To the Editor
Fish represents one of the important allergen sources responsible for IgE-mediated food allergy.1 Although many food allergies developed during childhood are frequently outgrown, allergy to fish often remains persistent, even in adulthood.2 Parvalbumin, a small calcium-binding protein, was identified as the major and highly cross-reactive fish allergen.3 Parvalbumins from several fish species have been produced as recombinant allergens, and in particular, recombinant carp parvalbumin, rCyp c 1, was found to contain the majority of fish-specific IgE epitopes and to show broad cross-reactivity with parvalbumins from a variety of fish species.4 A hypoallergenic derivative of carp parvalbumin has been developed for specific immunotherapy of fish allergy.5,6 The IgE reactivity and ability of this protein to induce activation of patients’ basophils in vitro was reduced by introducing point mutations in the calcium-binding domains of Cyp c 1. Furthermore, it was shown that immunization of animals with the Cyp c 1 mutant (mCyp c 1) induced IgG antibody responses specific for the wild-type Cyp c 1 allergen (wtCyp c 1), which blocked IgE binding to Cyp c 1 in patients with fish allergy. In this study we analyzed the in vivo allergenic activity of the Cyp c 1 mutant by comparing it with the wild-type allergen regarding the induction of immediate-type skin reactions in patients with fish allergy.
We investigated 12 children sensitized to fish who had experienced grade 2 to 4 reactions according to the grading system of Sampson7 that could be attributed to fish ingestion. Table I provides their demographic and clinical characterization, and Table E1 in this article’s Online Repository at www.jacionline.org provides their serologic characterization. They had positive skin prick test reactions to codfish, as well as sardine extract (see Table E2 in this article’s Online Repository at www.jacionline.org), and had IgE antibodies specific for codfish extract and/or the major codfish and carp allergens Gad c 1 and Cyp c 1, as determined by means of ImmunoCAP and ISAC chip (Thermo Fisher, Uppsala, Sweden). Childrens’ IgE reactivities to other allergens are shown in Fig E1 in this article’s Online Repository at www.jacionline.org. Cyp c 1–specific IgE levels determined by using ImmunoCAP ISAC measurements showed a good correlation with Cyp c 1–specific IgE levels determined by means of ImmunoCAP (R2 = 0.9558, see Fig E2 in this article’s Online Repository at www.jacionline.org). We further tested the children with fish allergy for IgE cross-reactivity using nitrocellulose-blotted extracts from carp, cod, and tuna (see Fig E3 in this article’s Online Repository at www.jacionline.org).3 All patients reacted to natural Cyp c 1 at approximately 10 kDa, whereas only 10 of 12 patients reacted to Gad c 1, and only 5 of 12 patients showed weak IgE reactivity to the major tuna allergen Thu a 1.
Table I. Demographic and clinical characterization of the children with fish allergy who underwent skin testing.
| Patient. no. | Sex | Age (y) | Family history of atopy | Fish allergy symptoms | Fish allergy systemic reaction (grade) | Other allergic symptoms | Therapies | sIgE to inhalant allergens | SPTs to inhalant allergens | Other IgE-mediated food allergies |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 5 | Y | S, R, G | 2 | AD, R, U | n/sCS, AH | + | + | W, S |
| 2 | M | 14 | Y | S, G | 2 | AD, R, A | n/sCS, ICS, AH, TCS | + | + | P, T |
| 3 | M | 6 | Y | S, R | 2 | AD, R, A | n/sCS, ICS, AH, TCS | + | + | P, T |
| 4 | M | 10 | N | S, R | 2 | AD, R, A | n/sCS, ICS, AH, TCS | + | + | None |
| 5 | F | 7 | Y | S, R, G | 3 | AD, R, A | n/sCS, ICS, AH | + | + | P, T, F |
| 6 | F | 6 | Y | S, R, G | 3 | AD, R, A | None | + | + | P, T |
| 7 | M | 12 | N | S, G | 2 | AD, R | n/sCS, AH | + | + | E |
| 8 | F | 12 | N | S, G | 2 | None | None | + | + | None |
| 9 | M | 3 | Y | S, R | 4 | AD, R, A | n/sCS, ICS, AH | + | + | P, T |
| 10 | F | 5 | N | S, R | 4 | AD | AH, TCS | − | − | E, P, T |
| 11 | M | 10 | N | S, G | 4 | AD | TCS | + | ND | E, W |
| 12 | M | 13 | Y | S, R, G | 2 | AD, R, A | n/sCS, ICS, AH | + | + | E |
Fish allergy systemic reactions were graded according to the method of Sampson (range, 1-5).7
F, Female; M, male; ND, not done.
Fish allergy symptoms: G, gastrointestinal (throat pruritus, nausea, emesis, diarrhea); R, respiratory (sneeze, nasal rhinorrhea-congestion, cough, wheezing, and dyspnea); S, skin (urticaria and flushing).
Other allergic symptoms: A, asthma; AD, atopic dermatitis; R, rhinitis; U, urticaria. Therapies: AH, oral antihistamine; ICS, inhaled corticosteroid; n/sCS, nasal spray corticosteroid; TCS, topical corticosteroid.
sIgE and SPTs to common inhalant allergens (sIgE to mite, grass pollen, olive pollen, weed pollen, and mold allergens was measured by ImmunoCAP d1, d2, g2, g6, t9, w19, m2, m3, m6): at least 1 positive response/negative response.
Food allergies (other than fish allergy): E, egg; F, fruit; P, peanut; S, shellfish; T, tree nuts; W, wheat.
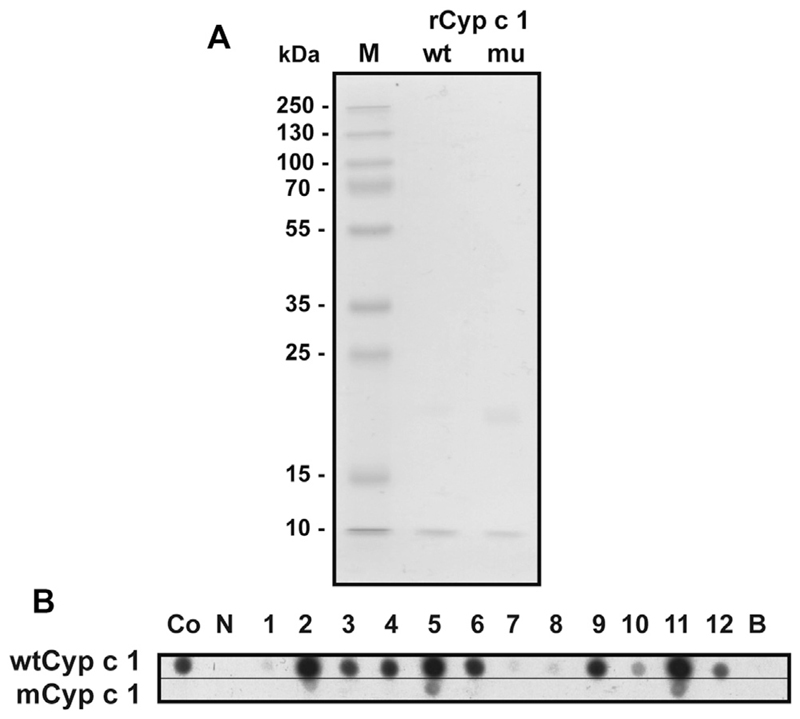
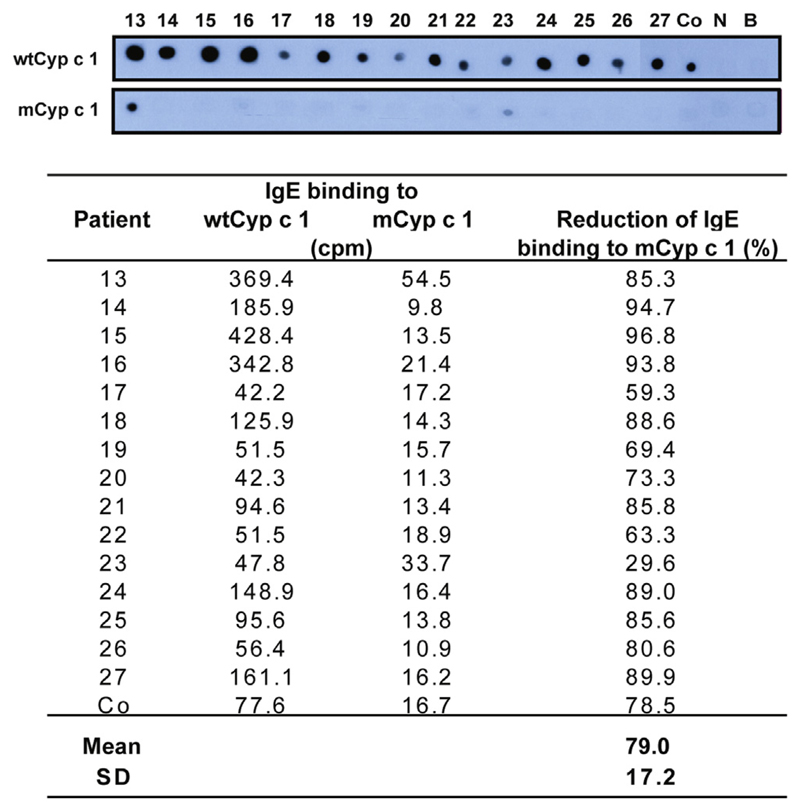
Recombinant wtCyp c 1 and mCyp c 1 were expressed in Escherichia coli and purified to homogeneity, as previously described (see Fig E4, A in this article’s Online Repository at www.jacionline.org).4,5 mCyp c 1 showed strongly reduced IgE reactivity when compared with wtCyp c 1, as determined by means of IgE dot blotting in 12 patients with fish allergy (see Fig E4, B). The reduction in IgE reactivity to mCyp c 1 was measured by using a quantitative nondenaturing RAST-based IgE assay in an additional 15 children, showing a mean reduction of 79% ± 17.2% (see Fig E5 in this article’s Online Repository at www.jacionline.org).
Skin prick tests with natural allergen extracts and purified wtCyp c 1 and mCyp c 1 were done during regular follow-up visits of the patients at the Allergy Department, 2nd Pediatric Clinic, University of Athens, ‘‘P&A Kyriakou’’ Children’s Hospital, with approval of the Institutional Ethics Committee after written informed consent was obtained from the parents and oral consent was obtained from the subjects. Skin prick tests were performed on forearms by using histamine hydrochloride (positive control), physiologic saline (negative control), sardine extract, cod extract (Stallergenes, Antony, France), and 4 concentrations (1, 4, 16, and 32 μg/mL) of purified wtCyp c 1 and mCyp c 1. In addition, 11 control children (7 male and 4 female; age, 6-12 years) sensitized to various food allergens, inhaled allergens, or both typical for the Mediterranean area other than fish were included. Wheal sizes were determined by using digital planimetry (ImageJ software, National Institutes of Health, open source). A wheal area of greater than 7.1 mm2 (corresponding to a mean diameter of >3 mm) was considered positive.
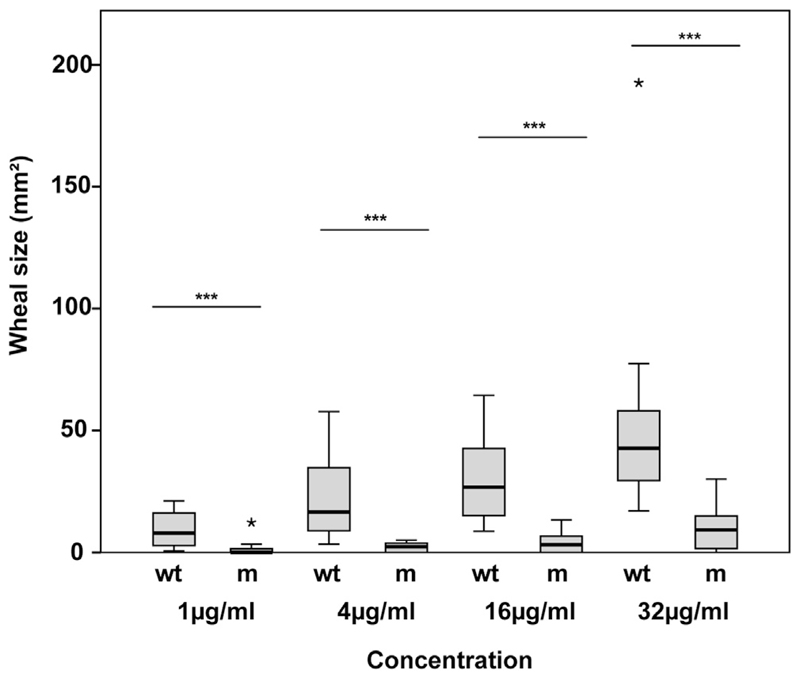
Codfish and sardine extract induced positive skin reactions in each of the 12 patients, yielding mean wheal areas between 7.22 mm2 (patient 10) and 114.28 mm2 (patient 6; ie, codfish) or between 30.46 mm2 (patient 1) and 116.38 mm2 (patient 2; ie, sardine; for detailed results, see Table E2). Histamine-induced positive reactions ranged from 16.52 mm2 (patient 10) to 59.29 mm2 (patient 6), whereas sodium chloride did not induce any reactions in any of the tested children. Table E2 specifies skin reactions to the 4 different concentrations of wtCyp c 1 and mCyp c 1. Six patients had positive reactions to wtCyp c 1 at 1 μg/mL, 4 had positive reactions at 4 μg/mL, and 2 had positive reactions at 16 μg/mL (see Table E2). Repeated-measures ANOVA was used to fit the mCyp c 1 and wtCyp c 1 wheal curves (Fig 1). Skin reactions to mCyp c 1 were significantly (P < .001) less than those to wtCyp c 1. In fact, the mean areas under the curve for mCyp c 1 and wtCyp c 1 were found to be 148.5 (SD, 117.9) and 1059.73 (SD, 730.42), respectively (P < .001). No positive skin reactions to codfish, sardine, saline, wtCyp c 1, and mCyp c 1 were found in the 11 children without fish allergy (data not shown). No local late reactions or generalized reactions were observed.
Fig 1.
Reduced allergenic activity of mCyp c 1 compared with wtCyp c 1 demonstrated by means of skin testing of patients with fish allergy. Twelve patients were pricked with increasing concentrations (x-axis: 1, 4, 16, and 32 μg/mL) of wtCyp c 1 (wt) and mCyp c 1 (m). Displayed are box plots representing wheal areas (y-axis: millimeter squared) for patients, with medians indicated by horizontal lines and outliers represented by asterisks (*). Significant differences (***P < .001) between wtCyp c 1 and mCyp c 1 are displayed for each tested concentration.
We found that both wtCyp c 1 and natural fish extracts induced positive skin reactions in the tested children with fish allergy. In fact, a concentration of 4 μg/mL wtCyp c 1 induced specific skin reactions in each of the children with fish allergy but not in the children without fish allergy, indicating that wtCyp c 1 can be used as a standardized allergen for skin testing of patients with fish allergy. In contrast, we observed a highly significant and consistent reduction of the in vivo allergenic activity of mCyp c 1 compared with wtCyp c 1, even in those children who had shown the most severe systemic allergic reactions to fish, according to the grading of Sampson7 (ie, patients 5, 6, and 9-11; Table I and see Table E2). Therefore it should be possible to formulate vaccines for fish allergy based on mCyp c 1 that allow the administration of much higher doses compared with vaccines based on wild-type allergens, and thus it should be possible to reach therapeutic maintenance doses with less side effects, particularly when the immunogen is adsorbed to an adjuvant that keeps it bound in local tissues. Thus the current study provides important safety information for the development of a fish vaccine for subcutaneous immunotherapy based on mCyp c 1, which is currently under development in the European Union project FAST.8
Extended Data
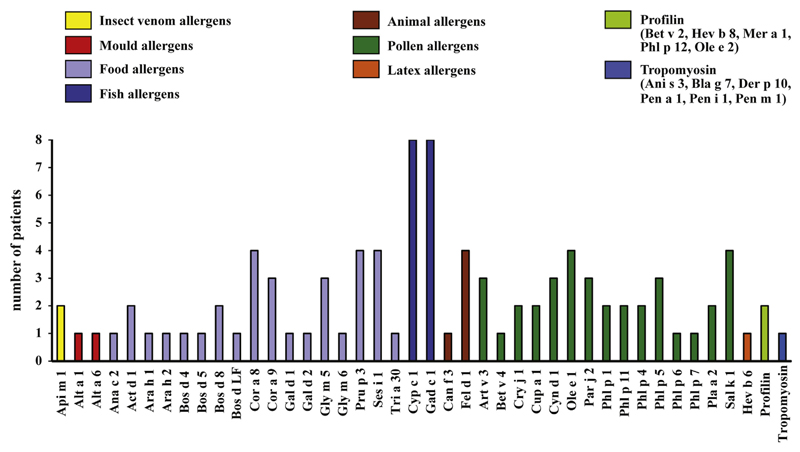
Fig E1.
IgE sensitization profile of the children with fish allergy determined by using ISAC. Sera from 8 of the patients were tested for IgE reactivity to 103 different allergens by using ISAC. The number of patients (y-axis) displaying IgE reactivity to the allergens (x-axis) is shown.
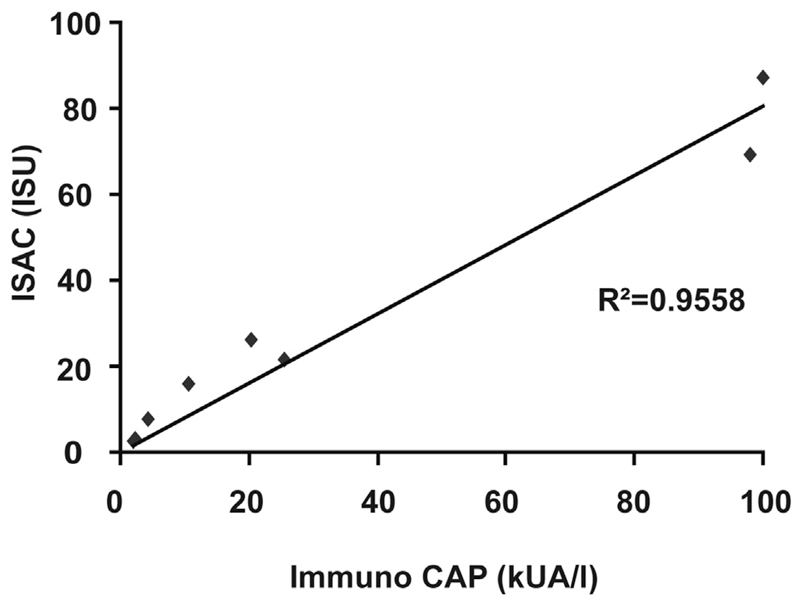
Fig E2.
Correlation of Cyp c 1–specific IgE levels measured by using ImmunoCAP (x-axis) and ISAC (y-axis).
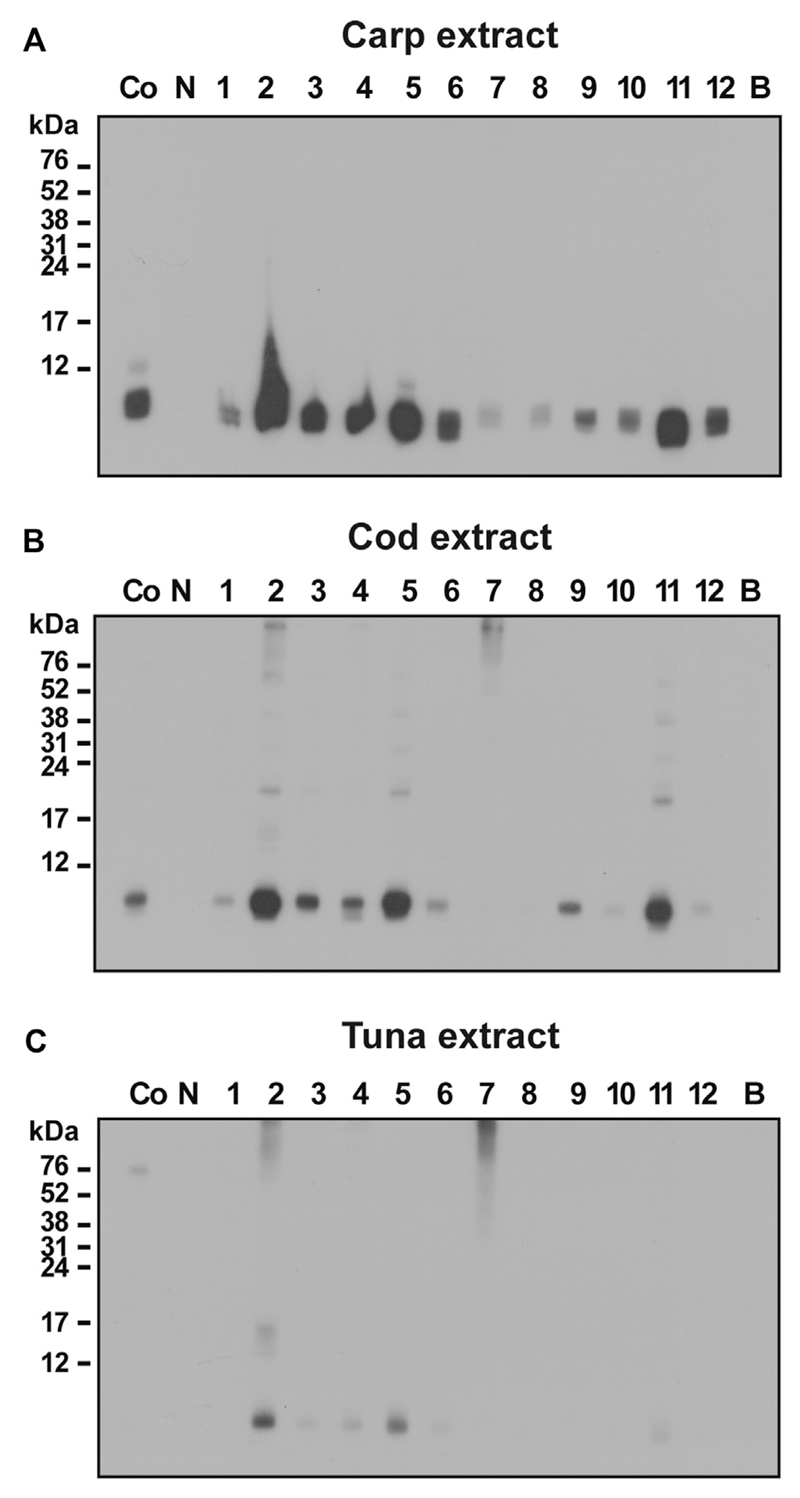
Fig E3.
IgE reactivity to natural fish allergens. Nitrocellulose-blotted natural allergen extracts from carp (A), cod (B), and tuna (C) were incubated with sera from 12 children with fish allergy (1-12; Co, positive control serum from a patient with fish allergy), serum from a nonallergic subject (N), or buffer alone (B). Bound IgE was detected with iodine125–labeled anti-IgE antibodies and visualized by means of autoradiography. Molecular weights (in kilodaltons) are displayed on the left margin.
Fig E4.
A, SDS-PAGE of purified recombinant wild-type Cyp c 1 (wt) and mutant Cyp c 1 (mu). A molecular weight marker (M; in kilodaltons) is shown on the left side. B, Comparison of dot-blotted wtCyp c 1 (upper part) with mCyp c 1 (lower part) regarding IgE reactivity as tested with sera from allergic patients (Co and 1-12), serum from a nonallergic subject (N), or buffer alone (B).
Fig E5.
Dot-blotted wtCyp c 1 and mCyp c 1 were tested regarding IgE reactivity with sera from patients with fish allergy (Co and 13-27), serum from a nonallergic subject (N), or buffer alone (B). IgE was detected with an iodine125–labeled anti-IgE antibody, and counts per minute values corresponding to wtCyp c 1– and mCyp c 1–bound IgE, as well as the percentage reduction of IgE binding to mCyp c 1, are shown.
Table E1. Serologic characterization of the children with fish allergy who underwent skin testing.
| Patient no. | Cyp c 1 ImmunoCAP (kUA/L) | Cyp c 1 ISAC (ISU) | Gad c 1 ISAC (ISU) | No. of sensitizations to other allergens (ISAC) | f3 ImmunoCAP (kUA/L) | ELISA (OD) Cyp c 1-specific | Total IgE (kU/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgM | IgG1 | IgG2 | IgG3 | IgG4 | |||||||
| 1 | 4.21 | 7.78 | 5.3 | 8 | 4.12 | 0.15 | 0.07 | 0.8 | 0.07 | 0.04 | 0.02 | 0.06 | 30 |
| 2 | >100 | 87.08 | 72.36 | 27 | 99.3 | 0.88 | 0.09 | 0.61 | 0.08 | 1.61 | 0.05 | 0.69 | 1392 |
| 3 | ND | ND | ND | ND | 5.89 | ND | ND | ND | ND | ND | ND | ND | 63 |
| 4 | 20.3 | 26.01 | 20.68 | 30 | 28 | 0.41 | 0.05 | 0.23 | 0.1 | 0.06 | 0.02 | 0.93 | 677 |
| 5 | ND | ND | ND | ND | 26.2 | ND | ND | ND | ND | ND | ND | ND | 467 |
| 6 | 10.5 | 15.73 | 8.34 | 5 | ND | 0.25 | 0.06 | 0.53 | 0.07 | 0.04 | 0.08 | 0.04 | ND |
| 7 | 2.08 | 2.76 | 2 | 1 | 9.34 | 0.08 | 0.07 | 0.45 | 0.03 | 0.04 | 0.02 | 0.08 | 248 |
| 8 | 2.16 | 2.87 | 1.95 | 10 | 1.99 | 0.19 | 0.1 | 0.55 | 0.06 | 0.03 | 0.04 | 0.07 | 208 |
| 9 | 25.4 | 21.54 | 14.1 | 9 | 11.2 | 0.41 | 0.04 | 0.47 | 0.15 | 0.04 | 0.02 | 0.03 | 105 |
| 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 38 |
| 11 | 98 | 68.99 | 51.57 | 14 | >100 | 0.88 | 0.06 | 0.52 | 0.43 | 0.12 | 0.02 | 0.17 | 1444 |
| 12 | ND | ND | ND | ND | 9.09 | ND | ND | ND | ND | ND | ND | ND | 278 |
| N | ND | ND | ND | ND | ND | 0.03 | 0.03 | 0.5 | 0.02 | 0.03 | 0.02 | 0.02 | ND |
N, Nonallergic adult; ND, not done.
Table E2. Wheal areas (in millimeters squared) in response to wtCyp c 1, mCyp c 1, cod, sardine, and histamine.
| Patient no. | Concentration (μg/mL) | wtCyp c 1 | mCyp c 1 | Cod extract | Sardine extract | Histamine |
|---|---|---|---|---|---|---|
| 1 | 1 | 6.5 | 0 | |||
| 4 | 10.97 | 2.97 | ||||
| 16 | 17.42 | 6.77 | ||||
| 32 | 40.1 | 10.36 | ||||
| 25.27 | 30.46 | 32.51 | ||||
| 2 | 1 | 15.55 | 0 | |||
| 4 | 57.7 | 0 | ||||
| 16 | 59.58 | 0 | ||||
| 32 | 77.53 | 30.09 | ||||
| 88.57 | 116.38 | 22.51 | ||||
| 3 | 1 | 2.57 | 0 | |||
| 4 | 7.01 | 0 | ||||
| 16 | 27 | 0.48 | ||||
| 32 | 45.31 | 3.27 | ||||
| 50.84 | 52.16 | 26.32 | ||||
| 4 | 1 | 21.19 | 1.4 | |||
| 4 | 38.6 | 4.66 | ||||
| 16 | 38.62 | 12.64 | ||||
| 32 | 49.04 | 12.23 | ||||
| 70.38 | 80.66 | 31.38 | ||||
| 5 | 1 | 10.11 | 12.25 | |||
| 4 | 14.08 | 4.49 | ||||
| 16 | 42.51 | 13.29 | ||||
| 32 | 67.08 | 17.85 | ||||
| 48.93 | 69.98 | 27.89 | ||||
| 6 | 1 | 16.81 | 3.37 | |||
| 4 | 52.12 | 4.99 | ||||
| 16 | 64.41 | 6.48 | ||||
| 32 | 192.65 | 0 | ||||
| 114.28 | 81.94 | 59.29 | ||||
| 7 | 1 | 0.54 | 0 | |||
| 4 | 3.37 | 0 | ||||
| 16 | 8.98 | 0 | ||||
| 32 | 17.01 | 0 | ||||
| 47.05 | 51.97 | 36.32 | ||||
| 8 | 1 | 19.19 | 0.85 | |||
| 4 | 9.55 | 2.63 | ||||
| 16 | 12.77 | 2.9 | ||||
| 32 | 17.79 | 8 | ||||
| 33.05 | 33.28 | 19.47 | ||||
| 9 | 1 | 2.96 | 1.54 | |||
| 4 | 30.99 | 1.94 | ||||
| 16 | 42.8 | 4.86 | ||||
| 32 | 39.11 | 17.57 | ||||
| 60.64 | 40.93 | 25.72 | ||||
| 10 | 1 | 9.31 | 0 | |||
| 4 | 19.07 | 0 | ||||
| 16 | 26.52 | 0 | ||||
| 32 | 35.03 | 7.64 | ||||
| 7.22 | 95.39 | 16.52 | ||||
| 11 | 1 | 4.82 | 0 | |||
| 4 | 19.01 | 3.16 | ||||
| 16 | 25.21 | 3.4 | ||||
| 32 | 48.19 | 11.05 | ||||
| 27.03 | 50.51 | 23.89 | ||||
| 12 | 1 | 1.96 | 0 | |||
| 4 | 8.11 | 0 | ||||
| 16 | 8.67 | 0 | ||||
| 32 | 23.85 | 0 | ||||
| 30.13 | 64.95 | 19.18 |
Wheal areas greater than 7.1 mm2 were considered positive and values are in boldface.
Table E3. Demographic, clinical, and serologic characterization of patients 13 to 27 tested for IgE reactivity to wtCyp c 1 and mCyp c 1.
| Patient no. | Age (y) | Family history | Fish allergy systemic
reactions (grade) |
SPT wheal diameter (mm) | ImmunoCAP (kUA/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cod | Tuna | Salmon | Sardine | Whitefish mix | Shrimp | Cod f3 | Tuna f40 | Salmon f41 | Sardine f61 | Shrimp f24 | Total IgE | ||||
| 13 | 10 | Y | 4 | 5.5 | 4 | 5.5 | 5 | 6 | 0 | 33.1 | 13.1 | 26.3 | ND | ND | 3980 |
| 14 | 7 | Y | 1 | 12.5 | 5.5 | 11 | 10 | 9.5 | 0 | >100 | 5.66 | >100 | ND | ND | 620 |
| 15 | 8 | Y | 4 | 9.5 | 7.5 | 9 | 10.5 | 7.5 | 0 | 17.3 | <0.35 | <0.35 | <0.35 | <0.35 | 578 |
| 16 | 7 | Y | 4 | 10.5 | 6 | 6.5 | 10 | 7 | 0 | 11.9 | ND | ND | ND | ND | 216 |
| 17 | 7 | Y | 4 | 14 | 5 | 5 | 9 | 8.5 | 0 | 14.1 | 1.82 | 13.9 | 0.4 | <0.35 | 429 |
| 18 | 9 | Y | 4 | 11 | 8 | 8 | 10.5 | 8 | 5 | 32 | ND | ND | ND | ND | 1382 |
| 19 | 9 | Y | 1 | 9.5 | 5.5 | 5 | 16 | 11 | 0 | 8.22 | 3.17 | 4.58 | 6.39 | <0.35 | 48.7 |
| 20 | 15 | N | 4 | 12 | 6.5 | 4.5 | 11.5 | 10 | 0 | 10.2 | ND | ND | ND | ND | 692 |
| 21 | 6 | Y | 3 | 6.75 | 3.5 | 4.5 | 5 | 5 | 0 | 36 | ND | ND | ND | <0.35 | 245 |
| 22 | 8 | Y | 1 | 11.5 | 4.5 | 6.5 | 8.5 | 7.5 | ND | 2.84 | 1.53 | 2.68 | 2.41 | <0.35 | 687 |
| 23 | 13 | Y | 1 | 12.5 | 4 | 4.5 | 3.5 | 9.5 | 0 | 3.08 | 1.82 | 3.19 | 3.27 | <0.35 | 620.5 |
| 24 | 7 | Y | 2 | 10 | 6 | 6.5 | 10.5 | 6.5 | 0 | 8.84 | 5.73 | 7.53 | 11 | <0.35 | 106.5 |
| 25 | 8 | Y | 4 | 12.5 | 5 | 3 | 10 | 12 | 0 | 4.02 | 0.75 | 1.8 | 3.41 | <0.35 | 385.1 |
| 26 | 8 | Y | 1 | 6 | 4 | 0 | 6 | 4 | 0 | 1.16 | 0.36 | 0.92 | 1.14 | ND | 240 |
| 27 | 10 | N | 1 | 12 | 7.5 | 4.5 | 9.5 | 10 | 0 | 35.3 | 6 | 7.65 | 21 | <0.35 | 1470 |
Fish allergy systemic reactions were graded according to Sampson (range, 1-5).7
ND, Not done.
Acknowledgments
Supported by the FAST project 201871 of the FP7 of the European Union and by the Austrian Science Fund (FWF) projects P23350-B11 and F4605.
Footnotes
Disclosure of potential conflict of interest: B. Linhart has received research support from the FAST Project (EU grant) and Austrian Science Fund (FWF) project P23350-B11. R. Valenta has received research support from the Austrian Science Fund (FWF) and the FPF Program “FAST” and serves as a consultant for Biomay AG, Vienna, Austria. N. G. Papadopoulos has received consultancy fees from AbbVie, Novartis, Menarini, Meda, ALK-Abelló, and GlaxoSmithKline; has received research support from Nestlé, Merck, and GlaxoSmithKline; has received lecture fees from Novartis, Uriach, GlaxoSmithKline, Allergopharma, Stallergenes, and MSD; and has received payment for development of educational presentations from AbbVie, Sanofi, Menarini, and Meda. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. J Allergy Clin Immunol. 2012;129:906–20. doi: 10.1016/j.jaci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Tsabouri S, Triga M, Makris M, Kalogeromitros D, Church MK, Priftis KN. Fish and shellfish allergy in children: review of a persistent food allergy. Pediatr Allergy Immunol. 2012;23:608–15. doi: 10.1111/j.1399-3038.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 3.Bugajska-Schretter A, Elfman L, Fuchs T, Kapiotis S, Rumpold H, Valenta R, et al. Parvalbumin, a cross-reactive fish allergen, contains IgE-binding epitopes sensitive to periodate treatment and Ca2+ depletion. J Allergy Clin Immunol. 1998;101:67–74. doi: 10.1016/S0091-6749(98)70195-2. [DOI] [PubMed] [Google Scholar]
- 4.Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, et al. Recombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergy. J Immunol. 2002;168:4576–84. doi: 10.4049/jimmunol.168.9.4576. [DOI] [PubMed] [Google Scholar]
- 5.Swoboda I, Bugajska-Schretter A, Linhart B, Verdino P, Keller W, Schulmeister U, et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J Immunol. 2007;178:6290–6. doi: 10.4049/jimmunol.178.10.6290. [DOI] [PubMed] [Google Scholar]
- 6.Zuidmeer-Jongejan L, Swoboda I, Valenta R, Linhart B, Rigby R, Versteeg SA, et al. Development of a hypo-allergenic recombinant parvalbumin for first-in-man SCIT of fish allergy. Int Arch Allergy Immunol. 2015;166:41–51. doi: 10.1159/000371657. [DOI] [PubMed] [Google Scholar]
- 7.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–8. [PubMed] [Google Scholar]
- 8.Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK, Neubauer A, Asturias J, Blom L, et al. FAST: towards safe and effective subcutaneous immunotherapy of persistent life-threatening food allergies. Clin Transl Allergy. 2012;2:5. doi: 10.1186/2045-7022-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]