Abstract
Purpose
Alzheimer's disease (AD) is the most common neurodegenerative disease, with a rising prevalence worldwide. Long noncoding RNAs (lncRNAs) have been found to play important roles in the development and treatment of AD. However, the exact role of lncRNA nuclear enriched abundant transcript 1 (NEAT1) in neuronal damage in AD is largely unknown.
Materials and Methods
The AD model was established in SH-SY5Y and SK-N-SH cells via treatment with amyloid β1−42 (Aβ). The expression of NEAT1 and microRNA-107 (miR-107) was measured by quantitative real-time polymerase chain reaction. Cell viability and apoptosis were detected by MTT assay, immunocytochemistry, and flow cytometry. The expression of phosphorylated tau protein (p-Tau) was measured by Western blot. The interaction between NEAT1 and miR-107 was explored by bioinformatics analysis, luciferase activity, and RNA immunoprecipitation assays.
Results
NEAT1 expression was enhanced in Aβ-treated SH-SY5Y and SK-N-SH cells, and its knockdown attenuated Aβ-induced inhibition of viability and promotion of apoptosis and p-Tau levels. NEAT1 was indicated as a decoy of miR-107. miR-107 abundance was reduced in Aβ-treated cells, and its overexpression reversed Aβ-induced injury. Moreover, interference of miR-107 abated silencing of NEAT1-mediated inhibition of neuronal damage in Aβ-treated SH-SY5Y and SK-N-SH cells.
Conclusion
LncRNA NEAT1 aggravated Aβ-induced neuronal damage by sponging miR-107, indicating a novel avenue for treatment of AD.
Keywords: Alzheimer's disease, NEAT1, miR-107, neuronal damage
INTRODUCTION
Alzheimer's disease (AD), as a major cause of neurodegenerative disease, poses a major healthcare challenges to older adults worldwide.1 The pathology of AD is characterized by cognitive loss and pathological hallmarks of amyloid and neurofibrillary tangles.2 Meanwhile, research has shown that amyloid β peptides (Aβ1–40 and Aβ1–42) and hyperphosphorylation of tau protein (p-Tau) contribute to AD development.3,4 Although great attention has been given to the diagnosis and treatment of AD, strategies for preventing AD progression remain limited.
Noncoding RNAs (ncRNAs), including long ncRNAs (lncRNAs) and microRNAs (miRNAs), have been implicated in the onset and pathogenesis of AD.5 Emerging evidence suggests lncRNAs as promising targets in the treatment, diagnosis, and prevention of neurodegenerative diseases, including AD.6 For example, lncRNA sex-determining region Y (SRY)-related HMG box (SOX) 21 antisense RNA 1 (SOX21-AS1) knockdown attenuated neuronal oxidative injury in mice with AD by regulating Wnt signaling via Frizzled 3/5 (FZD3/5).7 LncRNA early B cell factor 3 antisense RNA (EBF3-AS) facilitated neuronal apoptosis in an in vitro AD model.8 LncRNA nuclear enriched abundant transcript 1 (NEAT1) was indicated as a promising target in neurodegenerative diseases. Chanda, et al.9 reported that NEAT1 is upregulated in Huntington's disease and that its knockdown weakens the formation of aggregates. Furthermore, NEAT1 has been described as promoting neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced autophagy by regulating phosphatase and tensin homolog deleted on chromosome ten-induced kinase 1 (PINK1) in Parkinson's disease.10 Additionally, NEAT1 knockdown has been found to increase cell viability and to suppress apoptosis in a MPTP/MPP+-induced Parkinson's disease model,11 and research has indicated that NEAT1 is highly expressed in the temporal cortex and hippocampus of AD patients.12 However, the potential role of NEAT1 in AD progression and its underlying mechanism are largely unclear.
miRNAs are a class of small ncRNAs, and have been described as promising diagnostic and therapeutic tools for AD treatment. 13 miR-107 has been shown to be associated with pathogenesis in human diseases.14,15,16 Moreover, miR-107 is reported to be downregulated in AD and to play an essential role in AD pathology.17 Bioinformatics analysis has predicted the potential binding sites of NEAT1 and miR-107, indicating a potential interaction between them. Therefore, we hypothesized that miR-107 might be involved in NEAT1-mediated progression of AD. In this study, we established an AD model using SH-SY5Y and SK-N-SH cells treated with amyloid β1–42 (Aβ). Therein, we explored the effect of NEAT1 on Aβ-induced neuronal damage and its underlying mechanism.
MATERIALS AND METHODS
Cell culture and treatment
The human neuroblastoma cell lines (SH-SY5Y and SK-N-SH) and human embryonic kidney cells 293T were purchased from American Tissue Culture Collection (ATCC; Manassas, VA, USA). All cells were maintained in Dulbecco's Modified Eagle Medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (Gibco), 100 U/mL of penicillin, and 100 µg/mL of streptomycin (Gibco) at 37℃ and 5% CO2. For establishment of AD model in vitro, SH-SY5Y and SK-N-SH cells were treated with different concentrations (0, 5, 10, or 20 µM) of Aβ (purity: 95.64%; MedChemExpress, Monmouth, NJ, USA) in dimethyl sulfoxide (DMSO; Thermo Fisher, Wilmington, DE, USA) for 24 h or 10 µM Aβ for different treatment times (0, 12, 24, or 48 h).
Small interfering RNA (siRNA) against NEAT1 (si-NEAT1) (5′-GUGAGAAGUUGCUUAGAAACUUUCC-3′), siRNA negative control (si-NC) (5′-UUCUCCGAACGUGUCACGUTT-3′), NEAT1 overexpression vector (NEAT1) (Forward, 5′-CTTC CTCCCTTTAACTTATCCATTCAC-3′; Reverse, 5′-CTCTT CC TCCACCATTACCAACAATAC-3′), pcDNA empty vector (pcDNA), miR-107 mimic (miR-107) (Forward, 5′-AGCAGCAUUG UACAGGGCUAUCA-3′; Reverse, 3′-AUAGCCCUGUACAAU GCUGCUUU-5′), mimic negative control (miR-NC) (Forward, 5′-UUCUCCGAACGUGUCACGUTT-3′; Reverse, 3′-ACGUGA CACGUUCGGAGAATT-5′), miR-107 inhibitor (anti-miR-107) (5′-UGAUAGCCCUGUACAAUGCUGCU-3′), and inhibitor negative control (anti-miR-NC) (5′-CAGUA CUUUUGUGUA GUACA-3′) were synthesized by Genephar ma (Shanghai, China). Cell transfection was conducted in SH-SY5Y and SK-N-SH cells for 48 h using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) platform according to the manufacturer's instructions prior to Aβ treatment.
Quantitative real-time polymerase chain reaction
Total RNA extracted from cells using TRIzol reagent (Invitrogen) was used for cDNA synthesis by TransScript miRNA Firststand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer's instructions. Subsequently, cDNA was diluted and used for quantitative real-time polymerase chain reaction (qRT-PCR) with SYBR green (Applied Biosystems, Foster City, CA, USA) using the ABI 7300 system (Applied Biosystems). The relative expressions of NEAT1 and miR-107 were measured with GAPDH or U6 small RNA as internal control using 2–ΔΔCt method.18 The primers used in this study were as follows: NEAT1 (Forward, 5′-TGGCTAGCTCAG GGCTTCAG-3′; Reverse, 5′-TCTCCTT GCCAAGCTTCCTTC-3′), miR-107 (Forward, 5′-AGCAG CATT GTACAGGG-3′; Reverse, 5′-GTGCAGGGTCCGAGGT-3′), U6 (Forward, 5′-CTCGCTTC GGCAGCACA-3′; Reverse, 5′-AAC GCTTCACGAATTTGCGT-3′), GAPDH (Forward, 5′-TATGAT GATATCAAGAGGGTAGT-3′; Reverse, 5′-TGTATCCAAACTCATTGTCATAC-3′).
Cell viability
MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) assay was conducted to measure cell viability. SH-SY5Y and SK-N-SH cells (1×104 cells per well) were seeded into 96-well plates and treated with 10 µM Aβ for 24 h. Then cells were incubated with 0.5 mg/mL of MTT solution (Sigma, St. Louis, MO, USA) for another 4 h. Subsequently, 100 µL of DMSO was added to each well until the solubilization of formazan. Absorbance was measured at 490 nm using a microplate reader (Bio-Rad, Hercules, CA, USA). All samples were prepared in triplicate, and relative cell viability was normalized to non-Aβ group.
Immunocytochemistry
SH-SY5Y and SK-N-SH cells transfected with si-NEAT1 or si-NC were cultured in 24-well plates and exposed to 10 µM Aβ for 24 h. Cells were then fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton (Sigma). After blocked with 1% bovine serum albumin (Sigma), cells were incubated with Alexa Flour 488-conjugated primary antibodies against Ki67 (ab197234, Abcam, Cambridge, MA, USA). A DAPI solution (Sigma) was used for nuclear staining (blue).
Cell apoptosis
Cell apoptosis was measured using Annexin V-FITC/PI apoptosis detection kits (Solarbio, Beijing, China) via flow cytometry according to the manufacturer's instructions. After washing with PBS, SH-SY5Y and SK-N-SH cells were resuspended in binding buffer and then stained with 5 µL of Annexin V-FITC for 10 min and 5 µL of PI for 5 min in the dark at room temperature. Stained cells were analyzed using a flow cytometer (Becton Dickinson, San Jose, CA, USA). Samples from each group were prepared in triplicate, and experiments were repeated three times. The apoptotic rate comprised the percentage of cells in early apoptosis and last apoptosis or death.
Western blot
SH-SY5Y and SK-N-SH cells were washed with cold PBS and then lysed with RIPA lysis buffer (Beyotime Biotech, Shanghai, China). After centrifugation at 12000×g for 5 min, total proteins in supernatant were quantified using BCA protein assay kits (Thermo Fisher). Equal amounts of protein (25 µg) were denatured in SDS-PAGE sample loading buffer (Beyotime Biotech) at 100℃ for 5 min and then separated on SDS-PAGE gel. Polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) were used for transfer of protein and were blocked with 5% non-fat milk for 1 h at room temperature. Subsequently, the membranes were incubated with primary antibodies against p-Tau (ser396) (ab109390; Abcam) or β-actin (ab8227; Abcam) overnight at 4℃ and then interacted with horseradish peroxidase-conjugated secondary antibody (ab6721; Abcam) for 2 h at room temperature. Protein signaling was visualized using enhanced chemiluminescence chromogenic substrate (Beyotime Biotech) and was analyzed with β-actin as a loading control.
Bioinformatics analysis and luciferase activity assay
The putative binding sites of miR-107 and NEAT1 were predicted by bioinformatics analysis using StarBase online (http://starbase.sysu.edu.cn/). The 3′-UTR sequences of NEAT1 carrying wild-type (WT) or mutant (MUT) putative binding sites of miR-107 were amplified and cloned into pmirGLO vectors (Promega, Madison, WI, USA) to synthesize luciferase reporter vectors (NEAT1-WT or NEAT1-MUT). 293T cells were cotransfected with NEAT1-WT or NEAT1-MUT and miR-107 or miR-NC using Lipofectamine 2000 according to the manufacturer's protocols. After transfection for 48 h, cells were collected and luciferase activity was analyzed with luciferase assay kits (Promega) according to the manufacturer's instructions.
RNA immunoprecipitation
RNA immunoprecipitation (RIP) assay was conducted using Magna RNA immunoprecipitation kits (Millipore) according to the manufacturer's protocols. In brief, SH-SY5Y and SK-N-SH cells transfected with miR-107 or miR-NC were lysed in RIP immunoprecipitation buffer containing magnetic beads bound with antibody against Ago2 or IgG. The enrichment of NEAT1 on beads was measured by qRT-PCR.
Statistical analysis
Data are presented as means±standard deviations from three independent experiments. Differences between groups were measured by one-way analysis of variance using GraphPad Prism 7 Software (GraphPad Inc., La Jolla, CA, USA). p<0.05 was regarded as statistically significant.
RESULTS
NEAT1 expression is enhanced in Aβ-treated SH-SY5Y and SK-N-SH cells
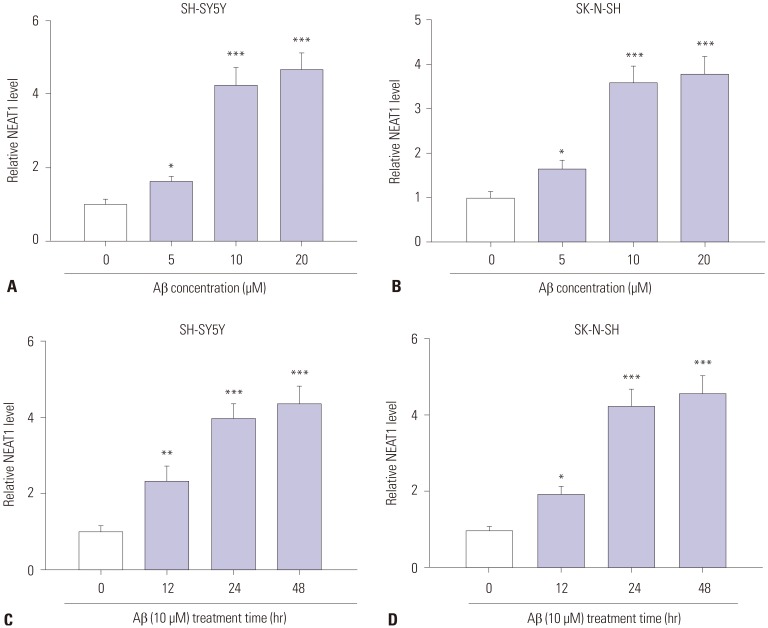
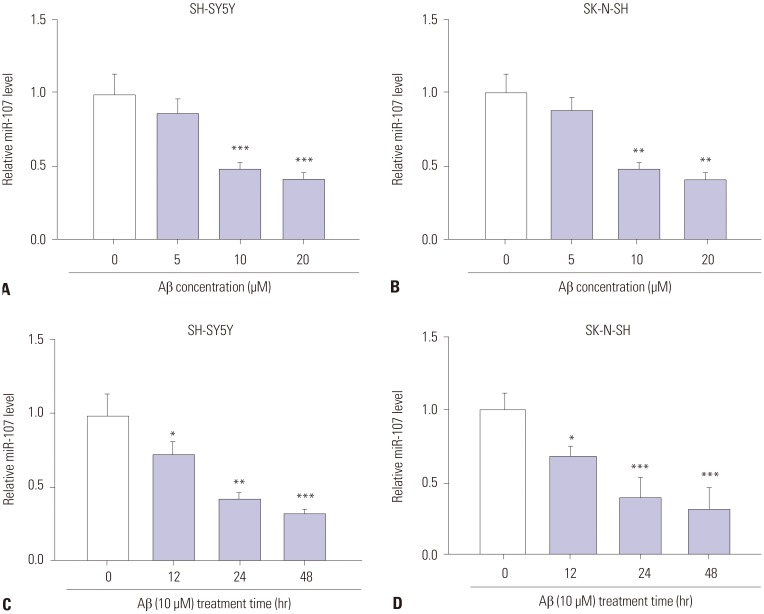
To explore the potential role of NEAT1 in AD progression, the expression of NEAT1 was measured in an Aβ-induced AD model in vitro. As shown in Fig. 1A and B, after treatment of Aβ for 24 h, NEAT1 levels significantly increased in SH-SY5Y and SK-N-SH cells in a concentration dependent manner, compared with those in the non-treated group. Moreover, an abundance of NEAT1 was obvious in 10 µM Aβ-treated SH-SY5Y and SK-N-SH cells in a time dependent manner, compared with the non-treated group (Fig. 1C and D). SH-SY5Y and SK-N-SH cells treated with 10 µM Aβ for 24 h were used for the following experiments because of the significant alteration of NEAT1 levels.
Fig. 1. Expression of NEAT1 is elevated in Aβ-treated SH-SY5Y and SK-N-SH cells. (A and B) The expression of NEAT1 was measured in SH-SY5Y and SK-N-SH cells after treatment of different concentrations (0, 5, 10, and 20 µM) of Aβ for 24 h by qRT-PCR. (C and D) The levels of NEAT1 were detected in SH-SY5Y and SK-N-SH cells after treatment of 10 µM Aβ for different treatment times (0, 12, 24, and 48 h) by qRT-PCR. *p<0.05, **p<0.01, ***p<0.001. NEAT1, nuclear enriched abundant transcript 1; Aβ, amyloid β1–42.
Knockdown of NEAT1 attenuates Aβ-induced neuronal damage in SH-SY5Y and SK-N-SH cells
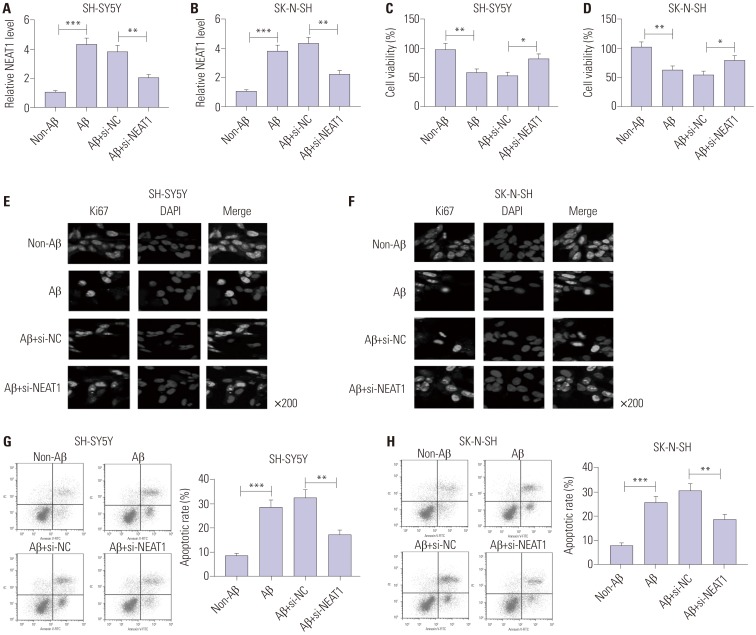
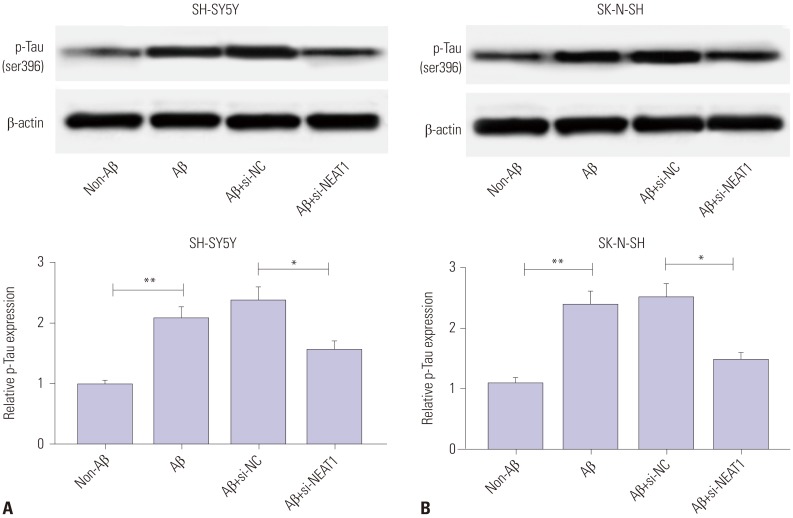
To investigate the effect of NEAT1 on AD progression, SH-SY5Y and SK-N-SH cells were transfected with si-NEAT1 or si-NC and then treated with 10 µM Aβ for 24 h. As displayed in Fig. 2A and B, the abundance of NEAT1 was effectively reduced in SH-SY5Y and SK-N-SH cells upon transfection of si-NEAT1, compared with the si-NC group, after treatment of Aβ. MTT assay showed that cell viability was markedly inhibited by treatment of Aβ in SH-SY5Y and SK-N-SH cells, which was reversed by depletion of NEAT1 (Fig. 2C and D). Meanwhile, immunocytochemistry analysis revealed that exposure of Aβ significantly decreased Ki67 expression, and this effect was weakened via silencing NEAT1 (Fig. 2E and F). Furthermore, exposure of Aβ led to a strong increase in apoptosis of SH-SY5Y and SK-N-SH cells, which was attenuated via down-regulation of NEAT1 (Fig. 2G and H). Furthermore, the effect of NEAT1 on accumulation of p-Tau was evaluated in Aβ-treated SH-SY5Y and SK-N-SH cells. Western blot analysis demonstrated that protein levels of p-Tau were obviously increased in SH-SY5Y and SK-N-SH cells after treatment of Aβ, compared with the non-treated group, while it was greatly abated by inhibition of NEAT1 (Fig. 3). These findings indicated that Aβ successfully induced neuronal damage, which was attenuated by NEAT1 silencing.
Fig. 2. Knockdown of NEAT1 attenuates Aβ-induced neuronal damage in SH-SY5Y and SK-N-SH cells. (A and B) The expression of NEAT1 was measured in SH-SY5Y and SK-N-SH cells transfected with si-NEAT1 or si-NC after treatment of 10 µM Aβ for 24 h by qRT-PCR. Cell viability (C and D), Ki67 expression (E and F), and apoptosis (G and H) were detected in SH-SY5Y and SK-N-SH cells transfected with si-NEAT1 or si-NC after treatment of 10 µM Aβ for 24 h by MTT, immunocytochemistry, and flow cytometry. *p<0.05, **p<0.01, ***p<0.001. NEAT1, nuclear enriched abundant transcript 1; Aβ, amyloid β1–42.
Fig. 3. Interference of NEAT1 weakens Aβ-induced phosphorylation of Tau in SH-SY5Y and SK-N-SH cells. The protein levels of phosphorylation of Tau (p-Tau) were measured in SH-SY5Y (A) and SK-N-SH (B) cells transfected with si-NEAT1 or si-NC after treatment of 10 µM Aβ for 24 h by Western blot. *p<0.05, **p<0.01. NEAT1, nuclear enriched abundant transcript 1; Aβ, amyloid β1–42.
MiR-107 is bound to NEAT1
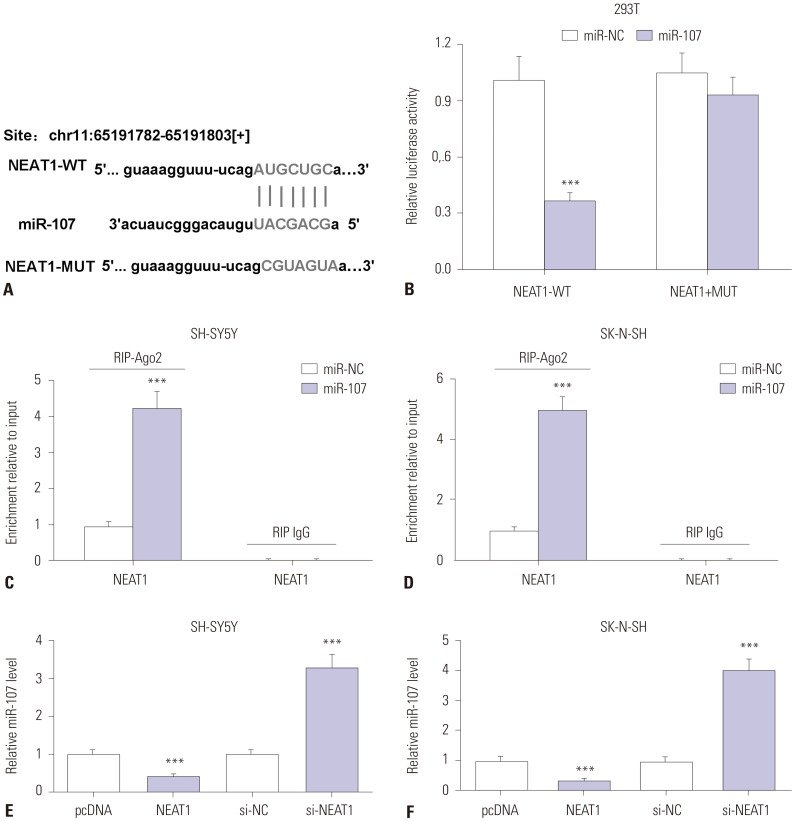
In order to elucidate the underlying mechanism accounting for NEAT1 involvement in AD development, we explored miRNA that bind to NEAT1 by bioinformatics analysis. StarBase online assay provided the putative binding sites of miR-107 and NEAT1, suggesting that miR-107 might be targeted by NEAT1 (Fig. 4A). To validate the prediction, luciferase activity and RIP assays were conducted. Luciferase activity was obviously inhibited in 293T cells transfected with miR-107, compared with that in cells treated with miR-NC in the NEAT1-WT group, while it was not affected in the NEAT1-MUT group (Fig. 4B). Moreover, the addition of miR-107 resulted in greater expression of NEAT1 enriched by Ago2 RIP in SH-SY5Y and SK-N-SH cells, whereas it showed little capacity for enrichment in the IgG RIP group (Fig. 4C and D). Then, the effect of NEAT1 on miR-107 expression was analyzed in SH-SY5Y and SK-N-SH cells. The results showed that overexpression of NEAT1 suppresses miR-107 levels in SH-SY5Y and SK-N-SH cells, compared with pcDNA transfection, while its knockdown causes an opposite effect on miR-107 abundance (Fig. 4E and F). These results revealed that NEAT1 is a sponge of miR-107.
Fig. 4. miR-107 is bound to NEAT1. (A) The putative binding sites of NEAT1 and miR-107 were predicted by StarBase. (B) Luciferase activity was measured in 293T cells co-transfected with NEAT1-WT or NEAT1-MUT and miR-107 or miR-NC. (C and D) The enrichment of NEAT1 was detected in SH-SY5Y and SK-N-SH cells transfected with miR-107 or miR-NC after RIP assay. (E and F) The expression of miR-107 was measured in SH-SY5Y and SK-N-SH cells transfected with pcDNA, NEAT1, si-NC or si-NEAT1 by qRT-PCR. ***p<0.001. NEAT1, nuclear enriched abundant transcript 1.
MiR-107 is lowly expressed in Aβ-treated SH-SY5Y and SK-N-SH cells
Having established that miR-107 is targeted by NEAT1, the abundance of miR-107 was measured in SH-SY5Y and SK-N-SH cells after treatment of Aβ. Compared with the non-treated group, treatment of Aβ for 24 h led to a marked reduction in miR-107 expression in the two types of cells in a concentration dependent manner (Fig. 5A and B). Furthermore, a progressive decrease in miR-107 levels was observed in SH-SY5Y and SK-N-SH cells after treatment of 10 µM Aβ in a time dependent manner (Fig. 5C and D). These data suggested that low expression of miR-107 might be associated with AD progression.
Fig. 5. The levels of miR-107 are decreased in Aβ-treated SH-SY5Y and SK-N-SH cells. (A and B) The expression of miR-107 was measured in SH-SY5Y and SK-N-SH cells after treatment of different concentrations of Aβ for 24 h by qRT-PCR. (C and D) The abundance of miR-107 was detected in SH-SY5Y and SK-N-SH cells after treatment of 10 µM Aβ for different treatment times by qRT-PCR. *p<0.05, **p<0.01, ***p<0.001. Aβ, amyloid β1–42.
NEAT1 mediates neuronal damage by sponging miR-107 in Aβ-treated SH-SY5Y and SK-N-SH cells
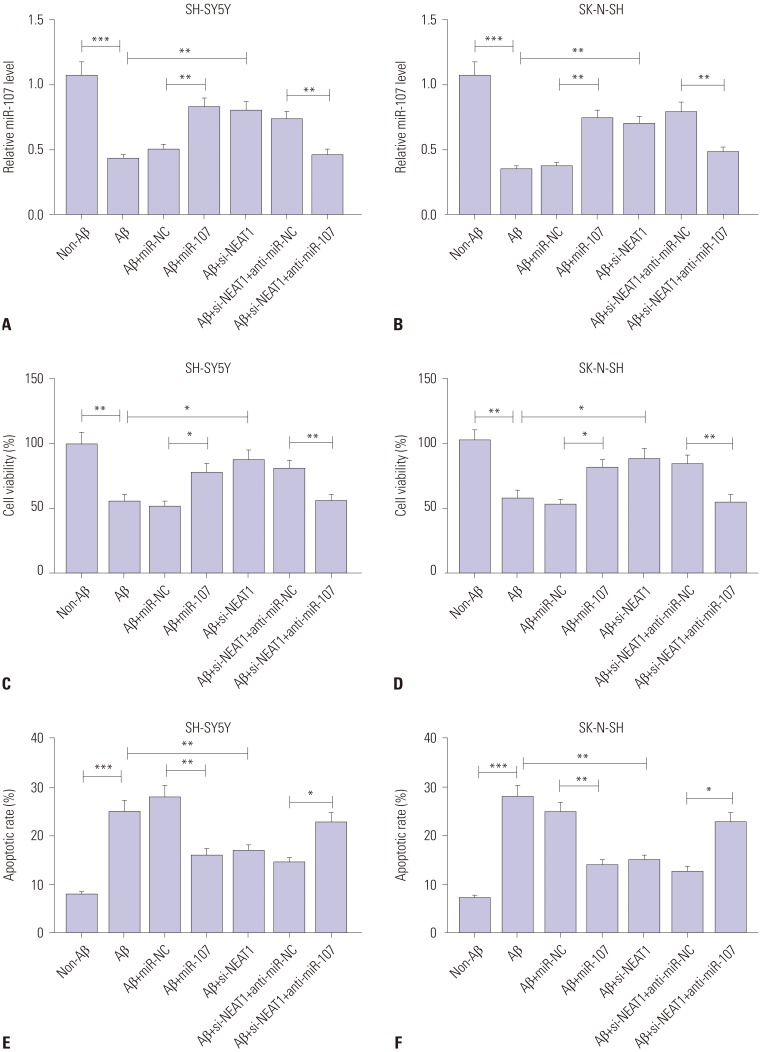
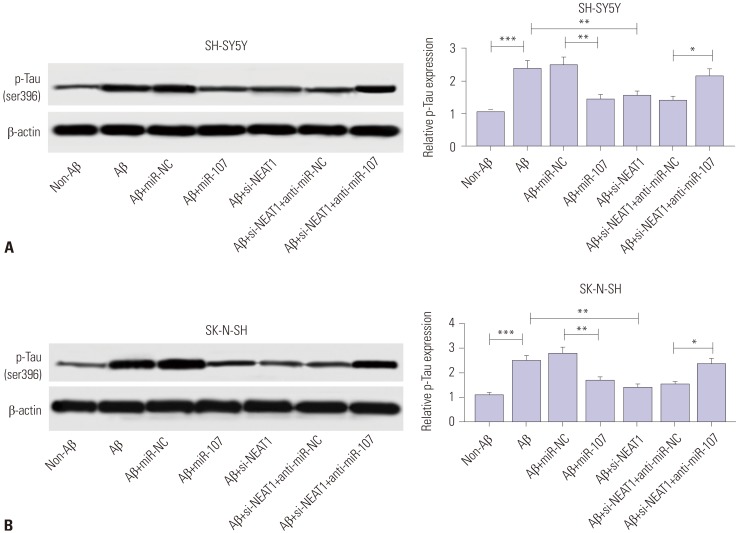
To analyze the potential role of miR-107 in AD progression, SH-SY5Y and SK-N-SH cells were transfected with miR-107 or miR-NC and then treated with 10 µM Aβ for 24 h. In result, the expression of miR-107 was effectively rescued by transfection of miR-107 mimic, compared with that in the miR-NC group, among Aβ-treated SH-SY5Y and SK-N-SH cells (Fig. 6A and B). Moreover, overexpression of miR-107 reversed Aβ-induced viability inhibition and apoptosis induction in SH-SY5Y and SK-N-SH cells (Fig. 6C–F). Meanwhile, knockdown of miR-107 aggravated Aβ-induced injury, while its knockdown alone showed little effect on cell viability and apoptosis in SH-SY5Y and SK-N-SH cells without Aβ treatment (Supplementary Fig. 1, only online). Furthermore, up-regulation of miR-107 abated Aβ-induced phosphorylation of Tau in SH-SY5Y and SK-N-SH cells, compared with miR-NC treatment (Fig. 7). These results highlighted the suppressive effect of miR-107 on Aβ-induced neuronal injury. Additionally, to explore whether miR-107 was associated with the regulatory effect of NEAT1 on AD development, SH-SY5Y and SK-N-SH cells were co-transfected with si-NEAT1 and anti-miR-107 or anti-miR-NC before treatment with Aβ. qRT-PCR assay showed that the abundance of miR-107 was obviously decreased in SH-SY5Y and SK-N-SH cells transfected with si-NEAT1 and anti-miR-107, compared with that in cells treated with si-NEAT1 and anti-miR-NC, after treatment of Aβ (Fig. 6A and B). Additionally, exhaustion of miR-107 alleviated the regulatory effect of NEAT1 knockdown on cell viability, apoptosis, and phosphorylation of Tau in Aβ-treated SH-SY5Y and SK-N-SH cells (Figs. 6C–F and 7). These results indicated that NEAT1 knockdown mitigates Aβ-induced neuronal damage by upregulating miR-107.
Fig. 6. Abrogation of miR-107 reverses the regulatory effect of NEAT1 knockdown on Aβ-induced neuronal damage in SH-SY5Y and SK-N-SH cells. (A and B) The expression of miR-107 was measured in SH-SY5Y and SK-N-SH cells transfected with miR-107, miR-NC, si-NEAT1, and anti-miR-NC or anti-miR-107 after treatment of 10 µM Aβ for 24 h by qRT-PCR. Cell viability (C and D) and apoptosis (E and F) were detected in SH-SY5Y and SK-N-SH cells transfected with miR-107, miR-NC, si-NEAT1, and anti-miR-NC or anti-miR-107 after treatment of 10 µM Aβ for 24 h by MTT or flow cytometry. *p<0.05, **p<0.01, ***p<0.001. NEAT1, nuclear enriched abundant transcript 1; Aβ, amyloid β1–42.
Fig. 7. miR-107 is involved in interference of NEAT1-mediated inhibition of phosphorylation of Tau (p-Tau) in Aβ-treated SH-SY5Y and SK-N-SH cells. The protein levels of p-Tau were detected in SH-SY5Y (A) and SK-N-SH (B) cells transfected with miR-107, miR-NC, si-NEAT1, and anti-miR-NC or anti-miR-107 after treatment of 10 µM Aβ for 24 h by Western blot. *p<0.05, **p<0.01, ***p<0.001. NEAT1, nuclear enriched abundant transcript 1; Aβ, amyloid β1–42.
DISCUSSION
Aβ-treated SH-SY5Y and SK-N-SH cells have been widely used for establishment of AD models in vitro.19,20 In the present study, we also established an AD model in SH-SY5Y and SK-N-SH cells via exposure of Aβ. Our results showed that treatment of Aβ leads to inhibition of cell viability and increases in apoptosis and p-Tau levels in SH-SY5Y and SK-N-SH cells, which supported the viability of our AD model. NEAT1, as a promising lncRNA, has been reported to contribute to neuronal injury in Huntington's disease and Parkinson's disease.9,11 On the basis of previous results, NEAT1 might play an important role in neurodegenerative disease. However, the role of NEAT1 in AD progression remains obscure. Here, we discovered that NEAT1 aggravates Aβ-induced neuronal damage in AD by sponging miR-107.
Previous research suggested that NEAT1 is highly expressed in AD patients, compared to control brain tissues.12 Similarly, we also found high expression of NEAT1 in our Aβ-induced AD model in vitro. In this work, silencing of NEAT1 attenuated Aβ-induced inhibition of cell viability and increases in apoptosis and phosphorylation of Tau in vitro, indicating NEAT1 as a potential neuro-regulator in AD progression. Nevertheless, the underlying mechanism by which NEAT1 participates in neuronal damage in AD needs to be further explored. Former efforts have suggested NEAT1 as novel target for diagnosis and treatment of human cancers by functioning as a sponge of miRNAs.21 Chen, et al.22 reported that NEAT1 promotes ox-LDL-induced inflammatory and oxidative stress injury by sponging miR-128 in macrophages. Zhou, et al.23 suggested that abrogation of NEAT1 suppresses migration and invasion by regulating miR-132 in glioma cells. In this study, using luciferase activity and RIP assays, we confirmed the regulatory network of NEAT1 and miR-107 in SH-SY5Y and SK-N-SH cells, which was also demonstrated as a main pathway in progression of glioma and laryngeal squamous cell cancer.24,25,26
We discovered that miR-107 expression was reduced in our in vitro Aβ-induced AD model, which is in agreement with previous works that reported low expression of miR-107 in AD brains.27,28 Liu, et al.29 reported that miR-107 prevented Aβ-induced disruption of the blood-brain barrier (BBB) and dysfunction of endothelial cells in AD by regulating endophilin-1, suggesting that miR-107 might play a neuroprotective role in AD progression. Meanwhile, we hypothesized that NEAT1 could promote BBB dysfunction in AD by regulating miR-107, which warrants further study in the future. Moreover, Wang, et al.30 suggested that miR-107 was decreased in AD and that it might increase vulnerability to AD. In this study, our results showed that addition of miR-107 inhibited Aβ-induced neuronal damage, which was consistent with research showing that miR-107 is negatively correlated with Aβ-induced reductions of cell viability and increases in apoptosis and phosphorylated Tau levels.31,32 These data demonstrate that miR-107 might be involved in a neuroprotective mechanism in AD progression. Meanwhile, we demonstrated that deficiency of miR-107 counteracted interference of NEAT1-mediated inhibition of neuronal damage in AD. This indicated that knockdown of NEAT1 plays a protective role in AD by regulating miR-107. Former efforts have suggested that rodent models are indispensable for research on AD.33 Hence, an animal model of AD and clinical experiments should be performed in the future to investigate the role of NEAT1 in vivo. Moreover, the potential targets of miR-107 and promising signaling pathways should be explored in further studies for better understanding the mechanism.
In conclusion, NEAT1 expression was increased in Aβ-treated SH-SY5Y and SK-N-SH cells. Knockdown of NEAT1 attenuated Aβ-induced neuronal injury, possibly via sponging miR-107, in an in vitro Aβ-induced AD model, indicating a novel avenue for treatment of AD.
ACKNOWLEDGEMENTS
This work was supported by City and College Strategic Cooperation Project of Nanchong (Grant No. 18SXHZ0576).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sha Ke, Fei Yang, Juan Tan, Bo Liao.
- Data curation: Juan Tan, Xiaoming Wang, Bo Liao, Fei Yang, Zhaohui Yang.
- Formal analysis: Sha Ke, Fei Yang, Juan Tan, Bo Liao.
- Funding acquisition: Sha Ke, Juan Tan, Xiaoming Wang, Bo Liao.
- Investigation: Bo Liao, Fei Yang.
- Methodology: Sha Ke, Bo Liao, Fei Yang.
- Project administration: Juan Tan, Fei Yang.
- Resources: Sha Ke, Bo Liao.
- Software: Sha Ke, Juan Tan.
- Supervision: Sha Ke, Fei Yang.
- Validation: Fei Yang, Bo Liao.
- Visualization: Sha Ke, Juan Tan.
- Writing—original draft: Sha Ke, Bo Liao, Fei Yang, Xiaoming Wang.
- Writing—review & editing: Sha Ke, Zhaohui Yang, Bo Liao.
SUPPLEMENTARY MATERIAL
The effect of miR-107 on Aβ-induced neuronal damage in SH-SY5Y and SK-N-SH cells. SH-SY5Y and SK-N-SH cells were transfected with or without anti-miR-107 and then treated with Aβ or not. Cell viability (A and B) and apoptosis (C and D) were measured in the two cell lines by MTT or flow cytometry. *p<0.05, **p<0.01, Aβ, amyloid β1–42; NS: not significant.
References
- 1.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Fu AKY, Ip NY. Synaptic dysfunction in Alzheimer's disease: mechanisms and therapeutic strategies. Pharmacol Ther. 2019;195:186–198. doi: 10.1016/j.pharmthera.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Nasica-Labouze J, Nguyen PH, Sterpone F, Berthoumieu O, Buchete NV, Coté S, et al. Amyloid β protein and Alzheimer's disease: when computer simulations complement experimental studies. Chem Rev. 2015;115:3518–3563. doi: 10.1021/cr500638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Götz J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discov. 2017;16:863–883. doi: 10.1038/nrd.2017.155. [DOI] [PubMed] [Google Scholar]
- 5.Idda ML, Munk R, Abdelmohsen K, Gorospe M. Noncoding RNAs in Alzheimer's disease. Wiley Interdiscip Rev RNA. 2018;9:e1463. doi: 10.1002/wrna.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang DQ, Fu P, Yao C, Zhu LS, Hou TY, Chen JG, et al. Long non-coding RNAs, novel culprits, or bodyguards in neurodegenerative diseases. Mol Ther Nucleic Acids. 2018;10:269–276. doi: 10.1016/j.omtn.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Fang Y, Cheng X, Lian YJ, Xu HL. Silencing of long noncoding RNA SOX21-AS1 relieves neuronal oxidative stress injury in mice with Alzheimer's disease by upregulating FZD3/5 via the Wnt signaling pathway. Mol Neurobiol. 2019;56:3522–3537. doi: 10.1007/s12035-018-1299-y. [DOI] [PubMed] [Google Scholar]
- 8.Gu C, Chen C, Wu R, Dong T, Hu X, Yao Y, et al. Long noncoding RNA EBF3-AS promotes neuron apoptosis in Alzheimer's disease. DNA Cell Biol. 2018;37:220–226. doi: 10.1089/dna.2017.4012. [DOI] [PubMed] [Google Scholar]
- 9.Chanda K, Das S, Chakraborty J, Bucha S, Maitra A, Chatterjee R, et al. Altered levels of long ncRNAs Meg3 and Neat1 in cell and animal models of Huntington's disease. RNA Biol. 2018;15:1348–1363. doi: 10.1080/15476286.2018.1534524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan W, Chen ZY, Chen JQ, Chen HM. LncRNA NEAT1 promotes autophagy in MPTP-induced Parkinson's disease through stabilizing PINK1 protein. Biochem Biophys Res Commun. 2018;496:1019–1024. doi: 10.1016/j.bbrc.2017.12.149. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Lu Z. Long non-coding RNA NEAT1 mediates the toxic of Parkinson's disease induced by MPTP/MPP+ via regulation of gene expression. Clin Exp Pharmacol Physiol. 2018;45:841–848. doi: 10.1111/1440-1681.12932. [DOI] [PubMed] [Google Scholar]
- 12.Spreafico M, Grillo B, Rusconi F, Battaglioli E, Venturin M. Multiple layers of CDK5R1 regulation in Alzheimer's disease implicate long non-coding RNAs. Int J Mol Sci. 2018;19:2022. doi: 10.3390/ijms19072022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez B, Peplow PV. MicroRNAs as diagnostic and therapeutic tools for Alzheimer's disease: advances and limitations. Neural Regen Res. 2019;14:242–255. doi: 10.4103/1673-5374.244784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finnerty JR, Wang WX, Hébert SS, Wilfred BR, Mao G, Nelson PT. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol. 2010;402:491–509. doi: 10.1016/j.jmb.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley NH, O'Neill LA. miR-107: a toll-like receptor-regulated miRNA dysregulated in obesity and type II diabetes. J Leukoc Biol. 2012;92:521–527. doi: 10.1189/jlb.0312160. [DOI] [PubMed] [Google Scholar]
- 16.Jiang ZP, Zhou TB. Role of miR-107 and its signaling pathways in diseases. J Recept Signal Transduct Res. 2014;34:338–341. doi: 10.3109/10799893.2014.896383. [DOI] [PubMed] [Google Scholar]
- 17.Fransquet PD, Ryan J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer's disease. Clin Biochem. 2018;58:5–14. doi: 10.1016/j.clinbiochem.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Xu B, Chen J, Sui Y, Ren L, Li J, et al. Micro-RNA-137 inhibits tau hyperphosphorylation in Alzheimer's disease and targets the CACNA1C gene in transgenic mice and human neuroblastoma SH-SY5Y cells. Med Sci Monit. 2018;24:5635–5644. doi: 10.12659/MSM.908765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Li X, Wang L, Zhang Y, Chen L. miR-98-5p acts as a target for Alzheimer's disease by regulating Aβ production through modulating SNX6 expression. J Mol Neurosci. 2016;60:413–420. doi: 10.1007/s12031-016-0815-7. [DOI] [PubMed] [Google Scholar]
- 21.Dong P, Xiong Y, Yue J, Hanley SJB, Kobayashi N, Todo Y, et al. Long non-coding RNA NEAT1: a novel target for diagnosis and therapy in human tumors. Front Genet. 2018;9:471. doi: 10.3389/fgene.2018.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen DD, Hui LL, Zhang XC, Chang Q. NEAT1 contributes to ox-LDL-induced inflammation and oxidative stress in macrophages through inhibiting miR-128. J Cell Biochem. 2018;120:2493–2501. doi: 10.1002/jcb.27541. [DOI] [PubMed] [Google Scholar]
- 23.Zhou K, Zhang C, Yao H, Zhang X, Zhou Y, Che Y, et al. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17:105. doi: 10.1186/s12943-018-0849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhen Y, Nan Y, Guo S, Zhang L, Li G, Yue S, et al. Knockdown of NEAT1 repressed the malignant progression of glioma through sponging miR-107 and inhibiting CDK14. J Cell Physiol. 2019;234:10671–10679. doi: 10.1002/jcp.27727. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Xiao Z, Du X, Huang L, Du G. Silencing of the long non-coding RNA NEAT1 suppresses glioma stem-like properties through modulation of the miR-107/CDK6 pathway. Oncol Rep. 2017;37:555–562. doi: 10.3892/or.2016.5266. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Wu T, Zhou H, Jin Q, He G, Yu H, et al. Long noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathway. J Exp Clin Cancer Res. 2016;35:22. doi: 10.1186/s13046-016-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta P, Bhattacharjee S, Sharma AR, Sharma G, Lee SS, Chakraborty C. miRNAs in Alzheimer disease - a therapeutic perspective. Curr Alzheimer Res. 2017;14:1198–1206. doi: 10.2174/1567205014666170829101016. [DOI] [PubMed] [Google Scholar]
- 28.Moncini S, Lunghi M, Valmadre A, Grasso M, Del Vescovo V, Riva P, et al. The miR-15/107 family of microRNA genes regulates CDK5R1/p35 with implications for Alzheimer's disease pathogenesis. Mol Neurobiol. 2017;54:4329–4342. doi: 10.1007/s12035-016-0002-4. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Cai H, Lin M, Zhu L, Gao L, Zhong R, et al. MicroRNA-107 prevents amyloid-beta induced blood-brain barrier disruption and endothelial cell dysfunction by targeting Endophilin-1. Exp Cell Res. 2016;343:248–257. doi: 10.1016/j.yexcr.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, et al. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao Y, Kong L, Yao Y, Li S, Tao Z, Yan Y, et al. Osthole decreases beta amyloid levels through up-regulation of miR-107 in Alzheimer's disease. Neuropharmacology. 2016;108:332–344. doi: 10.1016/j.neuropharm.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 32.Shu B, Zhang X, Du G, Fu Q, Huang L. MicroRNA-107 prevents amyloid-β-induced neurotoxicity and memory impairment in mice. Int J Mol Med. 2018;41:1665–1672. doi: 10.3892/ijmm.2017.3339. [DOI] [PubMed] [Google Scholar]
- 33.Götz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19:583–598. doi: 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of miR-107 on Aβ-induced neuronal damage in SH-SY5Y and SK-N-SH cells. SH-SY5Y and SK-N-SH cells were transfected with or without anti-miR-107 and then treated with Aβ or not. Cell viability (A and B) and apoptosis (C and D) were measured in the two cell lines by MTT or flow cytometry. *p<0.05, **p<0.01, Aβ, amyloid β1–42; NS: not significant.