy grass pollen extract, a dose-dependent degranulation was obtained (see Fig E4 in the Online Repository at www.jacionline.org).
Our results thus reveal an astonishing high frequency of IgE sensitization to nonallergenic, grass pollen–derived carbohydrate epitopes and the absence of IgE reactivity to highly allergenic grass pollen protein allergens in the Philippines, indicating that the local flora consisting of tropical grasses does not induce clinically relevant sensitizations. We consider these data important because they reveal that a certain environment (ie, the presence of certain plant pollens and a certain climate) seems to protect against clinically relevant sensitization to plants.
Extended Data
Fig E1.
Presence of allergens in timothy grass and tropical grasses. Pollen extracts were subjected to SDS-PAGE and silver stained (lanes a), and after Western blotting, analyzed by using rabbit antisera specific for grass pollen allergen groups (lanes a group 1, 2, 5, 6, 7, and 13), normal rabbit antibodies (lanes nra), or buffer (lanes b). Molecular weights are indicated on the left margins.
Fig E2.
IgE reactivity of individual sera (y-axis: ISU) to allergens on ImmunoCAP ISAC after periodate treatment (deglycosylated ISAC) or after exposure to buffer alone (untreated ISAC).
Fig E3.
Quantitative IgE binding (y-axis: kUA/L) of 11 nPhl p 4-positive symptomatic, 3 asymptomatic subjects, and 4 α-Gal–positive sera (positive controls) (x-axis) to α-Gal.
Fig E4.
Induction of rat basophil leukemia cell degranulation (y-axis) with different concentrations of timothy grass pollen allergens (x-axis) when cells were loaded with serum IgE from a patient allergic to timothy grass pollen versus lack of degranulation when serum from a nonallergic individals or buffer was used.
Table E1. List of purified allergen molecules of the ImmunoCAP ISAC 103, grouped according to allergen sources.
| Allergen Source | Allergen |
|---|---|
| Grass pollen | nCyn d 1 |
| rPhl p 1 | |
| rPhl p 2 | |
| nPhl p 4 | |
| rPhl p 5 | |
| rPhl p 6 | |
| rPhl p 7 | |
| rPhl p 11 | |
| rPhl p 12 | |
| Tree pollen | rBet v 1 |
| rBet v 2 | |
| rBet v 4 | |
| rAln g 1 | |
| rCor a 1.0101 | |
| nCry j 1 | |
| nCup a 1 | |
| nOle e 1 | |
| nOle e 2 | |
| rPla a 1 | |
| nPla a 2 | |
| Weed pollen | nAmb a 1 |
| nArt v 1 | |
| nArt v 3 | |
| rPar j 2 | |
| nSal k 1 | |
| rMer a 1 | |
| Mites | rDer f 1 |
| rDer f 2 | |
| nDer p 1 | |
| nDer p 2 | |
| rDer p 10 | |
| rEur m 2 | |
| Animals | rCan f 1 |
| rCan f 2 | |
| nCan f 3 | |
| nEqu c 3 | |
| rFel d 1 | |
| nFel d 2 | |
| rFel d 4 | |
| nMus m 1 | |
| Insect | nApi m 1 |
| nApi m 4 | |
| rBla g 1 | |
| rBla g 2 | |
| rBla g 4 | |
| rBla g 5 | |
| nBla g 7 | |
| Latex | rHev b 1 |
| rHev b 3 | |
| rHev b 5 | |
| rHev b 6 | |
| rHev b 8 | |
| Parasite | rAni s 1 |
| rAni s 3 | |
| Molds | rAlt a 1 |
| rAlt a 6 | |
| rAsp f 1 | |
| rAsp f 2 | |
| rAsp f 3 | |
| rAsp f 4 | |
| rAsp f 6 | |
| rCla h 8 | |
| Non-plant derived food | nBos d 4 |
| nBos d 5 | |
| nBos d 6 | |
| nBos d 8 | |
| nBos d (Bovine lactoferrin) | |
| nGal d 1 | |
| nGal d 2 | |
| nGal d 3 | |
| nGal d 5 | |
| rCyp c 1 | |
| rGad c 1 | |
| rPen a 1 | |
| nPen i 1 | |
| nPen m | |
| Plant food | nAct d 1 |
| nAct d 2 | |
| nAct d 5 | |
| nAct d 8 | |
| rApi g 1 | |
| rDau c 1 | |
| rMal d 1 | |
| rPru p 1 | |
| nPru p 3 | |
| rAna o 2 | |
| nAra h 1 | |
| nAra h 2 | |
| nAra h 3 | |
| rAra h 8 | |
| rBer e 1 | |
| rCor a 1.0401 | |
| rCor a 8 | |
| nCor a 9 | |
| rGly m 4 | |
| nGly m 5 | |
| nGly m 6 | |
| nSes i 1 | |
| nTri a 18 | |
| nTri a (Wheat Gliadin) | |
| rTri a 19.0101 | |
| nTri a a (Wheat trypsin inhibitor) | |
| Carbohydrate marker | nAna c 2 (Bromelain, N-linked glycan from pineapple) |
N, Natural; r, recombinant.
Supplementary Material
Acknowledgments
This study was supported by research grants from the Austrian Science Fund (FWF) F4605; the Christian Doppler Research Association; Biomay AG, Vienna, Austria; Thermofisher, Uppsala, Sweden; the Higher Education Development Program-Faculty Development Program, Commission on Higher Education, Philippines; the Swedish Research Council; and the Stockholm County Council.
Footnotes
Disclosure of potential conflict of interest: C. Lupinek has received one or more payments for lecturing from or is on the speakers’ bureau for Thermo Fisher Scientific. M. van Hage has received one or more payments for lecturing from or is on the speakers’ bureau for Thermo Fisher Scientific, Novartis, and ALK-Abell'o. R. Valenta has been supported by one or more grants from the Austria Science Fund, Biomay AG, and Thermo Fisher Scientific, and has received one or more consulting fees or honorarium from Biomag AG and Thermo Fisher Scientific. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Connett GJ, Gerez I, Cabrera-Morales EA, Yuenyongviwat A, Ngamphaiboon J, Chatchatee P, et al. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int Arch Allergy Immunol. 2012;159:384–90. doi: 10.1159/000338940. [DOI] [PubMed] [Google Scholar]
- 2.Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunology. 2010;126:324–31. doi: 10.1016/j.jaci.2010.06.003. 31, e1–7. [DOI] [PubMed] [Google Scholar]
- 3.Katelaris CH, Lai CK, Rhee CS, Lee SH, Yun WD, Lim-Varona L, et al. Nasal allergies in the Asian-Pacific population: results from the Allergies in Asia-Pacific Survey. Am J Rhinol Allergy. 2011;25:S3–15. doi: 10.2500/ajra.2011.25.3674. [DOI] [PubMed] [Google Scholar]
- 4.Cabauatan CR, Ramos JD. Immunoglobulin E-binding reactivities of natural pollen grain extracts from selected grass species in the Philippines. Asia Pac Allergy. 2012;2:136–43. doi: 10.5415/apallergy.2012.2.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linhart B, Jahn-Schmid B, Verdino P, Keller W, Ebner C, Kraft D, et al. Combination vaccines for the treatment of grass pollen allergy consisting of genetically engineered hybrid molecules with increased immunogenicity. Faseb J. 2002;16:1301–3. doi: 10.1096/fj.01-1012fje. [DOI] [PubMed] [Google Scholar]
- 6.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- 7.Burbach GJ, Heinzerling LM, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, et al. GA(2)LEN skin test study, II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy. 2009;64:1507–15. doi: 10.1111/j.1398-9995.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari R, Bhalla PL, Singh MB. Evaluation of molecular basis of cross reactivity between rye and Bermuda grass pollen allergens. Allergol Int. 2009;58:557–64. doi: 10.2332/allergolint.09-OA-0094. [DOI] [PubMed] [Google Scholar]
- 9.Arkestal K, Sibanda E, Thors C, Troye-Blomberg M, Mduluza T, Valenta R, et al. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-alpha 1,3-galactose. J Allergy Clin Immunol. 2011;127:1024–8. doi: 10.1016/j.jaci.2011.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.