Abstract
The active form of vitamin D3, 1,25-dihydroxyvitamin D3 (aVD3), is known to exert beneficial effects in the treatment of autoimmune diseases because of its immunosuppressive effects. However, clinical application of aVD3 remains limited because of the potential side effects, particularly hypercalcemia. Encapsulation of aVD3 within biodegradable nanoparticles (NPs) would enhance the delivery of aVD3 to antigen presenting cells, while preventing the potential systemic side effects of aVD3. In the present study, polymeric NPs containing ovalbumin (OVA) and aVD3 (NP[OVA+aVD3]) were prepared via the water-in-oil-in-water double emulsion solvent evaporation method, after which their immunomodulatory effects were examined. Bone marrow-derived immature dendritic cells (DCs) treated with NP(OVA+aVD3) did not mature into immunogenic DCs but were converted into tolerogenic DCs, which express low levels of co-stimulatory molecules and MHC class II molecules, produce lower levels of pro-inflammatory cytokines while increasing the production of IL-10 and TGF-β, and induce the generation of Tregs. Intravenous injection with NP(OVA+aVD3) markedly suppressed the generation of OVA-specific CTLs in mice. Furthermore, OVA-specific immune tolerance was induced in mice orally administered with NP(OVA+aVD3). These results show that biodegradable NPs encapsulating both antigen and aVD3 can effectively induce antigen-specific immune suppression.
Keywords: Polymeric nanoparticle, Vitamin D3, Dendritic cells, Treg cells, Antigen-specific immune suppression
INTRODUCTION
The biologically active form of vitamin D, 1,25-dihydroxyvitamin D3 (aVD3), exerts various immunoregulatory and anti-inflammatory effects, in addition to the classical hormonal effects on calcium and phosphate metabolism (1,2). Numerous studies have shown that aVD3 exerts immunomodulatory and anti-inflammatory effects primarily by modulating the function of dendritic cells (DCs) despite its direct immunosuppressive effects on T cells (3-6). DCs differentiated in the presence of aVD3 exhibit the characteristics of tolerogenic DCs (1,2,7). DCs generated using aVD3 express lower levels of MHC class II and co-stimulatory molecules and produce higher levels of IL-10 and lower levels of IL-12 and IL-6 compared to untreated normal DCs (8-10). Moreover, these DCs are poor activators of antigen-primed T cells but can stimulate the generation of Tregs (10,11). In addition, the tolerogenic DC-inducing activity of aVD3 has also been demonstrated both in diabetes-prone nonobese diabetic mice and normal mice (12-14).
Polymeric nanoparticles (NPs) generated from a biodegradable and biocompatible polymer, poly(D,L-lactide-co-glycolide) (PLGA), have been extensively explored as implantable reservoirs for sustained-release drug delivery. PLGA NPs have also been studied as vehicles for the delivery of antigens to phagocytes (15-18). PLGA NPs containing both antigens and drugs have remarkable advantages because they can specifically deliver the antigens and the drugs to phagocytes, such as DCs and macrophages, thereby reducing the potential systemic side effects of the drugs (19,20).
Although the beneficial effects of aVD3 are evident in many autoimmune disease models, numerous concerns have to be addressed before the clinical application of aVD3 for the treatment of autoimmune diseases. One of the major concerns is the severe hypercalcemia, which could develop in patients receiving excessive amounts of aVD3 (21,22). In the present study, PLGA NPs containing ovalbumin (OVA) and aVD3 (NP[OVA+aVD3]) were produced, with the goal of preferentially delivering OVA and aVD3 to phagocytes. Our results confirmed that (NP[OVA+aVD3]) could induce OVA-specific immune tolerance in mice that were intravenously injected or orally administered with the NPs.
MATERIALS AND METHODS
Animals
Female C57BL/6 and BALB/c mice (8 to 12 weeks old) were purchased from Kosa Bio Inc. (Seongnam, Korea). All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University, Cheongju, South Korea and performed in accordance with the IACUC guidelines and regulations.
Generation of DCs from bone marrow cells
DCs were generated as previously described (15). Briefly, bone marrow cells obtained from mouse femurs were cultured in 6-well plates (5×106 cells/well) containing culture medium supplemented with 40 ng/ml GM-CSF and 40 ng/ml IL-4 (both from CreaGene, Seongnam, Korea). After 3 days, the non-adherent cells were removed by gently shaking the plate and then replacing the medium. On day 4, the non-adherent cells were again removed by the same method. On day 6, the DCs were harvested by gentle pipetting and used for the subsequent experiments.
Preparation of PLGA NPs
PLGA NPs containing OVA (Sigma-Aldrich, St. Louis, MO, USA) and aVD3 (Sigma-Aldrich) were prepared following a previously described solvent evaporation method (23). Briefly, 600 μl of 80 mg/ml OVA in water was mixed by vortexing with 200 μl of 50% ethanol containing 400.0, 133.3, or 44.4 μg of aVD3. The resulting mixture was mixed with 4 ml of 100 mg/ml PLGA (Evonik Industries, Essen, Germany) in ethyl acetate (Sigma-Aldrich) and then homogenized at 20,000 rpm (T10 basic Homogenizer, IKA, Staufen, Germany) for 3 min. The homogenate was added with 11 ml of a 5% polyvinyl alcohol aqueous solution (PVA, Sigma-Aldrich) and homogenized at 20,000 rpm for 5 min. The double emulsion was added to 200 ml of a 0.1% PVA aqueous solution, and stirred at 500 rpm for 2 h to evaporate the organic solvent and solidify the NPs. The resulting NPs were centrifuged at 3,000 rpm for 20 min and washed twice with PBS. NPs containing only OVA (NP[OVA]) were prepared following the same method without adding aVD3 to the PLGA solution. The NPs were prepared immediately before use, or frozen in aliquots at −20°C for later use.
Characterization of PLGA NPs
The mean size of the NPs was measured using a particle size analyzer (ELS-Z, Otsuka, Japan). The OVA content was determined using a microbicinchoninic acid assay kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions after lysing the NPs in a lysis buffer containing 0.1% SDS and 0.1 N NaOH. For aVD3 quantitation, NPs were lysed in a 1:2 mixture of DMSO and methanol and then analyzed by HPLC analysis using a Waters HPLC system (Waters Corp., Milford, MA, USA) equipped with Waters 515 pumps, Waters 2996 photodiode array detector, and Waters Empower software using a YMC J'sphere ODS-H80 column (YMC America Inc., Allentown, PA, USA; 4 μm, 150×4.6 mm). Chromatographic separation was performed using a gradient solvent system of acetonitrile-water (ratio range, 20:80 to 100:0) for 30 min at a flow rate of 1.0 mL/min. aVD3 concentrations were determined based on the ultraviolet absorbance at 240 nm.
Phenotype analysis
Cells were stained with monoclonal antibodies against mouse cell surface markers, CD11c, H-2Kb, I-Ab, CD80, CD86, CD4, CD25, Foxp3, and an isotype-matched control antibody (BD Biosciences, San Jose, CA, USA) as previously described (23). For intracellular Foxp3 staining, cells were permeabilized using the BD Cytofix/Cytoperm Plus kit (BD Biosciences) according to the manufacturer's instructions. Subsequent analyses were performed using the FlowJo software (TreeStar, Ashland, OR, USA).
Cytokine production analysis
Immature DCs were harvested by gentle pipetting on day 6 and seeded in 24-well plates (1×106 cells/well). Then, the immature DCs were treated with the indicated NPs (10 μg/ml as OVA) for 48 h. The amounts of TNF-α, IL-1β, IL-6, IL-12p40, and IL-10 in the culture supernatants were measured using commercial ELISA kits (BD Biosciences). TGF-β1 levels were measured using an ELISA kit from R&D systems (Minneapolis, MN, USA) after treating the culture supernatant with the Sample Activation Kit 1 (R&D systems) according to the manufacturer's protocols.
MHC class II-restricted OVA presentation assay
DCs (1×105 cells/well) were incubated with the indicated NPs (50 μg/ml as OVA) for 2 h, washed with pre-warmed PBS, fixed with 1% paraformaldehyde, and then washed with PBS. The OVA-specific CD4 T cell stimulatory capacity of the DCs was measured using OVA-specific CD4+ T cell hybridoma DOBW cells, which recognize OVA323–339-I-Ad complexes and secrete IL-2 in response as previously described (23).
Isolation of T cells
Total T cells were purified from the spleens of BALB/c mice by adding the spleen cells to a nylon wool column and incubating for 1 h to remove adherent cells. CD4+CD25− T cells were isolated from the adherent cell-depleted spleen cells of BALB/c mice using a CD4+CD25− T cell isolation kit (Miltenyi Biotec Inc., Auburn, CA, USA).
Allogeneic T cell stimulatory activity
C57BL/6 bone marrow-derived DCs (1.25×104, 2.5×104, or 5×104 cells/well) were treated with the indicated NPs (10 μg/ml as OVA) for 48 h, added with T cells (5×105 cells/well) isolated from the spleens of BALB/c mice, and subsequently cultured for 96 h. DNA synthesis was measured by incorporating (3H)-thymidine, which was added before the final 18 h of culture.
Generation of Foxp3+ Tregs from naïve CD4+CD25− T cells
DCs (2×104 cells/well) were treated with the indicated NPs (10 μg/ml as OVA) for 48 h and then co-cultured with purified CD4+CD25− T cells (2×105 cells/well) for 4 days in a medium containing 100 U/ml recombinant human IL-2 (PeproTech Inc., Rocky Hill, NJ, USA). The cells were stained with monoclonal antibodies specific to mouse CD4, CD25, and Foxp3, after which the proportion of CD25+Foxp3+ T cells in the CD4+ T cell population was determined by flow cytometry.
In vivo OVA-specific CTL assay
C57BL/6 mice were intravenously (i.v.) immunized with PBS, or the indicated NPs (100 μg/mouse as OVA). After 7 days, OVA-specific CTL activity was assessed using an in vivo CTL assay, as previously described (23). Briefly, splenocytes from naïve syngeneic mice were pulsed with 1 μM OVA257–264 peptide for 1 h at 37°C and then labeled with a high concentration of CFSE (25 μM). The other control target population was syngeneic splenocytes labeled with a low concentration of CFSE (5 μM) without pulsing with the OVA peptide. A 1:1 mixture of each target cell population was injected via the tail vein into the immunized mice (1×107 cells/mouse). After 18 h, specific killing of OVA peptide-pulsed target cells was determined using spleen cells isolated from each mouse by flow cytometry.
OVA-specific IgG measurement
C57BL/6 mice were i.v. immunized with PBS or the indicated NPs (100 μg/mouse as OVA). After 7 days, sera were collected, and the amounts of OVA-specific IgG were measured by an ELISA. Briefly, 96-well immunoplates were coated overnight with OVA (2 mg/ml in PBS), blocked with 10% FBS in PBS, and loaded with 1:20,000 dilutions of serum. After 2 h of incubation, the plates were washed and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG antibodies (Sigma-Aldrich; 1:5,000) to allow specific binding, followed by washing and incubation with 3,30,5,50-tetramethybenzidine (BD Biosciences) substrate solution and 1 M H2SO4 stop solution. The absorbance was measured at the wavelengths of 450 and 570 nm using an ELISA plate reader.
Induction and assessment of OVA-specific oral tolerance
C57BL/6 mice was intragastrically administered with PBS or the indicated NPs (200 μg/mouse as OVA) on days 0 and 2. On day 9, mice were i.v. immunized with NP(OVA) (80 μg/mouse as OVA). OVA-specific CTL activity and OVA-specific IgG production were assessed at 7 days after i.v. immunization with NP(OVA) as described above.
Statistical analysis
One-way ANOVA analysis and Tukey post-hoc tests were performed to compare the significance of multiple groups. p≤0.05 was considered statistically significant.
RESULTS
Fabrication and characterization of NPs containing OVA and aVD3
NP(OVA) and NP(OVA+ aVD3) were fabricated with PLGA using the water-in-oil-in-water double emulsion solvent evaporation method. The OVA and aVD3 ratios (w/w) used to generate 3 types of NPs containing OVA plus aVD3 were 200:1, 600:1, and 1800:1, and the resultant NPs were designated as NP(200:1), NP(600:1), and NP(1,800:1). The physicochemical properties of the NPs were evaluated (Table 1). The mean diameters of the NP(OVA), NP(200:1), NP(600:1), and NP(1,800:1) were 724.1±98.4, 786.1±76.8, 838.4±94.3, and 800.8±82.4 nm, respectively. The average aVD3 contents in NP(200:1), NP(600:1), and NP(1,800:1) were 7.5, 2.8, and 0.08 μg/mg OVA. Thus, the average OVA: aVD3 ratios (w/w) in the NP(200:1), NP(600:1), and NP(1,800:1) were 133:1, 357:1, and 1,250:1, respectively.
Table 1. Characterization of PLGA-NPs.
| Nanoparticles | Size (nm) | OVA loading efficiency (%) | aVD3 loaded (µg/mg OVA) | OVA: aVD3 ratio in the NP |
|---|---|---|---|---|
| NP(OVA) | 724.8±98.4 | 10.8±0.7 | - | - |
| NP(200:1) | 786.1±76.8 | 12.9±0.8 | 7.5±0.06 | 133:1 |
| NP(600:1) | 838.4±94.3 | 13.8±0.7 | 2.8±0.02 | 357:1 |
| NP(1,800:1) | 800.8±82.4 | 13.3±0.6 | 0.8±0.01 | 1,250:1 |
DCs phagocytosed of NP(OVA+aVD3) exert properties of tolerogenic DCs
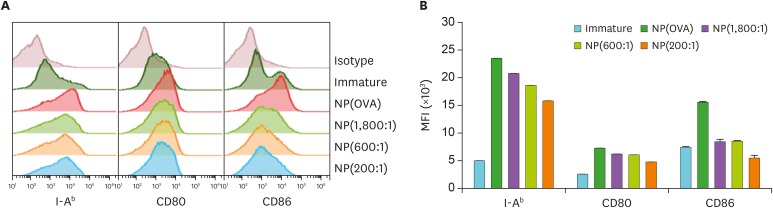
To examine the effects of NP(OVA+aVD3) on the maturation and function of DCs, immature DCs generated from mouse bone marrow cells were treated with NP(OVA+aVD3) for 2 days. Phenotypic analysis of the DCs showed that NP(OVA+aVD3)-treated DCs expressed considerably lower levels of MHC class II (I-Ab), CD80, and CD86 molecules, and the inhibitory effects of NP(OVA+aVD3) were dose-dependent on the concentrations of aVD3 in the NPs (Fig. 1).
Figure 1. Effects of NP(OVA+aVD3) on the expression of cell surface molecules in DCs. (A) Immature DCs generated from the bone marrow cells of C57BL/6 mice were treated with NP(OVA), NP(1,800:1), NP(600:1), or NP(200:1) (10 μg/ml as OVA) for 48 h. DCs were stained for CD11c, H-2Kb, I-Ab, CD80, and CD86. CD11c+ cells were gated and analyzed for the expression of cell surface molecules. The data shown are representative histograms of 4 independent experiments. (B) MFIs of NP-treated or untreated DCs. Data are presented as the mean±SD of 4 independent experiments.
MFI, mean fluorescence intensity.
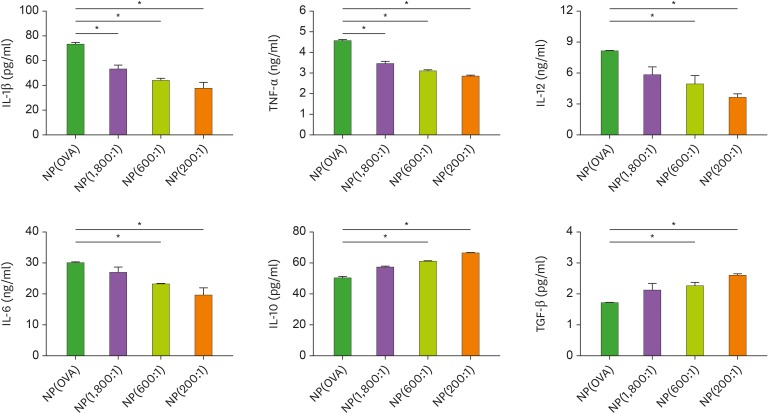
Phagocytosis of NPs by itself activates DC maturation and cytokine production. As shown in Fig. 2, DCs treated with NP(OVA) produced large amounts of pro-inflammatory cytokines, such as IL-1β, TNF-α, IL-12, and IL-6. The production of these inflammatory cytokines was significantly suppressed in NP(OVA+aVD3)-treated DCs, and the inhibitory effects were dependent on the amounts of aVD3 in the NPs (Fig. 2). By contrast, NP(OVA+aVD3)-treated DCs produced significantly higher amounts of IL-10 and TGF-β compared to DCs treated with NP(OVA), and these enhancing effects were also found to be dependent on the amounts of aVD3 in the NPs.
Figure 2. Effects of NP(OVA+aVD3) on cytokine production in DCs. Immature DCs generated from bone marrow cells of C57BL/6 mice were treated with NP(OVA), NP(1,800:1), NP(600:1), or NP(200:1) (10 μg/ml as OVA) for 48 h. Cytokine secretion in the culture supernatant was determined by ELISA. Data are presented as the mean±SD of 3 independent experiments.
*p<0.05.
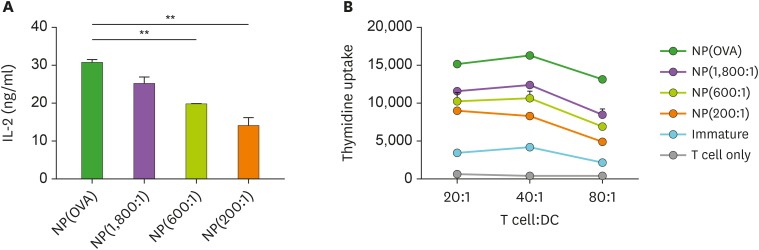
The capacity of NP-treated DCs to stimulate T cells was additionally investigated by performing MHC class II-restricted exogenous antigen presentation assay and allogeneic mixed lymphocyte reaction (MLR). In exogenous antigen presentation assays, DCs were incubated with the NPs for 2 h, washed, fixed, and then co-cultured with OVA-specific CD4+ T cell hybridoma, DOBW cells, which recognize OVA323−339-I-Ad complexes and secrete IL-2 (23). The MHC class II-restricted OVA peptide presentation capacity of NP(OVA+aVD3)-treated DCs was significantly lower compared to that of NP(OVA)-treated DCs, and the observed inhibitory effects were dependent on the amounts of aVD3 in the NPs (Fig. 3A). In addition, allogeneic T cell stimulation by NP(OVA+aVD3)-treated DCs was significantly weaker compared to that by NP(OVA)-treated DCs, and the degree of suppression was found to be dependent on the amounts of aVD3 in the NPs (Fig. 3B).
Figure 3. DCs treated with NP(OVA+aVD3) are impaired in both MHC class II-restricted exogenous antigen presentation and allogeneic T cell stimulatory capacity. (A) Immature DCs generated from bone marrow cells of BALB/c mice were treated with NP(OVA), NP(1,800:1), NP(600:1), or NP(200:1) (50 μg/ml as OVA) for 2 h. After washing and fixing, DCs were co-cultured with OVA323−339-specific DOBW cells. The supernatants were harvested, and IL-2 production was measured by ELISA. Data are presented as the mean±SD of 3 independent experiments. (B) Immature DCs generated from C57BL/6 mouse bone marrow cells were treated with NP(OVA), NP(1,800:1), NP(600:1), or NP(200:1) (10 μg/ml as OVA) for 48 h. DCs were then co-cultured with T cells isolated from the spleens of BALB/c mice at the indicated ratios for 96 h. T cell proliferation was measured by the incorporation of (3H)-thymidine added during the final 18 h of culture.
**p<0.01.
NP(OVA+aVD3)-treated DCs induce Foxp3+ Tregs
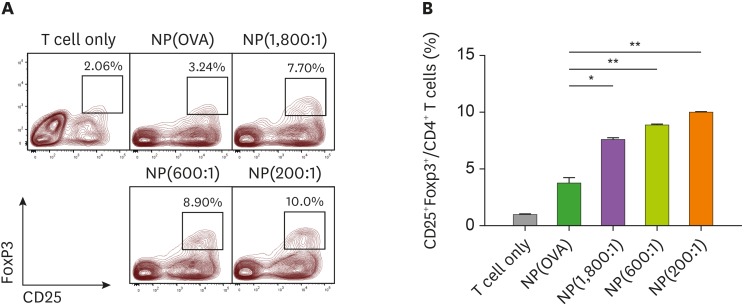
The ability of NP(OVA+aVD3)-treated DCs to induce the conversion of naïve CD4+ CD25− T cells into Foxp3+ Tregs was evaluated in vitro. To analyze the ability of NP(OVA+aVD3)-treated DCs to induce Tregs, immature DCs generated from C57BL/6 mouse bone marrow cells were treated with NP(OVA) or NP(OVA+aVD3) for 48 h. DCs were then co-cultured with CD4+CD25− T cells isolated from the spleens of BALB/c mice in the presence of recombinant human IL-2 (100 U/ml) for 4 days, after which CD25 and Foxp3 expression levels in the T cells were determined (Fig. 4A). The proportions of CD25+Foxp3+ Tregs were significantly higher in co-cultures with NP(1,800:1)-treated DCs (7.7%), NP(600:1)-treated DCs (8.9%), or NP(200:1)-treated DCs (10.0%), than those of co-cultures with NP(OVA)-treated DCs (3.24%) (Fig. 4B).
Figure 4. DCs treated with NP(OVA+aVD3) induce Foxp3+ Tregs from naïve CD4+CD25− T cells in allogeneic MLR. (A) Immature DCs generated from the bone marrow cells of C57BL/6 mice were treated with NP(OVA), NP(1,800:1), NP(600:1), or NP(200:1) (10 μg/ml as OVA) for 48 h. DCs were then co-cultured with CD4+CD25− T cells isolated from the spleens of BALB/c mice at a ratio of 1:10 in a medium containing recombinant human IL-2 (100 U/ml) for 4 days. Cells were gated on CD4+ cells, and the expression levels of CD25 and Foxp3 were analyzed. The data show representative histograms of 3 independent experiments. (B) The proportion of CD25+FoxP3+ Tregs in the CD4+ cell population of each experimental group is shown. Data are presented as the mean±SD of 3 independent experiments.
*p<0.05, **p<0.01.
Mice injected with NP(OVA+aVD3) show suppressed OVA-specific CTL responses
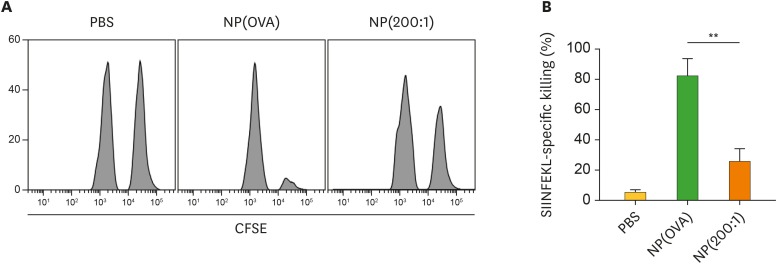
In this experiment, mice were i.v. injected with PBS, NP(OVA), or NP(200:1). After 7 days, OVA-specific CTL activity was assessed using CFSE-labeled syngeneic target cells. Representative histograms are shown in Fig. 5A. Mice that were injected with NP(OVA) showed potent OVA-specific CTL responses (specific killing, 82.8%). However, mice that injected with NP(200:1) showed significantly suppressed OVA-specific CTL responses (specific killing, 24.2%; Fig. 5B).
Figure 5. Effect of i.v. treatment of NP(OVA+aVD3) on OVA-specific CTL generation in vivo. (A) PBS, NP(OVA), or NP(200:1) (100 μg/mouse as OVA) was i.v. injected into C57BL/6 mice. After 7 days, OVA-specific cytotoxic activity was assessed by an in vivo CTL assay. The target cells were a 1:1 mixture of syngeneic cells pulsed with the OVA257−264 peptide and then labeled with a high concentration of CFSE and syngeneic cells non-pulsed and labeled with a low concentration of CFSE. The target cells were i.v. injected into recipient mice, and the specific cytotoxicity was evaluated after 18 h. The number of mice in each group was 5. (B) The proportion of killed target cells in each experimental group is shown. Data are presented as the mean±SD of 3 independent experiments.
**p<0.01.
Oral administration of mice with NP(OVA+aVD3) induces OVA-specific immune tolerance
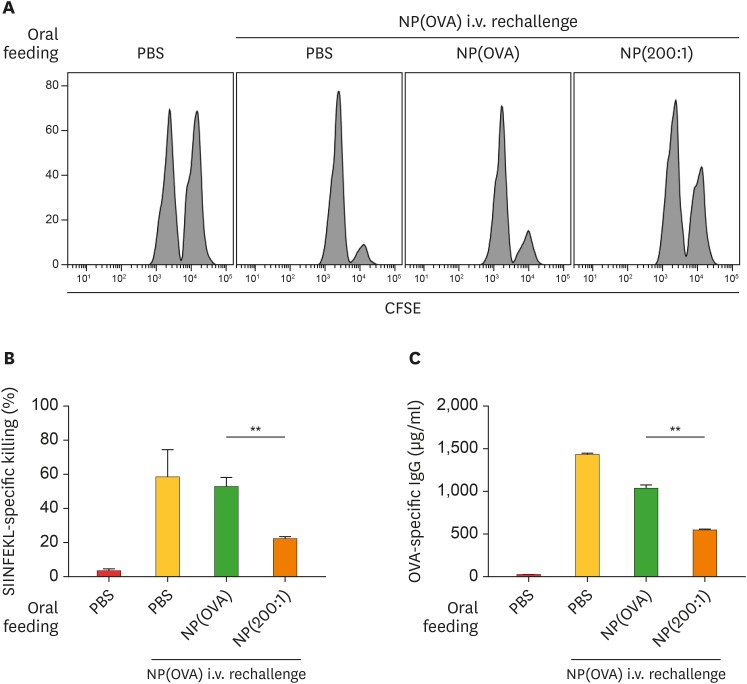
To investigate whether OVA-specific oral tolerance could be induced by oral administration of NP(OVA+aVD3), mice were intragastrically injected with PBS, NP(OVA), or NP(200:1) on days 0 and 2. On day 9, mice were i.v. immunized with PBS or NP(OVA). After 7 days, OVA-specific CTL activity was assessed using CFSE-labeled syngeneic target cells. Representative histograms are shown in Fig. 6A. Oral administration of mice with NP(OVA) did not induce OVA-specific T cell tolerance, as evidenced by the similar rate of target cell killing in PBS-administered mice following i.v. re-challenge with NP(OVA). However, oral administration of mice with NP(200:1) significantly induced OVA-specific T cell tolerance, as evidenced by the significantly reduced rate of target cell killing compared to NP(OVA)-administered mice following i.v. re-challenge with NP(OVA) (Fig. 6A and 6B). OVA-specific IgG production was also examined using sera collected from the mice described in Fig. 6A. The level of OVA-specific IgG in the serum was evidently lower in mice orally administered with NP(200:1) compared to that in NP(OVA)-administered mice (Fig. 6C).
Figure 6. Effect of oral treatment of NP(OVA+aVD3) on OVA-specific responses in vivo. (A) Induction of OVA-specific CTLs. PBS, NP(OVA), or NP(200:1) (200 μg/mouse as OVA) was intragastrically injected into C57BL/6 mice on days 0 and 2. On day 9, mice were i.v. injected with NP(OVA) (80 μg/mouse as OVA). After 7 days, OVA-specific cytotoxic activity was assessed using an in vivo CTL assay, as shown in Fig. 5. The number of mice in each group was 5. (B) The proportion of killed target cells in each experimental group is shown. Data are presented as the mean±SD of 3 independent experiments. (C) Production of OVA-specific IgG. PBS, NP(OVA), or NP(200:1) (200 μg/mouse as OVA) was intragastrically injected into C57BL/6 mice on days 0 and 2. On day 9, mice were i.v. injected with NP(OVA) (80 μg/mouse as OVA). After 7 days, the sera were collected from the mice, and OVA-specific IgG levels were measured by ELISA. Data are presented as the mean±SD of 3 independent experiments.
**p<0.01.
DISCUSSION
Induction of Ag-specific immune suppression or tolerance is one of the ultimate goals in the immunotherapy of autoimmune diseases and other harmful immunological reactions. In the present study, we showed that i.v. injection of biodegradable polymeric NPs containing antigen and the active form of aVD3 is a useful method for inducing antigen-specific immune suppression in mice. In addition, our results showed that oral feeding of biodegradable polymeric NPs containing antigen and aVD3 could also induce antigen-specific immune suppression in mice.
The 2 primary main sources of vitamin D3 are dietary uptake and synthesis in the skin (7,24). In the human skin, vitamin D3 is synthesized from 7-dihydroxycholesterol when exposed to ultraviolet B. In the liver, vitamin D3 is hydroxylated to 25-hydroxyvitamin D, which is the main circulating form of vitamin D3. 25-hydroxyvitamin D3 is further hydroxylated further into aVD3 by the kidney enzymes to exert its biological activity (7,25).
The active form of vitamin D3 has long been established to play an important role in the innate and adaptive immune responses (26-29). Low serum concentrations of aVD3 have been associated with higher rates of infections and the development of autoimmune diseases, such as multiple sclerosis, type 1 diabetes mellitus, and systemic lupus erythematosus (30-33). aVD3 exerts immunomodulatory activities both directly by activating T cells and indirectly via modification of antigen-presenting cell. aVD3 inhibits the secretion of proinflammatory cytokines from Th1, Th9, Th17, and Th22 cells, but promotes Th2 production of cytokines, such as IL-4, IL-5, and IL10, thereby skewing T cells towards Th2 polarization (3-6,33). In addition, aVD3 in combination with IL-2 promotes the development of Tregs expressing CTLA-4 and FoxP3 (3). Moreover, numerous studies have shown that aVD3 primarily exerts its immunomodulatory and anti-inflammatory effects by modulating the function of DCs. Both in vivo and in vitro experiments have demonstrated that DCs differentiated in the presence of aVD3 exhibit the properties of tolerogenic DCs characterized by downregulated CD40, CD80, and CD86 expression, low IL-12 production, and enhanced IL-10 secretion (2,7-10). Moreover, these DCs act as poor activators of antigen-primed T cells but stimulate the generation of Tregs, which play critical roles in the antigen-specific immune suppression (10-14,34). The above findings showed that aVD3 exerts beneficial effects on the immune function, particularly in the context of autoimmunity, via generation of tolerogenic DCs.
The clinical application of aVD3 is limited by its potential side effects, particularly hypercalcemia (35). In fact, the conversion of the circulating, inactive form of vitamin D3 to its active form (aVD3) is strictly regulated by the parathyroid hormone and the phosphaturic hormone fibroblast growth factor 23 (7). In this regard, encapsulation of aVD3 with a biodegradable polymer would not only reduce the potential systemic side effects of aVD3, but also preferentially target the delivery of aVD3 to phagocytes, such as macrophages and DCs. Our results showed that NP(OVA+aVD3)-treated DCs showed impaired antigen-specific T cell stimulation, expression of MHC class II and co-stimulatory molecules (CD80 and CD86), and secretion of pro-inflammatory cytokines (IL-1β, IL-6, IL-12, and TNF-α). However, the production of the immunosuppressive cytokine IL-10 was increased in NP(OVA+aVD3)-treated DCs. Furthermore, NP(OVA+aVD3)-treated DCs induced Foxp3+ Tregs from naïve CD4 T cells. These results demonstrated that the DCs treated with NP(OVA+aVD3) are converted into tolerogenic DCs.
Notably, our findings showed that NP(OVA+aVD3) can induce OVA-specific immune tolerance in mice orally administered or i.v. injected with the NPs. Mice i.v. injected with NP(OVA+aVD3) showed suppressed induction of OVA-specific cytotoxic T cells. In addition, mice that were orally administered with NP(OVA+aVD3) exhibited markedly impaired generation of OVA-specific cytotoxic T cells and OVA-specific IgG upon i.v. re-challenge with NP(OVA). These results demonstrated that NP(OVA+aVD3) can induce OVA-specific oral tolerance.
In summary, the present study showed that DCs treated with NP(OVA+aVD3) exhibit tolerogenic DC properties. Tolerogenic DCs generated by NP(OVA+aVD3) efficiently induced the differentiation of naïve T cells into regulatory T cells. Our findings also demonstrate that oral administration or intravenous injection of NP(OVA+aVD3) enhanced OVA-specific immune suppression in mice. Therefore, polymeric NPs encapsulating both antigen and aVD3 can be used to effectively induce antigen-specific immune tolerance, which is crucial for the treatment of autoimmune diseases.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2017R1A2B4006828, MRC-2017R1A5A2015541).
Abbreviations
- aVD3
1,25-dihydroxyvitamin D3
- DC
dendritic cell
- i.v.
intravenously
- MLR
mixed lymphocyte reaction
- NP
nanoparticle
- NP(OVA+aVD3)
nanoparticles containing ovalbumin and 1,25-dihydroxyvitamin D3
- OVA
ovalbumin
- PLGA
poly(D,L-lactide-co-glycolide)
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee CK.
- Data curation: Jung HH, Kim SH.
- Formal analysis: Jung HH, Kim SH, Moon JH.
- Funding acquisition: Lee CK.
- Investigation: Jeong SU, Jang S.
- Methodology: Park CS.
- Supervision: Lee CK.
- Validation: Moon JH, Jeong SU, Jang S, Park CS.
- Writing - original draft: Jung HH.
- Writing - review & editing: Lee CK.
References
- 1.O'Brien MA, Jackson MW. Vitamin D and the immune system: beyond rickets. Vet J. 2012;194:27–33. doi: 10.1016/j.tvjl.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology. 2016;148:227–236. doi: 10.1111/imm.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer MT, Lee YK, Maynard CL, Oliver JR, Bikle DD, Jetten AM, Weaver CT. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J Biol Chem. 2011;286:997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, et al. 1,25-dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012;71:50–61. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 7.Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7:8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 9.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1α,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3 . Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 11.Széles L, Keresztes G, Töröcsik D, Balajthy Z, Krenács L, Póliska S, Steinmeyer A, Zuegel U, Pruenster M, Rot A, et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, Van Belle TL, Pauwels F, Verstuyf A, Korf H, et al. 1,25-dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol. 2014;192:4210–4220. doi: 10.4049/jimmunol.1302350. [DOI] [PubMed] [Google Scholar]
- 13.Farias AS, Spagnol GS, Bordeaux-Rego P, Oliveira CO, Fontana AG, de Paula RF, Santos MP, Pradella F, Moraes AS, Oliveira EC, et al. Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS Neurosci Ther. 2013;19:269–277. doi: 10.1111/cns.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolic T, Roep BO. Regulatory multitasking of tolerogenic dendritic cells – lessons taken from vitamin D3-treated tolerogenic dendritic cells. Front Immunol. 2013;4:113. doi: 10.3389/fimmu.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YR, Lee YH, Im SA, Yang IH, Ahn GW, Kim K, Lee CK. Biodegradable nanoparticles containing TLR3 or TLR9 agonists together with antigen enhance MHC-restricted presentation of the antigen. Arch Pharm Res. 2010;33:1859–1866. doi: 10.1007/s12272-010-1119-z. [DOI] [PubMed] [Google Scholar]
- 16.Moni SS, Safhi MM, Barik BB. Nanoparticles for triggering and regulation of immune response of vaccines: perspective and prospective. Curr Pharm Biotechnol. 2013;14:1242–1249. doi: 10.2174/1389201015666140317120843. [DOI] [PubMed] [Google Scholar]
- 17.Rahimian S, Fransen MF, Kleinovink JW, Christensen JR, Amidi M, Hennink WE, Ossendorp F. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J Control Release. 2015;203:16–22. doi: 10.1016/j.jconrel.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Koerner J, Horvath D, Groettrup M. Harnessing dendritic cells for poly (D,L-lactide-co-glycolide) microspheres (PLGA MS)-mediated anti-tumor therapy. Front Immunol. 2019;10:707. doi: 10.3389/fimmu.2019.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22:2406–2412. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63:943–955. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PN, Davies JS. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br J Clin Pharmacol. 2018;84:1121–1127. doi: 10.1111/bcp.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D toxicity-a clinical perspective. Front Endocrinol (Lausanne) 2018;9:550. doi: 10.3389/fendo.2018.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Park CS, Jang S, Kim JW, Kim SH, Song S, Kim K, Lee CK. Tolerogenic dendritic cells are efficiently generated using minocycline and dexamethasone. Sci Rep. 2017;7:15087. doi: 10.1038/s41598-017-15569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gil Á, Plaza-Diaz J, Mesa MD. Vitamin D: classic and novel actions. Ann Nutr Metab. 2018;72:87–95. doi: 10.1159/000486536. [DOI] [PubMed] [Google Scholar]
- 25.Heaney RP. Vitamin D--baseline status and effective dose. N Engl J Med. 2012;367:77–78. doi: 10.1056/NEJMe1206858. [DOI] [PubMed] [Google Scholar]
- 26.Grad R. Cod and the consumptive: a brief history of cod-liver oil in the treatment of pulmonary tuberculosis. Pharm Hist. 2004;46:106–120. [PubMed] [Google Scholar]
- 27.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3 . FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 28.White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13:21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 29.Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018;19:2663. doi: 10.3390/ijms19092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 32.Dimeloe S, Nanzer A, Ryanna K, Hawrylowicz C. Regulatory T cells, inflammation and the allergic response—the role of glucocorticoids and vitamin D. J Steroid Biochem Mol Biol. 2010;120:86–95. doi: 10.1016/j.jsbmb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:217–226. doi: 10.1007/s12016-013-8361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JY, Kim JH, Patil AM, Kim SB, Uyangaa E, Hossain FM, Eo SK. Exacerbation of Japanese encephalitis by CD11chi dendritic cell ablation is associated with an imbalance in regulatory Foxp3+ and IL-17+CD4+ Th17 cells and in Ly-6Chi and Ly-6Clo monocytes. Immune Netw. 2017;17:192–200. doi: 10.4110/in.2017.17.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37:521–547. doi: 10.1210/er.2016-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]