Abstract
OBJECTIVES:
The effectiveness of stool-based colorectal cancer (CRC) screening is contingent on colonoscopy completion in patients with an abnormal fecal immunochemical test (FIT). Understanding system and patient factors affecting follow-up of abnormal screening tests is essential to optimize care for high-risk cohorts.
METHODS:
This retrospective cohort study was conducted in an integrated safety-net system comprised of 11 primary-care clinics and one Gastroenterology referral unit and included patients 50–75 years, with a positive FIT between April 2012 and February 2015.
RESULTS:
Of the 2,238 patients identified, 1,245 (55.6%) completed their colonoscopy within 1-year of the positive FIT. The median time from positive FIT to colonoscopy was 184 days (interquartile range 140–232). Of the 13% of FIT positive patients not referred to gastroenterology, 49% lacked documentation addressing their abnormal result or counseling on the increased risk of CRC. Of the patients referred but who missed their appointments, 62% lacked documentation following up on the abnormal result in the absence of a completed colonoscopy. FIT positive patients never referred to gastroenterology or who missed their appointment after referrals were more likely to have comorbid conditions and documented illicit substance use compared with patients who completed a colonoscopy.
CONCLUSIONS:
Despite access to colonoscopy and a shared electronic health record system, colonoscopy completion after an abnormal FIT is inadequate within this safety-net system. Inadequate follow-up is in part explained by inappropriate screening, but there is an absence of clear documentation and systematic workflow within both primary care and GI specialty care addressing abnormal FIT results.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States (1). Despite evidence that screening is effective in reducing CRC related mortality (2–4), screening remains underutilized in the general population (5), especially among racial/ethnic minorities and low-income populations (6,7). These patients are disproportionately cared for in safety-net health care settings (8,9) and because screening is low, CRC stage is often late when diagnosed (10). When screening is utilized in the safety-net population, evidence suggests a patient preference for non-invasive methods (11,12). Owing to both limited colonoscopy resources (13) and patient preference, many safety-net health systems have promoted non-invasive screening methods such as fecal immunochemical tests (FIT). Multiple population-based studies support the use of stool-based colon cancer screening to reduce CRC-related mortality (2–4,14–16).
Implicit in a stool-based screening strategy is that a positive test must be followed by a diagnostic colonoscopy (3,4), Given these patients carry a high risk of CRC (17,18), if a patient does not complete a colonoscopy, an informed discussion should occur with documentation of the risk of late cancer detection associated with non-adherence. California Public hospital Redesign and Incentives in Medi-Cal (PRIME), a pay-for-performance delivery system transformation and alignment program began including follow-up of abnormal stool tests as a novel quality metric in 2016 (ref. 19). We examined the colonoscopy completion rate 1 year following an abnormal FIT in an integrated safety-net system to understand the facilitators and barriers to colonoscopy completion. In addition, we sought to understand how care is coordinated between primary and specialty care and whether abnormal FIT results, in the absence of a colonoscopy, are addressed in the electronic health record (EHR).
METHODS
Study Design and Population
This retrospective cohort study was performed in a dynamic population served by the San Francisco Health Network (SFHN). SFHN is an integrated safety-net health system of 11 community-and hospital-based primary care clinics and one specialty medical center, Zuckerberg San Francisco General Hospital (ZSFG) (20), providing services to low-income populations in San Francisco. These clinics share an integrated EHR, a clinical laboratory, and one Gastroenterology (GI) referral unit at ZSFG. In 2014–2015, 68% of outpatients and 58% of inpatients were either uninsured or covered by Medi-Cal, California’s state health program for low-income and resource-limited residents (21).
The study cohort included men and women, 50–75 years old with an abnormal FIT from institution of FIT testing in 2012 until February 2015. Colonoscopy data were collected through February 2016, allowing at least 1 year of follow-up for each patient.
Data Sources
SFHN is supported by E-Clinical Works (eCW) as its primary EHR platform, which is linked to other data sources such as the clinical laboratory and gastroenterology procedures. Demographic information, clinic details, and laboratory data of individuals with abnormal FIT results were abstracted. Receipt of colonoscopy was confirmed using the endoscopy software ProVation (ProVation Medical Inc., Minneapolis, MN, USA).
Process for CRC Screening
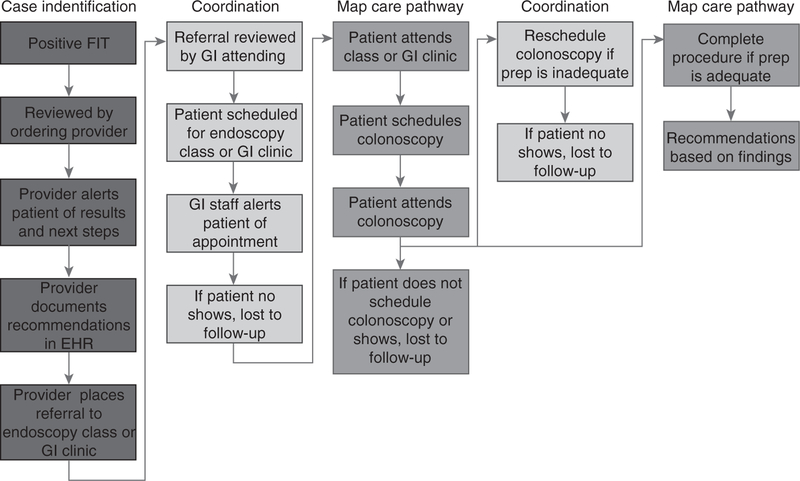
Since 2012, FIT has been promoted as the primary CRC screening modality for average-risk patients in SFHN, whereas colonoscopy is recommended for patients at increased risk for CRC (e.g., family history, personal history of polyps, etc.). In this CRC screening process (Figure 1), patients are provided a FIT kit with return postage at a point of care visit, perform the stool based screening test at home, and mail the FIT kit to a central laboratory located at ZSFG. The laboratory uses a qualitative FIT platform, OC-Sensor (Eiken Chemical Co., Tokyo, Japan) with a cutoff of 50 μg hemoglobin/g of stool. A positive FIT is automatically flagged and enters the primary care provider’s ‘inbox’ for review. Most results are routed to the primary care provider, but in some clinics, nurses or medical assistants provide panel management around CRC screening. This includes notifying patients of abnormal results and electronically referring patients to gastroenterology for colonoscopy under the supervision of the provider. Our study predates process of care changes implemented in September 2015 to minimize steps between positive FIT and colonoscopy.
Figure 1.
Abnormal fecal immunochemical test (FIT) follow-up workflow. Current workflow in addressing abnormal FIT results, from positive result until colonoscopy completion. A full color version of this figure is available at the American Journal of Gastroenterology journal online.
Referral Process for Positive FIT
SFHN uses an electronic referral platform for all GI clinic referrals (22–24), with few adverse outcomes reported in this communication process (25). A staff gastroenterologist evaluates referrals typically within 72 h, and the patients either attend a clinic appointment or a group colonoscopy class, as appropriate. For Spanish- and Cantonese-speaking patients who have a positive FIT, referring providers can request an appointment for a group class led by a GI nurse practitioner. The class, offered separately in English, Spanish, and Cantonese explains the meaning of the abnormal result, the risks and benefits of colonoscopy, and the steps to prepare for the procedure. The colonoscopy is scheduled at the conclusion of the nurse practitioner led group class.
Referring providers are asked to refer patients with comorbidities, increased cardiovascular or sedation risk, and those who speak languages not provided in the class to the gastroenterology clinic. A staff gastroenterologist reviews these referrals and the patient is scheduled for a one-on-one pre-procedure GI clinic visit to discuss the topics addressed in class, including the potential need for anesthesia. Patients are consented by the gastroenterologist and scheduled for colonoscopy with sedation method chosen by the provider.
Data Collection for Chart Review
After defining the process from positive FIT to colonoscopy completion, a random sample of 200 patients from the cohort were identified from the points of highest dropout using a Microsoft Access randomization tool. These were patients never referred for a colonoscopy after the abnormal result and patients who missed their colonoscopy appointment after referral. An additional 100 patients who completed a colonoscopy within 1 year of the positive FIT were also sampled. Two independent reviewers (SO & VL) manually reviewed medical records for these 300 patients and findings verified by RI. The medical record was abstracted for primary care clinic visits, documentation of the abnormal result and if counseling regarding cancer risk occurred. Counseling was defined as a provider-patient documented discussion about the abnormal FIT result, the importance of colonoscopy follow-up, and the increased risk for prevalent CRC following an abnormal FIT. Homelessness, polysubstance abuse, as well as comorbidities defined as the presence of any of the following: coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, human immunodeficiency virus/acquired immune deficiency syndrome, and active cancer were also abstracted.
Statistical Analysis
In the larger cohort as well as the random chart review, patient demographic information was described as proportions, except age, which was described using means and s.d.’s. Days to colonoscopy completion were described using medians and interquartile ranges (IQR). Differences between groups of patients were assessed using χ2 and Student’s t-test, as appropriate. Univariate logistic regression was performed with colonoscopy completion as the outcome. Multivariable analysis was performed to determine the factors associated with colonoscopy completion after adjusting for age, gender, race, marital status, insurance type, primary language and clinic. Accompanying odds ratios (OR), 95% confidence interval (CI), and P values were reported in all instances and P values <0.05 were considered statistically significant. We used Stata/SE (version 14.0; StataCorp LP, College Station, TX, USA) statistical software for all analyses.
RESULTS
Patient Demographics and Colonoscopy Completion Rate
A total of 2,238 patients with a positive FIT met inclusion criteria. The mean age was 59, 52% were male, and the cohort was racially diverse (38% Asian, 22% Caucasian, 20% Black, and 18% Hispanic). 34% were married and 44% identified English as their primary language. Of the cohort, 1,245 (55.6%) completed a colonoscopy within 1 year, ranging from 28–76% across the 11 clinics (Table 1).
Table 1.
Characteristics of FIT-positive patients who completed colonoscopy within 1 year from a positive FIT
| Overall | No colonoscopy N=993 (44%) | Colonoscopy N=1,245 (56%) | OR | 95%CI | P value | |
|---|---|---|---|---|---|---|
| Age, mean±s.d. | 59.6±6.0 | 59.8±6.4 | 59.4±5.8 | 0.99 | 0.98–1.00 | 0.19 |
| Gender, n (%) | ||||||
| Male | 1,165 (52.1%) | 584 (50.1%) | 581 (49.9%) | Ref. | – | – |
| Female | 1,073 (47.9%) | 409 (38.1%) | 664 (61.9%) | 1.63 | 1.38–1.93 | <0.01 |
| Marital status a | ||||||
| Single | 997 (44.5%) | 522 (52.4%) | 475 (47.6%) | Ref. | – | – |
| Married | 770 (34.4%) | 248 (32.2%) | 522 (67.8%) | 2.31 | 1.90–2.81 | <0.01 |
| Separated/divorced | 323 (14.4%) | 148 (45.8%) | 175 (54.2%) | 1.30 | 1.01–1.67 | 0.04 |
| Widowed | 110 (4.9%) | 47 (42.7%) | 63 (57.3%) | 1.47 | 0.99–2.19 | 0.05 |
| Race | ||||||
| White | 495 (22.2%) | 255 (51.5%) | 240 (48.5%) | Ref. | — | — |
| Asian | 852 (38.1%) | 289 (33.9%) | 563 (66.1%) | 2.07 | 1.65–2.60 | <0.01 |
| Hispanic | 399 (17.8%) | 182 (45.6%) | 217 (54.4%) | 1.27 | 0.97–1.65 | 0.08 |
| Black | 436 (19.5%) | 240 (55.1%) | 196 (44.9%) | 0.87 | 0.67–1.12 | 0.28 |
| Other | 56 (2.5%) | 27 (48.2%) | 29 (51.8%) | 1.14 | 0.66–1.98 | 0.64 |
| Language | ||||||
| English | 992 (44.3%) | 626 (50.2%) | 620 (49.8%) | Ref. | – | – |
| Non-English | 1246 (55.7%) | 367 (37.0%) | 625 (63.0%) | 1.72 | 1.45–2.04 | <0.01 |
| Insurance | ||||||
| Medicare | 668 (29.8%) | 327 (49%) | 341 (51%) | Ref. | – | – |
| Medi-Cal | 923 (41.2%) | 442 (47.9%) | 481 (52.1%) | 1.04 | 0.86–1.27 | 0.67 |
| Healthy Workerb | 390 (17.4%) | 101 (25.9%) | 289 (74.1%) | 2.74 | 2.09–3.60 | <0.01 |
| Healthy SFc | 166 (7.4%) | 76 (45.8%) | 90 (54.2%) | 1.13 | 0.81–1.60 | 0.46 |
| Commercial | 11 (0.50) | 1 (9.1%) | 10 (90.9%) | 9.59 | 1.22–75.3 | 0.03 |
| Uninsured | 80 (3.6%) | 46 (57.5%) | 34 (42.5%) | 0.71 | 0.44–1.13 | 0.15 |
| Clinic | ||||||
| A | 88 (3.9%) | 63 (71.6%) | 25 (28.4%) | Ref. | ||
| B | 187 (8.4%) | 120 (64.2%) | 67 (35.8%) | 1.41 | 0.81–2.44 | 0.23 |
| C | 171 (7.6%) | 105 (61.4%) | 66 (38.6%) | 1.58 | 0.91–2.76 | 0.11 |
| D | 389 (17.4%) | 184 (47.3%) | 205 (52.7%) | 2.81 | 1.70–4.65 | <0.01 |
| E | 363 (16.2%) | 160 (44.1%) | 203 (55.9%) | 3.20 | 1.92–5.31 | <0.01 |
| F | 107 (4.8%) | 47 (43.9%) | 60 (56.1%) | 3.22 | 1.76–5.86 | <0.01 |
| G | 133 (5.9%) | 58 (43.6%) | 75 (56.4%) | 3.26 | 1.83–5.79 | <0.01 |
| H | 188 (8.4%) | 71 (37.8%) | 117 (62.2%) | 4.15 | 2.40–7.19 | <0.01 |
| I | 298 (13.3%) | 101 (33.9%) | 197 (66.1%) | 4.91 | 2.92–8.28 | <0.01 |
| J | 138 (6.2%) | 41 (29.7%) | 97 (70.3%) | 5.96 | 3.31–10.7 | <0.01 |
| K | 176 (7.9%) | 43 (24.4%) | 133 (75.6%) | 7.79 | 1.38–13.9 | <0.01 |
CI, confidence interval; FIT, fecal immunochemical test; SF, San Francisco; OR, odds ratios.
Marital status missing for 38 patients.
Health insurance administered by San Francisco Health Plan.
Health insurance operated by the San Francisco Department of Public Health.
Process of Care After Positive FIT
Of all patients with an abnormal FIT (n=2,238), 13% were never referred to the integrated GI group at ZSFG for follow-up. Of the 87% of patients who were referred (n=1,947), 24.9% (n=485) were scheduled for a GI clinic consultation and the remaining 75.1% (n=1,462) were scheduled for direct access colonoscopy class. Among scheduled patients, 25% (n=486) missed their appointment and 75% (n=1,461) attended a GI encounter after an abnormal FIT. Of the patients who attended a GI encounter, 85% (n=1,245) completed a colonoscopy within 1 year of this result (Figure 2), which is 55.6% of the original cohort with an abnormal FIT. The median time to colonoscopy completion for these patients was 184 days (IQR 140–232).
Figure 2.
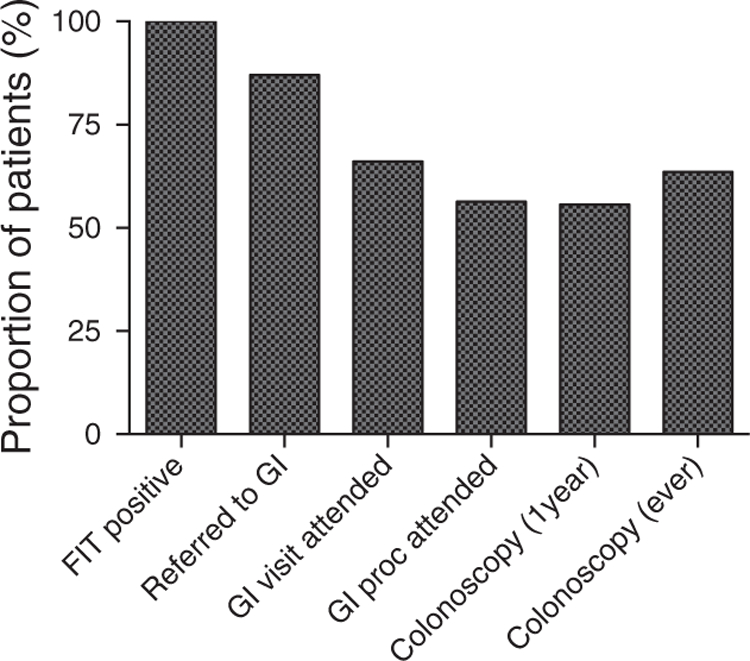
Proportion of patients by fecal immunochemical tests (FIT) process of care. Proportion of patients remaining after each step in the process of care from positive FIT to colonoscopy completion. A full color version of this figure is available at the American Journal of Gastroenterology journal online.
The median time to colonoscopy by type of referral completed was 186 days (IQR 143–232) for those referred to GI Clinic and 198 days (IQR 143–257) for those referred to direct access class. Despite a longer median time to colonoscopy, patients referred to direct access colonoscopy class were more likely than GI clinic referrals to complete their diagnostic colonoscopy within 1 year (61.2% vs. 54.9%, P<0.001). Non-English speaking patients were more likely to attend class after referral (57.4% vs. 51.7%, P=0.018) and also more likely to complete a colonoscopy if they showed to class (81.8% vs. 71.6%, P=<0.001).
Characteristics Associated with Colonoscopy Completion
Women were more likely than men to complete their colonoscopy within 1 year after an abnormal FIT (62% vs. 50%, OR 1.63, CI 1.38–1.93 p <0.001). Married patients were more likely to complete a colonoscopy compared to single patients (68% vs. 48%, OR 2.31, CI 1.90–2.81, P<0.001) or patients separated or divorced (68% vs. 54%, OR 1.30, CI 1.01–1.67, P=0.04). Asian patients had the highest proportion of follow-up colonoscopy compared with all other races (66% vs. 45–54%, OR 2.07, CI 1.65–2.59, P<0.001) and non-English speakers were more likely to complete their colonoscopy when compared to English speakers (63% vs. 50%, OR 1.72, CI 1.45–2.04, P<0.001; Table 1). The two highest performing clinics had colonoscopy completion rates of 70% and 76% and colonoscopy completion by race ranged from 61 to 81% in these clinics compared to 44–63% in the remaining 9 clinics, but this difference did not reach statistical significance (Supplementary Table 1).
After adjusting for the following variables (age, gender, race, marital status, insurance type, primary language and clinic), each additional year in age was associated with a 2.0% decreased odds of colonoscopy completion (OR 0.98, CI 0.96–0.99, P=0.014). Female patients had 1.38 times the odds of completing a colonoscopy compared with male patients (CI 1.14–1.66, P<0.001) and those who were married had 1.55 times the odds of completing a colonoscopy compared with those who were single, separated, or divorced (CI 1.21–1.99, P<0.001). Healthy Worker, an insurance program offered to select employees of the City and County of San Francisco, (OR 1.65, CI 1.20–2.25, P=0.002) and Asian race (OR 1.39, CI 1.03–1.86 P=0.027) remained positively associated with colonoscopy completion (Table 2).
Table 2.
Multivariate logistic regression of characteristics associated with colonoscopy completion 1 year from a positive FIT
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (years) | 0.98 | 0.96–0.99 | 0.014 |
| Gender | |||
| Male | Ref. | ||
| Female | 1.38 | 1.14–1.66 | 0.001 |
| Marital status | |||
| Single | Ref. | ||
| Married | 1.55 | 1.21–1.99 | <0.001 |
| Separated/divorced | 1.14 | 0.88–1.49 | 0.324 |
| Widowed | 1.15 | 0.75–1.76 | 0.513 |
| Race | |||
| White | Ref. | ||
| Asian | 1.39 | 1.03–1.86 | 0.027 |
| Hispanic | 1.21 | 0.89–1.66 | 0.226 |
| Black | 1.09 | 0.81–1.47 | 0.561 |
| Other | 1.24 | 0.68–2.26 | 0.471 |
| Language | |||
| English | Ref. | ||
| Non-English | 0.99 | 0.78–1.25 | 0.938 |
| Insurance | |||
| Medicare | Ref. | ||
| Medi-Cal | 0.99 | 0.78–1.24 | 0.906 |
| Healthy Workera | 1.65 | 1.20–2.25 | 0.002 |
| Healthy SFb | 0.82 | 0.56–1.21 | 0.327 |
| Commercial | 5.64 | 0.70–45.64 | 0.105 |
| Uninsured | 0.74 | 0.44–1.21 | 0.226 |
| Clinic | |||
| A | Ref. | ||
| B | 1.29 | 0.73–2.27 | 0.379 |
| C | 1.27 | 0.71–2.29 | 0.417 |
| D | 1.78 | 1.04–3.04 | 0.036 |
| E | 2.26 | 1.33–3.84 | 0.002 |
| F | 2.48 | 1.34–4.60 | 0.004 |
| G | 2.35 | 1.29–4.28 | 0.005 |
| H | 3.50 | 1.97–6.21 | <0.001 |
| I | 2.19 | 1.21–3.95 | 0.009 |
| J | 3.40 | 1.84–6.30 | <0.001 |
| K | 4.05 | 2.18–7.50 | <0.001 |
CI, confidence interval; FIT, fecal immunochemical test; SF, San Francisco; OR, odds ratios.
Health insurance administered by San Francisco Health Plan.
Health insurance operated by the San Francisco Department of Public Health.
Documented Reasons for Missed Colonoscopy after a Positive FIT
Charts from 300 patients (100 never referred to GI, 100 who were referred but missed their GI appointment, and 100 who completed their colonoscopy) were sampled for review. Of the patients who were never referred or who missed their appointment after referral, 84% were seen by a primary care provider within 1 year of the abnormal FIT and 60% of providers commented on this abnormal result in their note.
Of the patients never referred to GI after an abnormal FIT (n=100), 23% had documentation of counseling on the importance of follow up of colonoscopy, 9% completed the colonoscopy at an outside facility, 5% declined a colonoscopy, and the provider erroneously documented a positive test as negative in 4 cases. In addition, there were patients in whom CRC screening was not indicated and the patient was provided and completed a FIT in error (n=10). FIT was considered inappropriate if documented as such by the care provider in a follow-up visit or if the patient had a documented projected survival of <10 years. Excluding the reasons above (e.g., appropriate counseling, outside colonoscopy, declined colonoscopy, and documentation errors), 49% of patients not referred to GI after an abnormal FIT lacked documentation of counseling from the ordering provider regarding this result.
Of the sampled patients who missed their appointment after referral (n=100), 28% had documentation of counseling, 4% patients declined a colonoscopy and 1 patient had a colonoscopy completed at an outside facility. In 2 cases, the provider erroneously documented a positive test as negative, and 3 patients in whom CRC screening was not indicated received a FIT in error. Excluding the reasons above, 62% of patients who missed their GI appointment after referral lacked documentation of counseling regarding this abnormal result.
A total of 34 patients who were never referred and an additional 34 patients who missed their referral appointment had a documented comorbidity compared to 18 patients who completed a colonoscopy (P=0.005). Patients who never completed a colonoscopy were also more likely to have social issues (i.e. unstable housing and polysubstance abuse), an admission to the hospital after an abnormal FIT, and use chronic opiates when compared with patients who completed a colonoscopy (Table 3).
Table 3.
Comorbidities and social history of FIT positive patients not referred and or with missed appointments compared to patients who completed colonoscopy within 1 year from a positive FIT
| Colonoscopy N=100 (%) | No referral N=100(%) | Missed apt N=100 (%) | P value | |
|---|---|---|---|---|
| CHF | 0 | 6 | 2 | 0.028 |
| COPD | 4 | 8 | 8 | 0.424 |
| CAD | 2 | 10 | 5 | 0.047 |
| HIV/AIDS | 3 | 8 | 13 | 0.033 |
| Cancer | 9 | 7 | 13 | 0.343 |
| Admission post FIT | 7 | 20 | 18 | 0.021 |
| Homelessness | 2 | 6 | 6 | 0.302 |
| Substance abuse | 10 | 16 | 22 | 0.069 |
| Opiate use | 10 | 18 | 21 | 0.094 |
CAD, Coronary Artery Disease; CHF, Congestive Heart Failure; COPD, Chronic Obstructive Pulmonary Disease; FIT, fecal immunochemical test; HIV/AIDS, Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome.
DISCUSSION
Incomplete follow-up of patients with abnormal FITs is an important problem with reported cancer prevalence in these cohorts ranging from 3.4 to 6.1% (14,26). Despite this increased risk of CRC, inadequate colonoscopy completion has been noted in different settings (27–30) with limited data on safety-net systems, which often promote the use of non-invasive CRC screening (31). In this relatively robust integrated safety-net system, only 56% of FIT positive patients completed a colonoscopy within 1 year of their abnormal result. We found that being married (32), female (33), Asian and a non-English speaker were all positively associated with colonoscopy completion, but the significance of language did not persist in the multivariable analysis. The reported effect of Asian race and non-English speaking on CRC screening compliance varies in the literature, but prior data from this group support our findings (11,34). The observed effect of race and language on colonoscopy completion likely reflects a population of immigrants, who given the opportunity, are more likely to access any available resources.
By examining the cohort of patients with abnormal FIT tests, we discovered an absence of clear documentation addressing abnormal results and failure to close the loop of care suggesting opportunities for enhanced tracking, communication, and documentation to improve care quality. This work is timely because follow-up of abnormal stool tests is a novel quality metric tied to incentives in the 2016 California Public hospital Redesign and Incentives in Medi-Cal (PRIME), a pay-for-performance delivery system transformation and alignment program (19).
Inappropriate CRC screening also contributes to incomplete follow-up of abnormal results (35). In our study, 10% of patients not referred to GI for follow-up completed a FIT in error. Indeed, interventions at the patient-level should emphasize counseling and development of educational material that highlight the need for colonoscopy if FIT result is abnormal as these individuals carry ~3% risk of coexisting CRC. Improving follow-up of abnormal results will require a multilevel intervention approach (36). In an age of increasing uptake and efficiency of the EHR (37,38), strategies to address inappropriate screening and incomplete follow-up should include optimizing existing technologies to deliver personalized patient care (39). Incorporating prior lab results in panel management and outreach to minimize inappropriately screened patients is one example. In addition, EHR generated registries of abnormal results with periodic automated system updates shared with clinics would allow abnormal results to be reviewed by an entire practice. Doing so would create opportunities for system level improvements, which tied to incentives, could ultimately result in higher compliance. Enhanced utilization of EHR systems would be especially beneficial to patients who are referred but miss their scheduled follow-up, as they fall into a provider ownership gap and the lack of communication between primary and specialty care about these patients perpetuates incomplete follow-up of abnormal results.
Safety-net populations face financial challenges and resource limitations (40) that must be considered when addressing follow-up of abnormal results. On average, safety-net patients more frequently have complex medical conditions due to socioeconomic factors that lead to fragmented care (41,42). Our results support this notion with a study-defined comorbidity identified in 68% of patients without a diagnostic colonoscopy after a positive FIT. Patients in our cohort also had higher rates of polysubstance abuse and homelessness and median time to colonoscopy completion was 184 days. Although evidence suggests optimal timing for colonoscopy completion should be no longer than 6 months after an abnormal FIT (18), the time to colonoscopy varies significantly by health care system (31,43), Optimizing communication between primary and specialty care and their patients and root-cause analysis examining patient, provider, and system level factors should improve and lead to timely follow-up.
A cost-effective strategy that has been shown to improve follow-up of abnormal results is patient-assisted navigation to colonoscopy (44–46). In the two highest performing clinics within our health network, colonoscopy completion rates were higher for patients of all races when compared with the other nine clinics. Although traditionally described navigation has not been employed in this health system, a unique feature of these two clinics was an assigned nurse or medical assistant responsible for coordinating follow-up care which likely contributed to increased colonoscopy completion for patients of all races (47,48); indeed panel management by a dedicated team member harnesses the technology provided by a registry tool. However, lack of reimbursement by insurance companies may limit uptake of navigation-like programs and promote use of lower cost viable alternatives such as text messaging (49).
To our knowledge, our study is the largest to date to address follow-up of positive FIT within a safety-net population. The strengths of this study include a comprehensive evaluation of FIT follow-up in a large, diverse, urban community-based population with a mixed payer pool, a comprehensive approach for capturing follow-up diagnostic colonoscopy examinations, and detailed chart review of patients with an abnormal FIT. There are several potential limitations to this study. First, given the nature of patients utilizing safety-net systems, out-of-network utilization, especially in patients over 65 years who qualify for Medicare, may not have been completely captured in this study. In addition, due to a transition to electronic records during this study period, it is possible parallel nursing paper records are not reflected in our findings. We believe this applied to a minority of patients as our chart review revealed 84% of patients had electronic documentation of ongoing care within our system 1 year after their abnormal FIT. Second, as our study predates changes within our safety-net system implemented in September 2015, current patterns may differ. Third, medical records sometimes lack details discussed during patient encounters. However, the medical record remains the definitive document of issues addressed during a visit as well as medical-legal proceedings and should reflect as many of these details as possible.
In summary, low rates of diagnostic colonoscopy after an abnormal FIT within safety-net populations can occur despite an integrated health system. This along with inadequate documentation and counseling around abnormal results suggests deficiencies in care coordination and closure of the care loop, which will increase liability and decrease care quality. Although factors such as comorbidities and substance abuse may not be easily modifiable, identification of these issues may streamline the screening process and this research highlights multiple areas for quality improvement. These include minimizing inappropriate screening, better documentation of discussions about abnormal results which may be mediated by enhancing the utility of existing EHRs, and well-organized clinic programs addressing abnormal results. Quality improvement initiatives in CRC screening within safety-net systems should focus on these areas to increase rates of diagnostic colonoscopy completion after an abnormal FIT.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Colorectal cancer (CRC) screening is a quality metric and remains underutilized in the general population, especially among racial/ethnic minorities and low-income populations.
Stool-based CRC screening is promoted due to patient preference and limited resources in safety-net systems.
Risk of CRC is significantly higher after an abnormal stool test therefore a diagnostic colonoscopy is strongly recommended.
WHAT IS NEW HERE
Despite an integrated safety-net system with access to colonoscopy and a shared electronic health record, only 56% of all patients with a positive fecal immunochemical test (FIT) completed a colonoscopy within 1 year of this abnormal result.
Among FIT positive patients who were referred for diagnostic colonoscopy but missed their appointment, 62% of sampled patients lacked documentation addressing their abnormal result or counseling on the increased risk of prevalent CRC.
Given the risk associated with abnormal FIT test and its widespread use, measuring follow-up colonoscopy is a novel quality of care metric.
ACKNOWLEDGMENTS
This study was presented in development at the American College of Gastroenterology (Honolulu, HI; October 2015) and at Digestive Diseases Week (San Diego, CA, USA; May 2016). We appreciate the support of the University of California, San Francisco Clinical Data Research Consultations service, specifically Nelson Lee BS, who supported the data extraction. Clinical Data Research Consultations, University of California, San Francisco, San Francisco, CA, USA.
Financial support: This research was supported by the National Institute Of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health T32DK007007 (RI), the Jacobsohn Fund for Excellence (MS), the Center for Disease Control and Prevention (CDC) U48 DP004998 (MS), and the Agency for Healthcare Research and Quality 5P30HS023558 (US). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg.
CONFLICT OF INTEREST
Potential competing interests: None.
Guarantor of the article: Ma Somsouk, MD, MAS.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Baxter NN, Goldwasser MA, Paszat LF et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1–8. [DOI] [PubMed] [Google Scholar]
- 3.Mandel JS, Bond JH, Church TR et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993;328:1365–71. [DOI] [PubMed] [Google Scholar]
- 4.Selby JV, Friedman GD, Quesenberry CP Jr et al. Effect of fecal occult blood testing on mortality from colorectal cancer. A case-control study. Ann Intern Med 1993;118:1–6. [DOI] [PubMed] [Google Scholar]
- 5.Cyhaniuk A, Coombes ME Longitudinal adherence to colorectal cancer screening guidelines. Am J Manag Care 2016;22:105–11. [PubMed] [Google Scholar]
- 6.Etzioni DA, Ponce NA, Babey SH et al. A population-based study of colorectal cancer test use: results from the 2001 California Health Interview Survey. Cancer 2004. 101:2523–32. [DOI] [PubMed] [Google Scholar]
- 7.Liss DT, Baker DW Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med 2014;46:228–36. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Tong L, Allison JE et al. Screening for colorectal cancer in a safety-net health care system: access to care is critical and has implications for screening policy. Cancer Epidemiol Biomarkers Prev 2009;18:2373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klabunde CN, Cronin KA, Breen N et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev 2011;20:1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho C, Kornfield R, Vittinghoff E et al. Late presentation of colorectal cancer in a vulnerable population. Am J Gastroenterol 2013;108:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inadomi JM, Vijan S, Janz NK et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singal AG, Gupta S, Tiro JA et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety-net health system. Cancer 2016;122:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day LW, Bhuket T, Inadomi JM et al. Diversity of endoscopy center operations and practice variation across California’s safety-net hospital system: a statewide survey. BMC Res Notes 2013;6:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen CD, Corley DA, Quinn VP et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 2016;164:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu HM, Chen SL, Yen AM et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer 2015;121:3221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bibbins-Domingo K, Grossman DC, Curry SJ et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 17.Crouse AL, De Koning L, Sadrzadeh SM et al. Sensitivity and specificity of community fecal immunotesting screening for colorectal carcinoma in a high-risk canadian population. Arch Pathol Lab Med 2015;139:1441–5. [DOI] [PubMed] [Google Scholar]
- 18.Jensen CD, Quinn VP, Doubeni CA et al. Colonoscopy Delay After a Positive Fecal Test and Risk of Colorectal Cancer-Related Outcomes Paper presented at: Digestive Diseases; Week San Diego, CA, USA: 2016. [Google Scholar]
- 19.Attachment Q - PRIME Projects and Metrics Protocol. California Department of Health Care Services 2015;41: http://www.dhcs.ca.gov/provgovpart/Documents/MC2020_AttachmentQ_PRIMEProjectsMetrics.pdf Accessed on 12 August 2016. [Google Scholar]
- 20.Katz MH, Brigham TM Transforming a traditional safety net into a coordinated care system: lessons from healthy San Francisco. Health Aff (Millwood) 2011;30:237–45. [DOI] [PubMed] [Google Scholar]
- 21.San Francisco General Hospital & Trauma Center Annual Report Fiscal Year 2014–2015 2015, https://www.sfdph.org/dph/files/hc/HCAgen/HCAgen2015/Nov17/04bSFGH-AnnualReport2014-2015_draft20151110-1.pdf; accessed on 1 July 2016.
- 22.Chen AH, Kushel MB, Grumbach K et al. Practice profile. A safety-net system gains efficiencies through ‘eReferrals’ to specialists. Health Aff (Millwood) 2010;29:969–71. [DOI] [PubMed] [Google Scholar]
- 23.Chen AH, Murphy EJ, Yee HF Jr. eReferral--a new model for integrated care. N Engl J Med 2013;368:2450–3. [DOI] [PubMed] [Google Scholar]
- 24.Tuot DS, Murphy EJ, McCulloch CE et al. Leveraging an electronic referral system to build a medical neighborhood. Healthc (Amst) 2015;3:202–8. [DOI] [PubMed] [Google Scholar]
- 25.Price EL, Sewell JL, Hm Chen A et al. Minding the gaps: assessing communication outcomes of electronic preconsultation exchange. Jt Comm J Qual Patient Saf 2016;42:341–54. [DOI] [PubMed] [Google Scholar]
- 26.Chiu HM, Lee YC, Tu CH et al. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin Gastroenterol Hepatol 2013;11:832–8, e831–832. [DOI] [PubMed] [Google Scholar]
- 27.Baig N, Myers RE, Turner BJ et al. Physician-reported reasons for limited follow-up of patients with a positive fecal occult blood test screening result. Am J Gastroenterol 2003;98:2078–81. [DOI] [PubMed] [Google Scholar]
- 28.Crotta S, Segnan N, Paganin S et al. High rate of advanced adenoma detection in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clin Gastroenterol Hepatol 2012;10:633–8. [DOI] [PubMed] [Google Scholar]
- 29.Myers RE, Turner B, Weinberg D et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med 2004;38:375–81. [DOI] [PubMed] [Google Scholar]
- 30.Parente F, Boemo C, Ardizzoia A et al. Outcomes and cost evaluation of the first two rounds of a colorectal cancer screening program based on immunochemical fecal occult blood test in northern Italy. Endoscopy 2013;45:27–34. [DOI] [PubMed] [Google Scholar]
- 31.Oluloro A, Petrik AF, Turner A et al. Timeliness of colonoscopy after abnormal fecal test results in a safety net practice. J Community Health 2016;41:864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Haddad B, Dong F, Kallail KJ et al. Association of marital status and colorectal cancer screening participation in the USA. Colorectal Dis 2015;17:O108–14. [DOI] [PubMed] [Google Scholar]
- 33.von Euler-Chelpin M, Brasso K, Lynge E Determinants of participation in colorectal cancer screening with faecal occult blood testing. J Public Health (Oxf) 2010;32:395–405. [DOI] [PubMed] [Google Scholar]
- 34.Sewell JL, Kushel MB, Inadomi JM et al. Non-English speakers attend gastroenterology clinic appointments at higher rates than English speakers in a vulnerable patient population. J Clin Gastroenterol 2009;43:652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson CM, Kirby KA, Casadei MA et al. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med 2011;171:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zapka JM, Edwards HM, Chollette V et al. Follow-up to abnormal cancer screening tests: considering the multilevel context of care. Cancer Epidemiol Biomarkers Prev 2014;23:1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laxmisan A, Sittig DF, Pietz K et al. Effectiveness of an electronic health record-based intervention to improve follow-up of abnormal pathology results: a retrospective record analysis. Med Care 2012;50:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh H, Arora HS, Vij MS et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007;14:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoury MJ, Iademarco MF, Riley WT Precision public health for the era of precision medicine. Am J Prev Med 2016;50:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canin L, Wunsch B Specialty Care in the Safety Net: Efforts to Expand Timely Access 2009:20 http://www.chcf.org/~/media/MEDIALIBRARYFiles/PDF/PDFS/PDFSpecialtyCareOverview.pdf Accessed on 1 July 2016. [Google Scholar]
- 41.Gusmano MK, Fairbrother G, Park H Exploring the limits of the safety net: community health centers and care for the uninsured. Health Aff (Millwood) 2002;21:188–94. [DOI] [PubMed] [Google Scholar]
- 42.Woolf SH, Braveman P Where health disparities begin: the role of social and economic determinants--and why current policies may make matters worse. Health Aff (Millwood) 2011;30:1852–9. [DOI] [PubMed] [Google Scholar]
- 43.Chubak J, Garcia MP, Burnett-Hartman AN et al. Time to colonoscopy after positive fecal blood test in four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev 2016;25:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen LA, Santos S, Jandorf L et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol 2008;6:443–50. [DOI] [PubMed] [Google Scholar]
- 45.Ladabaum U, Mannalithara A, Jandorf L et al. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority individuals. Cancer 2015;121:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh H, Kadiyala H, Bhagwath G et al. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am J Gastroenterol 2009;104:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jandorf L, Braschi C, Ernstoff E et al. Culturally targeted patient navigation for increasing african americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2013;22:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braschi CD, Sly JR, Singh S et al. Increasing colonoscopy screening for Latino Americans through a patient navigation model: a randomized clinical trial. J Immigr Minor Health 2014;16:934–40. [DOI] [PubMed] [Google Scholar]
- 49.Baker DW, Brown T, Buchanan DR et al. Design of a randomized controlled trial to assess the comparative effectiveness of a multifaceted intervention to improve adherence to colorectal cancer screening among patients cared for in a community health center. BMC Health Serv Res 2013;13:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.