Abstract
Cell adhesion to the extracellular matrix (ECM) is fundamental to metazoan multicellularity and is accomplished primarily through the integrin family of cell-surface receptors. Integrins are internalised and enter the endo/exocytic pathway before being recycled back to the plasma membrane. The trafficking of this extensive protein family is regulated in multiple context-dependent ways to modulate integrin function in the cell. Here we discuss recent advances in understanding the mechanisms and cellular roles of integrin endocytic trafficking.
Introduction
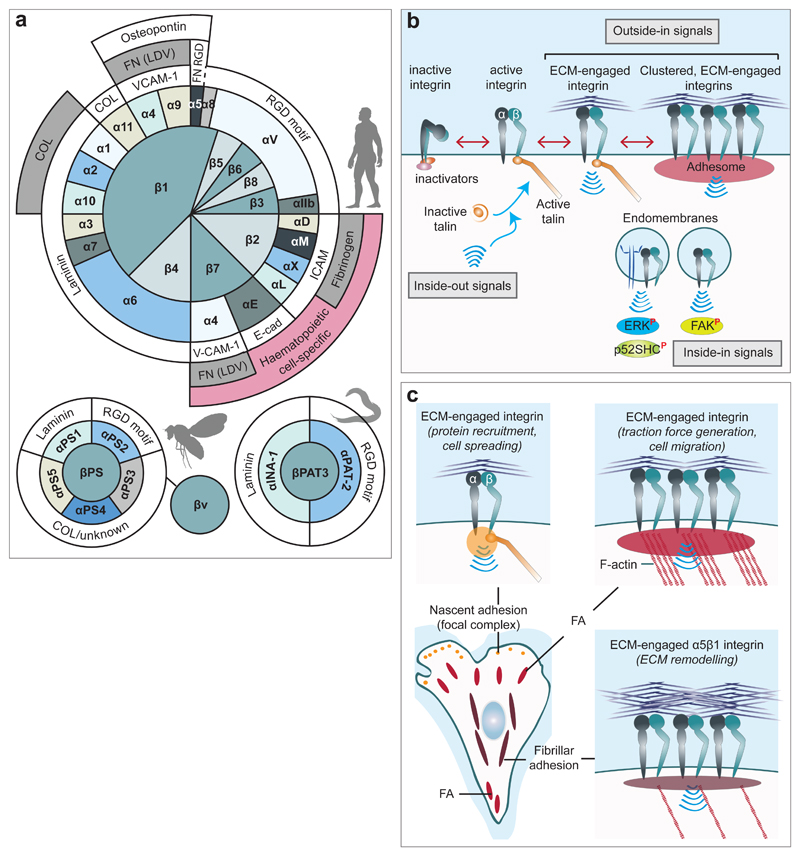
In mammals, stable non-covalent interaction between 18 α- and 8 β-subunits generates 24 functionally distinct integrin heterodimers, the majority of which contain the β1-subunit (Fig. 1a). Whereas some integrins, such as α5β1, interact with a limited number of ECM ligands, others, such as αvβ3 and α4β1, have multiple ECM binding partners. In addition, the same ECM ligand can be engaged by different integrins1 (Fig. 1a) and activate alternative signalling pathways. Therefore, the biological response to environmental cues is strongly influenced by which integrins are expressed and active on the plasma membrane. The extensive overlap in the signalling pathways of the mammalian integrin family, complicate the interpretation of loss-of-function experiments. Invertebrate model organisms have greatly broadened our understanding of integrin-driven processes in vivo as these express a smaller repertoire of integrins and integrin ligands2 (Fig. 1a). Integrin function is regulated through multiple mechanisms including conformational changes, protein-protein interactions and trafficking3–7. In most cell types integrin function depends on a delicate balance between active and inactive receptors on the cell surface8,9and spatiotemporal control of integrin activation is key for efficient adhesion formation and cell motility10. Integrins are activated through “inside-out” signals: an intracellular signal promoting the binding of proteins such as talin and kindlin to the β-integrin tail switching the receptor into an extended conformation with high affinity for ECM ligands11. Integrin binding to ECM ligands, in turn, triggers “outside-in” signals that recruit protein complexes (Fig. 1b), consisting of scaffolding/adaptor molecules, kinases and phosphatases, to regulate cell behaviour. The network of proteins recruited to integrin adhesion sites12,13, varies in composition and size giving rise to multiple classes of integrin-ECM adhesions (Fig. 1c).
Figure 1. Composition and function of the integrin family.
a, The reported pairing between integrin α and β subunits and the ECM ligand/s for each heterodimer are illustrated for mammals, Drosophila and C. elegans. In mammals, α5β1-integrin binds to the fibronectin RGD motif, whereas α4β1 binds to the fibronectin LDV motif. COL: collagen; E-cad: E-cadherin; FN: fibronectin; ICAM: intercellular cell adhesion molecule; VCAM-1: vascular cell adhesion molecule 1. For a more comprehensive/exhaustive list of integrin ligands, please refer to1.
b, Integrins are bidirectional signalling molecules. Inside-out signals regulate talin binding to integrin β-tails and thus tightly control integrin affinity for ECM ligands. Subsequent ECM binding triggers recruitment of protein complexes (scaffolding and adaptor proteins, kinases and phosphatases, etc.) to the integrin cytoplasmic tails to promote integrin downstream signalling (outside-in signalling). Integrins can also signal from within endosomes (inside-in signalling) to support FAK activity and suppress anoikis16 or to promote signalling downstream of co-trafficking MET to support anoikis resistance, tumour growth and cancer cell dissemination to lungs19. The superscripted P in red indicates phosphorylation. ERK, extracellular signal-regulated kinase; p52SHC, p52 isoform of SHC-transforming protein 1.
c, Integrin-ECM adhesions, in vitro, are defined based on localisation, components and maturation stage. Nascent adhesions (focal complexes) represent initial integrin receptor clustering in response to ECM engagement and recruitment of adaptor and signalling proteins to the integrin tails. These small protein assemblies mature into focal adhesions (FA), which serve to anchor actin stress fibres and are vital for generation of contractile force. Fibrillar adhesions are mature α5β1-integrin adhesions, and prominent sites of fibronectin fibrillogenesis, that result from the centripetal translocation of this specific integrin heterodimer towards the cell body.
Integrin adhesions are highly dynamic and undergo constant cycles of assembly and disassembly. The lifetime and composition of focal adhesions (FA) (described in Fig. 1c) strongly influences ECM-driven signalling and therefore cell behaviour. Adhesion turnover is regulated, in part, by integrin endocytosis and exocytosis back to the cell surface, typically referred to as recycling4,14,15 (Fig. 2a). Endocytosed integrins are also able to transmit intracytoplasmic “inside-in” signals either through recruitment of focal adhesion kinase (FAK), and possibly other effectors16,17, or by enhancing the signalling of co-trafficking growth factor receptors18–20 (Fig. 1b).
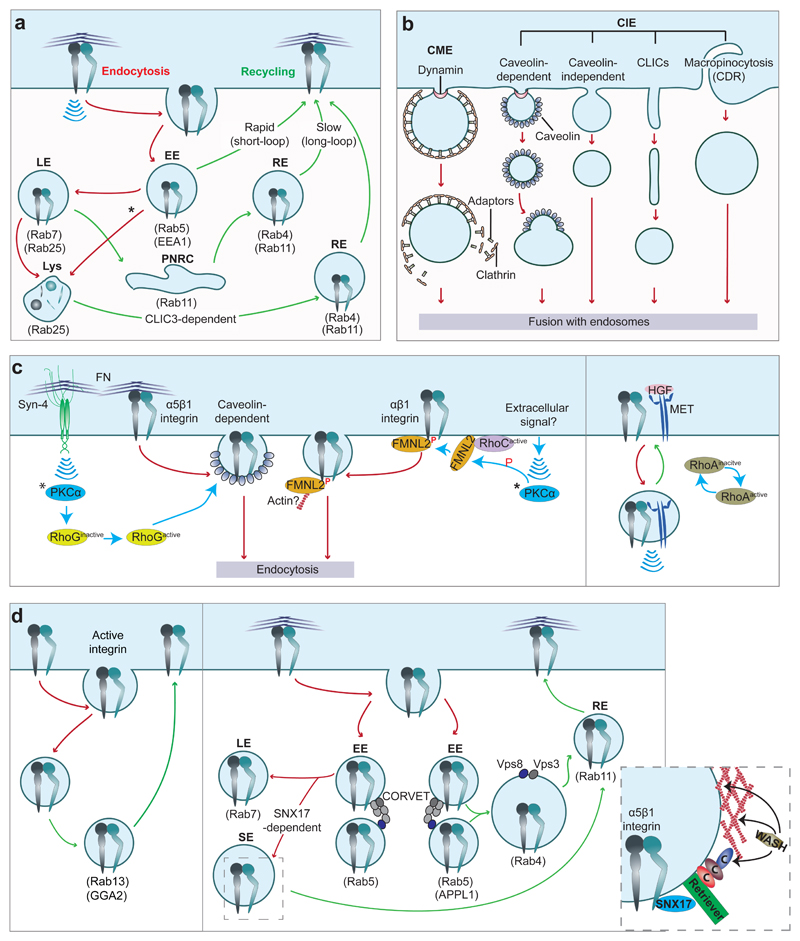
Figure 2. Fine-tuning integrin endocytosis and recycling.
a, Summary of integrin trafficking pathways. Integrins are internalised into Rab5-positive early endosomes (EE). EE mature into late endosomes (LE), which fuse with lysosomes (Lys) for integrin degradation. Under certain conditions (Rab25 and chloride intracellular channel protein 3 (CLIC3) expression), integrins exit LE/Lys compartments and are recycled back to the plasma membrane24. Integrins also traffic to multivesicular bodies (highlighted by *) and to the perinuclear recycling compartment (PNRC). This process occurs within 20 minutes, while degradation takes several hours133 resulting in the majority of integrins being recycled back to the cell surface. Each step requires a spatiotemporal hierarchy of interactions between integrins, endocytic adaptors, Ras and Arf GTPase family members and other molecules. The Rab GTPases involved are indicated.
b, Integrin receptor internalisation mechanisms, broadly classified as clathrin-mediated endocytosis (CME) or clathrin-independent endocytosis (CIE, including caveolin-dependent pathways, micropinocytosis and clathrin-independent carriers (CLICs)). CDR, circular dorsal ruffles.
c, Integrin endocytosis can be fine-tuned by extracellular-initiated signals. Left-hand panel: Syndecan-4(Syn-4)─fibronectin interaction activates PKCα and RhoG and promotes caveolin-dependent α5β1-integrin endocytosis, attenuated adhesion and increased cell migration34. PKCα also phosphorylate FMNL2 (recruited by RhoC). Phospho-FMNL2 (superscripted P in red indicates phosphorylation) interaction with the α-integrin GFFKR motif drives αβ1-integrin internalisation124. The role of actin nucleation and the extracellular signal regulating this pathway remain undefined. *PKCα activation occurs at the plasma membrane. Right-hand panel: MET receptor─β1-integrin interaction induces integrin endocytosis, collective mesenchymal cell migration, in a HIP1- (clathrin adaptor) and RhoA-dependent manner51, and “inside-in” signalling19 (see Fig. 1b).
d, Recruitment of specific endosomal adaptors imposes selectivity to integrin recycling pathways. Left-hand panel: GGA2, an Arf effector has been implicated in Rab13-mediated recycling of β1-integrin65 (a non-peer-reviewed study). Right-hand panel: Different populations of Rab5-positive EE, defined by the presence of Rab5 effectors EEA1 (not shown), APPL1 and the CORVET complex promote different recycling routes64,66,67. CORVET mediates fission and fusion of EE that mature into LE. Here, Rab5 is replaced by Rab7 and integrins enter LE. Integrins escape degradation by interaction with SNX17 (components in inset)60–62. CORVET components Vps3/Vps8 localise to Rab4-positive endosomes (fusion of APPL1-positive EE). Vps3/Vps8 vesicles deliver integrins to the plasma membrane through Rab11-positive recycling endosomes. SE, sorting endosomes.
Knowledge of the underlying mechanisms and the molecular specificity of integrin trafficking pathways is therefore fundamental in understanding integrin function. In this review, we will discuss recent insights into how integrins traffic and how integrin-trafficking pathways regulate cellular processes including animal development.
Integrin endocytic trafficking pathways
Unlike many other cell-surface receptors that undergo synchronized ligand-induced internalisation and degradation, integrins are constantly trafficked in cells. The Rab family of small GTPases are implicated in each step of this process4,14,21 (Fig. 2a). Integrins are initially endocytosed by different mechanisms (Fig. 2b) and routed to Rab5-positive early endosomes (EE) where sorting decisions are made. A large proportion of integrins are recycled back to the plasma membrane through two distinct pathways (reviewed in4,14). As a result, integrins are remarkably stable with a half-life of 12–24 hours22–24. The constant flux of integrins between the plasma membrane and intracellular pools allows cells to probe the environment for adhesive and migratory cues and to adapt their mechanical and morphological properties to mount appropriate responses.
Clathrin-mediated and -independent endocytosis of integrins
Clathrin-mediated endocytosis (CME) is the best-studied integrin internalisation route14,25. Over 50 proteins identified as key components of the CME machinery coordinate membrane invagination, formation of clathrin-coated pits, cargo recruitment and vesicle scission26 (Fig. 2b). In polarized cells, ECM-proximal integrins at the cell front are endocytosed via interaction with the clathrin adaptor Numb, the recruitment of which is inhibited by phosphorylation mediated by apical polarity determinants Par3-aPKC27. In mature disassembling FAs, kinesin KIF15, a microtubule motor, promotes the recruitment of clathrin adaptor Dab2 to integrins to trigger CME and FAK- and dynamin-dependent vesicle scission28–30. Following FA disassembly, endocytosed, ligand-unbound integrins can be maintained in an active state by talin and FAK within endosomes. This integrin pool transits from Rab5-positive EE to Rab11 recycling endosomes (RE) and is recycled to the cell front for polarised adhesion assembly and directional fibroblast cell migration17. Rab5- and FAK-dependent integrin endocytosis is also associated with ultrasound therapy-induced cell migration31, suggesting that this pathway may be a more general mechanism for regulating cell migration.
FAK also plays a role in clathrin-independent endocytosis (CIE) of integrins. Galectin-3, an extracellular carbohydrate binding lectin, induces the formation of clathrin-independent carriers and internalization of receptors including β1-integrins by recruiting intracellular GRAF1 in complex with phosphorylated FAK to the receptors32,33. Other routes of integrin CIE include internalisation from caveolae34–37, rapid macropinocytosis of unengaged integrins from the dorsal cell surface following PDGF stimulation38 and Rab21-dependent internalisation via a yet undefined mechanism39. Recently, accumulation of an LL5 (pleckstrin homology like domain, family B)–liprin-α1–ERC1 (ELKS/Rab6-interacting/CAST family member 1) ternary complex at the cell periphery was associated with FA turnover through vinculin and paxillin displacement and active integrin internalisation into endosomes40. Whereas ERC-1 was shown to partially colocalise with caveolin, a definitive role for caveolae was not investigated here41.
These internalisation pathways underscore the importance of precise and spatially controlled integrin uptake. However, they fail to explain how cells choose a particular endocytic pathway and/or discriminate between different integrin heterodimers or integrin activation states.
Fine-tuning integrin endocytosis
The majority of integrin receptors share a common β1-subunit (Fig. 1a) with a conserved β-tail NPxY/NxxY motif that enables interaction with CME adaptors and endocytosis accessory proteins such as epidermal growth factor receptor substrate 8 (EPS8), Dab2 and Numb42. Hence, all β1-containing heterodimers can potentially be endocytosed via the same mechanism.
However, it is becoming apparent that different molecular pathways are employed to distinguish between integrin heterodimers, activation states, and trafficking routes to regulate specific biological processes (Table 1). For example, in endothelial cells, internalisation of active β1-integrin from nascent adhesions into Rab5 endosomes, requiring R-Ras and Rin2, leads to Rac activation, lamellipodia formation and directional cell motility43. Conversely, selective traffic of inactive β1-integrins, containing the α1, α2 and α3, but not αv or α5, chains, from the dorsal plasma membrane to the ventral side by a Dab2─EPS15 CME module may be important for maintaining the reservoir of intracellular integrins during HeLa cell migration44,45. How these two pro-migratory pathways are consolidated in cells remains unknown and, perhaps, each requires a distinct extracellular cue and/or is cell-type specific.
Table 1. Recent examples of molecular pathways regulating specific integrin trafficking routes and/or biological processes.
Recent players in integrin trafficking from the last three years are highlighted in the table. FIP5, family-interacting protein 5; LRP1, prolow-density lipoprotein receptor-related protein 1; RN-tre, related to the N terminus of tre oncogene.; PAK, p21-activated kinase 1; ANKFY1, ankyrin repeat and FYVE domain containing 1; HUVEC, human umbilical vein endothelial cell; RPE-1, retinal pigmented epithelial cell.
| Molecule/modification | Mechanism and/or phenotype | Model system | Reference |
|---|---|---|---|
| Plasma membrane / endocytic pathways | |||
| ADAM9 | Binds to inactive β1-integrins and induces their endocytosis and intracellular degradation. This allows adhesion turnover and cancer cell migration. | Prostate cancer and fibrosarcoma cells | [49] |
| FMNL2 | Upon phosphorylation by PKCα, FMNL2 mediates actin assembly-dependent endocytosis of αβ1-integrins. Phospho-FMNL2 promotes invasion of melanoma cells. | Melanoma and HeLa cells | [124] |
| Hic-5 | HIc-5 interaction with tensin1 prevents β1-integrin internalization and lysosomal degradation and instead promotes fibrillar adhesion formation. | Fibroblasts | [91] |
| Liprin-α1, LL5 & ERC1 | Complex aids focal adhesion turnover by displacing adhesion components and inducing internalization of active β1-integrins into Rab7 endosomes. Drives protrusion formation at the leading edge during migration and invasion of breast cancer cells. | Breast cancer cells and fibroblasts | [40, 41] |
| N-glycosylation of α5- integrin | Glycosylation of the α5-integrin β-propeller (extracellular domain) promotes interactions with Syndecan-4, endocytosis of active α5β1-integrin and cell migration in cancer cells. No effect on cell spreading. | HeLa, breast cancer and glioblastoma cells | [52] |
| RN-TRE | RN-TRE, a Rab5 GTPase inactivating protein (GAP), is recruited by EPS8 to β1-integrin and inhibits receptor endocytosis | Fibroblasts | [125] |
| Tensin1-3 | Tensins bring ligand-occupied integrins from peripheral adhesions to subnuclear adhesions; promoting their endocytosis via Arf4 and subsequent degradation in lysosomes. | Ovarian cancer cells | [90] |
| Recycling pathways | |||
| Ankyrin B | Recruits RabGAP1L to PI3P positive endosomes to inactivate Rab22a. Aids integrin delivery to the cell front of fibroblasts and promotes polarized migration via α5β1-integrin. | Fibroblasts | [126] |
| APPL1 | Decreases internalization and promotes recycling of α5β1-integrin in a Rab5-dependent manner. Inhibits cell migration on fibronectin, but not collagen by decreasing Rac1 and PAK activity at the leading edge. | Fibrosarcoma and breast cancer cells | [64] |
| Cullin-3 & ANKFY1 | Together, they inhibit integrin recycling and mediate cell spreading and angiogenesis in vitro. | Endothelial cells and HUVECs | [127] |
| GGA2 | Arf-binding protein 2 (GGA2) associates with β1-integrins and promotes receptor recycling via Rab13, which boosts active cell migration. | Breast cancer cells | [65] |
| LRP1 | Mediates internalization of β1-integrins. In thyroid cancer cells, it binds to SNX17 and directs active and inactive integrins to the Rab11 recycling pathway. In fibroblasts, LRP1 bridges kindlin2 to β1-integrin, promoting its activation and lysosomal degradation. | Thyroid cancer, melanoma, glioblastoma, breast cancer cells, HUVECs, fibroblasts | [128, 129] |
| Rab7a | Controls the localization of β1-integrins to filopodia via Rac1 activation and in this way promotes cell migration. | Lung cancer cells | [130] |
| Rab34 | Mediates endocytosis of β3-integrin and prevents its lysosomal degradation, promoting cell adhesion, migration and invasion. | Breast cancer cells | [54] |
| Rab11-FIP5 | Controls Rab11-mediated recycling of α6β1 (but not α3β1) integrins and directs these integrins to cell-cell adhesions to promote migration on laminin. | Prostate cancer cells | [131] |
| Reggie-1/Flotillin 2 | Delivers integrins to new adhesions via Rab11. Involved in focal adhesion turnover, cell spreading and modulation of Rac1 activity. | HeLa and squamous cell carcinoma | [132] |
| Retriever & CCC complex | Together WASH, retriever and the CCC complex mediate recycling of α5β1-integrin. | RPE-1 and HeLa cells | [60] |
| Vps3 & Vps8 | Mediate fusion of early endosomes as part of CORVET and deliver integrins to recycling endosomes via association with Rab4-positive endosomes. Contribute to cell adhesion, spreading and migration. | HeLa cells | [67] |
While many adaptors have been associated with β1-integrin endocytosis, a few have also been attributed to the trafficking of other β-subunits. In oral squamous carcinoma cells, the cytoskeletal regulator HAX-1 interacts with αvβ6-integrin and induces its internalization via clathrin to promote cancer cell migration and invasion46. Integrin-heterodimer-specific internalisation can also be regulated by integrin α-subunits that harbour a common endocytosis motif (Yxxϕ) recognised by clathrin adaptor protein 2 (AP2)47. This conserved motif permits selective CME of integrins, containing the motif, over other receptors that lack it, but bind to the same ECM ligand. This could allow heterodimer-specific downstream signalling to initiate divergent responses to an ECM ligand.
Whereas the role for clathrin and/or its adaptors in integrin endocytosis is undisputed, there is some debate as to the precise conditions required for CME of integrins. A recent study highlighted a low-force microenvironment as a prerequisite for Dab2 recruitment to active β3 integrins48. Whether other clathrin adaptors are influenced by force is not known. Regardless, the cell clearly employs different mechanisms for selective CME of integrin heterodimers which may depend on extracellular cues, including ECM topology.
Integrin interaction with other cell-surface receptors, such as MET, Syndecan-4 and ADAM9, also influences endosomal traffic and cell adhesion and migration in vitro and in vivo18,19,34,49–51 (Fig. 2c). In addition, PKCα activation, in response to an extracellular cue, leads to phosphorylation of FMNL2, an actin nucleator/assembly factor and FMNL2-dependent integrin endocytosis (Fig. 2c). Further, N-glycosylation of the α5-integrin β-propeller, favours integrin association with Syndecan-4 and subsequent receptor internalization52. Thus, interactions and signals at the cell surface can be vital for driving integrin endocytosis.
Rab GTPases also provide selectivity for integrin traffic. Rab21, Rab25 and Rab34 directly interact with integrins. The ubiquitously expressed Rab21 binds to α-subunit tails to induce integrin endocytosis, regulate FA turnover39 and deliver active β1-integrin to FAK-positive signalling endosomes for suppression of anoikis and survival of disseminated cancer cells16. In contrast, epithelial-cell-specific Rab25 interacts with the β-subunit tail to recycle endocytosed α5β1-integrin locally within invasive protrusions in three-dimensional ECM53. In metastatic ovarian carcinoma cells, Rab25-dependent sorting of α5β1-integrin, endocytosed from the cell front, to lysosomes and exocytosis at the cell rear facilitates rear detachment by activating Src-mediated FA disassembly24. Rab34, a Golgi-associated protein, is upregulated in aggressive breast cancers and directly binds the cytoplasmic tail of β3-integrin. EGF stimulation recruits Rab34 to the plasma membrane and promotes Src-mediated tyrosine-phosphorylation of Rab34, which induces β3-integrin internalization and delivery to recycling compartments54. This protects β3-integrin from degradation and stimulates cancer cell migration and invasion54. In contrast, Rab35 appears to limit α5β1-integrin traffic as its inactivation by CLIC4 enhances α5β1-integrin endocytosis and Arf6-mediated recycling55. Concordantly, low Rab35 or elevated Arf6 expression enhances cell migration in some cancers56, placing Rab35 as a potential obstacle in cancer progression.
Receptor internalisation and subsequent transit to the Golgi, a process known as retrograde traffic, also appears to impart selectivity to integrin-dependent processes, depending on the accessory proteins involved. For example, a Golgi-SNARE complex, consisting of syntaxin-6 and VAMP3, promotes integrin-heterodimer-specific recycling in different cell types. In HeLa and prostate cancer cells, this complex drives α3β1-integrin-mediated chemotactic cell migration57, whereas in endothelial cells, syntaxin-6 is important for α5β1-integrin-mediated cell spreading and motility on fibronectin58. Interestingly, another SNARE complex consisting of syntaxin-16 is specific for the retrograde transport of inactive β1-integrins59. Thus, retrograde transport may be a broad mechanism for the trafficking of β1-integrin-containing heterodimers, and the ECM landscape and the composition of the SNARE complex determine the heterodimer and/or the specific receptor activation state that is a cargo for retrograde transport.
Sorting of integrins between late and recycling endosomes
Endocytic cargo can be recycled or shuttled through late endosomes to lysosomes for degradation. Integrins, however have the potential to bypass this protein downregulation machinery, which explains their generally low degradation rate mentioned above. An important regulator of endocytosed β1-integrin fate is sorting nexin 17 (SNX17), which binds to the membrane distal β-integrin NxxY motif, and in cooperation with the retriever and WASH (WASP and SCAR homologue) complexes diverts the receptor from degradation to recycling60–62 (Fig. 2d). This process depends on proper endosomal localization of SNX1763 and is fundamental for efficient cell adhesion and migration63.
Alternative integrin recycling routes are regulated by specific endocytic adaptor proteins such as APPL1, GGA2 and the CORVET complex64–67 (Fig. 2d). APPL1 inhibits internalization of α5β1-integrin and simultaneously promotes its recycling back to adhesion sites, resulting in increased cell-surface α5β1 and reduced adhesion dynamics64. However, the role of APPL1 may be dependent on cell type as in lung cancer cells, APPL1 depletion has no effect on internalization or endosomal signalling of integrins16. Another possible link between APPL1 and integrin traffic is the CORVET complex, which mediates fusion of APPL1-containing EE66. Furthermore, CORVET subunits Vps3 and Vps8 localise to Rab4- and Rab11-positive RE in HeLa cells and participate in integrin recycling. Correspondingly, Vps3/Vps8 knockdown inhibits cell adhesion, spreading and migration on fibronectin and collagen67 (Fig. 2d).
Similarly to internalisation, intracellular sorting of integrins is fine-tuned by other receptors and ECM ligands and this process impinges on efficient cell migration14,20. For example, the tyrosine phosphorylation status of Syndecan-4 acts as a switch controlling differential recycling of αvβ3- and α5β1-integrins, and the tightly balanced availability of these two receptors at the plasma membrane determines FA half-life and cell migration behaviour50. Association of inactive β1-integrins with ADAM9 metalloproteinase is required for optimal β1-integrin internalization, degradation and cell migration49. In fibroblasts, interaction between fibronectin and its receptor α5β1-integrin, promotes α5β1-integrin ubiquitination and routing towards lysosomal trafficking and degradation. This is suggested to limit intracellular accumulation of integrins destined for re-adhesion and thus allow more productive cell migration22. However, in cancer cells, it is the diversion of the α5β1-integrin receptor away from degradation towards recycling, in a process requiring WASH (Arp2/3 activator), that drives invasive cell migration68. Therefore, while it is clear that other cell-surface receptors and ECM cues actively participate in the regulation of integrin fate, the choice between integrin recycling and degradation, particularly during cell migration, appears to be context-dependent.
Altogether, these examples illustrate the complexity of integrin traffic and highlight the need to tightly control these events to modulate integrin-mediated functions in the organism.
Integrin traffic in developing tissues
Expanding evidence links integrin trafficking to the normal execution of cellular processes such as cell adhesion4,15, migration14,20,69, matrix remodelling70–72 and differentiation73,74. The concept that these pathways can be hijacked by cancer cells has been reviewed elsewhere4,75–77. Below we highlight the role of integrin trafficking pathways in regulating cellular processes fundamental for metazoan development and tissue homeostasis2,78.
Integrin trafficking determines cell polarity and drives ECM remodelling
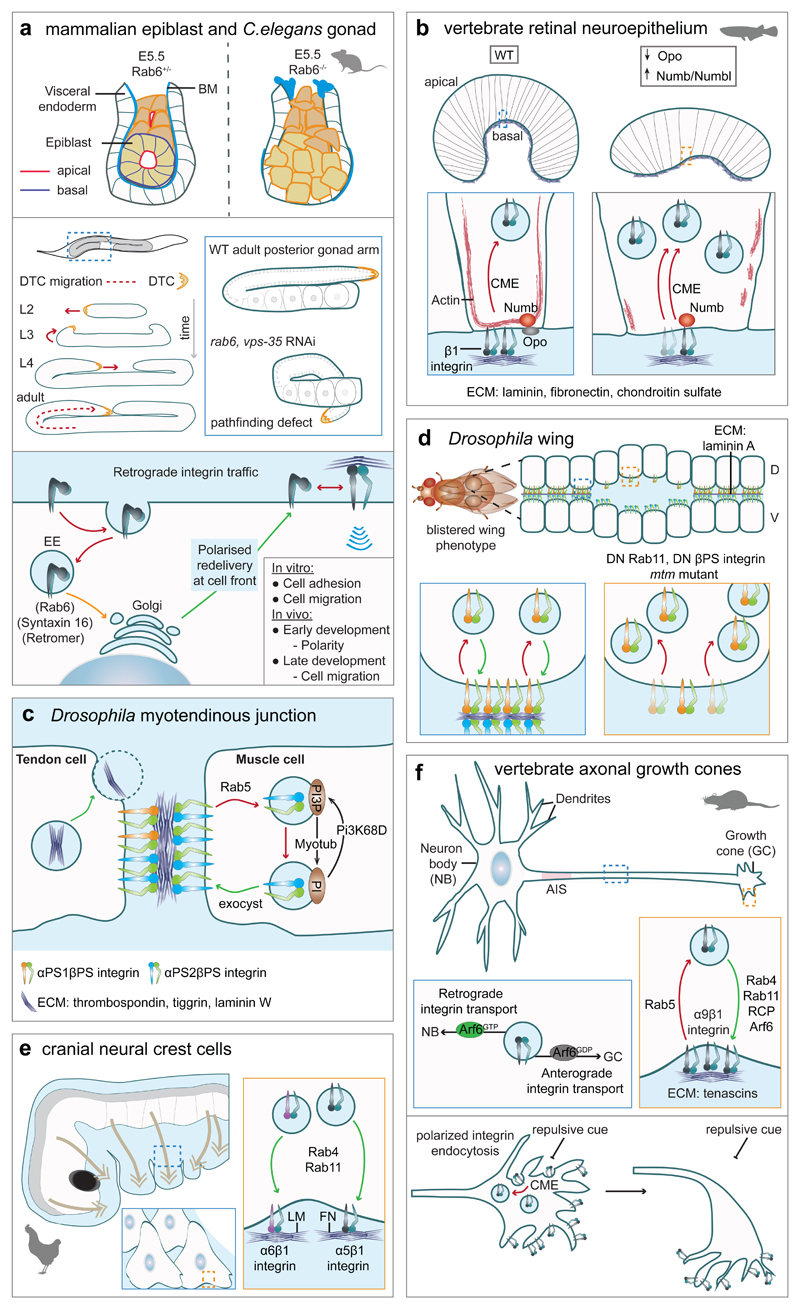
In polarised cell types, such as epithelial and endothelial cells, integrins control the orientation of the apico-basal axis by providing physical and mechanical cues (ECM engagement) and spatial positioning, and regulate molecular delivery to correct subcellular locations79–81. A striking example, is the peri-implantation stage of mouse embryos (E5.0), where the epiblast undergoes apico-basal polarisation and changes in cell morphology leading to formation of an epithelial rosette structure with a central cavity82,83. Interestingly, this polarization can be also recapitulated in stem cell colonies grown in Matrigel82. This process depends on ECM secreted by extraembryonic cell lineages and on selective, Rab6- and syntaxin-16-dependent, inactive β1-integrin retrograde traffic59 (Fig. 3a). In fish retinal neuroepithelium, regulated integrin-ECM interaction at the basal side of cells is required for bending this flat cell layer into a hemispherical optic cup. Here, the amount of integrin at the basal endfeet is regulated by direct competition between β1-integrin and a membrane protein Opo for binding to CME adaptors84. Loss of Opo triggers excess integrin endocytosis and failure in optic cup bending85 (Fig. 3b).
Figure 3. Integrin trafficking in development.
a, Retrograde transport of inactive β1-integrin (bottom) is required for polarized cell behaviours during development. Epiblast cell rosette structure fails to form in Rab6 mutant embryos59 (top). Integrin retrograde traffic is also required for persistent migration of a distal tip cell (DTC) in C. elegans gonad (middle). Knockdown of Rab6 or the retromer component Vps35 causes a DTC pathfinding error and morphogenetic defects59.
b, Integrin CME during optic cup formation is facilitated by binding of Numb and Numb-like to the β1-integrin NPxY motif and is inhibited by competitive binding of these adaptors to the NPxF motif of the membrane protein Ojoplano (Opo). Loss of Opo or overexpression of adaptors result in excess integrin endocytosis, decreased cortical actin in basal endfeet, failure in basal constriction and a flat retina84,85.
c, In MTJ, the muscle cell (αPS2βPS-integrins) is attached to the tendon cell (αPS1βPS- and αPS2βPS-integrins) indirectly through ECM. Integrin turnover in MTJ is increased by reduced availability of ECM and decreased by raised muscle tension, elevated integrin outside-in activation, or expression of Rab5 mutants95,96. Cleavage of PI(3)P by myotubularin (MTM1) prevents receptor accumulation in endosomal-related inclusions98.
d, Cellular layers forming Drosophila wing are held together by adhesion of αPS1βPS-integrin (dorsal, D) and αPS2βPS-integrin (ventral layer, V) to the ECM secreted in between. In wing imaginal disc, βPS-integrin trafficking is mediated by Rab11101, which when disrupted leads to increased apical cell area, intracellular βPS-integrin accumulation and disorganised actin cytoskeleton. The ensuing change in cell shape from columnar to cuboidal (not shown) leads to separation of cell layers and blisters. DN, dominant negative; mtm mutant, myotubularin mutant.
e, Recycling of α5β1- and α6β1-integrins is required for migration of cranial NCC104. Integrins are recycled through Rab4 and Rab11 pathways in a laminin substrate-dependent manner.
f, α9β1-integrin recycling within the growth cone and long-range axonal integrin traffic are required for efficient axon growth. Rab5-regulated endocytosis is followed by recycling through Rab4, Rab11 or Arf6 pathways. Activation of Arf6 promotes retrograde transport of integrin-containing vesicles towards the neuron body112. Chemorepellent cues trigger β1-integrin endocytosis on one side of the growth cone, which changes direction of growth cone migration134.
In endothelial cells, the removal of “old” fibronectin and the basolateral secretion of new fibronectin molecules are coupled to the cycle of Rab21-dependent α5β1-integrin recycling70. Fibronectin turnover is critical for fibronectin fibrillogenesis and vascular development71,86. Non-canonical interactions between angiopoietin-2 (Ang-2), a regulator of vascular homeostasis, and α5β1-integrin, leading to integrin activation, impacts fibronectin fibrillogenesis, in addition to destabilizing endothelial cell-cell junctions87. Ang-2 induces α5β1-integrin transition from peripheral FA to stable fibrillar adhesions (primary sites of fibronectin fibrillogenesis, Fig. 1b), positive for tensin1. In this study, the role of integrin traffic was not directly addressed; however, increased α5β1-integrin recycling into tensin1-positive adhesions has been linked to endothelial destabilization in sepsis88. Thus, dynamic intracellular movement of integrins and generation of fibrillar adhesions are important for both vascular development and maintenance of vascular homeostasis.
Links between metabolism and integrin trafficking
A cell’s metabolic state is intimately associated with integrin traffic and matrix remodelling72. In high-nutrient conditions, tensin expression is suppressed by the cellular metabolic sensor AMPK89. In contrast, nutrient depletion induces α5β1-integrin translocation to tensin-dependent fibrillar adhesions, from which ligand- and tensin-bound integrins and ECM components are endocytosed to lysosomes to provide cell nutrients90. In cancer-associated fibroblasts (CAFs), interactions between Src-phosphorylated Hic-5 (also known as TGB1I1), β1-integrin and tensin1 stabilise β1-integrin on the cell surface, preventing receptor internalisation to lysosomes91. In starved human mammary epithelial cells, enhanced β4-integrin expression and endocytosis expedite laminin uptake and degradation in LAMP1-positive vesicles to generate the nutrients required for mammalian target of rapamycin complex 1 (mTORC1) activation and cell survival92. Notably, in the mammary glands of dietary restricted mice, resident mammary fibroblasts are the primary source of laminin production, and fibroblast-conditioned medium supports survival of starved mammary epithelial cells92. An intriguing question is how metabolic stress creates both matrix-devouring (epithelial cells) and matrix-producing (fibroblasts) entities within the same tissue93.
Integrin traffic regulates cell adhesion in development
Drosophila models have provided important insights into the developmental roles of integrin traffic in vivo. Surprisingly, in apparently very stable structures, like the myotendinous junctions (MTJs) (Fig. 3c), integrins are dynamically turned over by clathrin- and Rab5-mediated endocytosis94. Although the dynamics of this process somewhat decrease as development proceeds, it is comparable to the reported values for integrin turnover in fibrillar adhesions in vitro94. Integrin dynamics in MTJs depends on muscle tension and on integrin activation state. Increased tension reduces integrin endocytosis, whereas loss in tension promotes both endocytosis and exocytosis95. In addition, whereas integrin inactivating mutations or reduced ECM levels trigger increased integrin turnover, integrin activation decreases integrin turnover96. It remains unknown whether tension increases integrin activity and whether muscle tension and integrin activation converge on the same pathway to regulate integrin dynamics.
Similar to other trafficking events97, integrin recycling in MTJs depends on phosphoinositide turnover in vesicle membranes. In Drosophila, loss of a lipid phosphatase Myotubularin, which cleaves the EE lipid PI(3)P, causes the accumulation of the β1-integrin ortholog, βPS integrin, (Fig. 1a) inside cells in large abnormal PI(3)P-positive endosomal compartments and leads to myofiber detachment from the cuticle98. Symptoms of Myotubularin deficiency can be alleviated by simultaneous depletion of the class II PI3-kinase Pi3K68D98. These observations can be explained by the need for PI(3)P cleavage before an endosomal cargo can undergo exocyst-mediated exocytosis99 (Fig. 3c). Myotubularin loss in Drosophila manifests only in late pupal stages and resembles the condition of Myotubularin mutation in humans, X-linked myotubular myopathy, where β1-integrin is mislocalized to the perinuclear compartment98. Myotubularin loss also causes wing blistering, in line with the requirement for integrin–ECM adhesion and integrin trafficking for Drosophila wing morphogenesis (Fig. 3d)100,101. In summary, integrin turnover occurs even in stable adhesive structures and allows for flexible response to changing conditions such as fluctuating muscle tension. The dynamic nature of adhesions is not surprising given that based on single molecule tracking, integrin-ligand binding events do not exceed ~80 s in human epithelial cells in vitro102. This flexible system might constitute a conserved homeostatic mechanism, and the routes taken by integrins at different developmental stages might be tightly regulated to exert specific functions as required.
Integrin trafficking impinges on cell migration in development
During Metazoan development cells actively migrate either individually or collectively. Integrin recycling is necessary for the migration of many cell types including distal tip cells (Fig. 3a), cranial neural crest cells (NCC) (Fig. 3e), neuronal growth cones (Fig. 3f), and immune cells (the latter reviewed in14,20,103). In NCC, integrin receptors for fibronectin and laminin are recycled through Rab4 and Rab11 pathways (Fig. 3e) and inhibition of integrin recycling impedes NCC migration in vitro104. Integrin trafficking is crucial also for collective cell migration or morphogenetic movements of cell sheets. During Xenopus gastrulation, the protein XGIPC1 (also known as kermit2) promotes Rab21-mediated α5β1-integrin endocytosis and fibronectin ECM assembly by directly binding to the cytoplasmic domain of α5-integrin105. Loss of XGIPC1 function disrupts fibronectin ECM in embryos and causes defects indicative of slower mesoderm cell migration105.
Whereas integrins are required for normal morphogenesis of the central nervous system106,107, integrin trafficking is dispensable for the glial-guided radial migration of neurons, a dominant migratory mode in the cerebral cortex and cerebellum. Instead, the tissue-layering defects caused by integrin perturbation, reflect the requirement for integrin-mediated adhesion and signalling earlier in development and for radial glia attachment to the ECM108,109. However, active integrin recycling plays a major role later in neuronal development110. Axonal growth cones use the Rab-coupling protein(RCP)-dependent integrin recycling pathway, also employed by invasive cancer cells for effective migration14,111. In NGF-differentiated PC12 cells and in dorsal root ganglion neurons, α9β1-integrin is trafficked through RCP, Rab11 and Arf6 RE (Fig. 3f)112,113 and perturbing this pathway reduces integrin cell-surface levels, resulting in inhibited axonal growth113. The use of particular recycling pathways seems to be neuron type-specific. Retinal ganglion cell axonal growth cones in Xenopus embryos require Rab4 and Rab5, but not Rab11, presumably for local integrin recycling within the growth cone to support migration114. Development of the axon initial segment in mature neurons precludes integrins and Rabs from entering the axon and axon regeneration115,116. Integrin traffic also influences dendrite morphogenesis. In hippocampal neurons, Ndr2 kinase phosphorylates the β1-integrin cytoplasmic domain and stimulates Rab5 and Rab11-dependent trafficking to increase active integrin at the cell surface, triggering premature dendritic branching117. Thus, whereas adhesion and ligand availability are important regulators of cell morphology and positioning within a developing tissue, integrin trafficking coordinates an integral mechanism for delivering integrins and effectors to drive the formation of highly organised tissues and may be an important factor in the regulation of stem cell niches.
Future Perspectives
Our knowledge of integrin trafficking pathways is rapidly expanding. However, how cells choose a specific internalization route or routes for different integrins is unclear. An important aspect is the composition of the plasma membrane surrounding integrins and the impact of the physical properties derived from lipid composition, membrane tension and curvature. The formation of nanoclusters of active and inactive integrins in the same FA and the different kinetics at which specific heterodimers are internalized may also be key in understanding pathway specificity118,119, but more work is needed to clarify these aspects of integrin behaviour. Whereas the precise spatial details of integrin redelivery to the cell surface remain to be elucidated, exocytosis of integrins 23 and other cargo seems to preferentially occur at focal adhesions120. Whether these hotspots of integrin exocytosis depend on integrin activation state, cellular signalling or ECM substrate are the next exciting questions. Furthermore, understanding why certain Rabs mediate the transport of specific integrins requires further investigation. Many of these questions may be answered by new imaging probes for integrins and super-resolution microscopy within more relevant in vivo models (BOX 1). Furthermore, integrin reconstitution on artificial liposomes121,122 may help in understanding the role of membrane composition on the recruitment of specific endocytic adaptors and to identify core complexes needed to direct heterodimer-specific traffic. Understanding integrin endocytosis is also of clinical importance. This route is being exploited as a mechanism for cytotoxic drug uptake in the development of anticancer therapies123. Finally, it is critical to not only understand how integrin traffic occurs at the cellular level but also how this complex process gives rise to distinct biological functions in tissues.
Box 1. New tools to study integrin trafficking.
Lattice light sheet microscopy (LLSM)
Integrin trafficking is a dynamic process and therefore best studied by live fluorescence microscopy techniques. Fast moving endocytic vesicles need to be captured at high spatio-temporal resolution while avoiding illumination-induced phototoxicity on the living sample135,136. LLSM fulfils these criteria as the new iteration of the method uses adaptive optics to correct for sample-induced image distortions and allows capture of intracellular events in a cell within intact tissues137,138. Measurements of the lifetime of endocytic events and vesicle trajectories within human stem cell-derived organoids and zebrafish embryos have demonstrated the power of this technique137. LLSM also enables imaging of fluorescently tagged endogenous proteins without the need for overexpression.
3D models
Studying integrins in physiologically relevant 3D model systems may reveal biological events not observed on stiff 2D substrates. For example, growing cells in soft 3D collagen matrices has unravelled clathrin tubular lattices as mediators of integrin-based cell adhesion during cell migration139.
Integrin tags
Ectodomain labelling of β1, β3 and αL-integrins, which preserves receptor functionality has been achieved23,102,140. Besides creating fusions with fluorescent proteins, ectodomain tagging also enables other applications. For example, the cell-surface population of integrin β1 has been selectively tagged by a cell non-permeable Halo tag ligand, which allowed the kinetics of β1 endocytosis to be measured23. Additionally, pH probes based on red fluorescent SNAP tag ligands141 could be used to capture endo and exocytosis, taking advantage of endosome acidification in comparison to the extracellular space.
Synchronised endocytosis
Unlike other membrane receptors, integrin endocytosis cannot be easily synchronized by addition of ligand, because ECM ligands are constantly present in cell culture conditions. Synchronized CME of integrins could be achieved by adapting the inducible system of recruiting clathrin-binding protein fragments to specific membrane proteins142, for instance to allow local optogenetic activation of integrin endocytosis with subcellular precision to steer cellular behaviour.
In summary, by combining these methods it is possible to capture integrin behaviour at the cytoplasmic membrane and in endosomes with improved spatiotemporal precision and to probe them in diverse experimental contexts.
Acknowledgements
We apologise to all colleagues whose work was not mentioned here due to space limitations. Work in the authors’ laboratory was supported by funding from the Academy of Finland, an ERC Consolidator Grant (no. 615258), the Sigrid Juselius Foundation and the Cancer Society of Finland. J. Icha is a member of the Turku Collegium of Science and Medicine and recipient of the EMBO Long-Term Fellowship ALTF 405-2018.
Footnotes
Financial and non-financial competing interests
The authors declare that they have no financial and non-financial competing interests.
References
- 1.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maartens AP, Brown NH. Current Topics in Developmental Biology. Vol. 112. Academic Press; Cambridge: 2015. Anchors and Signals: The diverse roles of integrins in development; pp. 233–272. 2015. [DOI] [PubMed] [Google Scholar]
- 3.Mould AP, Humphries MJ. Regulation of integrin function through conformational complexity: Not simply a knee-jerk reaction? Curr Opin Cell Biol. 2004;16:544–551. doi: 10.1016/j.ceb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 4.De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. Integrin traffic - the update. J Cell Sci. 2015;128:839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: The end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C, Ye F, Ginsberg MH. Regulation of Integrin Activation. Annu Rev Cell Dev Biol. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 7.Legate KR, Fassler R. Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 8.Arjonen A, Alanko J, Veltel S, Ivaska J. Distinct Recycling of Active and Inactive β1 Integrins. Traffic. 2012;13:610–625. doi: 10.1111/j.1600-0854.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieswandt B, Varga-Szabo D, Elvers M. Integrins in platelet activation. J Thromb Haemost. 2009;7:206–209. doi: 10.1111/j.1538-7836.2009.03370.x. [DOI] [PubMed] [Google Scholar]
- 10.Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: Balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14:432–444. doi: 10.1038/nrm3599. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Costell M, Fassler R. Integrin activation: talin, kindlin and a pinch of force. Nat Cell Biol. 2019 doi: 10.1038/s41556-018-0234-9. [DOI] [PubMed] [Google Scholar]
- 12.Horton ER, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17 doi: 10.1038/ncb3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton ER, et al. The integrin adhesome network at a glance. J Cell Sci. 2016;129:4159–4163. doi: 10.1242/jcs.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul NR, Jacquemet G, Caswell PT. Endocytic Trafficking of Integrins in Cell Migration. Current Biology. 2015;25 doi: 10.1016/j.cub.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Valdembri D, Serini G. Regulation of adhesion site dynamics by integrin traffic. Curr Opin Cell Biol. 2012;24:582–591. doi: 10.1016/j.ceb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Alanko J, et al. Integrin endosomal signalling suppresses anoikis. Nat Cell Biol. 2015;17:1412–1421. doi: 10.1038/ncb3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nader GPF, Ezratty EJ, Gundersen GG. FAK, talin and PIPKI 3 regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol. 2016;18 doi: 10.1038/ncb3333. [DOI] [PubMed] [Google Scholar]
- 18.Ivaska J, Heino J. Cooperation Between Integrins and Growth Factor Receptors in Signaling and Endocytosis. Annu Rev Cell Dev Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- 19.Barrow-McGee R, et al. Beta 1-integrin-c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat Commun. 2016;7 doi: 10.1038/ncomms11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson BJ, Allen JL, Caswell PT. Vesicle trafficking pathways that direct cell migration in 3D and in vivo. Traffic. 2018 doi: 10.1111/tra.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci. 2015;128:3171–3176. doi: 10.1242/jcs.166074. [DOI] [PubMed] [Google Scholar]
- 22.Lobert VH, et al. Ubiquitination of α5β1 Integrin Controls Fibroblast Migration through Lysosomal Degradation of Fibronectin-Integrin Complexes. Dev Cell. 2010;19:148–159. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Huet-Calderwood C, et al. Novel ecto-tagged integrins reveal their trafficking in live cells. Nat Commun. 2017;8 doi: 10.1038/s41467-017-00646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dozynkiewicz MA, et al. Rab25 and CLIC3 Collaborate to Promote Integrin Recycling from Late Endosomes/Lysosomes and Drive Cancer Progression. Dev Cell. 2012;22:131–145. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bridgewater RE, Norman JC, Caswell PT. Integrin trafficking at a glance. J Cell Sci. 2012;125:3695–3701. doi: 10.1242/jcs.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura T, Kaibuchi K. Numb Controls Integrin Endocytosis for Directional Cell Migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 29.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eskova A, et al. An RNAi screen identifies KIF15 as a novel regulator of the endocytic trafficking of integrin. J Cell Sci. 2014;127:2433–2447. doi: 10.1242/jcs.137281. [DOI] [PubMed] [Google Scholar]
- 31.Atherton P, Lausecker F, Harrison A, Ballestrem C. Low-intensity pulsed ultrasound promotes cell motility through vinculin-controlled Rac1 GTPase activity. J Cell Sci. 2017;130:2277–2291. doi: 10.1242/jcs.192781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakshminarayan R, et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat Cell Biol. 2014;16:592–603. doi: 10.1038/ncb2970. [DOI] [PubMed] [Google Scholar]
- 33.Doherty GJ, et al. The endocytic protein GRAF1 is directed to cell-matrix adhesion sites and regulates cell spreading. Mol Biol Cell. 2011;22:4380–4389. doi: 10.1091/mbc.E10-12-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bass MD, et al. A Syndecan-4 Hair Trigger Initiates Wound Healing through Caveolin- and RhoG-Regulated Integrin Endocytosis. Dev Cell. 2011;21:681–693. doi: 10.1016/j.devcel.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.del Pozo MA, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi F, Sottile J. Caveolin-1-dependent 1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci. 2008;121:2360–2371. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbri M, et al. Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the αL/β2 integrin, LFA-1, during leukocyte chemotaxis. Mol Biol Cell. 2005;16:5793–5803. doi: 10.1091/mbc.E05-05-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Z, Noss EH, Hsu VW, Brenner MB. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J Cell Biol. 2011;193:61–70. doi: 10.1083/jcb.201007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellinen T, et al. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of β1-integrins. J Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Astro V, et al. Liprin-α1 and ERC1 control cell edge dynamics by promoting focal adhesion turnover. Sci Rep. 2016;6:1–16. doi: 10.1038/srep33653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Astro V, Chiaretti S, Magistrati E, Fivaz M, de Curtis I. Liprin- 1, ERC1 and LL5 define polarized and dynamic structures that are implicated in cell migration. J Cell Sci. 2014;127:3862–3876. doi: 10.1242/jcs.155663. [DOI] [PubMed] [Google Scholar]
- 42.Calderwood DA, et al. Integrin cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc Natl Acad Sci. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandri C, et al. The R-Ras/RIN2/Rab5 complex controls endothelial cell adhesion and morphogenesis via active integrin endocytosis and Rac signaling. Cell Res. 2012;22:1479–1501. doi: 10.1038/cr.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teckchandani A, et al. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol. 2009;186:99–111. doi: 10.1083/jcb.200812160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teckchandani A, Mulkearns EE, Randolph TW, Toida N, Cooper JA. The clathrin adaptor Dab2 recruits EH domain scaffold proteins to regulate integrin 1 endocytosis. Mol Biol Cell. 2012;23:2905–2916. doi: 10.1091/mbc.E11-12-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsay AG, et al. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin αvβ6. Cancer Res. 2007;67:5275–5284. doi: 10.1158/0008-5472.CAN-07-0318. [DOI] [PubMed] [Google Scholar]
- 47.De Franceschi N, et al. Selective integrin endocytosis is driven by interactions between the integrin α-chain and AP2. Nat Struct Mol Biol. 2016;23:172–179. doi: 10.1038/nsmb.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu CH, et al. Integrin-beta3 clusters recruit clathrin-mediated endocytic machinery in the absence of traction force. Nat Commun. 2015;6:1–12. doi: 10.1038/ncomms9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mygind KJ, Schwarz J, Sahgal P, Ivaska J, Kveiborg M. Loss of ADAM9 expression impairs β1 integrin endocytosis, focal adhesion formation and cancer cell migration. J Cell Sci. 2018;131 doi: 10.1242/jcs.205393. jcs205393. [DOI] [PubMed] [Google Scholar]
- 50.Morgan MR, et al. Syndecan-4 Phosphorylation Is a Control Point for Integrin Recycling. Dev Cell. 2013;24:472–485. doi: 10.1016/j.devcel.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mai A, et al. Distinct c-Met activation mechanisms induce cell rounding or invasion through pathways involving integrins, RhoA and HIP1. J Cell Sci. 2014;127:1938–1952. doi: 10.1242/jcs.140657. [DOI] [PubMed] [Google Scholar]
- 52.Hang Q, et al. A key regulator of cell adhesion: Identification and characterization of important N-Glycosylation Sites on Integrin α5 for Cell Migration. Mol Cell Biol. 2017;37:e00558–16. doi: 10.1128/MCB.00558-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caswell PT, et al. Rab25 Associates with α5β1 Integrin to Promote Invasive Migration in 3D Microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Sun L, et al. Rab34 regulates adhesion, migration, and invasion of breast cancer cells. Oncogene. 2018:1–17. doi: 10.1038/s41388-018-0202-7. [DOI] [PubMed] [Google Scholar]
- 55.Argenzio E, et al. CLIC4 regulates cell adhesion and 1 integrin trafficking. J Cell Sci. 2014;127:5189–5203. doi: 10.1242/jcs.150623. [DOI] [PubMed] [Google Scholar]
- 56.Allaire PD, et al. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci. 2013;126:722–731. doi: 10.1242/jcs.112375. [DOI] [PubMed] [Google Scholar]
- 57.Riggs KA, et al. Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction. J Cell Sci. 2012;125:3827–3839. doi: 10.1242/jcs.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiwari A, et al. Endothelial cell migration on fibronectin is regulated by syntaxin 6-mediated α5β1 integrin recycling. J Biol Chem. 2011;286:36749–36761. doi: 10.1074/jbc.M111.260828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shafaq-Zadah M, et al. Persistent cell migration and adhesion rely on retrograde transport of β 1 integrin. Nat Cell Biol. 2016;18 doi: 10.1038/ncb3287. [DOI] [PubMed] [Google Scholar]
- 60.McNally KE, et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat Cell Biol. 2017;19:1214–1225. doi: 10.1038/ncb3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Böttcher RT, et al. Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β 1-integrin tail. Nat Cell Biol. 2012;14:584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- 62.Steinberg F, Heesom KJ, Bass MD, Cullen PJ. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol. 2012;197:219–230. doi: 10.1083/jcb.201111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratcliffe CDH, Sahgal P, Parachoniak CA, Ivaska J, Park M. Regulation of Cell Migration and β1 Integrin Trafficking by the Endosomal Adaptor GGA3. Traffic. 2016;17:670–688. doi: 10.1111/tra.12390. [DOI] [PubMed] [Google Scholar]
- 64.Diggins NL, Kang H, Weaver A, Webb DJ. α5β1 integrin trafficking and Rac activation are regulated by APPL1 in a Rab5-dependent manner to inhibit cell migration. J Cell Sci. 2018;131 doi: 10.1242/jcs.207019. jcs207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahgal P, et al. GGA2 and RAB13 promote activity-dependent β1-integrin recycling. (2018) doi: 10.1242/jcs.233387. Preprint at https://www.biorxiv.org/content/early/2018/06/22/353086. [DOI] [PubMed]
- 66.Perini ED, Schaefer R, Stöter M, Kalaidzidis Y, Zerial M. Mammalian CORVET is required for fusion and conversion of distinct early endosome subpopulations. Traffic. 2014;15:1366–1389. doi: 10.1111/tra.12232. [DOI] [PubMed] [Google Scholar]
- 67.Jonker CTH, et al. Vps3 and Vps8 control integrin trafficking from early to recycling endosomes and regulate integrin-dependent functions. Nat Commun. 2018;9:1–12. doi: 10.1038/s41467-018-03226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zech T, et al. The Arp2/3 activator WASH regulates α5β1-integrin-mediated invasive migration. J Cell Sci. 2011;124:3753–3759. doi: 10.1242/jcs.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacquemet G, Humphries MJ, Caswell PT. Role of adhesion receptor trafficking in 3D cell migration. Curr Opin Cell Biol. 2013;25:627–632. doi: 10.1016/j.ceb.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mana G, et al. PPFIA1 drives active α5β1 integrin recycling and controls fibronectin fibrillogenesis and vascular morphogenesis. Nat Commun. 2016;7 doi: 10.1038/ncomms13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamidi H, Ivaska J. Vascular Morphogenesis: An Integrin and Fibronectin Highway. Curr Biol. 2017;27:R158–R161. doi: 10.1016/j.cub.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 72.Rainero E. Extracellular matrix internalization links nutrient signalling to invasive migration. Int J Exp Pathol. 2018;99:4–9. doi: 10.1111/iep.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bridgewater RE, Streuli CH, Caswell PT. Extracellular matrix promotes clathrin-dependent endocytosis of prolactin and STAT5 activation in differentiating mammary epithelial cells. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-04783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du J, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci. 2011;108:9466–9471. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Hamidi H, Pietilä M, Ivaska J. The complexity of integrins in cancer and new scopes for therapeutic targeting. Br J Cancer. 2016;115:1017–1023. doi: 10.1038/bjc.2016.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018:1–16. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meighan CM, Schwarzbauer JE. Temporal and spatial regulation of integrins during development. Curr Opin Cell Biol. 2008;20:520–524. doi: 10.1016/j.ceb.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol. 2013;15:17–27. doi: 10.1038/ncb2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JL, Streuli CH. Integrins and epithelial cell polarity. J Cell Sci. 2014;127:3217–3225. doi: 10.1242/jcs.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bedzhov I, Zernicka-Goetz M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell. 2014;156:1032–1044. doi: 10.1016/j.cell.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shahbazi MN, Zernicka-Goetz M. Deconstructing and reconstructing the mouse and human early embryo. Nat Cell Biol. 2018;22 doi: 10.1038/s41556-018-0144-x. [DOI] [PubMed] [Google Scholar]
- 84.Bogdanović O, et al. Numb/Numbl-Opo Antagonism Controls Retinal Epithelium Morphogenesis by Regulating Integrin Endocytosis. Dev Cell. 2012;23:782–795. doi: 10.1016/j.devcel.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 85.Martinez-Morales JR, et al. Ojoplano-Mediated Basal Constriction Is Essential for Optic Cup Morphogenesis. Development. 2009;136:2165–2175. doi: 10.1242/dev.033563. [DOI] [PubMed] [Google Scholar]
- 86.Valdembri D, et al. Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hakanpaa L, et al. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat Commun. 2015;6:1–12. doi: 10.1038/ncomms6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hakanpaa L, et al. Targeting β1-integrin inhibits vascular leakage in endotoxemia. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1722317115. 201722317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Georgiadou M, et al. AMPK negatively regulates tensin-dependent integrin activity. J Cell Biol. 2017;216:1107–1121. doi: 10.1083/jcb.201609066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rainero E, et al. Ligand-Occupied Integrin Internalization Links Nutrient Signaling to Invasive Migration. Cell Rep. 2015;10:398–413. doi: 10.1016/j.celrep.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 91.Goreczny GJ, Forsythe IJ, Turner CE. Hic-5 regulates fibrillar adhesion formation to control tumor extracellular matrix remodeling through interaction with tensin1. Oncogene. 2018;37:1699–1713. doi: 10.1038/s41388-017-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muranen T, et al. Starved epithelial cells uptake extracellular matrix for survival. Nat Commun. 2017;8:1–12. doi: 10.1038/ncomms13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Georgiadou M, Ivaska J. Tensins: Bridging AMP-Activated Protein Kinase with Integrin Activation. Trends Cell Biol. 2017;27:703–711. doi: 10.1016/j.tcb.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Yuan L, Fairchild MJ, Perkins AD, Tanentzapf G. Analysis of integrin turnover in fly myotendinous junctions. J Cell Sci. 2010;123:939–946. doi: 10.1242/jcs.063040. [DOI] [PubMed] [Google Scholar]
- 95.Pines M, et al. Mechanical force regulates integrin turnover in Drosophila in vivo. Nat Cell Biol. 2012;14:935–943. doi: 10.1038/ncb2555. [DOI] [PubMed] [Google Scholar]
- 96.López-Ceballos P, Herrera-Reyes AD, Coombs D, Tanentzapf G. In vivo regulation of integrin turnover by outside-in activation. J Cell Sci. 2016;129:2912–2924. doi: 10.1242/jcs.190256. [DOI] [PubMed] [Google Scholar]
- 97.Wallroth A, Haucke V. Phosphoinositide conversion in endocytosis and the endolysosomal system. J Biol Chem. 2018;293:1526–1535. doi: 10.1074/jbc.R117.000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribeiro I, Yuan L, Tanentzapf G, Dowling JJ, Kiger A. Phosphoinositide regulation of integrin trafficking required for muscle attachment and maintenance. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ketel K, et al. A phosphoinositide conversion mechanism for exit from endosomes. Nature. 2016;529:408–412. doi: 10.1038/nature16516. [DOI] [PubMed] [Google Scholar]
- 100.Brower DL. Platelets with wings: The maturation of Drosophila integrin biology. Curr Opin Cell Biol. 2003;15:607–613. doi: 10.1016/s0955-0674(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 101.Bhuin T, Roy JK. Rab11 is required for cell adhesion, maintenance of cell shape and actin-cytoskeleton organization during Drosophila wing development. Int J Dev Biol. 2011;55:269–279. doi: 10.1387/ijdb.103149tb. [DOI] [PubMed] [Google Scholar]
- 102.Tsunoyama TA, et al. Super-long single-molecule tracking reveals dynamic-anchorage-induced integrin function. Nat Chem Biol. 2018;14:497–506. doi: 10.1038/s41589-018-0032-5. [DOI] [PubMed] [Google Scholar]
- 103.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 104.Strachan LR, Condic ML. Cranial neural crest recycle surface integrins in a substratum-dependent manner to promote rapid motility. J Cell Biol. 2004;167:545–554. doi: 10.1083/jcb.200405024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spicer E, Suckert C, Al-Attar H, Marsden M. Integrin α5β1 function is regulated by XGIPC/kermit2 mediated endocytosis during Xenopus laevis gastrulation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lilja J, Ivaska J. Integrin activity in neuronal connectivity. J Cell Sci. 2018;131 doi: 10.1242/jcs.212803. jcs212803. [DOI] [PubMed] [Google Scholar]
- 107.Clegg DO, Wingerd KL, Hikita ST, Tolhurst EC. Integrins in the development, function and dysfunction of the nervous system. Front Biosci. 2003:723–750. doi: 10.2741/1020. [DOI] [PubMed] [Google Scholar]
- 108.Franco SJ, Müller U. Extracellular matrix functions during neuronal migration and lamination in the mammalian central nervous system. Dev Neurobiol. 2011;71:889–900. doi: 10.1002/dneu.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frick A, et al. Proper cerebellar development requires expression of β1-integrin in Bergmann glia, but not in granule neurons. Glia. 2012;60:820–832. doi: 10.1002/glia.22314. [DOI] [PubMed] [Google Scholar]
- 110.Myers JP, Santiago-Medina M, Gomez TM. Regulation of axonal outgrowth and pathfinding by integrin-ecm interactions. Dev Neurobiol. 2011;71:901–923. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wojnacki J, Galli T. Membrane traffic during axon development. Dev Neurobiol. 2016;76:1185–1200. doi: 10.1002/dneu.22390. [DOI] [PubMed] [Google Scholar]
- 112.Eva R, et al. ARF6 Directs Axon Transport and Traffic of Integrins and Regulates Axon Growth in Adult DRG Neurons. J Neurosci. 2012;32:10352–10364. doi: 10.1523/JNEUROSCI.1409-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eva R, et al. Rab11 and Its Effector Rab Coupling Protein Contribute to the Trafficking of 1 Integrins during Axon Growth in Adult Dorsal Root Ganglion Neurons and PC12 Cells. J Neurosci. 2010;30:11654–11669. doi: 10.1523/JNEUROSCI.2425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Falk J, Konopacki FA, Zivraj KH, Holt CE. Rab5 and Rab4 Regulate Axon Elongation in the Xenopus Visual System. J Neurosci. 2014;34:373–391. doi: 10.1523/JNEUROSCI.0876-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koseki H, et al. Selective rab11 transport and the intrinsic regenerative ability of CNS axons. Elife. 2017;6:1–25. doi: 10.7554/eLife.26956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nieuwenhuis B, Haenzi B, Andrews MR, Verhaagen J, Fawcett JW. Integrins promote axonal regeneration after injury of the nervous system. Biol Rev. 2017 doi: 10.1111/brv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rehberg K, et al. The Serine/Threonine Kinase Ndr2 Controls Integrin Trafficking and Integrin-Dependent Neurite Growth. J Neurosci. 2014;34:5342–5354. doi: 10.1523/JNEUROSCI.2728-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Das L, et al. Characterization of Laminin Binding Integrin Internalization in Prostate Cancer Cells. J Cell Biochem. 2017;118:1038–1049. doi: 10.1002/jcb.25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spiess M, et al. Active and inactive β1 integrins segregate into distinct nanoclusters in focal adhesions. J Cell Biol. 2018 doi: 10.1083/jcb.201707075. jcb.201707075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eisler SA, et al. A Rho signaling network links microtubules to PKD controlled carrier transport to focal adhesions. eLife. 2018;7:e35907. doi: 10.7554/eLife.35907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Franceschi N, et al. ProLIF: quantitative integrin protein-protein interactions and synergistic membrane effects on proteoliposomes. J Cell Sci. 2018 doi: 10.1242/jcs.214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Streicher P, et al. Integrin reconstituted in GUVs: A biomimetic system to study initial steps of cell spreading. Biochim Biophys Acta - Biomembr. 2009;1788:2291–2300. doi: 10.1016/j.bbamem.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 123.Man YKS, et al. The Novel Oncolytic Adenoviral Mutant Ad5-3Δ-A20T Retargeted to αvβ6 Integrins Efficiently Eliminates Pancreatic Cancer Cells. Mol Cancer Ther. 2018 doi: 10.1158/1535-7163.MCT-17-0671. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, et al. Formin-like 2 Promotes β1-Integrin Trafficking and Invasive Motility Downstream of PKCα. Dev Cell. 2015;34:475–483. doi: 10.1016/j.devcel.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 125.Palamidessi A, et al. The GTPase-activating protein RN-tre controls focal adhesion turnover and cell migration. Curr Biol. 2013;23:2355–2364. doi: 10.1016/j.cub.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 126.Qu F, et al. Ankyrin-B is a PI3P effector that promotes polarized α5β1-integrin recycling via recruiting RabGAP1L to early endosomes. Elife. 2016;5:1–25. doi: 10.7554/eLife.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maekawa M, et al. Cullin-3 and its adaptor protein ANKFY1 determine the surface level of integrin β1 in endothelial cells. Biol Open. 2017;6:1707–1719. doi: 10.1242/bio.029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Theret L, et al. Identification of LRP-1 as an endocytosis and recycling receptor for β1-integrin in thyroid cancer cells. Oncotarget. 2017;8:78614–78632. doi: 10.18632/oncotarget.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wujak L, et al. Low density lipoprotein receptor-related protein 1 couples β1 integrin activation to degradation. Cell Mol Life Sci. 2018;75:1671–1685. doi: 10.1007/s00018-017-2707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Margiotta A, Progida C, Bakke O, Bucci C. Rab7a regulates cell migration through Rac1 and vimentin. Biochim Biophys Acta - Mol Cell Res. 2017;1864:367–381. doi: 10.1016/j.bbamcr.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 131.Das L, et al. Novel Regulation of Integrin Trafficking by Rab11-FIP5 in Aggressive Prostate Cancer. Mol Cancer Res. 2018 doi: 10.1158/1541-7786.MCR-17-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hülsbusch N, Solis GP, Katanaev VL, Stuermer CAO. Reggie-1/Flotillin-2 regulates integrin trafficking and focal adhesion turnover via Rab11a. Eur J Cell Biol. 2015;94:531–545. doi: 10.1016/j.ejcb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 133.Rainero E, Norman JC. Late endosomal and lysosomal trafficking during integrin-mediated cell migration and invasion: Cell matrix receptors are trafficked through the late endosomal pathway in a way that dictates how cells migrate. BioEssays. 2013;35:523–532. doi: 10.1002/bies.201200160. [DOI] [PubMed] [Google Scholar]
- 134.Hines JH, Abu-Rub M, Henley JR. Asymmetric endocytosis and remodeling of B1-integrin adhesions during growth cone chemorepulsion by MAG. Nat Neurosci. 2010;13:829–837. doi: 10.1038/nn.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Icha J, Weber M, Waters JC, Norden C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays. 2017;39:1–15. doi: 10.1002/bies.201700003. [DOI] [PubMed] [Google Scholar]
- 136.Laissue PP, Alghamdi RA, Tomancak P, Reynaud EG, Shroff H. Assessing phototoxicity in live fluorescence imaging. Nat Methods. 2017;14:657–661. doi: 10.1038/nmeth.4344. [DOI] [PubMed] [Google Scholar]
- 137.Liu TL, et al. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science. 2018;360:6386. doi: 10.1126/science.aaq1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Picco A, Kaksonen M. Quantitative imaging of clathrin-mediated endocytosis. Curr Opin Cell Biol. 2018;53:105–110. doi: 10.1016/j.ceb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Elkhatib N, et al. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science (80-. ) 2017;356 doi: 10.1126/science.aal4713. [DOI] [PubMed] [Google Scholar]
- 140.Nordenfelt P, et al. Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. Nat Commun. 2017;8:2047. doi: 10.1038/s41467-017-01848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martineau M, et al. Semisynthetic fluorescent pH sensors for imaging exocytosis and endocytosis. Nat Commun. 2017;8:1–10. doi: 10.1038/s41467-017-01752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wood LA, Larocque G, Clarke NI, Sarkar S, Royle SJ. New tools for hot-wiring clathrin-mediated endocytosis with temporal and spatial precision. J Cell Biol. 2017;216:4351–4365. doi: 10.1083/jcb.201702188. [DOI] [PMC free article] [PubMed] [Google Scholar]