Abstract
Background and Objectives
Conflicting data exist regarding the prognostic implication of ventricular conduction disturbance pattern in patients with heart failure (HF). This study investigated the prognostic impact of ventricular conduction pattern in hospitalized patients with acute HF.
Methods
Data from the Korean Acute Heart Failure registry were used. Patients were categorized into four groups: narrow QRS (<120 ms), right bundle branch block (RBBB), left bundle branch block (LBBB), and nonspecific intraventricular conduction delay (NICD). The NICD was defined as prolonged QRS (≥120 ms) without typical features of LBBB or RBBB. The primary endpoint was the composite of all-cause mortality or rehospitalization for HF aggravation within 1 year after discharge.
Results
This study included 5,157 patients. The primary endpoint occurred in 39.7% of study population. The LBBB group showed the highest incidence of primary endpoint followed by NICD, RBBB, and narrow QRS groups (52.5% vs. 49.7% vs. 44.4% vs. 37.5%, p<0.001). In a multivariable Cox-proportional hazards regression analysis, LBBB and NICD were associated with 39% and 28% increased risk for primary endpoint (LBBB hazard ratio [HR], 1.392; 95% confidence interval [CI], 1.152–1.681; NICD HR, 1.278; 95% CI, 1.074–1.520) compared with narrow QRS group. The HR of RBBB for the primary endpoint was 1.103 (95% CI, 0.915–1.329).
Conclusions
LBBB and NICD were independently associated with an increased risk of 1-year adverse event in hospitalized patients with HF, whereas the prognostic impacts of RBBB were limited.
Trial Registration
ClinicalTrials.gov Identifier: NCT01389843
Keywords: Cardiac conduction system disease, Bundle branch block, Prognosis, Heart failure
INTRODUCTION
Increased QRS width has been known to be associated with poor prognosis in patients with heart failure (HF).1) However, despite the similar degree of QRS prolongation, their prognosis might be different according to the patterns of ventricular conduction disturbance. Previously, the prognostic impact between left bundle branch block (LBBB) and right bundle branch block (RBBB) has been compared in patients with HF. However, the results were inconsistent and remain controversial until now.2),3),4),5)
Nonspecific intraventricular conduction delay (NICD) generally refers to QRS prolongation without a typical RBBB or LBBB pattern.6) The prevalence of NICD was reported in 3.8–5.8% of HF patients with reduced ejection fraction.6) Despite its considerable prevalence, the prognostic impact of NICD in patients with HF has not been well evaluated and compared with that of typical bundle branch blocks (BBBs), especially in East Asia. Therefore, we sought to investigate the prognostic impact of NICD and to compare that with those of narrow QRS, RBBB and LBBB in hospitalized patients with acute HF.
METHODS
Study population
We used data from the Korean Acute Heart Failure (KorAHF) registry, a prospective multicenter cohort study that consecutively enrolled 5,625 patients who were hospitalized for acute HF syndrome from 10 tertiary university hospitals throughout the country between March 2011 and December 2014. Detailed information on the study design and results has been previously reported elsewhere (ClinicalTrial.gov NCT01389843).7),8) In brief, patients with signs or symptoms of HF and either lung congestion, objective findings of left ventricular systolic dysfunction, or structural heart disease were eligible for the study. The mortality data for patients who were lost to follow-up were collected from the National Insurance data or National Death Records.
All patients with available baseline electrocardiography (ECG) data in the KorAHF registry were included in the present study. We excluded patients with a pacing rhythm (those with a permanent pacemaker or receiving cardiac resynchronization therapy [CRT]), ventricular rhythm, or pre-excitation, and patients who died or underwent urgent heart transplantation during initial hospitalization.
The study protocol was approved by the ethics committee or institutional review board at each hospital. The need for written informed consent was waived by the institutional review board. The study complied with the Declaration of Helsinki.
Study variables
Patients were categorized into four groups: narrow QRS (<120 ms), RBBB, LBBB, and NICD. The World Health Organization's criteria were used to define NICD, RBBB, and LBBB.9) NICD was defined as prolonged QRS (≥120 ms) without typical features of LBBB or RBBB.9) Standard 12-lead ECG was performed with the subject at rest using a paper speed of 25 mm/s and a calibration of 1 mV per 10 mm. Each electrocardiogram was reviewed by two independent cardiologists. The primary endpoint was the composite of all-cause mortality or rehospitalization for HF aggravation within 1 year after discharge.
Statistical analysis
Categorical variables are presented as numbers and frequencies, whereas continuous variables are presented as mean±standard deviation. Student's t-test or analysis of variance was used to compare continuous variables, and the χ2 test was used to compare categorical variables.
Kaplan-Meier curves were plotted and compared using the log-rank test. To adjust for covariates, a multivariable Cox proportional hazards regression model was used to predict the primary endpoint. Age, sex, body mass index, de novo HF, ischemic etiology, presence of hypertension, presence of diabetes mellitus, left ventricular ejection fraction (LVEF), presence of atrial fibrillation, serum hemoglobin level, serum creatinine level, high-sensitivity C-reactive protein level, beta-blocker use, renin-angiotensin-system inhibitor use, and mineralocorticoid receptor antagonist use were included in the multivariable model. Statistical tests were performed using SPSS, version 22 (IBM Corp., Armonk, NY, USA) and R programming version 3.5.1 (http://www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study population
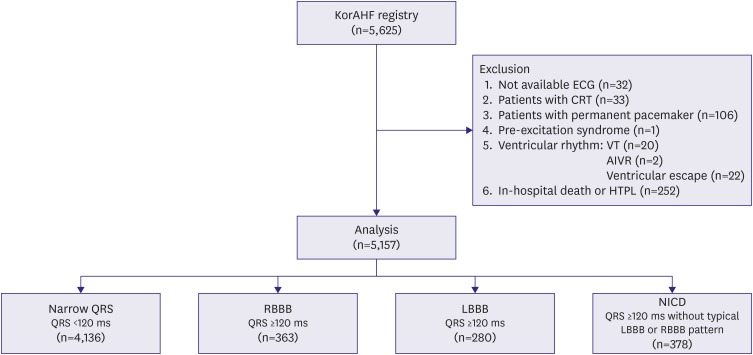
This study included 5,157 patients for the analysis. The flow chart of patient selection is depicted in Figure 1. The patients with narrow QRS accounted for 80.2% (n=4,136) of the total subjects, followed by NICD (7.4%, n=378), RBBB (7.0%, n=363), and LBBB (5.4%, n=280). Baseline characteristics of the study population were significantly different among the groups (Table 1). The patients with LBBB were the oldest (73.0±11.1 years), followed by those with RBBB (69.0±14.2 years), narrow QRS (68.3±14.8 years), and NICD (66.0±13.5 years, p<0.001). The LVEF was the lowest in patients with NICD (28.6±13.4%), followed by those with LBBB (29.7±12.4%), narrow QRS (40.1±15.6%), and RBBB (40.7±16.7%, p<0.01). The QRS width was the largest in patients with LBBB (155.8±19.1 ms), followed by those with RBBB (147.0±19.6 ms), NICD (138.8±24.1 ms), and narrow QRS (93.6±12.1 ms).
Figure 1. Flow chart of the patient selection.
AIVR = accelerated idioventricular rhythm; CRT = cardiac resynchronization therapy; ECG = electrocardiography; HTPL = heart transplantation; KorAHF = Korean Acute Heart Failure; LBBB = left bundle branch block; NICD = nonspecific intraventricular conduction delay; RBBB = right bundle branch block; VT = ventricular tachycardia.
Table 1. Baseline characteristics of the study population.
| Variables | Narrow QRS (n=4,136) | RBBB (n=363) | LBBB (n=280) | NICD (n=378) | p value | |
|---|---|---|---|---|---|---|
| Age (years) | 68.3±14.8 | 69.0±14.2 | 73.0±11.1 | 66.0±13.5 | <0.001 | |
| Male | 2,127 (51.4) | 217 (59.8) | 130 (46.4) | 257 (68.0) | <0.001 | |
| SBP at ADM (mmHg) | 133.6±30.1 | 129.1±29.8 | 128.1±26.1 | 124.5±30.4 | <0.001 | |
| DBP at ADM (mmHg) | 80.1±18.8 | 76.2±17.1 | 75.0±15.8 | 77.0±18.6 | <0.001 | |
| HR at ADM (bpm) | 93.8±25.7 | 87.1±24.5 | 91.7±22.1 | 80.1±25.2 | <0.001 | |
| BMI (kg/m2) | 23.3±3.9 | 23.6±3.9 | 23.0±3.5 | 23.6±3.6 | 0.169 | |
| NYHA class IV | 1,948 (47.1) | 173 (47.7) | 135 (48.2) | 171 (45.2) | 0.871 | |
| De novo HF | 2,334 (56.4) | 170 (46.8) | 115 (41.1) | 128 (33.9) | <0.001 | |
| Ischemic HF | 1,547 (37.4) | 121 (33.3) | 106 (37.9) | 153 (40.5) | 0.249 | |
| HFrEF (EF ≤40%) | 2,138 (66.7) | 187 (64.3) | 230 (95.4) | 296 (90.2) | <0.001 | |
| Past history | ||||||
| Hypertension | 2,469 (59.7) | 202 (55.6) | 165 (58.9) | 211 (55.8) | 0.253 | |
| DM type 2 | 1,430 (34.6) | 123 (33.9) | 118 (42.1) | 151 (39.9) | 0.014 | |
| MI | 657 (15.9) | 67 (18.5) | 39 (13.9) | 73 (19.3) | 0.145 | |
| COPD | 457 (11.1) | 45 (12.4) | 37 (13.2) | 35 (9.3) | 0.365 | |
| AF | 1,181 (28.6) | 132 (36.4) | 52 (18.6) | 101 (26.7) | <0.001 | |
| CKD | 566 (13.7) | 52 (14.3) | 42 (15.0) | 53 (14.0) | 0.923 | |
| LVEF (%) | 40.1±15.6 | 40.7±16.7 | 29.7±12.4 | 28.6±13.4 | <0.001 | |
| LVESD (mm) | 43.8±11.6 | 43.5±12.4 | 52.5±10.7 | 55.2±12.6 | <0.001 | |
| LVEDD (mm) | 56.4±9.5 | 56.3±10.0 | 62.3±9.2 | 65.1±11.0 | <0.001 | |
| Na (mmol/L) | 137.8±4.6 | 137.1±5.2 | 137.5±4.7 | 136.4±5.3 | 0.008 | |
| HsCRP (mg/L) | 2.2±4.1 | 2.5±4.9 | 1.9±3.1 | 2.4±4.3 | 0.277 | |
| Hb (g/dL) | 12.4±2.3 | 12.6±2.3 | 12.3±2.0 | 12.7±2.4 | 0.065 | |
| Uric acid (mg/dL) | 6.9±2.8 | 7.3±2.9 | 6.9±2.6 | 7.5±2.8 | 0.643 | |
| Albumin (g/dL) | 3.7±0.5 | 3.7±0.5 | 3.8±0.5 | 3.7±0.5 | 0.060 | |
| Cr (mg/dL) | 1.4±1.5 | 1.5±1.6 | 1.5±1.2 | 1.5±1.4 | 0.102 | |
| BNP (pg/mL) | 858 (461, 1,704) | 900 (441, 1,554) | 1,023 (538, 2,145) | 980 (539, 1,850) | <0.001 | |
| NT-proBNP (pg/mL) | 4,673 (2,024, 10,987) | 5,515 (2,527, 12,780) | 4,890 (2,560, 14,422) | 4,790 (2,432, 12,333) | <0.001 | |
| QRS width (ms) | 93.6±12.1 | 147.0±19.6 | 155.8±19.1 | 138.8±24.1 | <0.001 | |
| Discharge medication | ||||||
| Beta blocker | 2,223 (53.7) | 156 (43.0) | 158 (56.4) | 185 (48.9) | <0.001 | |
| ACEi or ARB | 2,834 (68.5) | 232 (63.9) | 215 (76.8) | 277 (73.3) | <0.001 | |
| MRA | 1,867 (45.1) | 179 (49.3) | 152 (54.3) | 207 (54.8) | <0.001 | |
Values are expressed as number (%) or mean±standard deviation or median (25th quartile, 75th quartile).
ACEi = angiotensin-converting enzyme inhibitor; ADM = admission; AF = atrial fibrillation; ARB = angiotensin II receptor blocker; BMI = body mass index; BNP = brain natriuretic peptide; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; Cr = creatinine; DBP = diastolic blood pressure; DM = diabetes mellitus; EF = ejection fraction; Hb = hemoglobin; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; HsCRP = high sensitivity C-reactive protein; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; MI = myocardial infarction; MRA = mineralocorticoid receptor antagonist; Na = sodium; NICD = nonspecific intraventricular conduction delay; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; NYHA = New York Heart Association; RBBB = right bundle branch block; SBP = systolic blood pressure.
Clinical outcomes
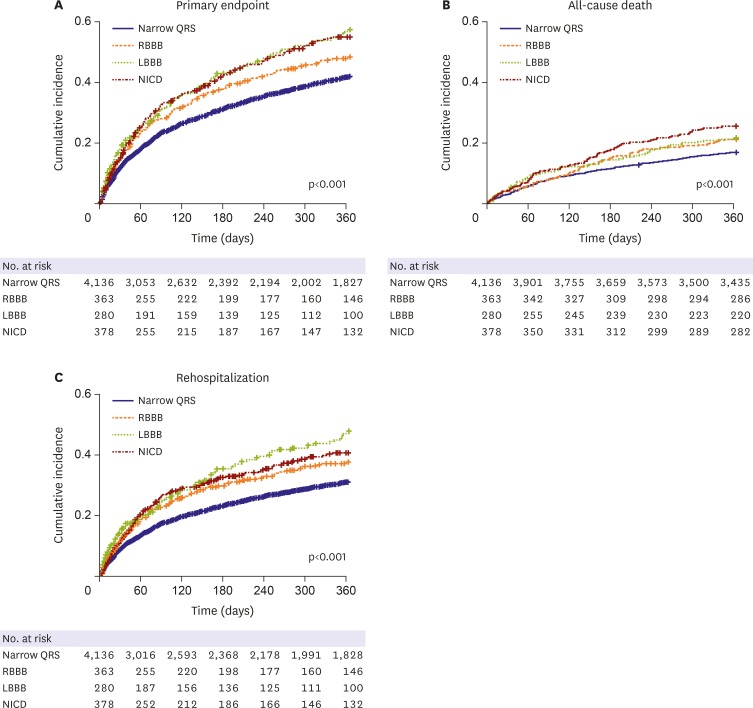
During the 1-year follow-up, primary endpoint occurred in 2,045 (39.7%) patients (All-cause death: 939, HF rehospitalization: 1,421). The incidence of the primary endpoint was the highest in patients with LBBB (n=147, 52.5%), followed by those with NICD (n=188, 49.7%), RBBB (n=161, 44.4%), and narrow QRS (n=1,549, 37.5%) (Table 2). All-cause mortality was the highest in NICD group (n=96, 25.4%) followed by LBBB (n=61, 21.8%), RBBB (n=77, 21.2%) and narrow QRS (n=705, 17%, p<0.001) while the rehospitalization rate was the highest in LBBB group (n=111, 39.6%) followed by NICD (n=127, 33.6%), RBBB (n=117, 32.2%) and narrow QRS (n=1,066, 25.8%, p<0.001).
Table 2. Incidence of study endpoints according to the ventricular conduction patterns.
| Study endpoint | Narrow QRS (n=4,136) | RBBB (n=363) | LBBB (n=280) | NICD (n=378) | p value | |
|---|---|---|---|---|---|---|
| Primary endpoint | 1,549 (37.5) | 161 (44.4) | 147 (52.5) | 188 (49.7) | <0.001 | |
| All cause death | 705 (17.0) | 77 (21.2) | 61 (21.8) | 96 (25.4) | <0.001 | |
| Rehospitalization | 1,066 (25.8) | 117 (32.2) | 111 (39.6) | 127 (33.6) | <0.001 | |
Values are expressed as number (%).
LBBB = left bundle branch block; NICD = nonspecific intraventricular conduction delay; RBBB = right bundle branch block.
In the Kaplan-Meier curve analysis, the RBBB, LBBB, and NICD groups showed a higher incidence of the primary composite endpoint than the narrow QRS group (p<0.001, Figure 2). The LBBB group showed the highest incidence of primary composite endpoints and HF rehospitalization, while NICD group showed the highest incidence of all-cause death among the study groups (Figure 2).
Figure 2. Kaplan-Meier curves for the incidence of study endpoints according to the different ventricular conduction patterns.
LBBB = left bundle branch block; NICD = nonspecific intraventricular conduction delay; RBBB = right bundle branch block.
In the multivariable Cox-proportional hazard regression analysis, LBBB and NICD were associated with 39% and 28% increased risk for primary endpoint (LBBB hazard ratio [HR], 1.392, 95% confidence interval [CI], 1.152–1.681; NICD HR, 1.278; 95% CI, 1.074–1.520) compared with the narrow QRS group, while RBBB showed modest association with the primary endpoint (HR, 1.103; 95% CI, 0.915–1.329; Table 3).
Table 3. Cox hazard regression analysis for the primary endpoint.
| Variables | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age | 1.022 (1.019–1.026) | <0.001 | 1.017 (1.013–1.022) | <0.001 | |
| Male | 0.905 (0.830–0.987) | 0.024 | 1.007 (0.906–1.118) | 0.902 | |
| BMI (kg/m2) | 0.958 (0.947–0.970) | <0.001 | 0.983 (0.970–0.997) | 0.018 | |
| De novo HF | 0.599 (0.548–0.654) | <0.001 | 0.755 (0.681–0.838) | <0.001 | |
| Ischemic HF | 1.297 (1.189–1.416) | <0.001 | 1.110 (0.996–1.236) | 0.058 | |
| LVEF (%) | 0.997 (0.994–1.000) | 0.029 | 0.994 (0.990–0.997) | 0.001 | |
| Hypertension | 1.356 (1.238–1.484) | <0.001 | 1.126 (1.009–1.257) | 0.034 | |
| DM | 1.366 (1.251–1.492) | <0.001 | 1.142 (1.028–1.268) | 0.013 | |
| AF | 1.233 (1.124–1.353) | <0.001 | 1.114 (0.995–1.247) | 0.060 | |
| Hb (g/dL) | 0.885 (0.869–0.902) | <0.001 | 0.932 (0.909–0.955) | <0.001 | |
| Cr (mg/dL) | 1.079 (1.056–1.103) | <0.001 | 1.056 (1.023–1.090) | 0.001 | |
| HsCRP (mg/dL) | 1.019 (1.009–1.029) | <0.001 | 1.011 (1.000–1.022) | 0.055 | |
| Narrow QRS (<120 ms) | 1 (Reference) | NA | 1 (Reference) | NA | |
| RBBB | 1.237 (1.051–1.454) | 0.010 | 1.103 (0.915–1.329) | 0.304 | |
| LBBB | 1.534 (1.295–1.816) | <0.001 | 1.392 (1.152–1.681) | 0.001 | |
| NICD | 1.454 (1.250–1.692) | <0.001 | 1.278 (1.074–1.520) | 0.006 | |
| ACEi or ARB | 0.772 (0.704–0.846) | <0.001 | 0.829 (0.746–0.922) | 0.001 | |
| BB | 0.699 (0.641–0.763) | <0.001 | 0.777 (0.704–0.857) | <0.001 | |
| MRA | 0.981 (0.899–1.070) | 0.660 | 1.078 (0.976–1.191) | 0.139 | |
ACEi = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin II receptor blocker; BB = beta blocker; BMI = body mass index; CI = confidence interval; Cr = creatinine; DM = diabetes mellitus; Hb = hemoglobin; HF = heart failure; HR = hazard ratio; HsCRP = high-sensitivity C-reactive protein; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NA = not applicable; NICD = nonspecific intraventricular conduction delay; RBBB = right bundle branch block.
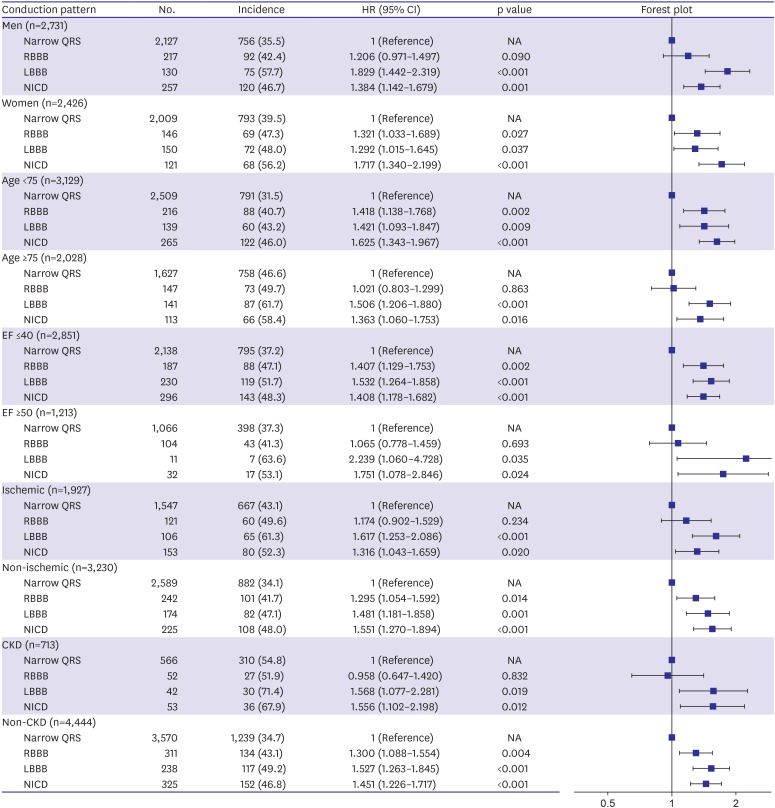
In the subgroup analysis, similar trends of worse outcome with LBBB and NICD were observed consistently regardless of age (age <75 years vs. age ≥75 years), sex, LVEF (LVEF ≤40% vs. LVEF ≥50%), HF etiology (ischemic vs. non-ischemic) and presence of renal dysfunction (Figure 3).
Figure 3. Subgroup analysis of the incidence of primary endpoint according to the different ventricular conduction patterns.
CI = confidence interval; CKD = chronic kidney disease; EF = ejection fraction; HF = heart failure; HR = hazard ratio; HTN = hypertension; LBBB = left bundle branch block; NICD = nonspecific intraventricular conduction delay; RBBB = right bundle branch block.
DISCUSSION
The present study demonstrated the prognostic impacts of different left ventricular conduction disturbances in patients with HF. Patients with LBBB and NICD showed the worse prognosis compared with those with narrow QRS. This study finding implies that examining the patterns of ventricular conduction disturbance is important for risk stratification of patients with HF.
Chronic ventricular dyssynchrony and its consequent ventricular remodeling thought to be a main mechanism of LBBB to worsens the course of HF over time.4) However, the pathophysiology of NICD is complex. Possible mechanisms include atypical LBBB, intraventricular parietal block, and peri-infarct block. In contrast to LBBB, the left ventricular activation of NICD is relatively fast through the Purkinje network, but followed by slow activation in the scarred region.10) This unique activation pattern of NICD is attributed to the poor response to the CRT. The substrates of heterogeneous slow ventricular activation could also play a role in the development of ventricular arrhythmia in NICD. We think these findings could partly explain the poor prognosis of the NICD group herein. The worse prognosis of NICD has been demonstrated previously in patients with structural heart disease and in a healthy population. According to Aro et al.'s study,11) patients with NICD were associated with a two-fold increased risk of all-cause death compared to those without NICD during a 30-year follow-up in a general population. Moreover, in the Multicenter Unsustained Tachycardia Trial, which enrolled patients with coronary artery disease, non-sustained ventricular tachycardia, and depressed ejection fraction (LVEF ≤40%), patients with NICD were associated with a lower LVEF and 1.5-fold increased risk of all-cause mortality compared with those without NICD.12) We believe our results are consistent with the results of these previous studies.
There are controversies regarding the prognostic impact of the type of conduction disturbance in patients with HF. In a previous study, the presence of LBBB was independently associated with a 10% increased risk of mortality compared to those with narrow QRS (<120 ms) when 9,082 patients with HF were followed up over 5 years.4) In that study, RBBB showed modest prognostic implication (HR, 1.10; 95% CI, 0.99–1.21). However conflicting data also exists. Barsheshet et al.13) reported that the RBBB was associated with a 29% increased risk of all-cause death compared with LBBB in 1888 patients with systolic HF (ejection fraction <50%) followed over 4 years. Similarly, in the KorAHF registry, RBBB was associated with a 2.6-fold risk of all-cause death or re-hospitalization compared with LBBB.3) However, all these studies were performed after excluding patients with NICD. To date, relatively sparse data exist comparing the prognosis of NICD, RBBB, and LBBB in the same cohort. Tolppanen et al.5) followed up 982 patients with HF for 3.9 years and revealed that only presence of RBBB and NICD (QRS ≥110 ms) were associated with 1.7-fold and 1.6-fold increased risks of mortality respectively. Since the definition of NICD (QRS ≥110 ms) is different from that in our study (QRS ≥120 ms), these results cannot be directly compared with present study.
The prevalence of the pattern of conduction disturbance varies in HF patients with different ethnicities. According to the published data from Western countries, the prevalence of LBBB was reported to be 13–24% in patients with HF.4),14),15),16),17) Conversely, in Asia, the prevalence of LBBB was much lower (3.3–4.6%).3),18) According to the Spanish chronic HF registry, the prevalences of NICD, LBBB, and RBBB were reported as 5.8%, 23.6%, and 6%, respectively.17) In the present study, the prevalences of NICD, LBBB, and RBBB were 7.3%, 5.4%, and 7.0%, respectively. The contribution of ethnic differences on the pattern of conduction disturbance in HF is not yet completely understood.
QRS width was related to worse outcomes in previous studies of patients with HF.1),19),20) However, the variables of bundle branch blocks (BBBs) and NICD were not included in the multivariable analysis of these studies. Since the worse outcome of patients with NICD and BBBs automatically indicates the worse outcome of prolonged QRS, we believe our results are consistent with those of previous studies.
Present study has several limitations. First, there were significant differences in baseline characteristics between the study groups. Notably, the LBBB and NICD group had lower LVEF and higher left ventricular end-diastolic diameter compared to other groups at baseline. However, worse prognosis of LBBB and NICD were also observed in HF patients with preserved LVEF (LVEF ≥50%) in subgroup analysis. Nevertheless, potential confounders may still remain despite adjustments for the significant covariates in the multivariable model. Seconds, the prognostic implication of RBBB group might be underpowered to demonstrate statistical significance due to the small number of subjects. And the trends of worse prognosis in patients with RBBB might be clearer in a longer-term follow-up study. Third, the worse prognosis of LBBB group might be overestimated because we excluded the patients with CRT at baseline. Because we initially focused on the prognostic implication of the intrinsic LV conduction patterns, we had to exclude all patients with pacing rhythm. Fourth, we did not include implantable cardioverter defibrillator (ICD) and CRT implantation in the multivariable model. The benefits of an ICD and CRT were only limited to the eligible patients (LVEF ≤35%). Furthermore, we excluded patients with CRT at baseline as we mentioned above. Thus, we thought these variables were unsuitable for the multi-variable analysis in present study. Meanwhile, the ICD implantation rates during the follow up were 1.4%, 4.1%, 5.4%, and 6.6% in the narrow QRS, RBBB, LBBB, and NICD groups. The CRT implantation rates during the follow-up were 0.2%, 1.1%, 5.0%, and 2.6% in the narrow QRS, RBBB, LBBB, and NICD groups, respectively.
In conclusion, LBBB and NICD was independently associated with an increased risk of all-cause mortality in hospitalized patients with acute HF syndrome. The prognostic impacts of RBBB were not very clear in this study. Further research is still required to assess the long-term prognosis according to the different conduction disturbance patterns.
Footnotes
Funding: This work was supported by the Research of Korea Centers for Disease Control and Prevention (2010-E63003-00, 2011-E63002-00, 2012-E63005-00, 2013-E63003-00, 2013-E63003-01, 2013-E63003-02, and 2016-ER6303-00).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Choi DJ, Lee JH, Park JJ, Cho Y, Oh IY.
- Data curation: Lee JH.
- Methodology: Choi DJ, Lee JH, Park JJ.
- Supervision: Choi DJ, Yoo BS, Kim JJ, Kim KH, Kang SM, Baek SH, Jeon ES, Cho MC, Chae SC, Oh BH.
- Validation: Lee JH.
- Visualization: Lee JH, Cho Y, Oh IY.
- Writing - original draft: Lee JH.
- Writing - review & editing: Choi DJ, Lee JH, Park JJ, Cho Y, Oh IY.
References
- 1.Wang NC, Maggioni AP, Konstam MA, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656–2666. doi: 10.1001/jama.299.22.2656. [DOI] [PubMed] [Google Scholar]
- 2.Mueller C, Laule-Kilian K, Klima T, et al. Right bundle branch block and long-term mortality in patients with acute congestive heart failure. J Intern Med. 2006;260:421–428. doi: 10.1111/j.1365-2796.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- 3.Hong SJ, Oh J, Kang SM, et al. Clinical implication of right bundle branch block in hospitalized patients with acute heart failure: data from the Korean Heart Failure (KorHF) Registry. Int J Cardiol. 2012;157:416–418. doi: 10.1016/j.ijcard.2012.03.155. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Qadir HM, Tu JV, Austin PC, Wang JT, Lee DS. Bundle branch block patterns and long-term outcomes in heart failure. Int J Cardiol. 2011;146:213–218. doi: 10.1016/j.ijcard.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Tolppanen H, Siirila-Waris K, Harjola VP, et al. Ventricular conduction abnormalities as predictors of long-term survival in acute de novo and decompensated chronic heart failure. ESC Heart Fail. 2016;3:35–43. doi: 10.1002/ehf2.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eschalier R, Ploux S, Ritter P, Haïssaguerre M, Ellenbogen KA, Bordachar P. Nonspecific intraventricular conduction delay: definitions, prognosis, and implications for cardiac resynchronization therapy. Heart Rhythm. 2015;12:1071–1079. doi: 10.1016/j.hrthm.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Lee SE, Cho HJ, Lee HY, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 8.Lee SE, Lee HY, Cho HJ, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF) Korean Circ J. 2017;47:341–353. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willems JL, Robles de Medina EO, Bernard R, et al. Criteria for intraventricular conduction disturbances and pre-excitation. J Am Coll Cardiol. 1985;5:1261–1275. doi: 10.1016/s0735-1097(85)80335-1. [DOI] [PubMed] [Google Scholar]
- 10.Derval N, Duchateau J, Mahida S, et al. Distinctive left ventricular activations associated with ECG pattern in heart failure patients. Circ Arrhythm Electrophysiol. 2017;10:e005073. doi: 10.1161/CIRCEP.117.005073. [DOI] [PubMed] [Google Scholar]
- 11.Aro AL, Anttonen O, Tikkanen JT, et al. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol. 2011;4:704–710. doi: 10.1161/CIRCEP.111.963561. [DOI] [PubMed] [Google Scholar]
- 12.Zimetbaum PJ, Buxton AE, Batsford W, et al. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110:766–769. doi: 10.1161/01.CIR.0000139311.32278.32. [DOI] [PubMed] [Google Scholar]
- 13.Barsheshet A, Goldenberg I, Garty M, et al. Relation of bundle branch block to long-term (four-year) mortality in hospitalized patients with systolic heart failure. Am J Cardiol. 2011;107:540–544. doi: 10.1016/j.amjcard.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 14.McCullough PA, Hassan SA, Pallekonda V, et al. Bundle branch block patterns, age, renal dysfunction, and heart failure mortality. Int J Cardiol. 2005;102:303–308. doi: 10.1016/j.ijcard.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Farwell D, Patel NR, Hall A, Ralph S, Sulke AN. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246–1250. doi: 10.1053/euhj.1999.1985. [DOI] [PubMed] [Google Scholar]
- 16.Tabrizi F, Englund A, Rosenqvist M, Wallentin L, Stenestrand U. Influence of left bundle branch block on long-term mortality in a population with heart failure. Eur Heart J. 2007;28:2449–2455. doi: 10.1093/eurheartj/ehm262. [DOI] [PubMed] [Google Scholar]
- 17.Cinca J, Mendez A, Puig T, et al. Differential clinical characteristics and prognosis of intraventricular conduction defects in patients with chronic heart failure. Eur J Heart Fail. 2013;15:877–884. doi: 10.1093/eurjhf/hft042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gijsberts CM, Benson L, Dahlström U, et al. Ethnic differences in the association of QRS duration with ejection fraction and outcome in heart failure. Heart. 2016;102:1464–1471. doi: 10.1136/heartjnl-2015-309212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park HS, Kim H, Park JH, et al. QRS prolongation in the prediction of clinical cardiac events in patients with acute heart failure: analysis of data from the Korean Acute Heart Failure Registry. Cardiology. 2013;125:96–103. doi: 10.1159/000348334. [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, On YK, Byeon K, et al. Short- and long-term outcomes depending on electrical dyssynchrony markers in patients presenting with acute heart failure: clinical implication of the first-degree atrioventricular block and QRS prolongation from the Korean Heart Failure registry. Am Heart J. 2013;165:57–64.e2. doi: 10.1016/j.ahj.2012.10.009. [DOI] [PubMed] [Google Scholar]