Abstract
Ovarian cancer (OC), the deadliest gynecological cancer, results in poor overall survival, urgently requiring a novel therapeutic approach. As cumulative exposures to endotoxins decreased OC risk epidemiologically, we evaluated if LPS, a Toll-like receptor 4 agonist known as active component of endotoxins, could increase survival in the murine peritoneal dissemination model of SKOV-3 OC cells. LPS significantly increased the mean survival time of more than 116 days compared with 63 days in the control. Furthermore, no tumor burden was present in three mice among eight LPS-treated mice. SKOV-3 cells were not responsive to LPS and showed unaltered chemokine signature. Rather than direct effects to OC cells, LPS was found to increase proinflammatory chemokines and cytokines, such as CXCL1, CXCL8, TNF, and IL-1B, in innate immune system. Taken together, LPS is likely to potentiate the cytotoxic-related innate immunogenicity via proinflammatory chemokines and cytokines, which attenuates the peritoneal dissemination of OC.
Keywords: Lipopolysaccharides, Chemokines, Cytokines, Innate immunity, Ovarian cancer
INTRODUCTION
Ovarian cancer (OC) is the fifth leading cause of cancer deaths among women in the US, with an estimated 22,240 new cases and 14,070 deaths in 2018 (1). OC alone accounts for 5% of cancer deaths and has frequently disseminated throughout the peritoneal cavity at diagnosis due to asymptomatic early-stage cancer (2). Five-year survival in women diagnosed with distant-stage OC is only 29% (3), urgently requiring a new and better therapeutic strategy. Epithelial OC occurs in 90% of OC, including the following subtypes: serous (68%–71%), endometrioid (9%–11%), clear cell (12%–13%), mucinous (3%), malignant Brenner tumors (1%), and mixed histologies (6%) (4). In particular, high-grade serous ovarian cancer (HGSOC) is highly-aggressive, is diagnosed at the advanced-stage, and has a poor prognosis compared to other subtypes (4,5). A frequent molecular alteration of HGSOC is closely associated with the genetic mutation in the tumor suppressor protein p53 (TP53) (6). We have shown that the frequent mutation of TP53 in OC enhances proinflammatory chemokines, leading to an inflammatory tumor microenvironment (7). The chemokine network appears to be involved in the progression, metastasis and survival of OC (8,9,10).
Endotoxins are widespread ubiquitously in ambient environmental settings, including air, water, and food. Endotoxins are an integral component of the outer wall of Gram-negative bacteria, and the active component, LPS, is liberated when the cell walls of bacteria break downs (11). LPS, a TLR4 agonist, is a potent inflammatory agent that causes TLR4-mediated signaling, inducing acute and chronic effects, such as fever, chills, septic shock, and respiratory symptoms (11). Despite these adverse effects, epidemiologic, laboratory and limited clinical trial evidence suggest that endotoxins are likely to prevent cancer progression (12). Interestingly, monophosphoryl lipid A, a detoxified endotoxin derivative marketed in Europe for allergy treatment, and approved by the US Food and Drug Administration for use in humans as part of Cervarix® (a vaccine against human papillomavirus 16 and 18; GlaxoSmithKline, London, UK) (13), reduces the risk of all site cancers (excluding skin) by 25% in both smokers and non-smokers, thereby appearing to be a chemopreventive agent (14). We demonstrated that LPS-induced proinflammatory chemokines in OC cells via TLR4-dependent NF-κB activation (15). Inflammation is a key contributor to OC cell seeding (16) and proinflammatory tumor microenvironment contributes to OC metastasis and chemoresistance (17).
Based on epidemiological studies revealing a decreased cancer risk, due to cumulative exposures of endotoxins and beneficial effects of LPS as a chemopreventive agent in many cancer types despite LPS-induced inflammatory reaction, we investigated how LPS could affect the murine peritoneal dissemination model of OC using SKOV-3 cells as described previously (9).
MATERIALS AND METHODS
Generation of stable SKOV-3 luciferase (SKOV3Luc) OC cell line and culture
SKOV3Luc cells were generated from parental human SKOV-3 OC cells purchased from the American Type Culture Collection (Manassas, VA, USA) by transfecting stably a luciferase vector (pGL4.51[luc2/CMV/Neo]; Promega Corporation, Madison, WI, USA) as described previously (9). Cells were cultured at 37°C in a water-saturated atmosphere of 95% air and 5% CO2 with Roswell Park Memorial Institute 1640 containing penicillin and streptomycin (each 100 U/ml) and 10% FBS. All liquid culture media were acquired from Invitrogen (Carlsbad, CA, USA). D-Luciferin was purchased from Cayman Chemical (Ann Arbor, MI, USA).
Mouse peritoneal dissemination model
A mouse peritoneal dissemination model was performed under guidelines approved by the Institutional Animal Care and Use Committee at Meharry Medical College and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. CD-1 nude (Crl:CD1-Foxn1nu) mice (stock No: 003814) having T cell deficiency were obtained from Charles River (Wilmington, MA, USA) for human cancer cell xenograft research. The mice were maintained in a specific pathogen-free animal housing facility at 22°C±2°C and 40%–60% humidity under a 12:12 light: dark cycle. SKOV3Luc OC cells (3×106 cells/mouse in a volume of 0.2 ml PBS) were injected intraperitoneally (i.p.) into mice. Four days after inoculation of OC cells, LPS (0.5 mg/kg of body weight) was administered i.p. 3 times per wk for 3 wk. Bioluminescence imaging was monitored for tumor metastasis in the peritoneal cavity at termination. Briefly, mice anesthetized with 3% isoflurane were administered D-Luciferin (125 mg/kg, i.p.) 5 min prior to the acquisition of images. Next, mice were placed in the chamber of an In-Vivo MS FX PRO optical imaging system (Carestream Health, Rochester, NY, USA), and photons were collected for a period of 1 min. The luminescent intensity of the region of interest were quantified using Molecular Imaging software (Carestream Health). We monitored body weights and terminated the mice upon irreversible accumulation of ascites (up to 8–10 ml). Mice were monitored three times weekly to assess animal health, such as hunched posture, lethargy, and inactivity, impaired ambulation, shallow or labored breathing, hair coat condition and change in the body weight. Mice showing clinical signs of ascites fluid production with constant increase of body weight and changes in appearance and activity were observed daily. When the body weight was increased by 20% and extensive ascites accumulation or sluggish activity was evident, animals were terminated for humane reasons. The survival time was compared between control and LPS-treated mice.
Oligo GEArray® microarray
The Oligo GEArray® microarrays for human chemokines (OHS-022) and the TrueLabeling-AMP™ Linear RNA Amplication Kit were purchased from Qiagen (Frederick, MD, USA) (15). The signal intensities were quantified by densitometry using Quantity One (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Data analysis was performed based on a Data Analysis Center (https://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overview-page/) provided by Qiagen.
Transient transfection and luciferase assays
SKOV-3 OC cells were transiently transfected with pNF-κB-Luc (Stratagene, Stockport, UK), mCXCL1 or hCXCL2 vectors overnight using Lipofectamine solution as described previously (7,18). After rinsing cells with ice-cold PBS and adding lysis buffer (Promega Corporation), cell lysates were used to determine luciferase activity using a microplate luminometer. Luciferase activity, expressed as relative light units, was normalized to measured protein levels.
Gene Expression Omnibus (GEO) data analysis
Data analysis was performed on publicly available microarray datasets that were deposited in the National Center for Biotechnology Information (NCBI) GEO (http://www.ncbi.nlm.nih.gov/geo/) database as described previously (9,10). We utilized Gitools 2.3.1 (http://www.gitools.org), which requires Oracle Java 7, an open-source tool to perform Genomic Data Analysis and Visualization for interactive heat-maps (19).
Cell proliferation
Cell proliferation assay was performed using the cleavage of MTT to a colored product as described previously (8).
Statistics
Data were analyzed using Student's t-test and one-way ANOVA as appropriate. If statistical significance (p≤0.05) was indicated by ANOVA, data were further analyzed using Tukey's pairwise comparisons to detect specific group differences.
RESULTS AND DISCUSSION
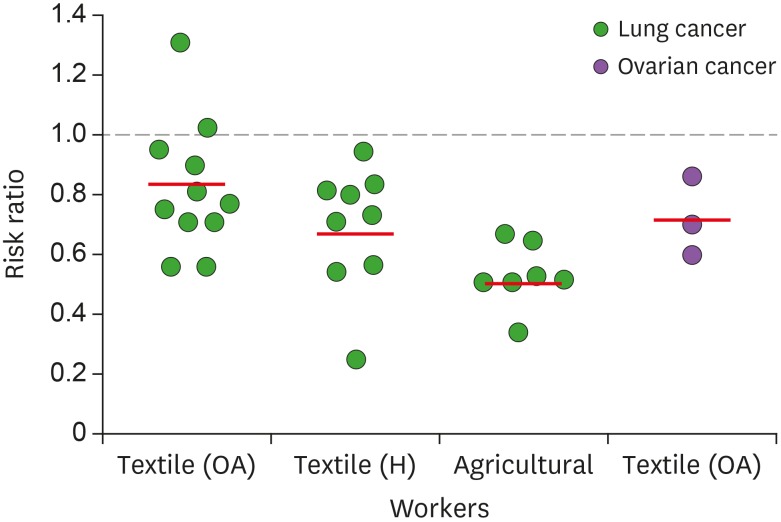
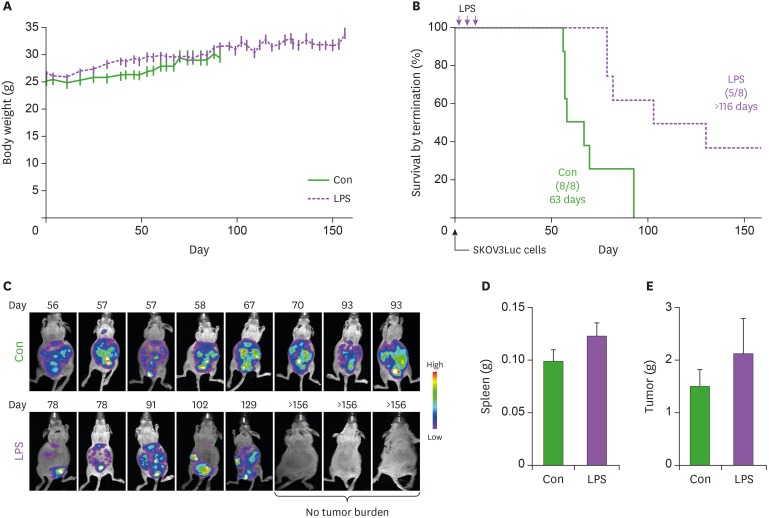
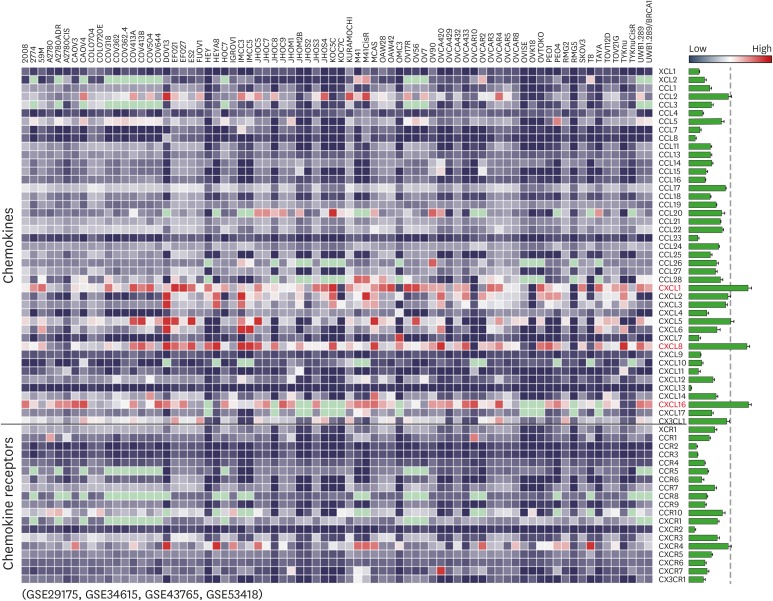
First, we evaluated epidemiologic links between endotoxins and OC through literature reviews. There were intense studies on relationship between exposure of endotoxins and lung cancer risk. Several occupational groups, including agricultural and cotton textile workers exposed to organic dusts contaminated by endotoxins, showed a reduced lung cancer risk (Fig. 1) (12,14,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40). On one hand, some reports on lung cancer indicated no change (41), while other reports indicated increased cancer risk (42,43). Epidemiologic evidence in OC revealed that cumulative exposures to endotoxins in textile workers reduced the risk of OC (Fig. 1) (20,21,44). Despite these limited data, endotoxins appear to reduce OC progression. We selected LPS, an active component of endotoxins, to clarify inhibitory effects of LPS on the progression of OC using the peritoneal dissemination model of SKOV3Luc OC cells as described (9). No differences in body weight between control and LPS-treated mice were noted (Fig. 2A). LPS-treated mice had significantly increased survival time of more than 116 days compared with 63 days in the control mice (Fig. 2B). Although detoxified Salmonella endotoxins had no effect on tumor growth, extracted fraction of cell-free Propionibacterium acnes, followed by detoxified endotoxins, resulted in long-term survival in a murine OC model (45). Further study requires clarifying whether anti-tumorigenicity between detoxified and toxified endotoxins is differential. Interestingly, there was no tumor burden in three mice out of eight LPS-treated mice, while all control mice showed peritoneal dissemination (Fig. 2C). No significant differences in spleen and tumor weight between control and LPS-treated mice were noted (Fig. 2D and E), which was expected at the terminal stage of OC. As CD-1 mice used in the present study are T cell-deficient, the antitumor effect of LPS is likely to involve directly an innate immune system rather than an adaptive immune system. Alternatively, addition of LPS as an adjuvant for dendritic cell immunotherapy of mouse ID8 OC had no survival benefit in C57BL/6 mice (46). These different results may be due to LPS-TLR4-dependent signaling in OC cells, as ID8 cells showed LPS-induced NF-κB activation, but not in SKOV-3 cells (15). In addition, differential immune response between CD-1 (defected adaptive immune system) and C57BL/6 mice (intact immune system) in OC may be responsible for causing the different LPS-responsive results. We analyzed chemokine signatures in human OC cell lines based on NCBI GEO dataset (GSE29175, GSE34615, GSE43765, and GSE53418). Chemokines, such as CXCL1, CXCL8, and CXCL16, highly expressed in a panel of 76 human OC cell lines (Fig. 3), indicating that proinflammatory tumor microenvironment may occur easily in OC.
Figure 1. Negative association between exposure to endotoxins and cancer risk. Summarized data are from 8 cohort, 1 case-cohort, and 2 case-control studies of cotton textile workers, and 15 cohort and 2 case-control studies of agricultural workers.
OA, overall; H, high exposure.
Figure 2. Prolonged effects of LPS on survival in mice bearing OC cells. (A) Body weight in control (Con) and LPS-treated CD-1 female mice. (B) Survival rate between Con and LPS-treated mice in the peritoneal dissemination model of OC cells. (C) The tumor burden imaging in the peritoneal cavity of SKOV3Luc cell bearing CD-1 mice measured by bioluminescence imaging prior to euthanasia due to the accumulation of ascites. (D) Spleen weight in Con and LPS-treated mice. (E) Tumor weight in Con and LPS-treated mice. Data values were expressed as the mean±SEM (n=8).
Con, control.
Figure 3. Heatmap for expression profiles of chemokines and chemokine receptors in 76 human OC cell lines from datasets deposited in the NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/) database (GSE29175, GSE34615, GSE43765, and GSE53418). The intensity of chemokine signature was also analyzed. Red letters in the heatmap indicate dominant chemokines, and light green colors indicate data not available.
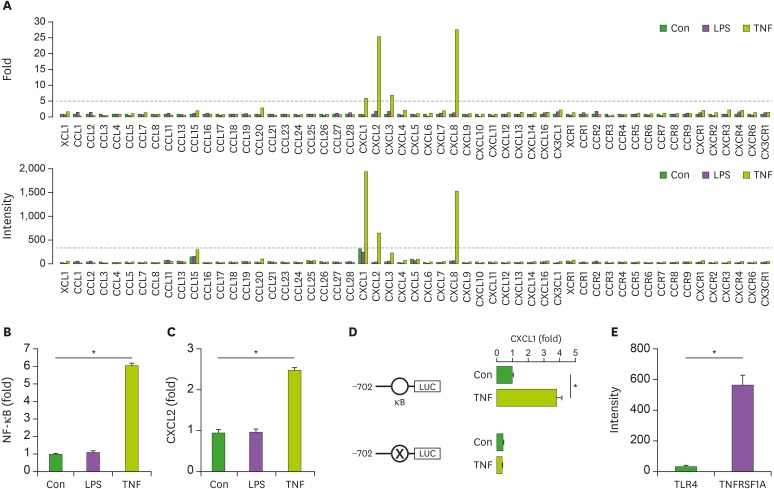
We discovered controversial reports in SKOV-3 cells, showing TLR4-positive (47) and -negative expression (15). We investigated LPS-induced chemokine signature in SKOV-3 cells compared with TNFα-induced effects as a positive control. While LPS had no effects on the chemokine signature in SKOV-3 cells (Fig. 4A), TNFα induced CXCL1, CXCL2, CXCL3, and CXCL8 (Fig. 4A), which is consistent with our previous study (18). LPS did not induce NF-κB promoter activity, whereas TNFα induced the activity (Fig. 4B). Promoter activity of CXCR2, one of highly-responsive TNFα-induced chemokines (Fig. 4A), was not affected by LPS but was induced by TNFα (Fig. 4C). Furthermore, we confirmed that TNFα-induced mouse CXCL1 promoter activity was NF-κB-dependent in SKOV-3 cells. Promoter activity of the mouse CXCL1-containing intact NF-κB was induced by TNFα, but its mutant promoter had no effects (Fig. 4D). Differential effects between LPS and TNFα in the chemokine signature may be due to expression levels of their receptors in SKOV-3 cells. Further, we confirmed that SKOV-3 cells expressed low levels of TLR4 but high levels of TNFRSF1A (Fig. 4E), resulting in no LPS-induced, but TNFα-induced, effects on chemokine signature (Fig. 4A). These findings indicate that LPS-induced anti-tumorigenicity in OC cells may be indirect effects through innate immune cells rather than direct effects to OC cells.
Figure 4. Involvement of NF-κB in LPS- and TNFα-induced chemokine signatures in SKOV-3 cells. (A) Fold change and intensity on LPS- and TNFα-induced chemokine and chemokine receptor signatures in SKOV-3 cells. (B) LPS- and TNFα-induced NF-κB promoter activity in SKOV-3 cells. (C) LPS- and TNFα-induced CXCL2 promoter activity in SKOV-3 cells. (D) Critical role of NF-κB on TNFα-induced CXCL1 promoter activity in SKOV-3 cells. (E) Expression intensity of TLR4 and TNFRSF1A in SKOV-3 cells. Data values were expressed as the mean ± SEM.
Con, control.
*Indicates significant increase (p<0.05) compared to its own control as calculated by the paired Student's t test, Student's t-test (D and E, n=3), and ANOVA using Tukey's pairwise comparisons (B and C, n=3).
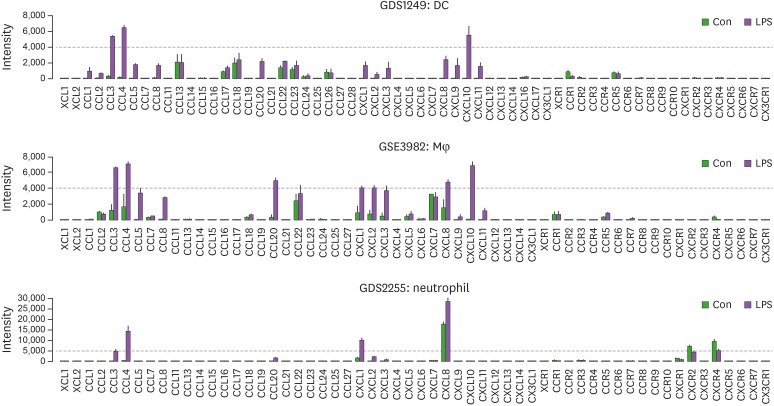
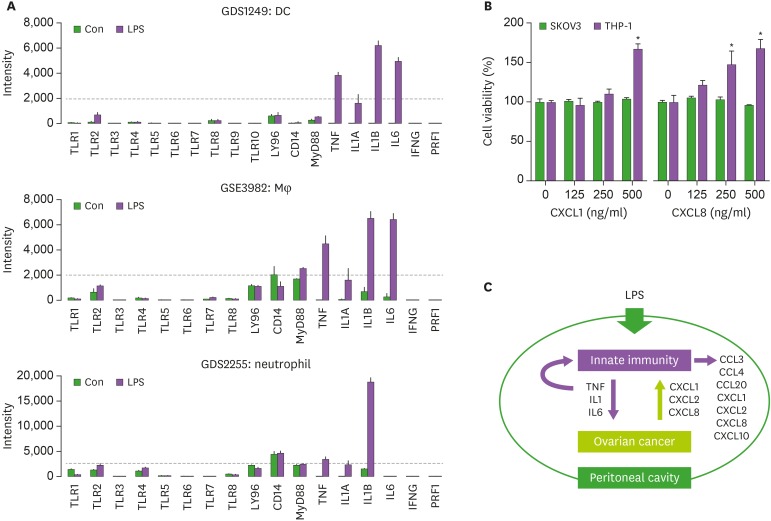
As CD-1 mice used in the present study were T cell-deficient, we analyzed LPS-induced chemokine signature in an innate immune system rather than adaptive immune system. High expression of LPS-induced chemokine signature in an innate immune system showed as follows: CCL3, CCL4, and CXCL10 in dendritic cells (DCs); CCL3, CCL4, CCL20, CXCL1, CXCL2, CXCL3, CXCL8, and CXCL10 in macrophages (Mφ); and CCL3, CCL4, CXCL1, and CXCL8 in neutrophils (Fig. 5). These results indicate that LPS may increase proinflammatory chemokines in an innate immune system, appearing anti-tumorigenicity via LPS-enhanced activation of innate immune cells. These LPS-induced proinflammatory chemokines are primarily regulated by IL-1- and TNFα-activated NF-κB in OC (7,15,18). Furthermore, we analyzed the LPS-induced TLR family and proinflammatory cytokine signatures in innate immune cells. DC, Mφ, and neutrophils expressed high levels of TNF, IL1A, and IL1B in response to LPS (Fig. 6A). Interestingly, IL6 was expressed highly in DCs and Mφ, but had low levels in neutrophils (Fig. 6A). These proinflammatory cytokines may be involved in antitumor effects of LPS, but not IFNG and PRF1 (Fig. 6A). As OC cells (Fig. 3), TNFα-treated SKOV-3 cells (Fig. 4A), and LPS-treated innate immune cells (Fig. 5) highly expressed CXCL1 and CXCL8, we investigated whether these chemokines could affect cell viability in SKOV-3 OC and THP-1 monocyte cells. Cell viability assay revealed that CXCL1 and CXCL8 had no effects on SKOV-3 cells but increased cell viability in THP-1 cells. CXCL8 antagonist (G31P) decreased cell viability, and LPS-induced CXCL8 protein, TNF, IL-1B, and IL6 mRNA in THP-1 cells (48), indicating the significant role of CXCL8 in THP-1 cell viability. LPS may activate an innate immune system by potentiating proinflammatory cytokines, such as TNFα, IL-1, and IL-6, which stimulate proinflammatory chemokines, such as CXCL1, CXCL2, and CXCL8, even in LPS-nonresponsive OC cells, and additionally increase CCL3, CCL4, CCL20, and CXCL10 in innate immune cells in an autocrine manner (Fig. 6C). Our current model indicates that the LPS-enhanced innate immunogenicity may exert anti-tumorigenicity to attenuate the peritoneal dissemination of OC in a cytotoxic-related proinflammatory tumor microenvironment.
Figure 5. LPS-induced chemokine signatures in DC, Mφ, and neutrophils.
Con, control.
Figure 6. LPS-induced TLR and proinflammatory cytokine signatures in main innate immune cells and roles of proinflammatory chemokines, CXCL1 and CXCL8, on cell viability. (A) Analysis of LPS-induced TLR and proinflammatory cytokine signatures in DC, Mφ, and neutrophils. (B) Effects of proinflammatory chemokines, CXCL1 and CXCL8, on cell viability in SKOV-3 and THP-1 cells. Data values were expressed as the mean±SEM. (C) Schematic representation of LPS-enhanced innate immunogenicity that blocks the peritoneal dissemination of OC in a cytotoxic-related proinflammatory tumor microenvironment.
Con, control.
*Indicates significant increase (p<0.05, n=3) compared to its control as calculated by ANOVA using Tukey's pairwise comparisons.
The present study has several limitations. As CD-1 mice are T cell deficient, antitumor effects of LPS in this study may not reflect the intact immune system, including T cell-mediated immunity, but mainly the innate immune system. In addition, as SKOV-3 cells are human OC and LPS-nonresponsive, antitumor effects of LPS in mice may have different roles in LPS-responsive human OC cells and in human beings. LPS used in this study may activate the cytotoxic immune system to kill OC cells (i.e., M1-like Mφ dominant), which may be disrupted in long-term cancer situation with loss of cytotoxic immune activity (i.e., M2-like Mφ dominant). As LPS induces side effects, such as fever, chills, septic shock, and respiratory symptoms (11), safety issues in using LPS may be a concern, requiring alternative agents, such as detoxified endotoxin derivatives. Further studies and additional data will be warranted to overcome these limitations.
In conclusion, LPS is likely to induce proinflammatory chemokines and cytokines in the innate immune system to establish a cytotoxic-related tumor microenvironment, followed by attenuated peritoneal dissemination of OC, thereby leading to LPS-based therapeutic potentials for OC.
ACKNOWLEDGEMENTS
This research was supported, in whole or in part, by National Institutes of Health as the following grants: R01ES024756 (E.L.), NIAID SC1AI089073 (D.S.), and NCI SC1CA200519 (D.S.). The research is solely the responsibility of the authors and does not necessarily represent the official views of NIH. Finally, we thank the Meharry Office of Scientific Editing & Publications for editorial assistance during preparation of this manuscript.
Abbreviations
- DC
dendritic cell
- GEO
Gene Expression Omnibus
- HGSOC
high-grade serous ovarian cancer
- i.p.
intraperitoneally
- Mφ
macrophages
- NCBI
National Center for Biotechnology Information
- NIH
National Institutes of Health
- OC
ovarian cancer
- SKOV3Luc
SKOV-3 luciferase
- TP53
tumor suppressor protein p53
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee ES, Son DS.
- Data curation: Ignacio RMC, Son DS.
- Formal analysis: Ignacio RMC, Son DS.
- Funding acquisition: Lee ES, Son DS.
- Investigation: Ignacio RMC, Lee ES, Son DS.
- Methodology: Ignacio RMC, Son DS.
- Supervision: Son DS.
- Validation: Ignacio RMC, Son DS.
- Visualization: Son DS.
- Writing - original draft: Son DS.
- Writing - review & editing: Lee ES, Son DS.
References
- 1.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doubeni CA, Doubeni AR, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Physician. 2016;93:937–944. [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Rojas V, Hirshfield KM, Ganesan S, Rodriguez-Rodriguez L. Molecular characterization of epithelial ovarian cancer: implications for diagnosis and treatment. Int J Mol Sci. 2016;17:E2113. doi: 10.3390/ijms17122113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurman RJ, Shih IM. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186:733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son DS, Kabir SM, Dong YL, Lee E, Adunyah SE. Inhibitory effect of tumor suppressor p53 on proinflammatory chemokine expression in ovarian cancer cells by reducing proteasomal degradation of IκB. PLoS One. 2012;7:e51116. doi: 10.1371/journal.pone.0051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong YL, Kabir SM, Lee ES, Son DS. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-κB activation via EGFR-transactivated Akt signaling. PLoS One. 2013;8:e83789. doi: 10.1371/journal.pone.0083789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignacio RM, Kabir SM, Lee ES, Adunyah SE, Son DS. NF-κB-mediated CCL20 reigns dominantly in CXCR2-driven ovarian cancer progression. PLoS One. 2016;11:e0164189. doi: 10.1371/journal.pone.0164189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignacio RM, Lee ES, Wilson AJ, Beeghly-Fadiel A, Whalen MM, Son DS. Chemokine network and overall survival in TP53 wild-type and mutant ovarian cancer. Immune Netw. 2018;18:e29. doi: 10.4110/in.2018.18.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielen S, Trischler J, Schubert R. Lipopolysaccharide challenge: immunological effects and safety in humans. Expert Rev Clin Immunol. 2015;11:409–418. doi: 10.1586/1744666X.2015.1012158. [DOI] [PubMed] [Google Scholar]
- 12.Lundin JI, Checkoway H. Endotoxin and cancer. Environ Health Perspect. 2009;117:1344–1350. doi: 10.1289/ehp.0800439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: experimental Toll-like receptor agonists for cancer therapy. OncoImmunology. 2012;1:699–716. doi: 10.4161/onci.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastrangelo G, Fadda E, Cegolon L. Endotoxin and cancer chemo-prevention. Cancer Epidemiol. 2013;37:528–533. doi: 10.1016/j.canep.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007;6:1302–1312. doi: 10.4161/cbt.6.8.4506. [DOI] [PubMed] [Google Scholar]
- 16.Jia D, Nagaoka Y, Katsumata M, Orsulic S. Inflammation is a key contributor to ovarian cancer cell seeding. Sci Rep. 2018;8:12394. doi: 10.1038/s41598-018-30261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savant SS, Sriramkumar S, O'Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel) 2018;10:E251. doi: 10.3390/cancers10080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son DS, Kabir SM, Dong Y, Lee E, Adunyah SE. Characteristics of chemokine signatures elicited by EGF and TNF in ovarian cancer cells. J Inflamm (Lond) 2013;10:25. doi: 10.1186/1476-9255-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Llamas C, Lopez-Bigas N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS One. 2011;6:e19541. doi: 10.1371/journal.pone.0019541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wernli KJ, Ray RM, Gao DL, Fitzgibbons ED, Camp JE, Astrakianakis G, Seixas N, Wong EY, Li W, De Roos AJ, et al. Occupational exposures and ovarian cancer in textile workers. Epidemiology. 2008;19:244–250. doi: 10.1097/EDE.0b013e31816339f9. [DOI] [PubMed] [Google Scholar]
- 21.Wernli KJ, Ray RM, Gao DL, Thomas DB, Checkoway H. Cancer among women textile workers in Shanghai, China: overall incidence patterns, 1989–1998. Am J Ind Med. 2003;44:595–599. doi: 10.1002/ajim.10265. [DOI] [PubMed] [Google Scholar]
- 22.Applebaum KM, Ray RM, Astrakianakis G, Gao DL, Thomas DB, Christiani DC, LaValley MP, Li W, Checkoway H, Eisen EA. Evidence of a paradoxical relationship between endotoxin and lung cancer after accounting for left truncation in a study of Chinese female textile workers. Occup Environ Med. 2013;70:709–715. doi: 10.1136/oemed-2012-101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agalliu I, Costello S, Applebaum KM, Ray RM, Astrakianakis G, Gao DL, Thomas DB, Checkoway H, Eisen EA. Risk of lung cancer in relation to contiguous windows of endotoxin exposure among female textile workers in Shanghai. Cancer Causes Control. 2011;22:1397–1404. doi: 10.1007/s10552-011-9812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenters V, Basinas I, Beane-Freeman L, Boffetta P, Checkoway H, Coggon D, Portengen L, Sim M, Wouters IM, Heederik D, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control. 2010;21:523–555. doi: 10.1007/s10552-009-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astrakianakis G, Seixas NS, Ray R, Camp JE, Gao DL, Feng Z, Li W, Wernli KJ, Fitzgibbons ED, Thomas DB, et al. Lung cancer risk among female textile workers exposed to endotoxin. J Natl Cancer Inst. 2007;99:357–364. doi: 10.1093/jnci/djk063. [DOI] [PubMed] [Google Scholar]
- 26.Lange JH, Mastrangelo G, Fedeli U, Fadda E, Rylander R, Lee E. Endotoxin exposure and lung cancer mortality by type of farming: is there a hidden dose-response relationship? Ann Agric Environ Med. 2003;10:229–232. [PubMed] [Google Scholar]
- 27.Mastrangelo G, Marzia V, Marcer G. Reduced lung cancer mortality in dairy farmers: is endotoxin exposure the key factor? Am J Ind Med. 1996;30:601–609. doi: 10.1002/(SICI)1097-0274(199611)30:5<601::AID-AJIM8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Merchant JA, Ortmeyer C. Mortality of employees of two cotton mills in North Carolina. Chest. 1981;79:6S–11S. doi: 10.1378/chest.79.4_supplement.6s. [DOI] [PubMed] [Google Scholar]
- 29.Hodgson JT, Jones RD. Mortality of workers in the British cotton industry in 1968-1984. Scand J Work Environ Health. 1990;16:113–120. doi: 10.5271/sjweh.1809. [DOI] [PubMed] [Google Scholar]
- 30.Levin LI, Gao YT, Blot WJ, Zheng W, Fraumeni JF., Jr Decreased risk of lung cancer in the cotton textile industry of Shanghai. Cancer Res. 1987;47:5777–5781. [PubMed] [Google Scholar]
- 31.Szeszenia-Dabrowska N, Wilczyńska U, Strzelecka A, Sobala W. Mortality in the cotton industry workers: results of a cohort study. Int J Occup Med Environ Health. 1999;12:143–158. [PubMed] [Google Scholar]
- 32.Mastrangelo G, Fadda E, Rylander R, Milan G, Fedeli U, Rossi di Schio M, Lange JH. Lung and other cancer site mortality in a cohort of Italian cotton mill workers. Occup Environ Med. 2008;65:697–700. doi: 10.1136/oem.2007.036327. [DOI] [PubMed] [Google Scholar]
- 33.Kuzmickiene I, Stukonis M. Lung cancer risk among textile workers in Lithuania. J Occup Med Toxicol. 2007;2:14. doi: 10.1186/1745-6673-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blair A, Sandler DP, Tarone R, Lubin J, Thomas K, Hoppin JA, Samanic C, Coble J, Kamel F, Knott C, et al. Mortality among participants in the agricultural health study. Ann Epidemiol. 2005;15:279–285. doi: 10.1016/j.annepidem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Laakkonen A, Pukkala E. Cancer incidence among Finnish farmers, 1995–2005. Scand J Work Environ Health. 2008;34:73–79. doi: 10.5271/sjweh.1167. [DOI] [PubMed] [Google Scholar]
- 36.Mastrangelo G, Grange JM, Fadda E, Fedeli U, Buja A, Lange JH. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol. 2005;161:1037–1046. doi: 10.1093/aje/kwi138. [DOI] [PubMed] [Google Scholar]
- 37.Pukkala E, Notkola V. Cancer incidence among Finnish farmers, 1979–93. Cancer Causes Control. 1997;8:25–33. doi: 10.1023/a:1018474919807. [DOI] [PubMed] [Google Scholar]
- 38.Reif J, Pearce N, Fraser J. Cancer risks in New Zealand farmers. Int J Epidemiol. 1989;18:768–774. doi: 10.1093/ije/18.4.768. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder JC, Tolbert PE, Eisen EA, Monson RR, Hallock MF, Smith TJ, Woskie SR, Hammond SK, Milton DK. Mortality studies of machining fluid exposure in the automobile industry IV: a case-control study of lung cancer. Am J Ind Med. 1997;31:525–533. doi: 10.1002/(sici)1097-0274(199705)31:5<525::aid-ajim5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Mastrangelo G, Fedeli U, Fadda E, Milan G, Lange JH. Epidemiologic evidence of cancer risk in textile industry workers: a review and update. Toxicol Ind Health. 2002;18:171–181. doi: 10.1191/0748233702th139rr. [DOI] [PubMed] [Google Scholar]
- 41.Checkoway H, Lundin JI, Costello S, Ray R, Li W, Eisen EA, Astrakianakis G, Seixas N, Applebaum K, Gao DL, et al. Possible pro-carcinogenic association of endotoxin on lung cancer among Shanghai women textile workers. Br J Cancer. 2014;111:603–607. doi: 10.1038/bjc.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuzmickiene I, Didziapetris R, Stukonis M. Cancer incidence in the workers cohort of textile manufacturing factory in Alytus, Lithuania. J Occup Environ Med. 2004;46:147–153. doi: 10.1097/01.jom.0000111601.85534.12. [DOI] [PubMed] [Google Scholar]
- 43.Baccarelli A, Khmelnitskii O, Tretiakova M, Gorbanev S, Lomtev A, Klimkina I, Tchibissov V, Averkina O, Rice C, Dosemeci M. Risk of lung cancer from exposure to dusts and fibers in Leningrad Province, Russia. Am J Ind Med. 2006;49:460–467. doi: 10.1002/ajim.20316. [DOI] [PubMed] [Google Scholar]
- 44.Wong EY, Ray R, Gao DL, Wernli KJ, Li W, Fitzgibbons ED, Feng Z, Thomas DB, Checkoway H. Reproductive history, occupational exposures, and thyroid cancer risk among women textile workers in Shanghai, China. Int Arch Occup Environ Health. 2006;79:251–258. doi: 10.1007/s00420-005-0036-9. [DOI] [PubMed] [Google Scholar]
- 45.Berek JS, Lichtenstein AK, Knox RM, Jung TS, Rose TP, Cantrell JL, Zighelboim J. Synergistic effects of combination sequential immunotherapies in a murine ovarian cancer model. Cancer Res. 1985;45:4215–4218. [PubMed] [Google Scholar]
- 46.Vindevogel E, Baert T, van Hoylandt A, Verbist G, Vande Velde G, Garg AD, Agostinis P, Vergote I, Coosemans AN. The use of Toll-like receptor 4 agonist to reshape the immune signature in ovarian cancer. Anticancer Res. 2016;36:5781–5792. doi: 10.21873/anticanres.11162. [DOI] [PubMed] [Google Scholar]
- 47.d'Adhemar CJ, Spillane CD, Gallagher MF, Bates M, Costello KM, Barry-O'Crowley J, Haley K, Kernan N, Murphy C, Smyth PC, et al. The MyD88+ phenotype is an adverse prognostic factor in epithelial ovarian cancer. PLoS One. 2014;9:e100816. doi: 10.1371/journal.pone.0100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walana W, Wang JJ, Yabasin IB, Ntim M, Kampo S, Al-Azab M, Elkhider A, Dogkotenge Kuugbee E, Cheng JW, Gordon JR, et al. IL-8 analogue CXCL8 (3-72) K11R/G31P, modulates LPS-induced inflammation via AKT1-NF-kβ and ERK1/2-AP-1 pathways in THP-1 monocytes. Hum Immunol. 2018;79:809–816. doi: 10.1016/j.humimm.2018.08.007. [DOI] [PubMed] [Google Scholar]