Abstract
Mucinous carcinoma (MC) is a rare subtype of breast cancer, which is composed of tumor cells floating in the abundant extracellular mucin. This form of cancer is usually estrogen receptor/progesterone receptor positive and human epidermal growth factor receptor 2 (HER2) negative. Here, we present a case of HER2-positive MC with an unusual signet ring cell differentiation. It is very rare that a breast tumor consists entirely of signet ring cells. The tumor showed pathologic complete response (pCR) after neoadjuvant chemotherapy with trastuzumab and pertuzumab. pCR of HER2-positive MC has rarely been described in literature. It is important to consider the biological heterogeneity of MCs for effective management.
Keywords: Breast neoplasms; Receptor, ErbB-2; Adenocarcinoma, mucinous; Neoadjuvant therapy; Carcinoma, signet ring cell
INTRODUCTION
Mucinous carcinoma (MC) is characterized by clusters of floating tumor cells in extracellular mucin pools [1]. A tumor comprised of > 90% of MC is called pure mucinous carcinoma (PMC). PMC is a rare subtype accounting for up to 2% of all breast cancer cases [1]. The typical immunoprofile for PMC is estrogen receptor (ER) and/or progesterone receptor (PR) positivity and human epidermal growth factor receptor 2 (HER2) negativity [1].
HER2 overexpression occurs in 15%–20% of invasive breast cancers [2]. However, HER2 overexpression is a rare event in PMC and has been reported in only 2.6%–9% cases [3,4,5,6]. Here, we describe a case of HER2-positive PMC with unusual histology of signet ring cell differentiation, which showed pathologic complete response (pCR) after neoadjuvant chemotherapy (NAC) with trastuzumab and pertuzumab.
CASE REPORT
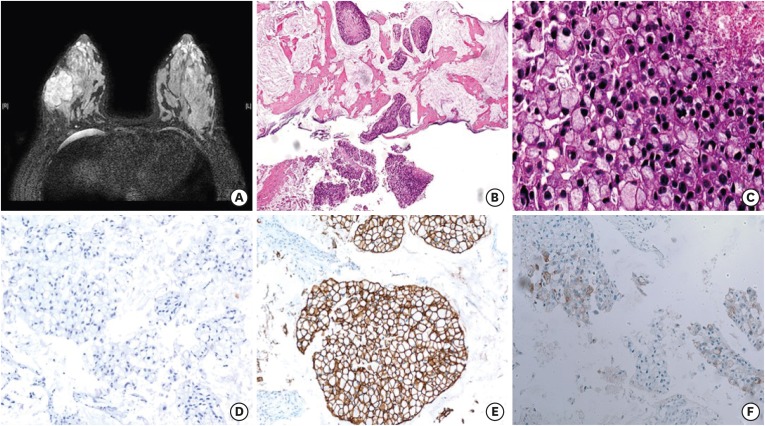
A 35-year-old woman visited a local hospital due to a palpable mass in her right breast. It had been initially discovered one year ago during her pregnancy. Her past medical history was unremarkable. Ultrasound examination showed a 5.5 cm mass in the lower outer quadrant of the right breast. The patient underwent core needle biopsy. She visited Samsung Medical Center for a second opinion. Magnetic resonance imaging (MRI) revealed a 5.5 cm mass with adjacent satellite lesions measuring 0.7 and 0.8 cm and multiple enhanced lymph nodes in the axillary levels I and II (Figure 1A). The initial clinical stage was characterized as T3N2. Microscopically, solid tumor cell nests were found floating in mucin pools (Figure 1B). The tumor consisted of signet ring cells with peripherally displaced nuclei due to intracellular mucin (Figure 1C). Tumor necrosis was present focally. Nuclei were hyperchromatic and showed marked size variation. Frequent mitosis was observed, up to 6 per 1 high power field. Tumor cells were ER negative (Figure 1D), PR weakly positive, and HER2 positive (3+) (Figure 1E). The Ki-67 labeling index was 30%. Tumor cells were positive for p53, weakly positive for epidermal growth factor receptor, and did not express cytokeratin 5/6. They also expressed E-cadherin and GATA binding protein 3 (GATA-3) as well as gross cystic disease fluid protein 15 (GCDFP-15), which was weakly positive in a few tumor cells (Figure 1F). An 18-fluorodeoxyglucose-positron emission tomography scan showed no significant uptake except in the breast mass and axillary lymph nodes. Fine needle aspiration of suspected lymph nodes was negative for malignancy. The final pathologic diagnosis made was that of mammary MC with signet ring cell differentiation.
Figure 1. Magnetic resonance imaging and microscopic findings before neoadjuvant treatment. (A) Axial T2-weighted image shows a well-enhanced mass in the right breast. (B) Core biopsy specimen shows tumor cell clusters floating in extracellular mucin pools (hematoxylin and eosin, magnification × 40) (C) Tumor showed extensive signet ring cell differentiation with high grade nuclear features, frequent mitosis, and tumor necrosis (hematoxylin and eosin, magnification × 400) (D, E, F). Tumor cells were negative for ER (D), positive for HER2 (E), and focally expressed GCDFP-15 (magnification × 200).
ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; GCDFP-15 = gross cystic disease fluid protein 15.
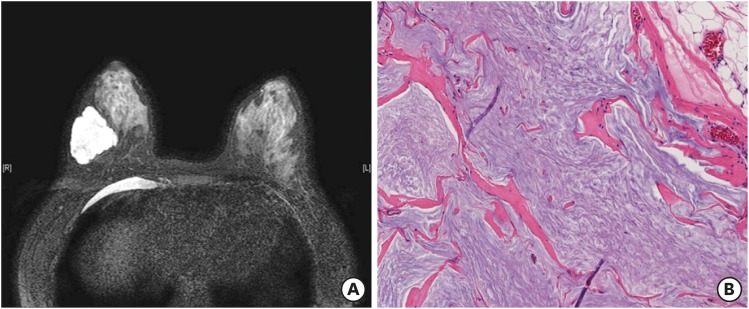
The patient received 6 cycles of docetaxel 75 mg/m2, carboplatin 600 mg (area under the curve 5), trastuzumab 6 mg/kg, and pertuzumab 420 mg with prophylactic pegylated granulocyte colony-stimulating factor. MRI after NAC revealed a 4.4 cm mass with extensive cystic changes and no enhanced solid portion in the right mid-outer breast (Figure 2A). She underwent right breast-conserving surgery with sentinel lymph node biopsy. The excised section of the surgical specimen showed a well-demarcated gelatinous mass, measuring 4.9 × 4.1 × 3.2 cm. The entire mass was embedded for pathologic examination. Microscopic examination revealed bluish mucinous material only with no viable tumor cells (Figure 2B). All resection margins were clear, and all six lymph nodes showed no metastasis. After the operation, she received adjuvant trastuzumab and tamoxifen treatment, in addition to radiation therapy. Currently, she is well with no signs of recurrence at 19 months after operation.
Figure 2. Magnetic resonance imaging and microscopic findings after neoadjuvant chemotherapy with trastuzumab and pertuzumab. (A) T2-weighted imaging shows a mass showing cystic change. (B) Surgical specimen reveals only mucin pools with no viable tumor cells (hematoxylin and eosin, magnification × 200).
DISCUSSION
MC is characterized by nests of tumor cells floating in lakes of extracellular mucin [1]. There are 2 subtypes of MC according to the proportion of the mucinous component [1]. PMC is composed of > 90% of the MC component, whereas mixed mucinous carcinoma (MMC) contains 50%–90% of the MC component [1]. PMC usually occurs in patients above 55 years of age and is associated with good prognosis, whereas MMC has poor prognosis [1]. Histologically, PMC is typically low grade, positive for ER and/or PR, and negative for HER2 [1,6]. However, there are rare cases of high grade, ER/PR negative, or HER2-positive PMCs. There are several studies that have shown that ER/PR negativity and high grade are associated with lymph node metastasis and poor prognosis in PMC [7,8]. HER2 overexpression is a rare event in PMC, with only 2.6% to 9% prevalence [3,4,5,6]. In a recent genomic analysis of stage IV PMC, 5 of 22 (23%) patients exhibited genomic alteration of HER2 (3 with amplification, 1 with both amplification and mutation, and 1 with mutation), a statistically higher rate than non-metastatic PMC [9].
In the present case, histologic features were unusual. Almost the entire tumor from the core biopsy consisted of signet ring cells. According to the recent World Health Organization classification, carcinoma with signet ring cell differentiation is no longer a single independent entity [1]. Signet ring cell differentiation can be found, usually focally, in invasive lobular carcinoma, invasive ductal carcinoma, MC, and metaplastic carcinoma [1,10]. Earlier studies suggested poor prognosis for signet ring cell carcinoma of the breast [11]. However, there had been no strict criteria for signet ring cell carcinoma. In a case series analysis of carcinoma with signet ring cell differentiation, it was seen that the proportion of signet ring cells in MC was focal, ranging between 8%–17% of the entire tumor [10]. It is very rare for a breast tumor to consist exclusively of signet ring cells. In such a case, care should be taken to rule out the possibility of metastasis, especially from gastric signet ring cell carcinoma. Evaluation of GATA-3 and GCDFP-15, which are expressed in breast cancers, are necessary for differential diagnosis. In this case, the presence of diffuse GATA-3 staining and focal GCDFP-15 staining supported a mammary origin.
We found 2 previous reports with similar histologic features [12,13]. All three cases, including this case, were histologically PMC with extensive signet ring cell differentiation, of high grade, negative or weakly positive for ER and/or PR, strong HER2 expression, and a high Ki-67 proliferation index. With these unusual features, MC with signet ring cell differentiation can be considered as an aggressive variant of MC. Similarly, recent studies have revealed that mucinous micropapillary carcinoma is an aggressive variant of MC [5].
The patient achieved pCR after NAC with trastuzumab and pertuzumab. Baretta et al. [14] reported resistance to trastuzumab in 2 advanced cases of HER2-positive MC and suggested that mucin might have acted as a barrier to trastuzumab. However, they used a different criterion of more than 50% of MC component and two cases consisted of invasive ductal carcinoma components as well. In this case, the tumor achieved pCR in spite of abundant extracellular mucin. Although the clinical settings are much different, the pCR in this case might suggest that mucin might not act as a barrier, at least, in some of the HER2-expressing MC. A recent study has also demonstrated markedly decreased cellularity after NAC in MC, although abundant mucin pools remained [15].
In summary, we described a PMC which showed unusual histologic as well as biologic features, i.e. signet ring cell differentiation, high grade, HER2 positivity and high proliferation. pCR was observed after NAC with trastuzumab and pertuzumab. It is important to consider the biologic heterogeneity of PMCs to ensure adequate treatment.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Investigation: Jang Y.
- Project administration: Cho SY.
- Supervision: Cho EY, Cho SY.
- Writing - original draft: Jang Y.
- Writing - review & editing: Cho EY, Cho SY.
References
- 1.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 2.Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 3.Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011;14:308–313. doi: 10.4048/jbc.2011.14.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan B, Yao R, Shi J, Xu QQ, Zhou YD, Mao F, et al. Prognosis of subtypes of the mucinous breast carcinoma in Chinese women: a population-based study of 32-year experience (1983–2014) Oncotarget. 2016;7:38864–38875. doi: 10.18632/oncotarget.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010;63:1043–1047. doi: 10.1136/jcp.2010.082495. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010;222:282–298. doi: 10.1002/path.2763. [DOI] [PubMed] [Google Scholar]
- 7.Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical nomogram for predicting survival outcomes in early mucinous breast cancer. PLoS One. 2016;11:e0164921. doi: 10.1371/journal.pone.0164921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avisar E, Khan MA, Axelrod D, Oza K. Pure mucinous carcinoma of the breast: a clinicopathologic correlation study. Ann Surg Oncol. 1998;5:447–451. doi: 10.1007/BF02303864. [DOI] [PubMed] [Google Scholar]
- 9.Ross JS, Gay LM, Nozad S, Wang K, Ali SM, Boguniewicz A, et al. Clinically advanced and metastatic pure mucinous carcinoma of the breast: a comprehensive genomic profiling study. Breast Cancer Res Treat. 2016;155:405–413. doi: 10.1007/s10549-016-3682-6. [DOI] [PubMed] [Google Scholar]
- 10.Bartosch C, Mendes N, Rios E, Rodrigues M, Eloy C, Reis CA, et al. Morphological features and mucin expression profile of breast carcinomas with signet-ring cell differentiation. Pathol Res Pract. 2015;211:588–595. doi: 10.1016/j.prp.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Hull MT, Seo IS, Battersby JS, Csicsko JF. Signet-ring cell carcinoma of the breast: a clinicopathologic study of 24 cases. Am J Clin Pathol. 1980;73:31–35. doi: 10.1093/ajcp/73.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Kim HM, Kim EK, Koo JS. Mucinous carcinoma with extensive signet ring cell differentiation: a case report. J Pathol Transl Med. 2017;51:176–179. doi: 10.4132/jptm.2016.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung KM, Yeoh GP, Chan JK, Cheung PS, Chan KW. Ductal type signet ring cell carcinoma of breast with growth pattern of pure mucinous carcinoma. Pathology. 2011;43:282–284. doi: 10.1097/PAT.0b013e3283437cac. [DOI] [PubMed] [Google Scholar]
- 14.Baretta Z, Guindalini RS, Khramtsova G, Olopade OI. Resistance to trastuzumab in HER2-positive mucinous invasive ductal breast carcinoma. Clin Breast Cancer. 2013;13:156–158. doi: 10.1016/j.clbc.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Didonato R, Shapiro N, Koenigsberg T, D'Alfonso T, Jaffer S, Fineberg S. Invasive mucinous carcinoma of the breast and response patterns after neoadjuvant chemotherapy (NAC) Histopathology. 2018;72:965–973. doi: 10.1111/his.13451. [DOI] [PubMed] [Google Scholar]