We show that spores incubated at 37°C on sporulation plates for up to 98 days have lost almost all mRNAs and rRNAs, yet the aged spores germinated and outgrew as well as 2-day spores, and all these spores had identical viability. Thus, it is unlikely that spore mRNA, rRNA, or protein synthesis is important in spore germination. Spores incubated for 47 to 98 days also had much higher wet heat resistance than 2-day spores, suggesting that spore “age” should be considered in generating spores for tests of sterilization assurance. These data are the first to show complete survival of hydrated spores for ∼100 days, complementing published data showing dry-spore survival for years.
KEYWORDS: Bacillus, endospores, germination, heat resistance
ABSTRACT
Bacillus spores incubated on plates for 2 to 98 days at 37°C had identical Ca-dipicolinic acid contents, exhibited identical viability on rich- or poor-medium plates, germinated identically in liquid with all germinants tested, identically returned to vegetative growth in rich or minimal medium, and exhibited essentially identical resistance to dry heat and similar resistance to UV radiation. However, the oldest spores had a lower core water content and significantly higher wet heat and NaOCl resistance. In addition, 47- and 98-day spores had lost >98% of intact 16S and 23S rRNA and 97 to 99% of almost all mRNAs, although minimal amounts of mononucleotides were generated in 91 days. Levels of 3-phosphoglyceric acid (3PGA) also fell 30 to 60% in the oldest spores, but how the 3PGA was lost is not clear. These results indicate that (i) translation of dormant spore mRNA is not essential for completion of spore germination, nor is protein synthesis from any mRNA; (ii) in sporulation for up to 91 days at 37°C, the RNA broken down generates minimal levels of mononucleotides; and (iii) the lengths of time that spores are incubated in sporulation medium should be considered when determining conditions for spore inactivation by wet heat, in particular, in using spores to test for the efficacy of sterilization regimens.
IMPORTANCE We show that spores incubated at 37°C on sporulation plates for up to 98 days have lost almost all mRNAs and rRNAs, yet the aged spores germinated and outgrew as well as 2-day spores, and all these spores had identical viability. Thus, it is unlikely that spore mRNA, rRNA, or protein synthesis is important in spore germination. Spores incubated for 47 to 98 days also had much higher wet heat resistance than 2-day spores, suggesting that spore “age” should be considered in generating spores for tests of sterilization assurance. These data are the first to show complete survival of hydrated spores for ∼100 days, complementing published data showing dry-spore survival for years.
INTRODUCTION
Sporulation of Bacillus species leads ultimately to the release of a spore from a mother cell, and these spores are metabolically dormant and extremely resistant to all manner of harsh treatments (1, 2). Because of spores’ ubiquity in the environment and their dormancy and resistance, foodstuffs invariably contain significant spore loads. Consequently, spores can often germinate in foodstuffs, and depending on the specific strain and species, the resultant growing cells can cause food spoilage or even foodborne diseases such as anthrax, botulism, or food poisoning (3). Thus, there is much interest in spore survival in various environments from an applied perspective, as well as basic interest in how an organism can survive even in the absence of nutrients. Indeed, because of their dormancy and resistance, spores of various species can survive for very long times, and there are reports of survival of spores for many hundreds of years in desiccated soil (4), as well as several reports that spores can survive for tens to hundreds of million years in special environments (5, 6). While the latter reports were not controlled, one experiment using purified dry Bacillus subtilis spores is in progress to measure both survival and changes in spore properties over 500 years; the first data from this experiment have recently been published, and the dry spores incubated at room temperature exhibited full survival after 2 years (7). However, there are no such studies on the survival of hydrated spores over very long periods, something that might also be pertinent to thinking about long-term survival of other types of organisms.
While, as noted above, there have been experiments in which the survival of purified spores under various conditions has been studied, there has been minimal attention paid to the fate of hydrated spores during very long incubation in sporulation medium and at sporulation temperatures long after the spores have been released from the sporangium. Presumably, this situation is one that is not infrequent when spores are formed in nature. Notably, since >99% of all 16S and 23S rRNA in highly purified spores is broken down in ∼5 days at 37°C (8), it is not unreasonable that there might be some loss in spore viability during very long incubations in spent sporulation medium at 37°C. In addition, there are a number of important questions that can be asked about spores that have undergone such extended incubations after their release from sporangia, including the following. (i) Does spore viability decrease appreciably? (ii) How are spore germination and outgrowth affected? (iii) How is spore resistance to stresses such as heat, UV radiation, and chemicals affected, as there could well be aging-related changes that altered spore resistance factors? (iv) What is the fate of the spore rRNA, which is known to be cleaved during incubation of highly purified spores at 37 to 50°C for extended periods of time? (v) Last, what are the effects of extended incubation on the stability of the dormant spore mRNAs (8–16)? Notably, there is one report that incubation of sporulating cultures at various temperatures following spore release from sporangia results in spores with very different rates of germination, although with essentially identical viability (8). In this work, we have examined these questions using Bacillus subtilis spores prepared on sporulation agar at 37°C for up to 98 days, with spore release from sporangia complete after ∼36 h. The results from this work indicate that spores can survive well under these conditions for up to 98 days, even after losing ≥99% of all their mRNA and intact rRNA. In addition, there are significant increases in spore resistance to several agents, most notably, wet heat. Presumably, long-term spore survival under such conditions has been strongly selected for in evolution.
RESULTS
Incubation of sporulating cells for 2 or up to 98 days and the viability and Ca-DPA and core water contents of purified spores.
Previous work on the effects of extended incubation of purified B. subtilis spores showed that there were no major changes in spore small molecules upon incubation of purified spores for up to 25 days at 37 or 50°C (17, 18). However, purified B. subtilis spores incubated for these long times normally exhibited high levels of germination. Consequently, the work showing no changes in spore small molecules described above could be carried out only with spores of strain FB73 (19), which lacks all inner membrane (IM) germinant receptors (GRs). Since FB73 spores germinate extremely poorly, it is difficult to determine these spores’ viability. Consequently, to easily examine properties of B. subtilis spores incubated at 37°C for long periods, incubation times for wild-type spores prepared on plates were extended for as long as needed. Unfortunately, the literature is not helpful in determining how long an incubation would be useful, as no analyses of spores incubated for long times under sporulation conditions have been reported. In preliminary experiments, there were no major decreases in spore viability, Ca-dipicolinic acid (Ca-DPA) content, or the ability to grow out when plates were incubated at 37°C for 20 days before spores were isolated and purified (data not shown).
In view of the preliminary results described above, the incubation times at 37°C were extended to 98 days, and spores were also harvested after 2, 6, 15, 47, 51, or 91 days of incubation on sporulation plates at 37°C. Notably, in the plates incubated for 2 days, <1% of the spores released from the sporangia had germinated, but ∼15% of the 47-day spores and ∼60% of the 98-day spores had germinated, as determined in examination by phase-contrast microscopy. However, purification of these preparations removed all germinated spores and gave spore preparations of the 98-day spores that were as pure as the 2-day spores. With purified spores incubated for these different times now available, their viability and Ca-DPA content were determined (Table 1). Notably, all of these spores had essentially the same viability on rich medium agar plates, and where tested, they had the same Ca-DPA content and the same viability on minimal medium plates (Table 1). Analysis of the core wet density of the 2-, 6-, 15-, 51-, and 91-day spores showed that this value increased with longer incubation at 37°C (Table 1), suggesting that the increase in spore wet heat resistance in older spores (see below) is due at least in part to the decreased core water content in the older spores (20).
TABLE 1.
Viability, Ca-DPA content, and core wet density of B. subtilis spores harvested after 2, 6, 15, 47, 51, 91, or 98 daysa
| Spore age (days) | Viability (% of 2-day spores) on: |
Ca-DPA content | Core wet density (g/ml) | |
|---|---|---|---|---|
| LB medium | Spz medium | |||
| 2 | 100 | 100 | 100 ± 35 | 1.33 |
| 6 | 114 | ND | ND | 1.34 |
| 15 | 114 | ND | ND | 1.35 |
| 47 | 95 | 95 | 117 ± 16 | |
| 51 | ND | ND | ND | 1.37 |
| 91 | ND | ND | ND | 1.39 |
| 98 | 105 | 121 | 123 ± 16 | ND |
Spores of B. subtilis harvested after various times and highly purified were tested for their viability on LB agar and Spizizen’s (Spz) minimal medium agarose plates as described in Materials and Methods. Ca-DPA content and core wet density were also measured as described in Materials and Methods. Values for 2-day spores’ viability and Ca-DPA content were set at 100%, and values for all older spores are expressed relative to the values for the 2-day spores determined in parallel. ND, not determined.
Germination and outgrowth of 2- to 98-day spores.
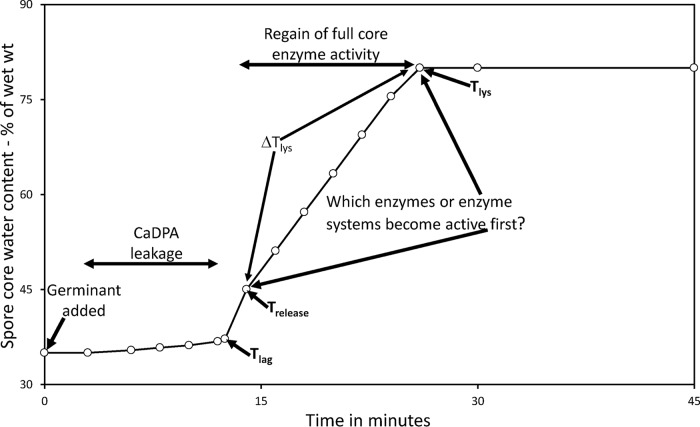
Given that spores incubated for longer times, even for 98 days, had viabilities that were identical to those of spores harvested after only 2 days of incubation on the sporulation plates, the percentages of the aged spores that germinate with IM GR-dependent germinants must be similar to those of the 2-day spores. To examine the relative rates of spore germination via the major B. subtilis spore GR, GerA, we incubated these spores with the GerA-dependent germinant l-valine and monitored spore germination by measuring Ca-DPA release (Fig. 1A). Notably, 2-, 47-, and 98-day spores all exhibited very similar rates of Ca-DPA release in germination with l-valine, and this was also true for the 6- and 15-day spores (data not shown).
FIG 1.
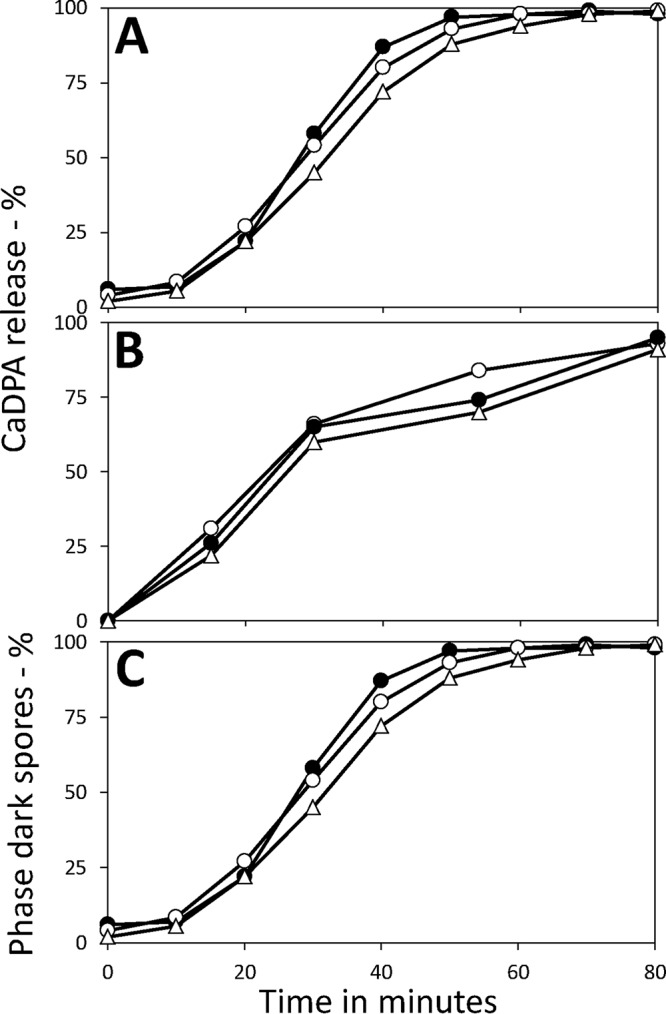
Germination of B. subtilis 2-, 47-, and 98-day spores with various germinants. Spores were harvested from sporulation plates incubated for 2, 47, or 98 days, highly purified as described in Materials and Methods, and germinated with either l-valine (A), dodecylamine (B), or Ca-DPA (C). Spore germination was monitored by measuring either Ca-DPA release by fluorometry (A and B) or generation of dark spores in phase-contrast microscopy (C), with 100 spores examined at all time points for each spore preparation. The symbols used are as follows: ○, 2-day spores; ●, 47-day spores; and ▵, 98-day spores. This experiment was carried out twice, with essentially identical results.
In addition to germination via IM GRs, spores can also germinate with compounds that do not act via GRs. Two such germinants are Ca-DPA, which activates the spore cortex lytic enzyme CwlJ, and dodecylamine, which activates the SpoVA protein channel for the release of Ca-DPA (21). Again, the 2-, 47-, and 98-day spores germinated very similarly with Ca-DPA and dodecylamine (Fig. 1B and C), and this was also true for the 6- and 15-day spores (data not shown).
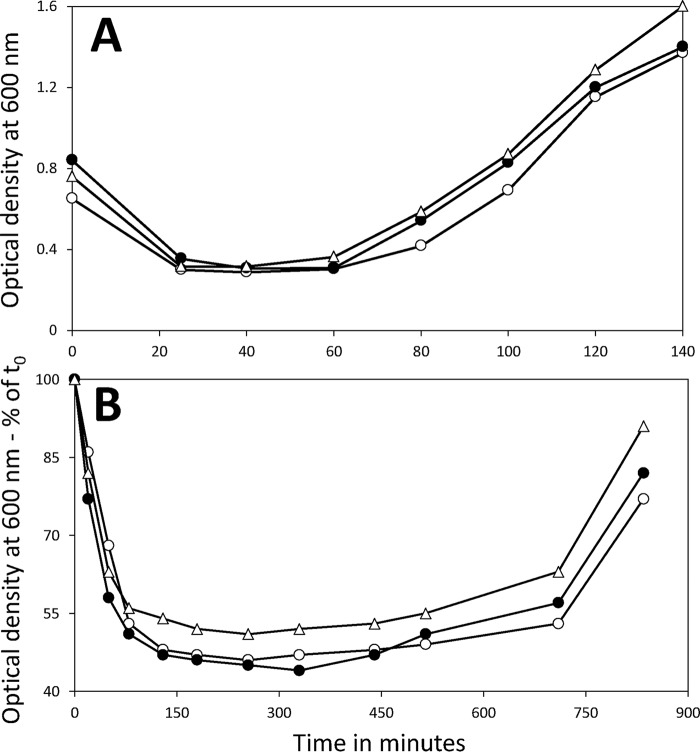
While germination levels with all germinants tested were similar with the 2-, 47-, and 98-day spores, it was possible that spore outgrowth would be slower with the 47- and 98-day spores. To test this possibility, spores were incubated in rich L broth medium to allow rapid cell growth and with l-valine to stimulate spore germination. The germination and outgrowth of these cultures were then followed by monitoring the culture optical density at 600 nm (OD600). This value will decrease ∼50% as germination is completed, remain constant as outgrowth begins, rise slowly in the transition between spore outgrowth and vegetative growth, and then rise rapidly during vegetative growth (21). Given that spore rRNA will likely be fragmented in spores incubated for long periods at 37°C (8), these rRNA fragments will have to be degraded soon after spores germinate and the mononucleotides be used for new rRNA synthesis to allow protein synthesis in spore outgrowth. While this might delay spore outgrowth relative to that with 2-day spores, this was not the case with purified spores incubated at several elevated temperatures giving almost complete rRNA fragmentation (17). Similarly, when the behavior of 2-, 47-, and 98-day spores in this rich medium was examined, all three types of spores exhibited essentially identical behavior, not only in germination but also in outgrowth (Fig. 2A). This was also the case when germination and outgrowth in Spizizen’s minimal medium were examined (Fig. 2B), indicating that the energy required for new rRNA synthesis soon after spore germination does not slow spore outgrowth in a minimal medium appreciably.
FIG 2.
Germination and outgrowth of B. subtilis 2-, 47-, and 98-day spores in rich or minimal medium. Spores harvested from sporulation plates after 2, 47, or 98 days were purified as described in Materials and Methods. Spores were then incubated at 37°C in either L broth plus l-valine (A) or Spizizen’s minimal medium plus l-alanine (B) as described in Materials and Methods, and spore germination, outgrowth, and vegetative growth were followed by measuring the OD600 of the cultures. The symbols used are as follows: ○, 2-day spores; ●, 47-day spores; and ▵, 98-day spores. This experiment was carried out twice, with essentially identical results.
Resistance to wet and dry heat, NaOCl, and 254-nm UV radiation of 2- to 98-day spores.
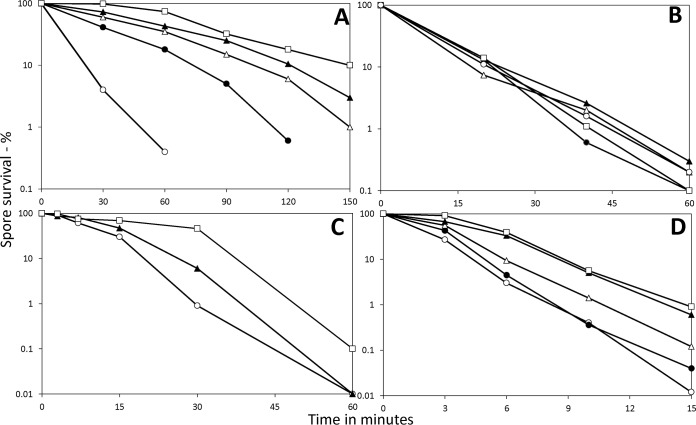
Although the levels of germination, outgrowth, and viability of 2- to 98-day spores were all quite similar, it was possible that the resistance properties of these spores would differ. Indeed, there are reports that spores undergo changes in resistance as well as some chemical properties soon after the spores are released from the sporangium (22, 23). Consequently, we examined the resistance of the 2-, 6-, 15-, 47-, and 98-day spores to the commonly used sporicidal treatments, wet heat, dry heat, 254-nm UV radiation, and NaOCl (Fig. 3). The 2-day spores exhibited the lowest wet heat resistance, which increased dramatically in 6-day spores and then further, with the highest resistance seen in 98-day spores (Fig. 3A), while the dry heat resistance levels for the spores of all different ages were relatively similar (Fig. 3B). The 47- and 98-day spores also had higher NaOCl resistance than did 2-day spores, with the 98-day spores having the highest NaOCl resistance (Fig. 3C). Forty-seven- and 98-day spores also exhibited higher UV resistance than did 2- or 6-day spores, with 15-day spores exhibiting intermediate resistance (Fig. 3D).
FIG 3.
Resistance properties of B. subtilis spores incubated for 2, 47, or 98 days. Spores harvested from sporulation plates incubated for 2, 6, 15, 47, or 98 days were purified as described in Materials and Methods. The purified spores’ survival when exposed to wet heat (A), dry heat (B), hypochlorite (C), or UV radiation (D) was then measured as described in Materials and Methods, and essentially identical results were obtained in duplicate experiments. The symbols for the ages of the spores used are as follows: ○, 2 days; ●, 6 days; ▵, 15 days; ▲, 47 days; and ☐, 98 days.
rRNAs and phosphorylated small molecules in 2-, 6-, 15-, 47-, and 98-day spores.
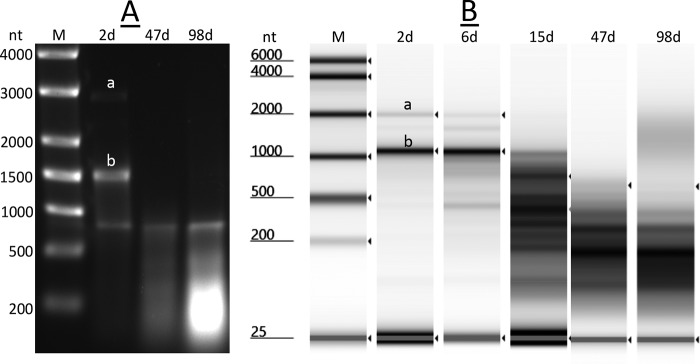
Previous work has shown that spores of B. subtilis FB73 that lack all functional IM GRs lose almost all 23S and 16S rRNA during incubation for as little as 5 days at 37°C (8, 17). This phenomenon was also observed with wild-type B. subtilis spores upon incubation at higher temperatures for shorter times (8). This loss in rRNAs was also seen upon incubation of sporulation plates for extended times at 37°C (Fig. 4), when RNA was run either on agarose gel electrophoresis or an Agilent TapeStation 4200 automated gel electrophoresis system (high-sensitivity RNA assay). Note that for unknown reasons, most 23S rRNA was fragmented even in 2-day spores, as seen previously (17). However, while significant rRNA, in particular, 16S rRNA, remained in 6-day spores, all but a small fraction was gone in 15-day spores, and all was gone in 47-day spores. It was notable that the overall RNA pattern shifted only slightly to smaller fragments between 47 and 91 days.
FIG 4.
Electrophoretic analysis of RNA in B. subtilis spores incubated for 2 to 98 days (d). Spores were harvested from plates incubated for 2 to 98 days and were purified as described in Materials and Methods. RNA was extracted from the purified spores and analyzed either by agarose gel electrophoresis with coelectrophoresed nucleotide size markers (A) or on an Agilent TapeStation 4200 automated gel electrophoresis system (high-sensitivity RNA assay) using in silico size markers (B), as described in Materials and Methods. Bands labeled “a” and “b” in panels A and B denote the migration positions of intact 23S and 16S rRNA, respectively; note the software sizing of detected rRNA peaks in panel B is not accurate compared to the expected size of the bands detected in panel A. Samples run were from separate experiments and had RNA from two separate spore preparations harvested after 2 days. (B) The 47- and 98-day RNA lanes were from a different TapeStation run than the 2-day, 6-day, and 15-day RNA lanes.
Previous work has shown that the rRNA lost from purified dormant B. subtilis or Bacillus megaterium spores incubated for 25 days at 37°C or even shorter times at higher temperatures does not show up as free nucleotides (17). It was certainly possible that even longer incubations would generate significant levels of mononucleotides from rRNA breakdown. However, this was not the case for spores from sporulation plates incubated for 2 to 91 days at 37°C (Fig. 5; data not shown), as levels of mononucleotides, in particular, AMP, in the older spores were comparable to those in 2-day spores and, as found previously, in highly purified spores incubated for ∼20 h at 75 to 80°C, which leads to the breakdown of essentially all rRNA (22). If all the rRNA and mRNA (see below) lost in 47- and 91-day spores had been converted to mononucleotides, levels of at least AMP in 51- and 91-day spores would have been >5 times higher than levels of 3-phosphoglyceric acid (3PGA) in 2-day spores (13, 17). Levels of 3PGA did decrease in older spores, and while these values fell only ∼30% in 6- and 47-day spores, there was no concomitant increase in inorganic phosphate (Pi) (Fig. 5); however, there was an ∼50% decrease in the 3PGA level in 91-day spores. The levels of Pi did decrease significantly in 6-day spores, as was seen previously (17), as much Pi in newly harvested spores appears to be in outer layers, where it can slowly diffuse away. However, Pi levels were relatively similar in 6-, 15-, and 51-day spores but rose significantly in 91-day spores (Fig. 5; data not shown).
FIG 5.
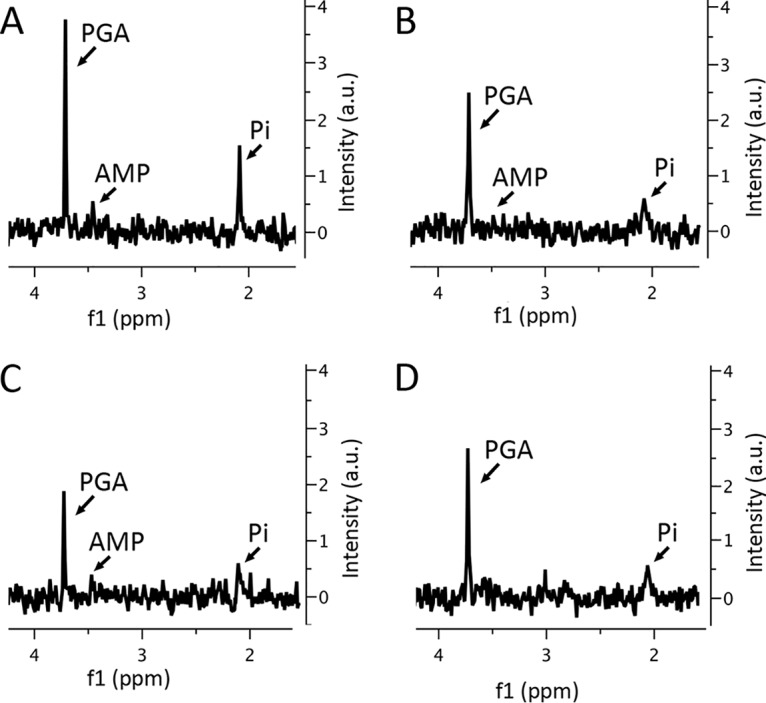
31P NMR spectra of small molecules extracted from 2-day (A), 6-day (B), 51-day (C), and 91-day (D) spores. Small molecules were extracted from spores of various ages, and extracts were processed and examined by 31P NMR, as described in Materials and Methods. Labeled peaks are from 3PGA, AMP, and Pi, and all spectra are shown at the same scale. There appear to be slightly broader peaks for Pi and 3PGA in spectra from 6- and 51-day spores, perhaps because of less efficient Mn2+ removal from these samples, and this could contribute to lower peak heights in these samples. a.u., arbitrary units.
Fate of dormant spore mRNAs and rRNAs during extended incubation of sporulation plates.
Previous work has shown that dormant B. subtilis spores contain only ∼46 mRNA species at levels such that all spores could have at least 1 copy of each mRNA (14). Some of these mRNAs are found at high levels in spores, and almost all the abundant spore mRNAs are transcribed by RNA polymerase with the final forespore-specific RNA polymerase σ factor, σG. Given the high levels of some mRNAs in spores, an obvious question is whether these mRNAs are stable in spores during long sporulation incubations, especially since previous work reported that many spore mRNAs are degraded when spores are held at 37 or 50°C (8). However, increases in levels of some spore mRNAs were also reported during spore storage at various temperatures (8). To examine effects on spore mRNA level of extended spore incubation at 37°C, total RNA from 2-, 47-, and 98-day spores was analyzed by RNA sequencing (RNA-seq) with ∼100 million reads per sample, and without prior rRNA depletion. The relative abundance of these spore mRNAs was determined and expressed as reads per mRNA transcript relative to reads for 16S plus 23S rRNA, as total reads for these two rRNAs were quite similar with all 3 RNA samples (Table 2). Strikingly, the levels of the 46 most abundant spore mRNAs decreased greatly as spores aged, by ∼97% in 47 days and ∼99% in 98 days (Table 2). Thus, the abundant mRNAs were all almost completely fragmented in 98-day spores. This was also true of the less abundant mRNAs, which were all almost completely gone even from 47-day spores (see Table S1 in the supplemental material; also data not shown). However, some spore mRNAs were more stable than bulk spore mRNAs, most notably sspE mRNA, although the reason for this greater mRNA stability is not clear.
TABLE 2.
Relative levels of mRNAs in spores aged for 2, 47, or 98 daysa
| Gene | No. of individual mRNA reads/total no. of reads for 23S + 16S rRNAs × 106 by spore age |
||
|---|---|---|---|
| 2 days | 47 days | 98 days | |
| sspA | 68 | 6 | 4 |
| sspE | 249 | 83 | 62 |
| sspF | 872 | 15 | 4 |
| sspJ | 131 | 9 | 3 |
| sspK | 191 | 2 | 0 |
| sspM | 61 | 1 | 0 |
| sspN | 148 | 15 | 2 |
| tlp | 150 | 8 | 3 |
| sspO | 34 | 3 | 0 |
| sspP | 389 | 1 | 1 |
| yfhD | 1,289 | 29 | 7 |
| yhcN | 320 | 1 | 1 |
| yhcQ | 192 | 7 | 3 |
| yhcV | 1,546 | 84 | 16 |
| yhdB | 1,458 | 4 | 1 |
| yizC | 719 | 27 | 4 |
| ykzE | 353 | 20 | 4 |
| ykzP | 697 | 38 | 1 |
| ymfJ | 421 | 15 | 1 |
| yozQ | 13 | 2 | 1 |
| ypzF | 1,791 | 25 | 10 |
| ypzG | 3,646 | 84 | 16 |
| yqfX | 1,080 | 18 | 0 |
| yrzQ | 293 | 2 | 0 |
| yrzR | 303 | 2 | 1 |
| ytzC | 504 | 61 | 12 |
| ytzL | 950 | 7 | 1 |
| yuzA | 72 | 2 | 0 |
| yxeD | 27 | 3 | 1 |
| sspB | 31 | 1 | 3 |
| sspH | 20 | 0 | 0 |
| yhcM | 576 | 1 | 1 |
| yraE | 52 | 0 | 0 |
| yrrD | 264 | 15 | 0 |
| yusG | 11 | 0 | 0 |
| yusN | 4 | 0 | 0 |
| pdhA | 12 | 0 | 0 |
| sscA | 1 | 0 | 0 |
| sspI | 19 | 0 | 0 |
| yhcO | 112 | 2 | 0 |
| ykzD | 9 | 0 | 0 |
| yoyE | 79 | 2 | 0 |
| ypzI | 3 | 0 | 0 |
| yraD | 12 | 1 | 0 |
| yrzB | 1 | 0 | 0 |
| yutC | 13 | 0 | 0 |
| Total no. of mRNA reads/no. of 23S + 16S rRNA reads × 106 (%)b | 19,041 (100) | 566 (3) | 163 (0.9) |
| No. of 23S + 16S rRNA reads | 94,820,503 | 77,821,429 | 83,264,436 |
Spores of various ages were prepared and purified, RNA was extracted, RNA-seq was carried out, and the data were analyzed, as described in Materials and Methods.
Values in parentheses are the percentages of total number of mRNA reads/number of 23S + 16S rRNA reads relative to the value in 2-day spores.
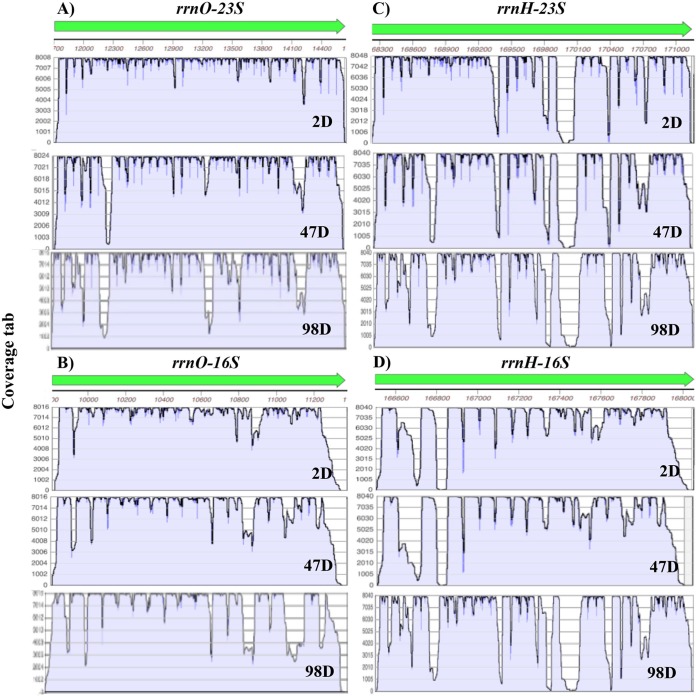
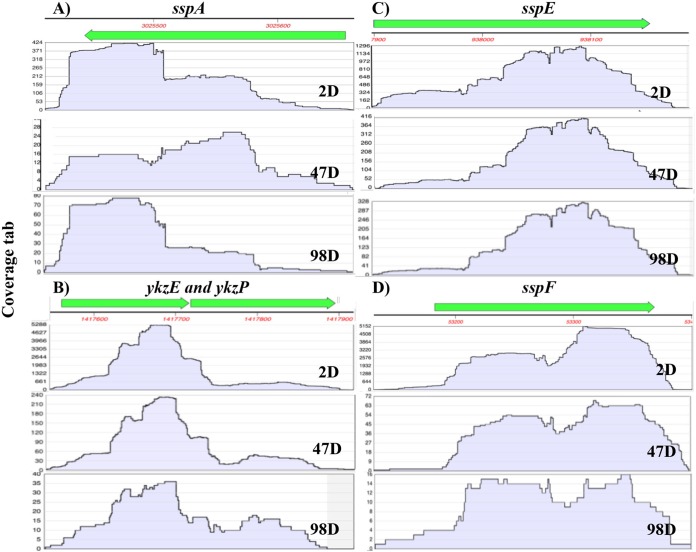
Analysis of the coverage of both rRNAs and abundant mRNAs in 2- to 98-day spores in RNA-seq analysis without ribo depletion found that, as seen previously (13), coverage of mRNAs in the RNA-seq reads from 2-day-spore RNA was relatively complete for most genes, even though there were significantly fewer reads from the mRNAs, since the most abundant reads were from rRNA; the coverage of the 16S and 23S rRNA from 2-day spores was also complete, although some partial gaps were present (Fig. 6 and 7 and data not shown). The coverage of reads of the two rRNAs expressed from all rrn operons remained fairly complete even with 98-day-spore RNA, although gaps present even in the 2-day-spore rRNA gaps became much larger as spores aged (Fig. 6 and data not shown). In contrast, the coverages of the most abundant mRNAs were rather similar in most 2- to 98-day-spore RNA, but invariably, the numbers of mRNA reads dropped off precipitously as spores aged (Fig. 7).
FIG 6.
Coverage of RNA-seq reads from 16S and 23S rRNAs from 2-, 47-, and 98-day spores. RNA-seq data for two sets of rRNA genes, rrnO and rrnH, which exhibited lower (rrnO) and higher (rrnH), respectively, loss of reads in regions of 47- and 98-day spore rRNA are shown, and coverage of reads was determined as described in Materials and Methods. The vertical axes are coverage in arbitrary units (a.u.). Note that the scales of the vertical axes are identical for 2-, 47-, and 98-day spore rRNA.
FIG 7.
Coverage of RNA-seq reads from 8 abundant mRNAs from 2-, 47-, and 98-day spores. RNA-seq data using RNA that was not ribo-depleted for 8 abundant mRNAs in 2-day spores, as well as in 47-day and 98-day spores, were obtained, and coverage of reads was determined as described in Materials and Methods. The vertical axes are coverage in arbitrary units (a.u.). Note the large differences in the scales of the vertical axes in the 2- to 98-day samples.
DISCUSSION
As is not surprising, spores of B. subtilis exhibited a high level of survival when incubated on nutrient plates for up to 98 days. The presumption is of course that the spores were formed and released from sporangia in 2 days and had then sat in the 37°C environment and were not continually being generated by successive waves of sporulation. Indeed, there was no evidence for continued sporulation in the aged cultures, as observed by microscopy. However, there was significant germination of spores as culture age increased, with ∼60% of the released spores having germinated in the 98-day cultures, although there was minimal subsequent growth of germinated spores as observed by microscopy. This observation indicates that the spores that were ultimately purified from the oldest spore preparations represent a slight minority of the total spores produced. Given that spore properties such as germination rate and heat resistance vary significantly between individuals in spore populations (21, 24), it is possible that the 2- and 98-day spores differ in properties that might have been selected against during up to 98 days at 37°C. However, detailed analysis of this possibility will require further work. It is also notable that with purified wild-type spores incubated in solution at 37°C, the great majority of spores germinated in 1 week (17; data not shown). Thus, the germination of purified spores during extended incubation at 37°C was much greater than that with spores incubated on sporulation plates. Presumably, some components in the spent sporulation medium are greatly slowing spore germination. Although the identities of these inhibitory components are not clear, this inhibition seems reasonable in light of the low nutrient levels in this medium that initially triggered spore formation, as presumably, further growth of any spores that germinated would be minimal.
Whatever the fate of the germinated spores noted above, when the spores that remained dormant were purified, the levels of viability, germination, and outgrowth of these spores incubated on plates for 2 to 98 days were essentially identical, both in and on rich and minimal media. These findings indicate that any differences between the spores of different ages had minimal effects if any on the spores’ ability to germinate and grow out and, thus, on spore viability. This is most significant considering that (i) almost all intact 23S and 16S rRNA had disappeared from the 47- and 98-day spores, and (ii) levels of normally abundant and less abundant spore mRNAs had fallen ≥98% in 98-day spores. Consequently, it seems extremely unlikely that intact dormant spore rRNA and mRNA and, thus, protein synthesis from any spore mRNAs play an essential role in spore germination, as was suggested previously (14). It was somewhat surprising that the extra energy demand in aged spores for resynthesis of >99% of all rRNA and assembly of ribosomes in aged spores before protein synthesis can take place did not slow aged spore outgrowth appreciably compared to that of 2-day spores. Possibly, experiments using even poorer medium for spore outgrowth, perhaps a medium without glucose, would reveal differences in outgrowth between aged and 2-day spores.
The fragmentation of spore RNAs as spores age raises the question of how this RNA cleavage takes place; is it enzymatic, and if so, what enzymes are involved, or is it simply slow water catalysis? There is evidence for both of these possibilities (8, 17). This is an important question to address experimentally in the future. What is surprising is that this RNA fragmentation seems to give mononucleotides only very slowly, as was seen previously when purified spores were incubated at 70°C for 24 to 48 h or at 37°C for many weeks (17). Indeed, only in 91-day spores did AMP levels go up slightly. However, this increase could be an underestimate of mononucleotide generation, as Pi levels also increased in 91-day spores, and this increase could have been by dephosphorylation of the mononucleotides. However, whether this scenario is correct will require future work with even older spores, and perhaps, analysis of nucleoside levels in these spores. Levels of 3PGA, which is used for ATP generation soon after spores germinate, also decreased by ∼30% in 6- and 51-day spores and by ∼60% in 91-day spores. However, the mechanism for the decreases in 3PGA levels is not clear, since at least in 51-day spores there was no increase in spore Pi equal to the loss in 3PGA. It is also known that 3PGA is not released from spores upon extended storage, at least at 4°C (25).
Notably, total RNA-seq reads for 16S and 23S rRNA changed only slightly between 2- and 47- and 98-day spores, and the total rRNA size distributions also changed minimally between 47 and 98 days (Fig. 4). Thus, rRNA seems to be broken down only into large fragments, possibly due to protection by binding of ribosomal proteins. However, RNA-seq reads from individual abundant spore mRNAs underwent decreases of 80 to 99% by 98 days, indicating that most mRNAs were cleaved into fragments too small to be seen in RNA-seq analysis. This could be because the 46 most abundant B. subtilis spore mRNAs are quite small, averaging only ∼383 nucleotides (nt) (14).
In contrast to levels of intact spore RNAs, other spore properties, such as Ca-DPA and core water contents and resistance to dry heat, were, like spore viability, germination, and outgrowth, essentially identical in spores aged 2 to 98 days. Two exceptions were UV resistance, which was slightly greater in the two oldest spores, and NaOCl resistance, which was also greater in the older spores. Spore resistance to NaOCl is due primarily to the spore coat layer, and spores with a variety of coat defects exhibit much lower NaOCl resistance than do wild-type spores (1, 2). It is also known that spore NaOCl resistance increases as spores mature (23), possibly because of coat modifications that take place after spores are released from the sporangium (26–28). However, how such coat modifications might increase spore NaOCl resistance is not known, but if these changes increase coat pigmentation, this might also increase spore UV resistance. It is also possible that the loss of some spores by germination as spores age is selectively removing spores with the lowest UV and NaOCl resistances, but this seems unlikely to explain the magnitude of the difference in NaOCl resistance seen between old and young spores.
The much higher wet heat resistance in aged spores was the most striking change in spore resistance and has been seen previously in spores as they age for ∼24 h, but not to the extent seen in the current work (22). The reason for the increased wet heat resistance in the aged spores is not completely clear but could be due to the lower core water content in the older spores, as was seen in the 51- and 91-day spores, as lower core water content is correlated with higher spore wet heat resistance (20). The increased wet heat resistance of the aged spores could also be due to changes in the spore coat layer, but what this change is and why this increases spore wet heat resistance are not clear. Importantly, if the finding that aged spores have higher wet heat resistance than young spores is true for spores of all species, this would indicate that great care must be taken in deciding on (i) an appropriate heat treatment to kill spores by wet heat in an applied setting, as this could well differ for spores of different ages, and (ii) precisely how to prepare spores used as biological indicators for sterilization assurance.
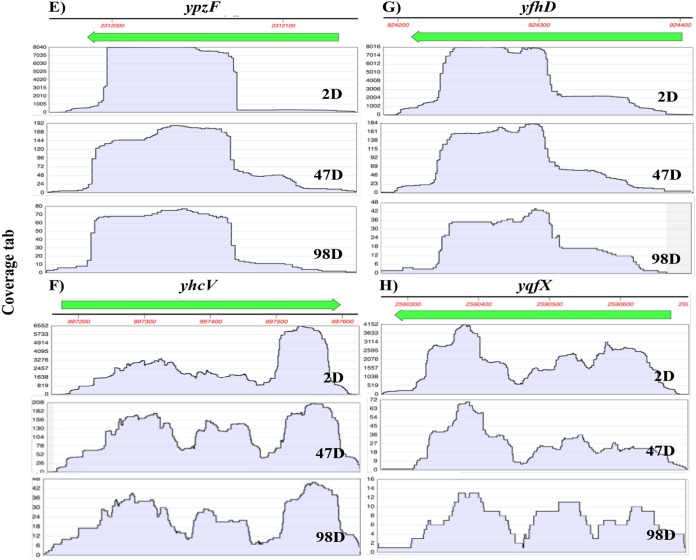
Additionally, if, as seems likely from the results in this communication, all abundant spore mRNAs can be lost with no effects on spore viability or return to growth, this suggests that the synthesis of proteins from spore mRNAs in outgrowth is not essential. This is not surprising for the abundant spore mRNAs, such as sspA mRNA, that encode DNA binding small acid-soluble spore proteins, as the presence of these proteins in outgrowing spores results in the death of these spores (29). This observation, as well as the fact that the dormant and early outgrowing spore is auxotrophic for synthesis of purine and pyrimidine nucleotides due to the loss of biosynthetic enzymes from the developing spore (30), strongly suggests that the role of most of the abundant spore mRNAs is to be degraded when spores germinate to provide nucleotides for new RNA synthesis. Note that fragmented rRNA would also need to be broken down and resynthesized before protein synthesis can begin in spore outgrowth, and this is known to take place soon after spore germination is triggered (17). An obvious question is how these degradative and biosynthetic events might be coordinated. One parameter that could play a role in the coordination of events in the early minutes of spore germination and outgrowth is the water content in the spore core, which changes from a value of ∼35% of wet weight in a dormant B. subtilis spore to a value of 80% of wet weight in a fully germinated spore (21). As noted above, Ca-DPA release increases the core water content to ∼45% of wet weight in 1 to 2 min for an individual spore, and this event triggers cortex peptidoglycan hydrolysis with accompanying core water uptake and core swelling. During the latter process, the core water content in an individual spore increases slowly from ∼45% to 80% of wet weight in 6 to 15 min in a period termed ΔTlys (21, 31) (Fig. 8). During the processes of Ca-DPA release and cortex hydrolysis, it seems possible that simple enzymatic reactions, such as RNA degradation, could begin at a lower core water content than more complicated processes such as RNA and protein synthesis. Thus, during Ca-DPA release and cortex hydrolysis, there might be a large, albeit transient, increase in the mononucleotide level in the spore core prior to the onset of energy metabolism and rapid RNA synthesis. Indeed, such a large increase in mononucleotides was seen when Bacillus megaterium spores were germinated in the presence of actinomycin D to prevent RNA synthesis (30). Assessing the magnitude and kinetics of changes in mononucleotide levels during the ΔTlys period might indicate when both RNA breakdown and RNA synthesis begin in this period in spore germination and the transition to spore outgrowth.
FIG 8.
Model showing changes in spore core water content in the early minutes of germination of an individual wild-type B. subtilis spore. Data from references 21 and 31 were used to generate the times for the various events shown in this model. wt, weight; Tlag, time when rapid Ca-DPA release begins; Trelease, time when all Ca-DPA has been released.
Finally, the decreases in spore core water content and increases in spore wet heat resistance as spores age at 37°C suggest the possibility that spores have adapted to respond to these incubation conditions to maximize their long-term survival by decreasing their core water content and increasing their wet heat resistance. There are certainly much data indicating that purified spores with higher resistance to temperatures such as 90°C survive better under more moderate temperatures, such as 70°C (2, 20). Although direct comparisons of spore wet heat resistance at 90°C and 37°C have not been made, it seems logical that spores with higher wet heat resistance at 90°C would survive better at 37°C than do spores with lower 90°C resistance. Spore water content is the major factor, the modification of which can alter spore wet heat resistance and, presumably, long-term survival (20). It would thus seem unreasonable that spores might have at least some control of their core water content in environments where a decreased core water content might be advantageous. The question then is, how could dormant spores decrease their core water content in the absence of ATP generation? One process which does take place in the absence of ATP is hydrolysis of rRNA and mRNA, and each phosphodiester bond hydrolyzed would use up a water molecule. The increase in ionic species accompanying phosphodiester bond hydrolysis would also further sequester free water. How much this type of water usage decreases spore core water content is not at all clear, but it would not take much to have a significant effect on core resistance (20). Consequently, this might be one reason that the degradation of spore RNA actually takes place during spore storage, as it increases spore survival under some extreme conditions and was therefore actually selected for. This is definitely a possibility to be tested going forward.
MATERIALS AND METHODS
B. subtilis strains used and spore preparations and purification.
The strain used for all work was B. subtilis PS533 (32), a 168 strain that carries plasmid pUB110 encoding resistance to kanamycin (10 μg/ml). Spores were prepared by spreading vegetative cells growing in L broth (10 g/liter tryptone, 5 g/liter yeast extract, 10 g/liter NaCl) on double-strength Schaeffer’s-glucose medium agar plates (33, 34). The plates were incubated at 37°C in plastic bags that were lightly sealed and contained a petri dish with water to prevent agar plates from drying out during long incubations, and the water was replenished frequently. Spores were ≥95% released from sporangia by 2 days and were harvested by scraping from plates after incubation for 2 to 98 days and suspended in 4°C water. Spores isolated at 2 days contained <1% germinated spores, while spore preparations incubated for 47 or 98 days contained ∼20 and ∼60% germinated spores, respectively, as determined by phase-contrast microscopy. The spores were purified over ∼7 days at 4°C by repeated rounds of centrifugation, water washes, and sonication, with removal of debris on the surface of spore pellets by gentle streams of water (35). Finally, spores were centrifuged in a microcentrifuge through a high-density solution of 50% Histodenz (Sigma Chemical Co., St. Louis, MO) in which the purified dormant spores pellet and debris and germinated spores float (35). The spore pellets were washed with water to remove Histodenz and were suspended and stored in 4°C water protected from light. All spores used in this work were >98% free of growing cells, germinated spores, or debris, as seen by phase-contrast microscopy.
Measurements of spore viability, Ca-DPA and core water content, germination, and outgrowth.
For measurement of spore viability, spores at an optical density at 600 nm (OD600) of 1 were serially diluted 10-fold in 4°C water. Ten-microliter aliquots were spotted in duplicate on a grid on either an L broth plate (L broth plus 15 g/liter agar) with 10 μg/ml kanamycin or on a Spizizen’s minimal medium plate without Casamino Acids (36) prepared with 15 g/liter ultrapure agarose instead of agar, and with 1 mM l-alanine present to trigger spore germination; l-alanine was used instead of l-valine for germination here, as l-alanine triggers spore germination at much lower concentrations than l-valine. The ultrapure agarose was used instead of agar, as agar commonly has low levels of nutrients. The plates were incubated initially at 30°C for ∼20 h and then for an additional 28 h until colonies were big enough to count but not so big that they grew together, and colonies were counted. No additional colonies appeared after 48 h.
The Ca-DPA contents of individual 2-, 47-, and 98-day spores were determined by laser tweezers Raman spectroscopy of spores in water that were randomly trapped by a 780-nm laser beam (37). Raman spectra of the trapped spore were captured by a charge-coupled device, and spore Ca-DPA contents were determined from the intensity of the 1,017 cm−1 Ca-DPA-specific Raman band in these spectra, with reference to that of Ca-DPA standards. The relative core water contents in 2-, 6-, 15-, 51-, and 91-day spores were determined by centrifugation of ∼100 μl of chemically decoated (13) spores at an OD600 of ∼20 in 30% Histodenz layered on a 2-ml step gradient of 56 to 75% Histodenz with 100-μl steps differing by 1%, as described previously (38). These density gradients were centrifuged at 20°C and for 45 min at 15,000 × g in a TLS-55 swinging bucket rotor, the Histodenz concentration at which the spores banded was determined by inspection of the tubes, and the Histodenz wet density was determined by weighing 2 ml of 75% Histodenz.
Spores were germinated with three small molecules, each of which triggers B. subtilis spore germination by a different mechanism (21). One was l-valine that triggers spore germination via the spore inner membrane (IM) GerA germinant receptor (GR), as does l-alanine, which germinates spores at lower concentrations than l-valine but can be converted to the germination-inhibiting d-alanine by a spore-associated alanine racemase which does not act on l-valine (21). With this germinant, spore germination was potentiated by a heat activation treatment for 30 min at 75°C, and then spores were cooled on ice. Spores were germinated at an OD600 of ∼0.5 (∼108 spores/ml) in 200 μl of 25 mM K-HEPES buffer (pH 7.4) with 10 mM l-valine and 50 μM TbCl3. Germination was performed in duplicate in wells of a 96-well plate in a fluorescence plate reader, and spore germination was measured by monitoring the fluorescence of released spore Ca-DPA by DPA’s fluorescence with Tb3+, as described previously (39). To determine the percentage of total Ca-DPA in the spores used in germination experiments, total Ca-DPA was extracted from spores at an OD600 of 1 by boiling for 30 min and then centrifuged in a microcentrifuge. Aliquots of the supernatant fluid (50 to 100 μl) were then made 25 mM in K-HEPES (pH 7.4) and 50 μM in TbCl3, and Tb3+-DPA fluorescence was measured, all as described previously (39). Spores without heat activation were also germinated with two germinants, Ca-DPA and dodecylamine, which do not require heat activation (21). Ca-DPA (50 mM, made pH 7.4 with dry Tris base) germination was at room temperature with spores at an OD600 of 1, and spore germination was measured at various times by examining ∼100 spores by phase-contrast microscopy to determine the percentage of spores that had become phase dark and had thus completed spore germination. Dodecylamine germination was also with spores at an OD600 of 1 with 1 mM dodecylamine in 25 mM Tris-HCl buffer (pH 8.4) and at 45°C. At various times, aliquots were diluted ∼1/1 and then made 50 μM in TbCl3, and Ca-DPA release was measured fluorometrically as described above.
For measurement of spore germination, outgrowth, and subsequent vegetative growth, spores were first heat activated and then cooled in ice, as described above. The activated spores were added to ∼15 ml of prewarmed (37°C) L broth plus 10 mM l-valine to give an initial OD600 of ∼0.7; the culture was incubated at 37°C in a rotary shaker to ensure good aeration, and at various times, the OD600 of the culture was measured. The OD600 value at time zero falls ∼50% as spore germination is completed, remains relatively constant during outgrowth, and then rises as cells grow. Measurement of spore germination and outgrowth at 37°C in Spizizen’s minimal medium without Casamino Acids, but with 1 mM l-alanine to trigger spore germination, was also performed as described above.
Measurements of spore resistance.
Spore resistance to wet heat was carried out with spores at an OD600 of 1 at 90°C. Aliquots of these incubations were diluted 1/10 in room temperature sterile water and then further serially diluted 10-fold. Aliquots (10 μl) of the dilutions were spotted in a grid on L broth plates with kanamycin (10 μg/ml), plates were incubated, and colonies were counted as described above.
Spore resistance to NaOCl (10 to 15% available chlorine) was carried out at 23°C with spores at an OD600 of 1 in water. At various times, aliquots of these incubations were diluted 1/10 in 10 g/liter sodium thiosulfate to neutralize the NaOCl. After ∼10 min, the initial dilutions were further serially diluted 10-fold in water. Aliquots (10 μl) of these dilutions were spotted in duplicate on L broth plates with kanamycin and incubated, and colonies were counted, all as described above. Spore resistance to dry heat (120°C) and UV radiation at 254 nm was determined as described previously (22, 32), and levels of spore killing were determined as described above.
Extraction and analysis of spore small molecules and RNA.
Spore small molecules were extracted in boiling 80% 1-propanol, extracts were processed, Mn2+ that would interfere with subsequent nuclear magnetic resonance (NMR) analyses was removed, and extracts were analyzed by 31P NMR, as described previously (17, 18).
RNA from 2- to 98-day spores was extracted and purified as described previously (13, 17). By measurements of the OD260 of the RNA isolated, all these spores were found to contain similar amounts of RNA. These RNA preparations were run and analyzed either on agarose gel electrophoresis, as described previously (17), or on a TapeStation 4200 automated gel electrophoresis system (high-sensitivity RNA assay), as described by the instrument manufacturer (Agilent Technologies, Santa Clara, CA).
Analysis of mRNAs in 2-, 47-, and 98-day spores.
Levels of mRNAs in dormant spores were determined in spores incubated for 2, 47, and 98 days by RNA-seq, and these data were processed as described previously (14). RNA for these analyses was isolated from 2-, 47-, and 98-day spores, as described above. In previous RNA-seq analyses of mRNA in spores, rRNA was depleted from RNA preparations prior to RNA-seq (14). However, given the fragmentation of spore rRNA during the incubations at 37°C for 47 or 98 days, this was not possible for the RNAs from the older spores. Instead, total spore RNA from the spores of the different ages was used without rRNA depletion for RNA-seq but with 90 to 100 million reads instead of the ∼15 million reads used previously for analyzing ribo-depleted dormant spore mRNA (14). Illumina TruSeq libraries were prepared, and RNA-seq data were obtained and analyzed as done previously (14). Fortunately, even though 23S and 16S rRNAs were fragmented in 47- and 98-day spore RNAs, the numbers of reads for these rRNAs were similar to those with 2-day spore rRNA (see Results). Consequently, reads for individual spore mRNAs were expressed relative to those for 23S plus 16S rRNA. The coverages for both 16S and 23S rRNAs as well as a number of the abundant mRNAs in 2 days and aged spores were obtained as described previously (14).
Supplementary Material
ACKNOWLEDGMENTS
The work in this paper was supported by a grant from the U.S. Department of Defense (RDECOM) Army Research Office (ARO) (grant W911NF-18-1-0024). M.J.C. was supported by the NIH/NIAD under award numbers R01AI029735, R21AI128379, R21AI126146, and R21AIAI139940.
We are grateful to Wendy W. K. Mok for helpful suggestions on the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00231-19.
REFERENCES
- 1.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 2.Setlow P. 2016. Spore resistance properties, p 201–215. In Eichenberger P, Driks A (ed), The bacterial spore: from molecules to systems. ASM Press, Washington, DC. [Google Scholar]
- 3.Setlow P, Johnson EA. 2012. Spores and their significance, p 45–79. In Doyle MP, Buchanan R (ed), Food microbiology, fundamentals and frontiers, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 4.Kennedy MJ, Reader SL, Swierczynski LM. 1994. Preservation records of microorganisms: evidence of the tenacity of life. Microbiology 140:2513–2529. doi: 10.1099/00221287-140-10-2513. [DOI] [PubMed] [Google Scholar]
- 5.Vreeland RH, Rosenzweig WD, Powers DW. 2000. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407:897–900. doi: 10.1038/35038060. [DOI] [PubMed] [Google Scholar]
- 6.Cano RJ, Borucki MK. 1995. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science 268:1060–1064. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich NJ, Nagler K, Laue M, Cockell CS, Setlow P, Moeller R. 2018. Experimental studies addressing the longevity of Bacillus subtilis spores–the first data from a 500-year experiment. PLoS One 13:e0208425. doi: 10.1371/journal.pone.0208425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segev E, Smith Y, Ben-Yehuda S. 2012. RNA dynamics in aging bacterial spores. Cell 148:139–149. doi: 10.1016/j.cell.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Bassi D, Cappa F, Cocconcelli PS. 2013. Array-based transcriptional analysis of Clostridium sporogenes UC9000 during germination, cell outgrowth and vegetative life. Food Microbiol 33:11–23. doi: 10.1016/j.fm.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Bassi D, Colla F, Gazzola S, Puglisi E, Delledonne M, Cocconcelli PS. 2016. Transcriptome analysis of Bacillus thuringiensis spore life, germination and cell outgrowth in a vegetable-based food model. Food Microbiol 55:73–85. doi: 10.1016/j.fm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, Zhang X, Diaz LA Jr, Velculescu VE, Parmigiani G, Kinzler KW, Vogelstein B, Zhou S. 2006. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol 24:1573–1580. doi: 10.1038/nbt1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dembek M, Stabler RA, Witney AA, Wren BW, Fairweather NF. 2013. Transcriptional analysis of temporal gene expression in germinating Clostridium difficile 630 endospores. PLoS One 8:e64011. doi: 10.1371/journal.pone.0064011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keijser BJF, Ter Beek A, Rauwerda H, Schuren F, Montijn R, van der Spek H, Brul S. 2007. Analysis of temporal gene expression during Bacilllus subtilis spore germination and outgrowth. J Bacteriol 189:3624–3634. doi: 10.1128/JB.01736-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korza G, Camilleri E, Green J, Robinson J, Nagler K, Moeller R, Caimano MJ, Setlow P. 2019. Analysis of messenger RNAs in spores of Bacillus subtilis. J Bacteriol 201:e00007-19. doi: 10.1128/JB.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagler K, Krawczyk AO, De Jong A, Madela K, Hoffmann T, Laue M, Kuipers OP, Bremer E, Moeller R. 2016. Identification of differently expressed genes during Bacillus subtilis spore outgrowth in high-salinity environments using RNA sequencing. Front Microbiol 7:1564. doi: 10.3389/fmicb.2016.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Melis CC, Nierop Groot MN, Tempelaars MH, Moezelaar R, Abee T. 2011. Characterization of germination and outgrowth of sorbic acid-stressed Bacillus cereus ATCC 14579 spores: phenotype and transcriptome analysis. Food Microbiol 28:275–283. doi: 10.1016/j.fm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Korza G, Setlow B, Rao L, Li Q, Setlow P. 2016. Changes in Bacillus spore small molecules, rRNA, germination and outgrowth after extended sub-lethal exposure to various temperatures: evidence that protein synthesis is not essential for spore germination. J Bacteriol 198:3254–3264. doi: 10.1128/JB.00583-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S, Korza G, Maciejewski M, Setlow P. 2015. Analysis of metabolism in dormant spores of Bacillus species by 31P-NMR of low molecular weight compounds. J Bacteriol 197:991–1001. doi: 10.1128/JB.02520-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paidhungat M, Setlow P. 2000. Role of Ger-proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J Bacteriol 182:2513–2519. doi: 10.1128/JB.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerhardt P, Marquis RE. 1989. Spore thermoresistance mechanisms, p 43–63. In Smith I, Slepecky RA, Setlow P (ed), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC. [Google Scholar]
- 21.Setlow P, Wang S, Li Y-Q. 2017. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol 71:459–477. doi: 10.1146/annurev-micro-090816-093558. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Salas JL, Setlow B, Zhang P, Li YQ, Setlow P. 2011. Maturation of released spores is necessary for acquisition of full spore heat resistance during Bacillus subtilis sporulation. Appl Environ Microbiol 77:6746–6754. doi: 10.1128/AEM.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abhyankar W, Pandey R, Ter Beek A, Brul S, de Koning LJ, de Koster CG. 2015. Reinforcement of Bacillus subtilis spores by cross-linking of outer coat proteins during maturation. Food Microbiol 45:54–62. doi: 10.1016/j.fm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Zhang P, Li Y-Q, Setlow P. 2009. Superdormant spores of Bacillus species have elevated wet heat resistance and temperature requirements for heat activation. J Bacteriol 191:5584–5591. doi: 10.1128/JB.00736-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson DL, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism in sporulation. J Biol Chem 245:1137–1145. [PubMed] [Google Scholar]
- 26.Ragkousi K, Setlow P. 2004. Transglutaminase-mediated cross-linking of GerQ in the coats of Bacillus subtilis spores. J Bacteriol 186:5567–5575. doi: 10.1128/JB.186.17.5567-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwana R, Okuda N, Takamatsu H, Watabe K. 2006. Modification of GerQ reveals a functional relationship between Tgl and YabG in the coat of Bacillus subtilis spores. J Biochem 139:887–901. doi: 10.1093/jb/mvj096. [DOI] [PubMed] [Google Scholar]
- 28.Takamatsu H, Imamura A, Kodama T, Asai K, Ogasawara N, Watabe K. 2000. The yabG gene of Bacillus subtilis encodes a sporulation specific protease which is involved in the processing of several coat proteins. FEMS Microbiol Lett 192:33–38. doi: 10.1111/j.1574-6968.2000.tb09355.x. [DOI] [PubMed] [Google Scholar]
- 29.Hayes CS, Setlow P. 2001. An α/β-type small, acid-soluble spore protein which has a very high affinity for DNA prevents outgrowth of Bacillus subtilis spores. J Bacteriol 183:2662–2666. doi: 10.1128/JB.183.8.2662-2666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow P, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XXIII. Nucleotide metabolism during spore germination. J Biol Chem 245:3645–3652. [PubMed] [Google Scholar]
- 31.Ramirez-Peralta A, Zhang P, Li Y-Q, Setlow P. 2012. Effects of sporulation conditions on the germination and germination protein levels of spores of Bacillus subtilis. Appl Environ Microbiol 78:2689–2697. doi: 10.1128/AEM.07908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom. [Google Scholar]
- 34.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512. doi: 10.1128/JB.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setlow P. 2019. Observations on research with spores of Bacillales and Clostridiales species. J Appl Microbiol 126:348–358. doi: 10.1111/jam.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spizizen J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci U S A 44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S-S, Chen D, Pelczar PL, Vepachedu VR, Setlow P, Li Y-Q. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J Bacteriol 189:4681–4687. doi: 10.1128/JB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindsay JA, Beaman TC, Gerhardt P. 1985. Protoplast water content of bacterial spores determined by buoyant density centrifugation. J Bacteriol 163:735–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.