Abstract
Purpose
Breast cancer (BC) is one of the most common malignancies globally, and millions of women worldwide are diagnosed with BC every year. Up to 70% of BC patients are estrogen receptor (ER)-positive. Numerous studies have shown that tamoxifen has a significant therapeutic effect on both primary and metastatic ER-positive BC patients. Although tamoxifen is currently one of the most successful therapeutic agents for BC, a significant proportion of patients will eventually become resistant to tamoxifen, leading to tumor recurrence and metastasis. Knowledge about the development of tamoxifen resistance in BC patients is still limited.
Methods
We applied a loss-and-gain method to study the biological functional role of cell division cycle associated 8 (CDCA8) in tamoxifen resistance in BC cells.
Results
We found that CDCA8 was significantly elevated in tamoxifen-resistant BC cells. Knockdown of CDCA8 expression significantly inhibited the proliferation of tamoxifen-resistant BC cells and reduced their resistance to tamoxifen. In contrast, overexpression of CDCA8 promoted the growth of tamoxifen-sensitive BC cells and induced their resistance to tamoxifen.
Conclusion
In this study, we reported that CDCA8 is a key regulator of tamoxifen resistance in BC, suggesting that CDCA8 may serve as a potential therapeutic target for BC treatment.
Keywords: Apoptosis; Breast neoplasms; Cell cycle; CDCA8 protein, human; Tamoxifen
INTRODUCTION
Breast cancer (BC) is the most common cancer among women and the second leading cause of cancer related death among women in the USA [1]. The incidence of BC has increased rapidly in recent years, and BC has now become the most common cancer in females in some major cities in China [2]. Although the underlying mechanism of BC development has been extensively studied, BC patients are still facing low survival rates. Therefore, development of novel and effective therapeutic treatments and drugs is very crucial. Tamoxifen, as a partial agonist of the estrogen receptor (ER), is an important component for the treatment of ER-positive BC [3]. It has been clinically used for the last 30 years and is currently available as a chemopreventive agent for women with high risk for BC. Like with most chemotherapy drugs, the most challenging issue with tamoxifen use is the development of resistance in an initially responsive breast tumor. Understanding the mechanism of tamoxifen resistance has important theoretical and clinical significance for development of new strategies for treating BC.
Unregulated cell proliferation and cell death (i.e., apoptosis, necroptosis, and pyroptosis) are known to contribute to tumorigenesis [4,5]. Cell division errors in cancer cells usually lead to aneuploidy [6]. Based on examination of mouse models with induced cell cycle disorder, increasing evidence suggests that chromosome missegregations may increase cancer occurrence [7]. The human cell division cycle associated 8 (CDCA8) gene is a member of the chromosomal passenger complex (CPC) and is indispensable for segregation of the chromosome during cell division [8]. During cytokinesis, the CPC, which is accumulated at the inner centromeres for bipolar spindle formation, promotes spindle midzone organization, regulates central spindle formation, and specifies the cleavage furrow [9,10]. Therefore, CDCA proteins play an important role in the regulation of cellular processes like growth, differentiation, tumor progression, and apoptosis [11,12]. CDCA8 is believed to be a putative oncogene that has been reported to be up-regulated in many types of cancer tissues (e.g., blade, lung, melanoma, and breast), but has a very low or absent expression in normal tissues [13,14,15,16]. Overexpression and nuclear accumulation of CDCA8 are linked to poor prognosis for cancer patients and are important for growth, survival, and the malignant nature of lung cancer [14], gastric cancer [13], and cutaneous melanoma [17]. In the present study, we found that CDCA8 was significantly elevated in tamoxifen-resistant BC cells (MCF-7/TamR and T47D/TamR), and knockdown of CDCA8 expression significantly inhibited cell proliferation and tamoxifen resistance of BC cells. On the contrary, CDCA8 overexpression significantly promoted proliferation of tamoxifen-sensitive BC cells (MCF-7 and T47D) and induced their resistance to tamoxifen. Taken together, these results indicate that CDCA8 is a key regulator of BC cellular resistance to tamoxifen.
METHODS
Cell culture
MCF-7 and T47D BC cells were obtained from the American Type Culture Collection (Manassas, USA). MCF-7 cells were cultured in Dulbecco's Modified Eagle's Medium (Sigma, St. Louis, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, USA). T47D cells were maintained in RPMI-1640 medium (Sigma) supplemented with 10% FBS. Cells were cultured at 37°C in an incubator with 5% CO2.
Establishment of tamoxifen-resistant cells
Tamoxifen-resistant MCF-7 and T47D cells (MCF-7/TamR and T47D/TamR, respectively) were established as previously reported [18,19]. Cells were cultured in phenol red-free RPMI-1640 containing 5% charcoal-stripped steroid depleted FBS (Gibco). Aliquots of 0.1 μM 4-OH tamoxifen were added to the medium after 24 hours culture. The concentration of 4-OH tamoxifen was gradually increased up to 1 μM. Tamoxifen-resistant cell lines were established when the growth of the cells could not be inhibited with 1 μM 4-OH tamoxifen for around 6 months of culture.
Western blotting
The cells were homogenized in chilled RIPA lysis buffer containing 10 mM Tris-HCl, 1% NP-40, 0.1% deoxycholic acid, 0.1% sodium dodecyl sulphate, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, and protease inhibitors (Sigma). The protein concentration was measured via BCA protein assay. Proteins (20–50 μg) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and subsequently transferred to a nitrocellulose membrane. Membranes were blocked with 5% non-fat dry milk for 1 hour and incubated with primary antibodies. Protein bands were detected by incubation with horseradish peroxidase conjugated antibodies and visualized with an enhanced chemiluminescence reagent. Antibodies for CDCA8 and β-actin detection were purchased from Abcam (Cambridge, USA).
Cell proliferation and soft agar colony formation assay
For cell proliferation assays, cells were trypsinized, counted, and plated into triplicate wells of 12-well plates at 1 × 104 cells per well with or without tamoxifen. Total cell number was counted using a hemocytometer once a day for 5 days.
For the colony formation assay, 1 mL of 0.5% solidified SeaPlaque agarose mixed with or without tamoxifen (Sigma) was added to each well of 6-well plates; then, 5 × 103 cells were mixed with 2 mL of 0.5% SeaPlaque mixed with or without tamoxifen and added onto the top of the wells. After 14 days of culture, colonies were fixed with 100% methanol and stained with 0.1% crystal violet. Colonies with a diameter of more than 1.5 mm were counted.
Establishment of stable cell lines
To establish stable cell lines, T47D and MCF-7 cells were infected with lentivirus carrying shRNA targeting CDCA8 (shCDCA8) or negative control precursor (Genecopoeia, Rockville, USA). Cells were selected by puromycin to obtain stable cell lines. To establish stable cell lines overexpressing CDCA8, T47D and MCF-7 cells were infected with pBABE retrovirus vector alone (or carrying CDCA8) followed by the selection with puromycin to obtain stable cell lines.
Cell cycle and apoptosis analysis
For cell cycle analysis, cells fixed with 70% ethanol and treated with RNase A were incubated in PBS containing propidium iodide (PI; ThermoFisher Scientific, Waltham, USA) on ice for 30 minutes. DNA content of each cell preparation was analyzed by flow cytometry with the CellQuest analysis program (BD Bioscience, Franklin Lakes, USA). For the cell apoptosis analysis, cells were suspended in staining buffer containing PI and fluorescein isothiocyanate-conjugated Annexin V (ThermoFisher Scientific) for 15 minutes. Annexin V-positive cells were counted as apoptotic cells.
Real-time polymerase chain reaction (PCR) assay
Total RNA was collected using Trizol Reagent (Life Technologies, Pleasanton, USA) and treated with DNase I. Next, 1 μg of total RNA was reverse transcribed in a 20-μL reaction using TaqMan Reverse Transcription Reagents (Life Technologies). Real-time PCR assay to detect CDCA8 mRNA levels was performed using the SYBR Green-qPCR Master Mix (Life Technologies).
Statistical analysis
The results were analyzed using SPSS 13 statistical software (SPSS., Chicago, USA). Quantitative variables were analyzed using Student's t-test between two groups or one- or two-way analysis of variance among multiple groups. The differences were considered significant at p < 0.05.
RESULTS
CDCA8 is upregulated in tamoxifen-resistant BC cell lines
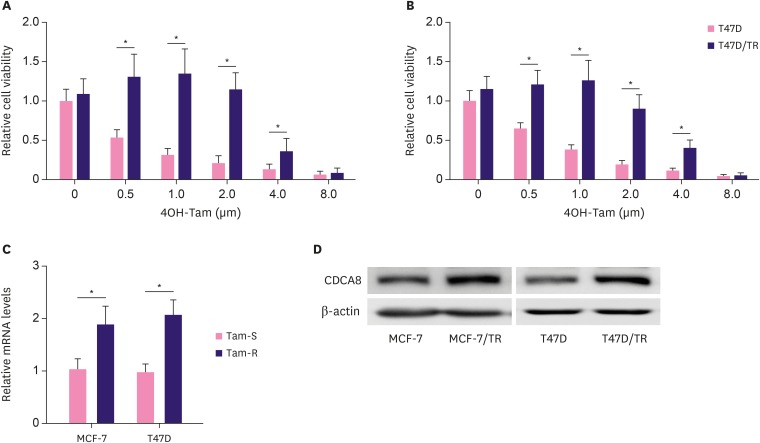
To establish tamoxifen-resistant BC cell lines, human BC cell lines, MCF-7 and T47D, were exposed to gradually increasing concentrations of 4OH-tamoxifen continuously for around 6-months and were maintained in 4OH-tamoxifen at a concentration of 1 μm, which is consistent with doses used in the clinical setting. The surviving cultures were named MCF-7/TR and T47D/TR. The IC50 of 4OH-tamoxifen-sensitive cell lines (MCF-7 and T47D) and 4OH-tamoxifen-resistant cell lines (MCF-7/TR and T47D/TR) was assessed by MTT assay. The IC50 of MCF-7 and T47D to 4OH-tamoxifen was 0.5 µm and 0.75 µm, respectively. In contrast, the IC50 of the resistant cell lines, MCF-7/TR and T47D/TR, was 3.8 μm and 4 μm, respectively (Figure 1A and B). Interestingly, we found that CDCA8 mRNA and protein expression levels were significantly upregulated in MCF-7/TR and T47D/TR than in MCF-7 and T47D (Figure 1C and D).
Figure 1. Upregulation of CDCA8 in Tam-resistant BC cells. (A, B) MCF-7, MCF-7/TR, T47D, and T47D/TR cell proliferation was determined by the MTT assay after 4OH-Tam treatment for 96 hr. (C) Quantitative reverse transcription-polymerase chain reaction analysis of CDCA8 mRNA levels in BC cells. Glyceraldehyde-3-phosphate dehydrogenase served as loading controls. (D) Western blotting analysis of CDCA8 levels in BC cells. β-actin served as loading controls. Data are presented as mean ± standard deviation from three independent experiments.
CDCA8 = cell division cycle associated 8; BC = breast cancer; Tam = tamoxifen.
*p < 0.01.
CDCA8 knockdown inhibits cell growth and decreases tamoxifen resistance of BC
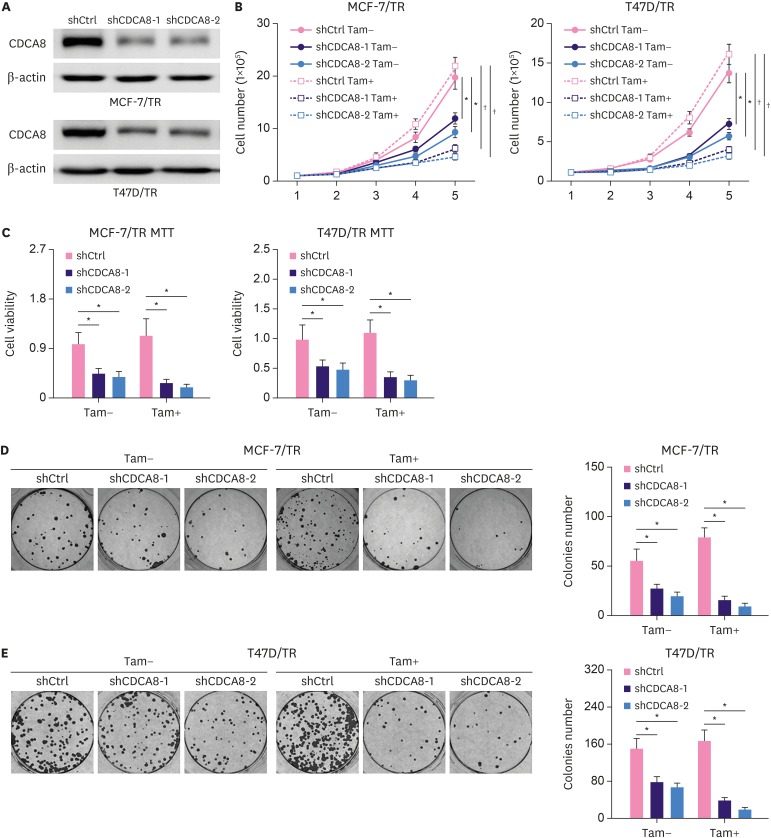
To test the effect of CDCA8 knockdown on 4OH-tamoxifen-resistant BC cell lines, MCF-7/TR and T47D/TR cells were infected with lentiviral carrying shCDCA8, and positive targeted cells were enriched by puromycin selection. A non-targeting shRNA sequence was used as control. Total protein was extracted from the cells and examined by western blotting analysis. The results demonstrated that shCDCA8 significantly reduced CDCA8 protein expression levels in MCF-7/TR and T47D/TR cells, and the knockdown efficiency was over 70% (Figure 2A). The function of CDCA8 in 4OH-tamoxifen resistance was explored by cell proliferation and colony formation assays. MCF-7/TR-shRNA control (shCtrl), MCF-7/TR-shCDCA8-1, MCF-7/TR-shCDCA8-2, T47D/TR-shCtrl, T47D/TR-shCDCA8-1, and T47D/TR-shCDCA8-2 cells were cultured with or without 4OH-tamoxifen. For the cell proliferation assay, the total number of cells in each group was counted every day for 5 days (Figure 2B), and MTT assay was performed on day 5 (Figure 2C). For the colony formation assay, cells were maintained in soft gel for 14 days, and the total cell colonies in each group were counted (Figure 2D and E). The results demonstrated that knockdown of CDCA8 in MCF-7/TR and T47D/TR decreased cell proliferation and colony formation rate and reduced the MCF-7/TR and T47D/TR resistance to 4OH-tamoxifen (Figure 2B-E).
Figure 2. Reduction of Tam resistance by knockdown of CDCA8 in Tam-resistant BC cells. (A) Western blotting assay for CDCA8 levels in Tam-resistant BC cells stably expressing shCtrl or shCDCA8. β-actin served as loading controls. (B) BC cells were grown in 6-well plates in media containing 10% serum and the cell number was determined at the indicated days with or without knockdown of CDCA8. (C) Cell proliferation of Tam-resistant BC cells was determined by the MTT assay after 4OH-Tam treatment for 5 days. (D) MCF-7/TR and (E) T47D/TR cells were subjected to cell colony formation assay. Tam+ and Tam− represents the culture media with and without 1 μM Tam, respectively. Data are presented as mean ± standard deviation from three independent experiments.
CDCA8 = cell division cycle associated 8; BC = breast cancer; shCtrl = shRNA control; shCDCA8 = shRNA targeting CDCA8; Tam = tamoxifen.
*p < 0.05; †p < 0.01.
Suppression of CDCA8 promotes cell apoptosis and cell cycle arrest
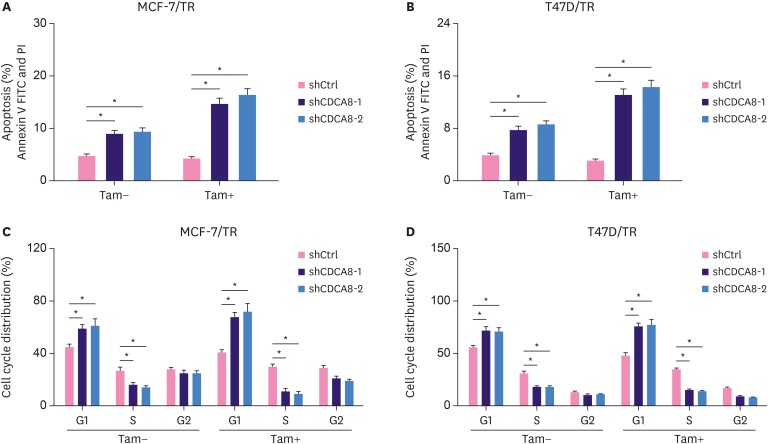
To investigate the effect of suppression of CDCA8 expression on cell apoptosis and cell cycle changes, MCF-7/TR-shCtrl, MCF-7/TR-shCDCA8-1, MCF-7/TR-shCDCA8-2, T47D/TR-shCtrl, T47D/TR-shCDCA8-1, and T47D/TR-shCDCA8-2 cells were cultured with or without 4OH-tamoxifen. Total cells were collected and subjected to flow cytometry analysis. Apoptosis results showed that knockdown of CDCA8 in MCF-7/TR and T47D/TR cells increased cell apoptosis from 5% and 4% to 10% and 8%, respectively. Moreover, when the MCF-7/TR- and T47D/TR-CDCA8 knockdown cell lines were treated with 4OH-tamoxifen, the cell apoptosis increased from 4.8% and 3.8% to 16% and 15%, respectively (Figure 3A and B). Similarly, the CDCA8 knockdown cells exhibited a clear tendency to accumulate in the G1 phase, and their number in the S phase of cell growth reduced compared with that in control groups. We observed a slight increase in cell arrest in the G1 phase and reduction of S phase cell populations when cells were treated with 4OH-tamoxifen (Figure 3C and D). The G2 phase cell populations among all groups were not significantly affected by CDCA8 knockdown treatment or CDCA8 knockdown plus 4OH-tamoxifen treatment.
Figure 3. Induction of cell apoptosis and cell cycle arrest in Tam-resistant BC cells by CDCA8 knockdown. MCF-7/TR-shCtrl, MCF-7/TR-shCDCA8-1, MCF-7/TR-shCDCA8-2, T47D/TR-shCtrl, T47D/TR-shCDCA8-1, and T47D/TR-shCDCA8-2 cells were cultured with or without 4OH-Tam. Total cells were collected and subjected to apoptosis (A, B) and cell cycle (C, D) analysis. Data represent mean ± standard deviation from three independent experiments with each measured in triplicate.
BC = breast cancer; CDCA8 = cell division cycle associated 8; shCtrl = shRNA control; shCDCA8 = shRNA targeting CDCA8; Tam = tamoxifen; FITC = fluorescein isothiocyanate.
*p < 0.01.
CDCA8 overexpression enhances cell growth and increases tamoxifen resistance of BC
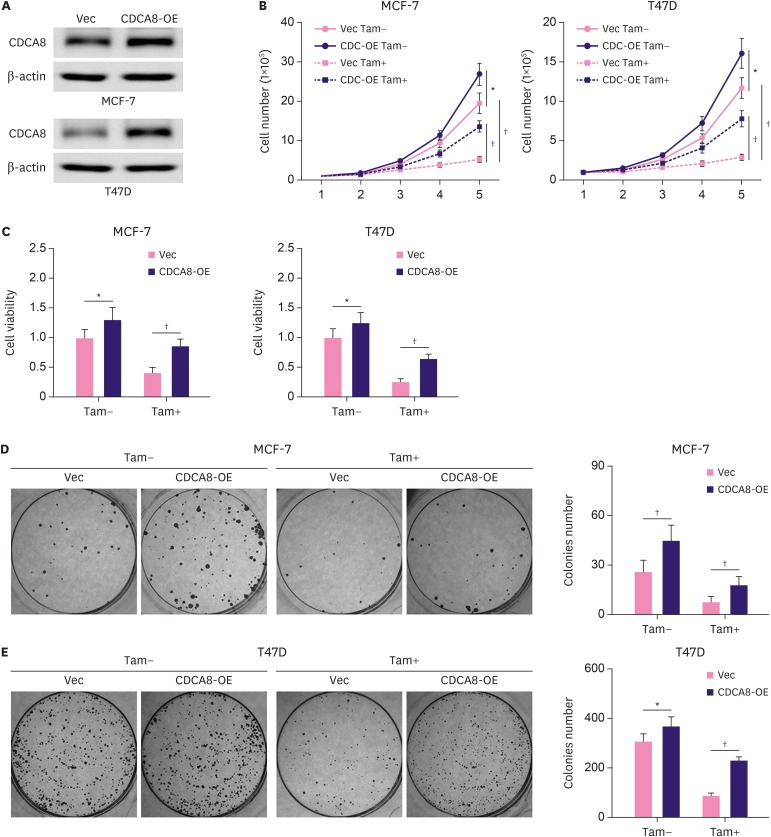
To study the effect of CDCA8 overexpression on cell growth and tamoxifen resistance of BC cells, MCF-7 and T47D cells were infected with retrovirus carrying CDCA8 or an empty vector as a control. The CDCA8 overexpression stable-cell lines were enriched by puromycin selection. Western blotting results showed that CDCA8 expression was robustly upregulated in MCF-7-CDCA8-OE and T47D-CDCA8-OE cells compared with that in control cells (MCF-7-Vec and T47D-Vec) (Figure 4A). The MCF-7-Vec, MCF-7-CDCA8-OE, T47D-Vec, and T47D-CDCA8-OE cells were subjected to cell growth assay and colony formation assay. The results (Figure 4B-E) demonstrated that overexpression of CDCA8 strongly promoted MCF-7 and T47D cell proliferation and colony formation, and also increased their resistance to 4OH-tamoxifen.
Figure 4. Induction of Tam resistance in BC cells by forced expression of CDCA8. MCF-7 and T47D cells were infected with retrovirus vector alone or carrying CDCA8. (A) Western blotting analysis of CDCA8 expression in MCF-7 and T47D cells. (B) BC cells were grown in 6-well plates in media containing 10% serum and the cell number was determined at the indicated days with or without forced expression of CDCA8. (C) MCF-7 and T47D cell proliferation was determined by the MTT assay after 4OH-Tam treatment for 5 days. (D) MCF-7 and T47D cells were subjected to cell colony formation assay. Tam+ and Tam− represent the culture media with and without 1 μM Tam, respectively. Data are presented as mean ± standard deviation from three independent experiments.
BC = breast cancer; CDCA8 = cell division cycle associated 8; Tam = tamoxifen.
*p < 0.05; †p < 0.01.
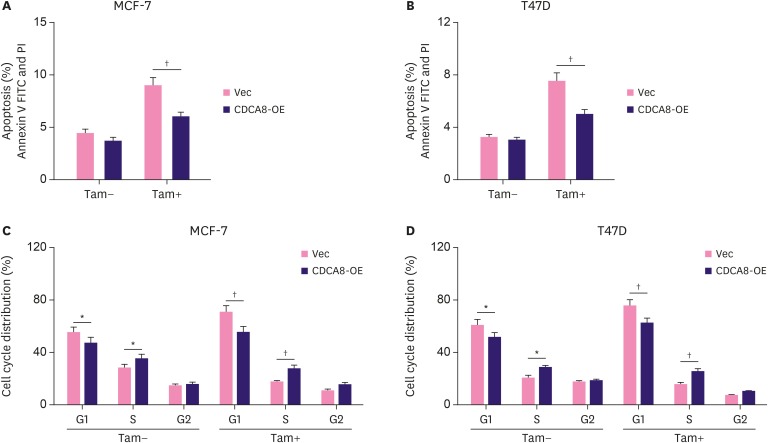
CDCA8 overexpression protects BC cells from tamoxifen-induced cell apoptosis and cell cycle arrest
The effect of CDCA8 overexpression on BC cell apoptosis and cell cycle changes was analyzed by flow cytometry assays. MCF-7-Vec, MCF-7-CDCA8-OE, T47D-Vec, and T47D-CDCA8-OE cells were cultured with or without 4OH-tamoxifen. Flow cytometry analysis showed that the apoptosis rate did not change in 4OH-tamoxifen untreated groups but significantly decreased in 4OH-tamoxifen-treated groups from 9% in MCF-7-Vec to 6% in MCF-7-CDCA8-OE, and from 7.5% in T47D-Vec to 4.5% in T47D-CDCA8-OE (Figure 5A and B). Cell cycle analysis showed that MCF-7-CDCA8-OE and T47D-CDCA8-OE cells exhibited more S phase population enrichment than MCF-7-Vec and T47D-Vec cells. Collectively, these results suggested that overexpression of CDCA8 protected MCF-7 and T47D against 4OH-tamoxifen-induced cell apoptosis and promoted cell cycle progression (Figure 5C and D).
Figure 5. Protection of Tam-sensitive BC cells from apoptosis and cell cycle arrest by forced expression of CDCA8. MCF-7-Vec, MCF-7-CDCA8-OE, T47D-Vec, and T47D-CDCA8-OE cells were cultured with or without 4OH-Tam. Total cells were collected and subjected to apoptosis (A, B) and cell cycle (C, D) analysis. Data represent mean ± standard deviation from three independent experiments which each measured in triplicate.
BC = breast cancer; CDCA8 = cell division cycle associated 8; Tam = tamoxifen; FITC = fluorescein isothiocyanate.
*p < 0.01; †p < 0.01.
DISCUSSION
BC is a hormone-dependent tumor, and estrogen, the primary female steroid hormone, is known to play a major role in the initiation and progression of BC. Since nearly 70% of breast tumors express ER and/or progesterone receptor, targeting the ER using ER antagonists or antiestrogens has been a reliable therapeutic measure for all stages of BC patients [3]. ER-mediated hormonal activity stimulates cell proliferation and rates of cell division, resulting in the induction of aneuploidy and accumulation of genetic damages. These events disrupt normal and proper cellular functions leading to carcinogenesis [20,21]. Tamoxifen, a selective ER that acts as an antagonist of ER, is the most commonly used chemotherapeutic agent for patients with ER-positive BC. In hormone-sensitive cases, tamoxifen impairs ER function by competing with estrogen for binding to the receptor, leading to inhibition of estrogenic effects, which are responsible for cancer cell growth and proliferation [22]. However, a substantial proportion of BC patients with localized disease or advanced disease who initially respond to tamoxifen develops de novo or acquired tamoxifen resistance. Although the molecular mechanisms of tamoxifen resistance in BC are not fully understood, different theories have been proposed. Loss of ER expression is the primary mechanism of tamoxifen resistance in some ER-positive BC patients who received prolonged ER antagonist treatment [23,24]. The differential pharmacological and metabolic activation of tamoxifen [25], the presence of cancer stem cells [26], and dysregulated tamoxifen-induced cell cycle progression [27] also contribute to the development of de novo or acquired tamoxifen resistance in BC patients.
CDCA8 overexpression is detected in various malignant tumors, including BC, and has been suggested to be closely associated with cancer cell proliferation and cell cycle progression [10,11]. However, whether CDCA8 is associated with tamoxifen resistance in BC has not been established. Initially, we observed that CDCA8 was significantly up-regulated in tamoxifen-resistant BC cells and CDCA8 expression could be induced by tamoxifen treatment. This observation led us to hypothesize that CDCA8 may be a crucial mediator of tamoxifen-resistant BC cells. The effect of inhibiting CDCA8 expression on the growth of tamoxifen-resistant cells or on tamoxifen-induced cell cycle progression has not been evaluated previously. In this study, we found that knockdown of CDCA8 in tamoxifen-resistant BC cells reduced cell proliferation and colony formation and restored cell sensitivity to tamoxifen. Moreover, we established the role of CDCA8 in the acquisition of tamoxifen resistance. We found that stable expression of CDCA8 not only enhanced the rate of cell proliferation and colony formation of BC cells but also promoted BC cells to acquire resistance to tamoxifen. The mechanism responsible for the phenomena of CDCA8-mediated cell proliferation and tamoxifen resistance is, at least, partially due to the effect of CDCA8 expression on inhibition of cell apoptosis and promotion of cell cycle progression. Our results showed that inhibition of CDCA8 expression led to increases in tamoxifen-resistant BC cell apoptosis and cell cycle arrest under tamoxifen treatment. On the contrary, forced expression of CDCA8 partially prevented tamoxifen-sensitive BC cell apoptosis and cell cycle arrest. In summary, these data together suggest that CDCA8 plays a key role in the cell growth and tamoxifen resistance of ER-positive BC cells. Blocking CDCA8 expression may represent a more specific and less toxic approach for treating ER-positive BC and preventing both tamoxifen flares and development of tamoxifen resistance.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Yu D.
- Data curation: Yu D, Bu Y.
- Formal analysis: Shi L, Bu Y.
- Funding acquisition: Shi L.
- Writing - original draft: Li W.
- Writing - review & editing: Li W.
References
- 1.Seely JM, Alhassan T. Screening for breast cancer in 2018-what should we be doing today? Curr Oncol. 2018;25:S115–S124. doi: 10.3747/co.25.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu TY, Liu YL, Chung S. Improving breast cancer outcomes among women in China: practices, knowledge, and attitudes related to breast cancer screening. Int J Breast Cancer. 2012;2012:921607. doi: 10.1155/2012/921607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang M. Tamoxifen resistance in breast cancer. Biomol Ther (Seoul) 2012;20:256–267. doi: 10.4062/biomolther.2012.20.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Labi V, Erlacher M. How cell death shapes cancer. Cell Death Dis. 2015;6:e1675. doi: 10.1038/cddis.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giam M, Rancati G. Aneuploidy and chromosomal instability in cancer: a jackpot to chaos. Cell Div. 2015;10:3. doi: 10.1186/s13008-015-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ly P, Cleveland DW. Interrogating cell division errors using random and chromosome-specific missegregation approaches. Cell Cycle. 2017;16:1252–1258. doi: 10.1080/15384101.2017.1325047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 9.Haase J, Bonner MK, Halas H, Kelly AE. Distinct roles of the chromosomal passenger complex in the detection of and response to errors in kinetochore-microtubule attachment. Dev Cell. 2017;42:640–654.e5. doi: 10.1016/j.devcel.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol. 2012;13:789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan NN, Wang CY, Li KL, Chen CF, Chiao CC, Yu HG, et al. Distinct expression of CDCA3, CDCA5, and CDCA8 leads to shorter relapse free survival in breast cancer patient. Oncotarget. 2018;9:6977–6992. doi: 10.18632/oncotarget.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi Y, Chen S, Jiang J, Yao J, Wang G, Zhou Q, et al. CDCA8 expression and its clinical relevance in patients with bladder cancer. Medicine (Baltimore) 2018;97:e11899. doi: 10.1097/MD.0000000000011899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JL, Chen TH, Wang CF, Chiang YH, Huang YL, Wong FH, et al. Borealin/Dasra B is a cell cycle-regulated chromosomal passenger protein and its nuclear accumulation is linked to poor prognosis for human gastric cancer. Exp Cell Res. 2006;312:962–973. doi: 10.1016/j.yexcr.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Hayama S, Daigo Y, Yamabuki T, Hirata D, Kato T, Miyamoto M, et al. Phosphorylation and activation of cell division cycle associated 8 by aurora kinase B plays a significant role in human lung carcinogenesis. Cancer Res. 2007;67:4113–4122. doi: 10.1158/0008-5472.CAN-06-4705. [DOI] [PubMed] [Google Scholar]
- 15.Ju W, Yoo BC, Kim IJ, Kim JW, Kim SC, Lee HP. Identification of genes with differential expression in chemoresistant epithelial ovarian cancer using high-density oligonucleotide microarrays. Oncol Res. 2009;18:47–56. doi: 10.3727/096504009789954672. [DOI] [PubMed] [Google Scholar]
- 16.Narayan G, Bourdon V, Chaganti S, Arias-Pulido H, Nandula SV, Rao PH, et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer. 2007;46:373–384. doi: 10.1002/gcc.20418. [DOI] [PubMed] [Google Scholar]
- 17.Ci C, Tang B, Lyu D, Liu W, Qiang D, Ji X, et al. Overexpression of CDCA8 promotes the malignant progression of cutaneous melanoma and leads to poor prognosis. Int J Mol Med. 2019;43:404–412. doi: 10.3892/ijmm.2018.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Liu Y, Zhang C, Chu J, Wu Y, Li Y, et al. Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nat Commun. 2018;9:1595. doi: 10.1038/s41467-018-03951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lü M, Ding K, Zhang G, Yin M, Yao G, Tian H, et al. MicroRNA-320a sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by targeting ARPP-19 and ERRγ. Sci Rep. 2015;5:8735. doi: 10.1038/srep08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53:217–227. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 22.Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di GH, et al. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007;9:R76. doi: 10.1186/bcr1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viedma-Rodríguez R, Baiza-Gutman L, Salamanca-Gómez F, Diaz-Zaragoza M, Martínez-Hernández G, Ruiz Esparza-Garrido R, et al. Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review) Oncol Rep. 2014;32:3–15. doi: 10.3892/or.2014.3190. [DOI] [PubMed] [Google Scholar]
- 24.Bostner J, Karlsson E, Pandiyan MJ, Westman H, Skoog L, Fornander T, et al. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res Treat. 2013;137:397–406. doi: 10.1007/s10549-012-2376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hultsch S, Kankainen M, Paavolainen L, Kovanen RM, Ikonen E, Kangaspeska S, et al. Association of tamoxifen resistance and lipid reprogramming in breast cancer. BMC Cancer. 2018;18:850. doi: 10.1186/s12885-018-4757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ojo D, Wei F, Liu Y, Wang E, Zhang H, Lin X, et al. Factors promoting tamoxifen resistance in breast cancer via stimulating breast cancer stem cell expansion. Curr Med Chem. 2015;22:2360–2374. doi: 10.2174/0929867322666150416095744. [DOI] [PubMed] [Google Scholar]
- 27.Kilker RL, Planas-Silva MD. Cyclin D1 is necessary for tamoxifen-induced cell cycle progression in human breast cancer cells. Cancer Res. 2006;66:11478–11484. doi: 10.1158/0008-5472.CAN-06-1755. [DOI] [PubMed] [Google Scholar]