Abstract
Purpose
Cancer cells develop acquired resistance induced by chemotherapeutic drugs. In this study, we investigated the effects of brief treatment with cytotoxic drugs on the phenotype of breast cancer cells.
Methods
Breast cancer cells MCF7 and BT-474 were briefly treated with paclitaxel or doxorubicin. Clonogenic, migration, and invasion assays were performed on the treated cells. Western blot analysis and RhoA activity assay were also performed.
Results
Breast cancer cells when briefly treated with paclitaxel or doxorubicin showed reduced clonogenic ability. Doxorubicin, but not paclitaxel, augmented cell migration and invasion. The invasion-promoting effects of doxorubicin were lost when the two drugs were sequentially used in combination. Myosin light chain (MLC) 2 phosphorylation and RhoA activity were upregulated by doxorubicin and downregulated by paclitaxel. Pretreatment with RhoA inhibitors abolished the migration- and invasion-promoting effects of doxorubicin.
Conclusion
Doxorubicin activates the RhoA/MLC pathway and enhances breast cancer cell migration and invasion. Therefore, this pathway might be explored as a therapeutic target to suppress anthracycline-enhanced tumor progression.
Keywords: Breast, Carcinoma, Cell movement, Doxorubicin
INTRODUCTION
Breast cancer is the second most frequently diagnosed cancer worldwide (after lung cancer) and the leading cause of cancer deaths in women [1]. Several molecular subtypes of breast cancer have been identified by molecular profiling, and a distinct subtype might correspond to a moderate difference in treatment efficacy among chemotherapy regimens [2]. Nonetheless, accumulating data from 100,000 women in 123 randomized trials of chemotherapy confirm that chemotherapy can reduce breast cancer mortality not only in the estrogen receptor (ER)-negative but also in the ER-positive disease and suggests potential benefits of taxane-based therapy over standard anthracycline chemotherapy [3]. These findings led a National Institutes of Health consensus panel to recommend adjuvant chemotherapy for most patients, a practice that has contributed to declining breast cancer mortality [4]. Anthracyclines and taxanes have been standard chemotherapeutic agents for breast cancer in the past decade.
Doxorubicin is considered as one of the most active, single cytotoxic agents for breast cancer, although the concern regarding congestive cardiomyopathy requires limiting the cumulative lifetime dose in order to minimize the risk of its toxicity [5]. Taxanes are a class of anticancer agents that bind to and stabilize microtubules, causing cell-cycle arrest and cell death. The best results in terms of disease-free and/or overall survival in breast cancer patients are achieved with combination chemotherapy containing anthracyclines and taxanes [3]. It is well recognized that suboptimal doses of chemotherapeutic agents or intrinsic resistance to standard dosing may trigger adaptive cellular response mechanisms that result in the induction of acquired drug resistance [6]. Studies have shown that the tumorigenic cancer cells remaining after chemotherapy had unique properties of enhanced self-renewal and might display an increased propensity for tumor formation [7].
To further characterize the phenotype of breast cancer cells subjected to sublethal doses of chemotherapeutic agents, we designed the present study in which breast cancer cells were briefly treated with paclitaxel or doxorubicin. The results of this study might have clinical implications in the chemotherapy sequences for the treatment of breast cancer.
METHODS
Cell culture and reagents
Human breast cancer cell lines (MCF7 and BT-474) and a human ovarian carcinoma cell line (ES-2) were obtained from the American Type Culture Collection (ATCC; Manassas, USA). MCF7 cells were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum (FBS), 0.1 mM non-essential amino acids and 1.0 mM sodium pyruvate. BT-474 cells were cultured in ATCC Hybri-Care Medium (ATCC 46-X) supplemented with 10% FBS and 30 ng/mL epidermal growth factor. ES-2 cells were maintained in McCoy's 5a Medium supplemented with 10% FBS. Cells were maintained at 37°C in a humidity-controlled incubator with 5% CO2. Paclitaxel and doxorubicin were obtained from Sigma-Aldrich (St. Louis, USA).
Clonogenic assay
Clonogenic assay is a versatile tool for in vitro screening of drugs for antineoplastic activity [8]. The clonogenic ability is an estimate of the reproductive viability of cancer cells (capacity of a single-cell suspension to produce progeny; or a single cell to form a colony of 50 or more cells) under different circumstances. We performed the colony-forming assay as previously described [9]. MCF7 cells were briefly treated with paclitaxel (30 nM) for 1 hour, doxorubicin (200 nM) for 3 hours or vehicle control. Cells were re-suspended and a total of 700 cells were seeded into 6-well plates containing standard culture media. After 10 days, colonies were stained with 3% crystal violet and examined by microscopy. Colonies containing at least 50 individual cells were counted.
Cell migration and invasion
This assay was carried out using modified Boyden chambers as previously described [10]. Cells were cultured in 6-well plates and were treated with paclitaxel for 1 hour, doxorubicin for 3 hours, or a combination of both in different sequences. Cells were then re-suspended and plated into the transwell insert. Transwell filters were polycarbonate membranes with 8-μm pores (Corning Life Sciences, Corning, USA). For invasion assay, the upper surfaces of the transwell membranes were coated with Matrigel matrix. Cells (MCF7, 8 × 104 cells/insert; BT-474, 2 × 105 cells/insert; ES-2, 1 × 104 cells/insert) in the transwell insert were allowed to migrate/invade toward the underside of the membrane for an additional 48 hours (MCF7 and BT-474 cells) or 18 hours (ES-2 cells). Non-migrating/non-invading cells were removed by wiping the upper side of the membrane, and cells on the lower surface of the filter were stained with Diff-Quick (Sysmex, Kobe, Japan). Number of migrated/invaded cells were counted from five random fields and averaged from at least three independent experiments.
Immunoblot assay
MCF7 cells were treated with paclitaxel (30 nM) for 1 hour or doxorubicin (200 nM) for 3 hours. Thereafter, the media was replaced and cells were maintained in standard culture media further for 24 to 48 hours. Whole cell lysates were prepared using the M-PER Protein Extraction Reagent (Thermo Fisher Scientific, Waltham, USA), and the protein concentrations were determined using the Bradford assay kit (Bio-Rad, Hercules, USA). Aliquots of 30 μg of total protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. The membranes were blocked in 5% (w/v) non-fat milk in TBST buffer, followed by primary antibody incubation at 4°C overnight. The primary antibodies used were anti-phospho-IκB kinase (IKK) α/β (Ser176/180) (#2697; Cell Signaling Technology, Danvers, USA), anti-E-cadherin (#5296; Cell Signaling Technology), anti-Twist1 (sc-15393; Santa Cruz Biotechnology, Dallas, USA), and phospho-myosin light chain (MLC) 2 (Ser19) (#3671; Cell Signaling Technology). Anti-β-actin antibody (Sigma-Aldrich) was used as the loading control. Immunoreactive proteins were detected by using horseradish peroxidase-conjugated secondary antibodies and were visualized by using the enhanced chemiluminescence system (Merck Millipore, Burlington, USA) [11].
RhoA activity assay
MCF7 cells were treated with paclitaxel (30 nM) or doxorubicin (200 nM) for 30 minutes. Cell lysates were prepared and RhoA activity assay was performed using the RhoA G-LISA Activation Assay Kit (Cytoskeleton, Inc., Denver, USA) according to the manufacturer's instructions. RhoA activity was quantified by determining the absorbance reading at 490 nm.
Rescue experiments
Two RhoA inhibitors, Y16 and rhosin/G04, were obtained from MedChemExpress (Monmouth Junction, USA). These inhibitors have been reported to specifically inhibit RhoA activity and RhoA-mediated signaling functions [12]. Cells were pretreated with or without Y16 (50 μM) or rhosin/G04 (50 μM) for 24 hours, and then incubated with doxorubicin (MCF7, 200 nM; BT-474, 800 nM) or vehicle control for an additional 3 hours. Then, cells were re-suspended and migration and invasion assays were performed as previously described.
Statistical analysis
Experiments were performed in triplicates, and data were reported as the mean ± standard error of the mean. Comparisons were made by using the two-tailed Student's t-test. Data were analyzed using GraphPad Prism version 6.01 for Windows (GraphPad Software, La Jolla, USA). Statistical significance was determined at a probability value of < 0.05.
RESULTS
Doxorubicin promotes migration and invasion of breast cancer cells
Anthracyclines and taxanes have been established as the cornerstones of chemotherapy in the management of breast cancer. To investigate the sublethal effects of these two agents, first we determined the half maximal inhibitory concentrations (IC50) of paclitaxel and doxorubicin by dose-response cell viability curve. The IC50 values of paclitaxel and doxorubicin in MCF7 cells were 30 nM and 200 nM, respectively. These concentrations were used in subsequent experiments.
In our institution, paclitaxel was administered weekly, in a standard manner of one-hour infusion. Doxorubicin was administered by three-hour slow infusions instead of bolus administration to reduce cardiac exposure to and penetration by anthracyclines. Slow infusion is a well-established strategy which may prevent or diminish the risk of early or delayed cardiotoxicity [13]. Therefore, in this study, the exposure time was based on our clinical practice.
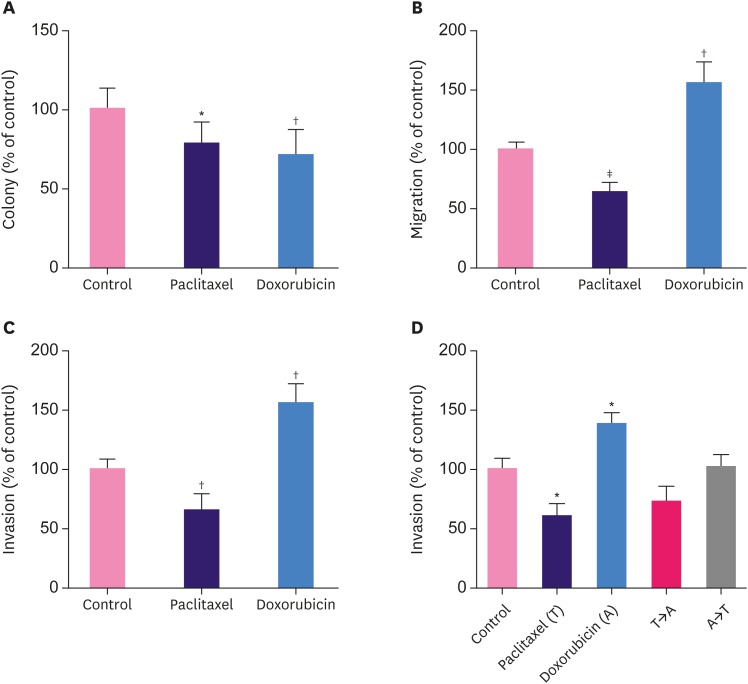
MCF7 cells, following a brief treatment with paclitaxel or doxorubicin, exhibited reduced clonogenic ability (Figure 1A). Colony numbers were 78.2% ± 3.6% and 70.7% ± 4.1% compared to the control in the paclitaxel and doxorubicin group, respectively. This suggests that a brief treatment with these cytotoxic drugs is sufficient to compromise the cell growth of breast cancer cells.
Figure 1. Effects of brief treatment with cytotoxic drugs on the phenotype of MCF7 breast cancer cells. Colony formation (A), cell migration (B) and invasion (C) assays were performed in MCF7 cells treated briefly with paclitaxel (30 nM) for 1 hour, doxorubicin (200 nM) for 3 hours, or vehicle control. (D) Invasion assay of MCF7 cells treated with paclitaxel (30 nM) for 1 hour, doxorubicin (200 nM) for 3 hours, or a combination of both in different sequences.
*p < 0.05 versus control; †p < 0.01; ‡p < 0.001.
Cell migration in the extracellular matrix is a multistep process involving dynamic changes in the cytoskeleton, cell-substrate adhesion, and the extracellular matrix components. Strikingly, the migratory ability of breast cancer cells was enhanced following a brief treatment with doxorubicin but not paclitaxel (Figure 1B). There was a 1.56-fold increase in the migratory ability of MCF7 cells following a three-hour treatment with doxorubicin. Conversely, the migratory ability decreased to 64.2% ± 4.0% compared to control after one-hour treatment with paclitaxel.
Next, we determined the invasive capacity by adding a layer of Matrigel matrix on top of the transwell membrane to mimic the process of extracellular matrix invasion and extravasation of breast cancer cells. Consistent with the results of the migration assay, the invaded cells were 65.2% ± 7.0% and 155.8% ± 15.9% compared to control following treatments with paclitaxel and doxorubicin, respectively (Figure 1C).
The effects of combination treatment in different sequences were also examined. The invasion ability of MCF7 cells was 72.9% ± 12.5% compared to control after one-hour paclitaxel treatment followed by three-hour doxorubicin treatment (Figure 1D). In the other group with a reverse sequence of treatment, such as, doxorubicin followed by paclitaxel, the invasion ability was 101.7% ± 10.4% of the control. This observation suggests that the invasion-inhibiting effect of paclitaxel may surpass the invasion-promoting effect of doxorubicin when the two agents are sequentially used in combination. Furthermore, although the difference was not statistically significant (p = 0.11), the invasion-promoting effect of doxorubicin tends to be more prominent during sequential treatment of cells with doxorubicin followed by paclitaxel than paclitaxel followed by doxorubicin.
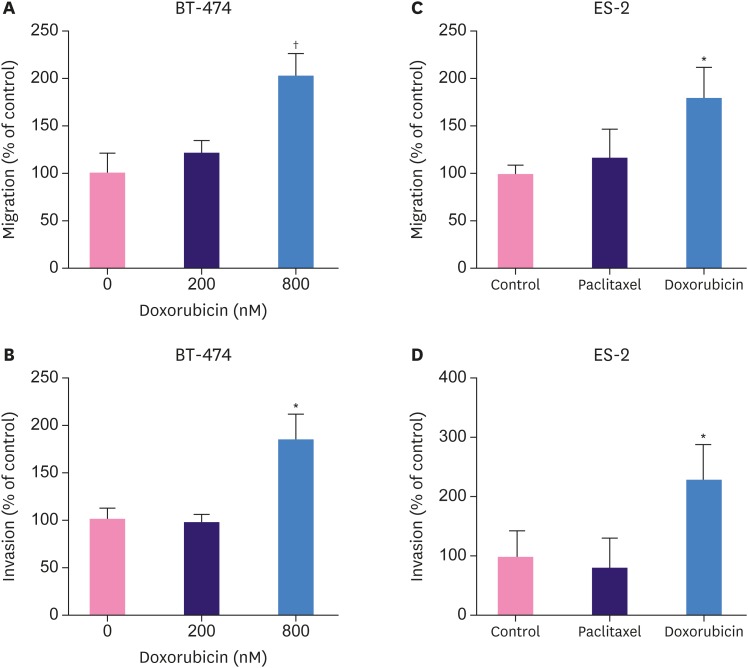
To corroborate our findings in another breast cancer cell line, BT-474 cells were treated with doxorubicin for three-hours and migration and invasion assays were subsequently performed. As shown in Figure 2A and B, brief treatment with doxorubicin (200 nM) did not significantly change the migratory or invasive capacity of BT-474 cells. Nonetheless, a brief treatment with 800 nM doxorubicin significantly augmented the migration and invasion of BT-474 cells. Taken together, a brief treatment with doxorubicin suppressed the clonogenic ability but enhanced the migratory and invasive capacity of BT-474 breast cancer cells.
Figure 2. Effects of brief treatment with doxorubicin or paclitaxel on cell migration (A, C) and invasion (B, D) in BT-474 breast cancer cells (A, B) and ES-2 ovarian cancer cells (C, D). Cells were pretreated with paclitaxel (30 nM) for 1 hour, doxorubicin (200 nM or 800 nM) for 3 hours, or vehicle control and migration and invasion assays were performed.
*p < 0.05 versus control; †p < 0.01.
We further examined whether these phenomena were limited to breast cancer cells or could be observed in other cancer cells. ES-2 ovarian cancer cells exhibited IC50s for paclitaxel and doxorubicin similar to those of MCF7 cells (data not shown). Interestingly, a brief treatment with paclitaxel did not affect the migratory or invasive capacity, but doxorubicin treatment promoted cell migration or invasion in ES-2 ovarian cancer cells (Figure 2C and D). This suggests that the migration- and invasion-promoting effects induced by doxorubicin were not unique to breast cancer cells.
Doxorubicin upregulates MLC2 phosphorylation and RhoA activity
To identify the mechanisms which might account for the increase in cell migration and invasion in doxorubicin-treated breast cancer cells, the expression of pertinent cell motility factors was determined. Doxorubicin treatment in breast cancer cells has been shown to activate an NF-кB transcription signature [14]. Furthermore, doxorubicin might induce epithelial-mesenchymal transition by downregulation of E-cadherin and upregulation of Twist1 [15]. However, when cells were briefly treated with paclitaxel or doxorubicin, there was no significant change observed in IKK phosphorylation or Twist1 expression (Figure 3A). The expression of E-cadherin increased at 24 hours and returned to the baseline following either paclitaxel or doxorubicin treatment.
Figure 3. Effects of brief treatment with cytotoxic drugs on the expression of cell motility factors and RhoA activity in MCF7 breast cancer cells. (A) Cells were treated with paclitaxel (30 nM) for 1 hour or doxorubicin (200 nM) for 3 hours. The media were replaced, and cells were maintained in standard culture medium further for 24 to 48 hours. Cell lysates were prepared and western blotting was performed. (B) Cells were treated with paclitaxel (30 nM) or doxorubicin (200 nM) for 30 minutes. Cell lysates were prepared and RhoA activity was determined.
IKK = IκB kinase; MLC = myosin light chain.
*p < 0.01 versus control.
MLC is directly and indirectly phosphorylated by Rho-associated coiled-coil-containing protein kinase (ROCK) at Ser19, thereby increasing the actomyosin contractile force and cell protrusions, resulting in cell migration [16]. A recent study showed that doxorubicin treatment caused the formation of long filopodia-like protrusions in MCF7 spheroid cells [17]. Interestingly, we found that the expression of phosphorylated MLC2 was significantly downregulated following one-hour treatment with paclitaxel, while MLC2 phosphorylation increased progressively from 24 to 48 hous following a three-hour treatment with doxorubicin (Figure 3A).
ROCK is a RhoA effector downstream of RhoA activation [18]. We next investigated whether the upstream RhoA activity was enhanced after treatment with doxorubicin. RhoA activity in MCF7 cells was determined following a brief treatment with paclitaxel or doxorubicin. Notably, paclitaxel decreased RhoA activity whereas doxorubicin significantly increased RhoA activity (Figure 3B), consistent with the changes in MLC2 phosphorylation.
RhoA inhibitors rescue the invasion-promoting effect of doxorubicin
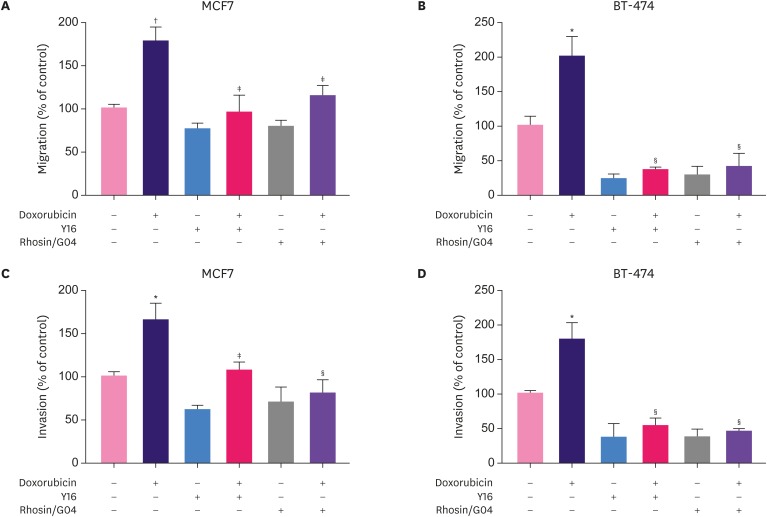
Rescue experiments were performed to investigate the significance of RhoA-GTPase activity in relation to our findings. Pretreatment with RhoA inhibitors, Y16 or rhosin/G04, significantly abolished the increase in cell migration (Figure 4A and B) and invasion (Figure 4C and D) induced by doxorubicin treatment in MCF7 and BT-474 breast cancer cells. This finding suggests that the migration- and invasion-promoting effects induced by a brief treatment with doxorubicin are mediated, at least in part, by the upregulation of the RhoA/MLC pathway.
Figure 4. Rescue effects of RhoA inhibitors on cell migration and invasion promoted by doxorubicin in breast cancer cells. MCF7 (A, C) and BT-474 (B, D) cells were pretreated with or without Y16 (50 μM) or rhosin/G04 (50 μM) for 24 hours and incubated with doxorubicin (MCF7, 200 nM; BT-474, 800 nM) or vehicle control for an additional 3 hours. Then, cells were re-suspended and migration and invasion assays were performed.
*p < 0.05 versus control; †p < 0.01; ‡p < 0.05 versus doxorubicin only; §p < 0.01.
DISCUSSION
Although most patients who present with localized breast cancer may be rendered disease-free with locoregional therapy, distant recurrence remains common and is the primary cause of death from the disease. The widespread use of adjuvant systemic therapies is effective in reducing the risk of distant and local recurrence, as well as the mortality rates. Initial chemotherapy choices for the treatment of newly diagnosed and metastatic breast cancer have dramatically changed over the past four decades, evolving from single alkylating agents to polychemotherapy regimens incorporating anthracyclines and/or taxanes.
Despite such advances in chemotherapy, many women diagnosed with breast cancer eventually succumb to the disease. Many potential agents have been investigated in clinical trials with the hope of improving patient's response to therapy, duration of response, and patient survival. Unfortunately, majority of these agents fail to show benefit and/or produce unacceptable toxicities. Thus far, sequential anthracycline-cyclophosphamide and taxane treatments have been shown to be the most effective adjuvant therapy regimen for early-stage breast cancer regardless of hormone receptor status [19].
Although anthracyclines are usually administered before taxanes (or in combination), the practice is based on the administration sequence used in clinical trials rather than strong research-based evidence. Administration of a taxane first, followed by an anthracycline, might be more effective and less toxic in line with the Norton-Simon hypothesis [20]. Sporadic evidence suggests that better outcomes may be achieved by simply changing the sequence of these two kinds of drugs [21,22,23]. Increased addition of the cytotoxic drug (gemcitabine) to paclitaxel, epirubicin, and cyclophosphamide chemotherapy did not improve pathological complete response (pCR). Nonetheless, improved pCR could be achieved by using taxane-first sequence during neoadjuvant chemotherapy [24]. Therefore, taxane-first sequence could be regarded as a reasonable option for chemotherapy in breast cancer.
In the present study, breast cancer cells were treated briefly with paclitaxel (1 hour) or doxorubicin (3 hours), which mirrors the infusion time in clinical practice. It is noteworthy that the invasion-promoting effects appear to be more prominent during the sequential treatment with doxorubicin followed by paclitaxel than paclitaxel followed by doxorubicin (Figure 1D). In addition, the sequence of chemotherapy might affect the emergence of cross-resistance. This hypothesis has been tested in vitro in MCF7 cell line which was rendered resistant to paclitaxel. The cell line showed limited cross-resistance to doxorubicin, but when the same cell line was first made resistant to doxorubicin, it exhibited a 4,700-fold cross-resistance to paclitaxel [25]. Therefore, sensitivity to paclitaxel might strongly be compromised by prior exposure to doxorubicin.
Cancer cell migration and invasion are the initial steps in metastasis. Malignant cancer cells utilize their intrinsic migratory ability to invade adjacent tissues and vasculature, and ultimately metastasize. Cell migration is the sum of multi-step processes initiated by the formation of membrane protrusions in response to migratory and chemotactic stimuli [26]. Doxorubicin has been shown to enhance tumor metastasis by increasing TGF-β levels and boosting circulating cancer cells [27]. Furthermore, doxorubicin may activate IKKs and Myc, thereby increasing invasiveness and tumorigenesis of breast cancer cells [28]. In this study, we identified that the RhoA/MLC pathway also participates in the invasion-promoting properties of doxorubicin.
Rho and its downstream targets are involved in regulating actin cytoskeleton dynamics, and therefore are responsible for cell migration and motility. Actin participates in a range of events in cellular locomotion, including cell motility, polarity, cell junction function, and chemotaxis [29]. It has been reported that a key factor in stress fiber formation is the phosphorylation of MLC2 [30]. Cells have a complicated signaling network modulating MLC2 phosphorylation, among which the Rho-associated pathway is one of the most crucial mechanisms in stimulating cross-linking of actin by myosin, leading to enhanced cancer cell contractility. In this study, we provided evidence that doxorubicin increased MLC2 phosphorylation probably through the activation of RhoA, and the suppression of RhoA activity might block the increased invasive capacity induced by doxorubicin. Targeting the RhoA/MLC pathway to inhibit cancer cell migration/invasion during doxorubicin therapy might be a useful strategy which deserves further investigation.
In conclusion, we showed that the standard chemotherapy drug, doxorubicin but not paclitaxel, activates the RhoA/MLC pathway and, in turn, enhances breast cancer cell migration and invasion. Regardless of the sequence of combination therapy, paclitaxel-containing regimen could abolish the stimulation of invasion induced by doxorubicin treatment. The RhoA/MLC pathway might have therapeutic potential in breast cancer treatment by suppressing anthracycline-enhanced tumor progression.
Footnotes
Funding: This work was supported by research grants (MMH-E-107-10 and MMH-E-108-10) from MacKay Memorial Hospital, Taipei, Taiwan.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Cheng SP, Chang YC.
- Data curation: Chen MJ, Lin JC, Chen SN.
- Investigation: Liu CL, Chen MJ, Lin JC, Chang YC.
- Methodology: Lin CH.
- Project administration: Chang YC.
- Resources: Chen MJ.
- Supervision: Chang YC.
- Visualization: Huang WC.
- Writing - original draft: Liu CL, Chang YC.
- Writing - review & editing: Cheng SP, Chang YC.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Hart CD, Sanna G, Siclari O, Biganzoli L, Di Leo A. Defining optimal duration and predicting benefit from chemotherapy in patients with luminal-like subtypes. Breast. 2015;24(Suppl 2):S136–S142. doi: 10.1016/j.breast.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;106:dju289. doi: 10.1093/jnci/dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Asola R, et al. Adjuvant capecitabine in combination with docetaxel and cyclophosphamide plus epirubicin for breast cancer: an open-label, randomised controlled trial. Lancet Oncol. 2009;10:1145–1151. doi: 10.1016/S1470-2045(09)70307-9. [DOI] [PubMed] [Google Scholar]
- 6.Wurz GT, DeGregorio MW. Activating adaptive cellular mechanisms of resistance following sublethal cytotoxic chemotherapy: implications for diagnostic microdosing. Int J Cancer. 2015;136:1485–1493. doi: 10.1002/ijc.28773. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 8.Hanauske AR, Hanauske U, Von Hoff DD. The human tumor cloning assay in cancer research and therapy: a review with clinical correlations. Curr Probl Cancer. 1985;9:1–50. doi: 10.1016/s0147-0272(85)80026-x. [DOI] [PubMed] [Google Scholar]
- 9.Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014;9:e89563. doi: 10.1371/journal.pone.0089563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang YC, Chen CK, Chen MJ, Lin JC, Lin CH, Huang WC, et al. Expression of 3β-hydroxysteroid dehydrogenase type 1 in breast cancer is associated with poor prognosis independent of estrogen receptor status. Ann Surg Oncol. 2017;24:4033–4041. doi: 10.1245/s10434-017-6000-6. [DOI] [PubMed] [Google Scholar]
- 11.Chang YC, Liu CL, Chen MJ, Hsu YW, Chen SN, Lin CH, et al. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg. 2014;118:116–124. doi: 10.1213/ANE.0b013e3182a94479. [DOI] [PubMed] [Google Scholar]
- 12.Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem Biol. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menna P, Salvatorelli E. Primary prevention strategies for anthracycline cardiotoxicity: a brief overview. Chemotherapy. 2017;62:159–168. doi: 10.1159/000455823. [DOI] [PubMed] [Google Scholar]
- 14.Dalmases A, González I, Menendez S, Arpí O, Corominas JM, Servitja S, et al. Deficiency in p53 is required for doxorubicin induced transcriptional activation of NF-кB target genes in human breast cancer. Oncotarget. 2014;5:196–210. doi: 10.18632/oncotarget.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15:2657–2665. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- 16.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebata T, Mitsui Y, Sugimoto W, Maeda M, Araki K, Machiyama H, et al. Substrate stiffness influences doxorubicin-induced p53 activation via ROCK2 expression. BioMed Res Int. 2017;2017:5158961. doi: 10.1155/2017/5158961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zandvakili I, Lin Y, Morris JC, Zheng Y. Rho GTPases: anti- or pro-neoplastic targets? Oncogene. 2017;36:3213–3222. doi: 10.1038/onc.2016.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii T, Le Du F, Xiao L, Kogawa T, Barcenas CH, Alvarez RH, et al. Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: a systematic review and network meta-analysis. JAMA Oncol. 2015;1:1311–1318. doi: 10.1001/jamaoncol.2015.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon R, Norton L. The Norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol. 2006;3:406–407. doi: 10.1038/ncponc0560. [DOI] [PubMed] [Google Scholar]
- 21.Wildiers H, Dirix L, Neven P, Prové A, Clement P, Squifflet P, et al. Delivery of adjuvant sequential dose-dense FEC-Doc to patients with breast cancer is feasible, but dose reductions and toxicity are dependent on treatment sequence. Breast Cancer Res Treat. 2009;114:103–112. doi: 10.1007/s10549-008-9970-z. [DOI] [PubMed] [Google Scholar]
- 22.Piedbois P, Serin D, Priou F, Laplaige P, Greget S, Angellier E, et al. Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol. 2007;18:52–57. doi: 10.1093/annonc/mdl355. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso F, Ferreira Filho AF, Crown J, Dolci S, Paesmans M, Riva A, et al. Doxorubicin followed by docetaxel versus docetaxel followed by doxorubicin in the adjuvant treatment of node positive breast cancer: results of a feasibility study. Anticancer Res. 2001;21:789–795. [PubMed] [Google Scholar]
- 24.Earl HM, Vallier AL, Hiller L, Fenwick N, Young J, Iddawela M, et al. Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high-risk early breast cancer (Neo-tAnGo): an open-label, 2×2 factorial randomised phase 3 trial. Lancet Oncol. 2014;15:201–212. doi: 10.1016/S1470-2045(13)70554-0. [DOI] [PubMed] [Google Scholar]
- 25.Guo B, Villeneuve DJ, Hembruff SL, Kirwan AF, Blais DE, Bonin M, et al. Cross-resistance studies of isogenic drug-resistant breast tumor cell lines support recent clinical evidence suggesting that sensitivity to paclitaxel may be strongly compromised by prior doxorubicin exposure. Breast Cancer Res Treat. 2004;85:31–51. doi: 10.1023/B:BREA.0000021046.29834.12. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–1313. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh PY, Lu YS, Ou DL, Cheng AL. IκB kinases increase Myc protein stability and enhance progression of breast cancer cells. Mol Cancer. 2011;10:53. doi: 10.1186/1476-4598-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–186. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh K, Kano Y, Noda Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. J R Soc Interface. 2011;8:305–311. doi: 10.1098/rsif.2010.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]