Abstract
Pathogens often rely on their host for dispersal. Yet, maximizing fitness via replication can cause damage to the host and an associated reduction in host movement, incurring a trade-off between transmission and dispersal. Here, we test the idea that pathogens might mitigate this trade-off between reproductive fitness and dispersal by taking advantage of sexual dimorphism in their host, tailoring responses separately to males and females. Using experimental populations of Daphnia magna and its bacterial pathogen Pasteuria ramosa as a test-case, we find evidence that this pathogen can use male hosts as a dispersal vector, and the larger females as high-quality resource patches for optimized production of transmission spores. As sexual dimorphism in dispersal and body size is widespread across the animal kingdom, this differential exploitation of the sexes by a pathogen might be an unappreciated phenomenon, possibly evolved in various systems.
Keywords: host–parasite, trade-off, virulence, meta-populations, range expansion, Daphnia magna, Pasteuria ramosa
1. Introduction
Many pathogens rely on their host for dispersal, yet maximizing the transmission benefits of within-host replication is often at odds with the need to disperse and encounter new hosts, since sick hosts are generally unlikely to move large distances [1]. Such a trade-off places limits on the evolution of pathogens in spatially explicit settings, as a pathogen's long-term fitness is highly dependent on their ability to disperse once the local pool of susceptible hosts has been exhausted [2,3]. Thus, for non-vectored pathogens, host movement, and how this is altered by infection [4], is essential for understanding any constraints on the epidemiology and evolution of infectious disease across space [5,6]. If infection reduces host dispersal, for example, selection is expected to favour more prudent host exploitation strategies at the front of an expanding host population [6].
Predictions for pathogen evolution in a patchy landscape often centre on a pathogen's dispersal ability relative to that of a generic host [7,8], implying that there is one optimal dispersal strategy. In nature, however, hosts commonly vary in their dispersal capacity and provided resources, with one common source of host heterogeneity being sexual dimorphism. Each sex often differs in size, dispersal [9,10] and in the prevalence and severity of disease [11–13]. Studies have captured the interplay between these key fitness components by linking one sex to the spatial distribution of a pathogen [14] and revealing how investment in dispersing life-stages [15] or modes of transmission [16] depends on host sex. Based on these findings, it is clear that sexual dimorphism influences both the evolution of a trade-off between within host replication and virulence [17–20] and the potential redistribution of pathogens [7,14]. Yet, it remains to be tested if a pathogen can optimize both transmission and dispersal simultaneously by tailoring their infection strategies separately to males and females.
Here, we propose the idea that pathogens may be able to mitigate the trade-off between dispersal and within-host replication by taking advantage of sexual dimorphism of their host. In particular, we suggest that sexual dimorphism in host dispersal and size will play a crucial role in mitigating any trade-offs for a pathogen. For example, where there is strong sexual dimorphism in size—often a proxy for the resources that a host provides to a pathogen—we might expect pathogens to maximize production of transmission propagules in the larger host. Likewise, with sexual dimorphism in dispersal, pathogens may maximize dispersal via the more dispersive sex. Where these two situations align, such that the sex providing the fewer resources is also the more dispersive, we would expect to see particularly strong opportunities for pathogens to exploit host sexual dimorphism.
In this study, we test this idea by using Daphnia magna and its pathogen Pasteuria ramosa. Daphnia are freshwater crustaceans, reproducing via cyclical parthenogenesis (females can produce genetically identical male and female offspring). Transmission of P. ramosa occurs exclusively horizontally, and infection is facilitated by filter-feeding, after which the pathogen sterilizes and kills its host [21]. Males are smaller and have a shorter lifespan than females [22,23] and display behavioural differences in mate-finding [24]. Males are also more resistant to infection, constrain the production of transmission spores and suffer from less pathogen-induced reduction in lifespan relative to females [19,22,23]. With this system, we then explored how host sex and infection interact to shape host dispersal behaviour in two different contexts: (i) the probability of dispersal from a crowded habitat and (ii) the rate and magnitude of any potential dispersal events. Overall, our experimental data suggest that pathogens are able to utilize male hosts as a vector for dispersal, and the female host as a high-quality patch for optimized spore production.
2. Material and methods
We performed two experiments to investigate host sex and infection differences in (i) probability of dispersal from a crowded habitat (two-patch microcosms), and (ii) movement capacity of individuals (unlimited continuous microcosms). The host genotype used for these experiments originated from Hungary (HUHO-2), and the two P. ramosa genotypes originated from Russia (C1) and Germany (C19) and have previously been shown to vary in transmission and virulence strategies [19,25]. For both experiments, animals were prepared by collecting female Daphnia from stock cultures and rearing following standard conditions (20°C, 16 L : 8 D). Males were generated via hormone treatment ([23], see electronic supplementary material). To produce infected individuals, each animal was exposed to 20 000 spores of one of the two pathogen genotypes (C1 or C19) or an equivalent placebo-solution (i.e. unexposed and uninfected controls) at age 3 and 4 days (40 000 spores individual−1).
(a). Experiment 1: The probability of dispersal from a crowded habitat
Two-patch microcosms were built by interconnecting two 950-ml containers with PVC-piping (15 mm diameter) and a closing valve between patches. We have previously shown that high-density conditions in a local patch directly induce Daphnia dispersal [26] and that info-chemicals contained in ‘crowded’ water influence Daphnia life-history [27]. To stimulate dispersal, we simulated a high-density environment in the first patch using crowded-conditioned water (electronic supplementary material) and introduced 20 infected individuals (by pathogen C1 only) and 20 unexposed controls (i.e. uninfected) of each sex (2 sexes × 2 treatments (infected and uninfected) × 20 individuals × 15 replicates = 1200).
After a 24 h acclimatization period, we opened the valves and allowed for dispersal to the second patch, containing fresh Daphnia media. Six days later, we quantified the number of animals of each sex and treatment (infected or uninfected) in patch 2. With this population-level approach, we cannot completely rule out differences in mortality between males and females (nor uninfected or infected animals) contributing to the observed results, but our approach does allow us to focus on the biologically meaningful aspect for this study: the probability of dispersal to a new patch.
(b). Experiment 2: The rate and magnitude of individual dispersal
To assay the movement capacity of individual Daphnia, we used continuous microcosms built from 50 ml falcon-tubes interconnected by 10 cm silicon tubing (internal diameter 8 mm and outer diameter 12 mm). For both host sexes, infected (pathogen C1 and C19) and uninfected animals were introduced individually into the first patch of a continuous microcosm system at 20 days post-infection. Each treatment was replicated 20 times (2 sexes × 3 treatments ([2] pathogens + controls] × 20 replicates = 120 animals) and checked daily for mortality and dispersal (back-dispersal was prevented by closing the tube after any dispersal event). At death, individual Daphnia were frozen in 500 μl RO for later spore count, using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA) following standard procedures [22].
(c). Statistical analyses
All data analyses were performed in R (ver. 3.3.3; R Development Core Team). For Experiment 1, we analysed the probability of dispersal using a generalized linear model with a binomial distribution and logit-link function, with infection treatment (C1, C19 and uninfected), host sex and their interaction as fixed effects. For Experiment 2, we analysed dispersal rate by fitting a linear mixed model using an accumulated number of patches as the response variable, time as a covariate, host sex, infection treatment and their interaction as fixed effects. We used a two-factor analysis of variance to analyse total distance covered and spore loads (both response values square root transformed before analysis), with pathogen treatment (both pathogen genotypes, including uninfected when analysing total distance), host sex and their interactions as fixed effects.
3. Results
(a). The probability of dispersal from a crowded habitat
The probability of dispersal from a crowded patch to a neighbouring patch depended on an interaction between host sex and infection status (d.f. = 1, χ2 = 16.22, p < 0.001; electronic supplementary material, table S1). In general, males appeared less likely to disperse from crowded conditions than females (figure 1), but the impact of infection reversed dispersal behaviour within each sex. Infected females were three times less likely to disperse than uninfected females (d.f. = 1, χ2 = 28.80, p < 0.001). By contrast, infection in males substantially increased their probability of dispersal from a crowded patch (d.f. = 1, χ2 = 6.91, p = 0.009).
Figure 1.
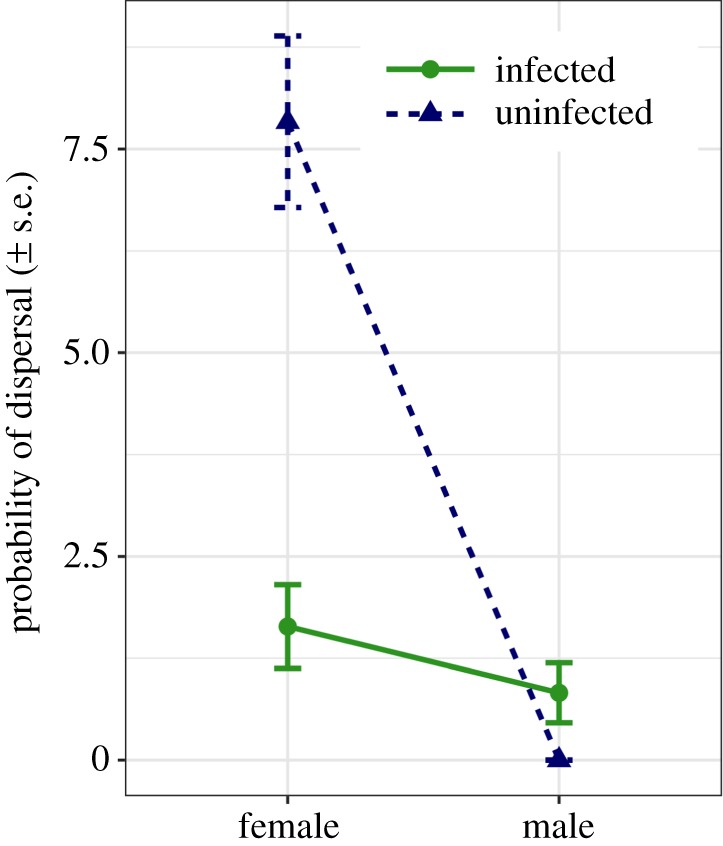
Probability of dispersal (mean ± s.e.) from a crowded habitat to a neighbouring uninhabited habitat for infected (solid line and circles) and uninfected (dashed line and triangles) male and female Daphnia. (Online version in colour.)
(b). The rate and magnitude of individual dispersal
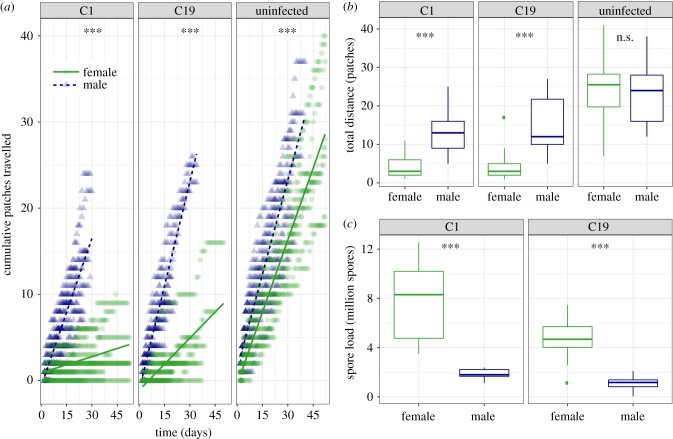
Both the rate of dispersal (patches travelled per time, figure 2a) and total patches travelled by an individual (figure 2b) were affected by an interaction between host sex and infection treatment (dispersal rate: χ2 = 666.5, p < 0.001; total distance: F2,93 = 13.49, p < 0.001; electronic supplementary material). For uninfected animals, dispersal rate was only marginally higher in males compared to females (slopes: male = 0.73 versus female = 0.56, p < 0.001), and with no difference in the total number of patches travelled (p = 0.612). When infected with each pathogen, however, females moved substantially more slowly than males (figure 2a) and travelled less far (fewer patches; figure 2b). Finally, the number of spores that a pathogen would release at host death in the final patch was four times higher in females, albeit influenced by pathogen genotype (F1,35 = 14.962, p = 0.001; see electronic supplementary material for details on size-corrected spore loads).
Figure 2.
Individual dispersal in interconnected microcosms. (a) The accumulated number of patches moved over time for male (blue triangles and dashed line) and female (green circles and solid line) Daphnia, infected with pathogen C1, C19 and uninfected. Coloured dots represent individual replicates and solid lines a fitted linear model. (b) Total distance (number of patches). (c) Pathogen spore load (millions) at death. Post hoc tests for difference between males and females in slope (a) or traits (b, c) are indicated at the top of each panel (ns. p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001).
4. Discussion
Sexual dimorphism manifests across a range of taxonomic groups, with sex differences in body size and dispersal being among the most commonly observed [9,10]. The ubiquity of sexual dimorphism may present pathogens with an opportunity to specialize the tasks of optimizing fitness and dispersal to different host sexes. Such specialization potentially allows pathogens to mitigate the trade-off between within-host replication and dispersal, such that one sex can be exploited for dispersal, and the other for maximizing transmission. Despite the ubiquity of host sexual dimorphism, the possibility that pathogens might tailor exploitation strategies has gone largely unexplored in the literature (but see [9,19,24]). Here, we explored this possibility using D. magna and its bacterial pathogen P. ramosa.
In line with our hypothesis, pathogens produced up to four times more spores in female hosts (figure 2c), but in doing so caused a substantial reduction in host dispersal. Infected females were less likely to leave crowded conditions and suffered a severe reduction in both dispersal rate (figure 2a) and total distance (figure 2b). For a pathogen, this should maximize the chance for secondary infections, as the release of a large number of transmission spores from a dying female is likely to coincide with a high-density population of (non-dispersing) animals. By contrast, while males allow for the production of fewer spores, infection increased the likelihood of dispersal (figure 1), and infected males showed dispersal rates and total distances comparable to uninfected males.
The differences we observe in dispersal behaviour between infected males and females appear to be a direct result of how the host sexes interact with the pathogen, and not an inherent property of each sex. Uninfected males and females, for example, had very similar rates and magnitudes of individual dispersal (figure 2a,b) and females were more likely to disperse from crowded habitats (figure 1). However, both patterns disappear once each sex is infected. The reduced dispersal of uninfected males from high-density conditions may be caused by the presence of females in these populations, since males might invest more energy in mating relative to dispersal (high-density conditions are one of the triggers for sexual reproduction in Daphnia [24,28]). In this situation, the increase in dispersal probability in infected males could be a direct pathogen-induced manipulation (as observed in other species [4,29]), or an indirect outcome of the partial sterilization [30].
Other studies have investigated how host sex may impact on the spatial spread of disease. The spread of vampire bat rabies between genetically isolated host populations, for example, is facilitated by dispersing male bats alone [14]. Mixed dispersal strategies for each sex also arise when microsporidia infect Aedes mosquitoes and invest a larger proportion of dispersing life-stages in females relative to males ([15], see also [16]). However, by tracking pathogen proliferation and dispersal simultaneously, we here connect these two observations and show how a pathogen can leverage the sex of its host to maximize the transmission benefits of within-host replication, but also achieve dispersal within and between host patches.
In summary, motivated by isolated examples linking sex differences to the mode of pathogen transmission and the spread of disease [14–16], our test-case formally explored how sex differences would alleviate the impact of pathogens on the movement of sick hosts. Whatever the proximate mechanisms, our results are consistent with the prediction that Pasteuria favours the production of spores in females, and host-assisted dispersal in males. This suggests that the sexes offer a mixed dispersal strategy, and a form of bet hedging for the pathogen [15,16]. However, the degree to which our prediction holds in other systems will be sensitive to the direction of sexual dimorphism and the relative density of the two host sexes. As of yet, any theory on pathogen evolution in sexually dimorphic hosts is currently limited to the evolution of virulence and proliferation alone [18,19] and does not account for dispersal in a spatial setting. Our work shows that pathogens can exploit this variation in their environment and suggests that pathogen strategies tailored to sexual dimorphism might be widespread and have important implications for disease dynamics.
Supplementary Material
Acknowledgements
We thank the Hall group for valuable discussions.
Data accessibility
Datasets supporting the conclusions of this article are available via a figshare repository: https://dx.doi.org/10.26180/5c8045f3e97dd [31].
Authors' contributions
L.S.N., B.L.P. and M.D.H. conceived of and designed the study. L.S.N. performed the experiments. M.D.H. and L.S.N. analysed the data. All authors drafted the manuscript, gave final approval and are accountable for accuracy.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by an Australian Research Council grant (DP160101730) to B.L.P. and M.D.H.
References
- 1.Binning SA, Shaw AK, Roche DG. 2017. Parasites and host performance: incorporating infection into our understanding of animal movement. Integr. Comp. Biol. 57, 267–280. ( 10.1093/icb/icx024) [DOI] [PubMed] [Google Scholar]
- 2.Gandon S. 2002. Local adaptation and the geometry of host–parasite coevolution. Ecol. Lett. 5, 246–256. ( 10.1046/j.1461-0248.2002.00305.x) [DOI] [Google Scholar]
- 3.Gandon S, Michalakis Y. 2002. Local adaptation, evolutionary potential and host–parasite coevolution: interactions between migration, mutation, population size and generation time. J. Evol. Biol. 15, 451–462. ( 10.1046/j.1420-9101.2002.00402.x) [DOI] [Google Scholar]
- 4.Moore J. 2002. Parasites and the behaviour of animals. Oxford series in ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Daversa DR, Fenton A, Dell AI, Garner TWJ, Manica A. 2017. Infections on the move: how transient phases of host movement influence disease spread. Proc. R. Soc. B 284, 20171807 ( 10.1098/rspb.2017.1807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osnas EE, Dobson AP. 2011. Evolution of virulence in heterogeneous host communities under multiple trade-offs. Evolution 66, 391–401. ( 10.1111/j.1558-5646.2011.01461.x) [DOI] [PubMed] [Google Scholar]
- 7.Lion S, Van Baalen M, Wilson WG. 2006. The evolution of parasite manipulation of host dispersal. Proc. R. Soc. B 273, 1063–1071. ( 10.1098/rspb.2005.3412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker DJ, Snedden CE, Altizer S, Hall RJ. 2018. Host dispersal responses to resource supplementation determine pathogen spread in wildlife metapopulations. Am. Nat. 192, 503–517. ( 10.1086/699477) [DOI] [PubMed] [Google Scholar]
- 9.Fairbairn DJ. 1997. Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annul. Rev. Ecol. Syst. 28, 659–687. ( 10.1146/annurev.ecolsys.28.1.659) [DOI] [Google Scholar]
- 10.Li X-Y, Kokko H. 2018. Sex-biased dispersal: a review of the theory. Biol. Rev. 94, 721–736. ( 10.1111/brv.1247541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulin R. 1996. Sexual inequalities in helminth infections: a cost of being male? Am. Nat. 147, 287–295. ( 10.1086/285851) [DOI] [Google Scholar]
- 12.Schalk G, Forbes MR. 1997. Male biases in parasitism of mammals: effects of study type, host age, and parasite taxon. Oikos 78, 67–74. ( 10.2307/3545801) [DOI] [Google Scholar]
- 13.Sheridan LAD, Poulin R, Ward DF, Zuk M. 2000. Sex differences in parasitic infections among arthropod hosts: is there a male bias? Oikos 88, 327–334. ( 10.1034/j.1600-0706.2000.880211.x) [DOI] [Google Scholar]
- 14.Streicker DG, et al. 2016. Host–pathogen evolutionary signatures reveal dynamics and future invasions of vampire bat rabies. Proc. Natl Acad. Sci. USA 113, 10 926–10 931. ( 10.1073/pnas.1606587113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soghigian J, Livdahl T. 2017. Differential response to mosquito host sex and parasite dosage suggest mixed dispersal strategies in the parasite Ascogregarina taiwanensis. PLoS ONE 12, e0184573 ( 10.1371/journal.pone.0184573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fellous S, Koella JC. 2009. Different transmission strategies of a parasite in male and female hosts. J. Evol. Biol. 22, 582–588. ( 10.1111/j.1420-9101.2008.01665.x) [DOI] [PubMed] [Google Scholar]
- 17.Gipson SAY, Hall MD. 2016. The evolution of sexual dimorphism and its potential impact on host–pathogen coevolution. Evolution 70, 959–968. ( 10.1111/evo.12922) [DOI] [PubMed] [Google Scholar]
- 18.Cousineau SV, Alizon S. 2014. Parasite evolution in response to sex-based host heterogeneity in resistance and tolerance. J. Evol. Biol. 27, 2753–2766. ( 10.1111/jeb.12541) [DOI] [PubMed] [Google Scholar]
- 19.Hall MD, Mideo N. 2018. Linking sex differences to the evolution of infectious disease life-histories. Phil. Trans. R. Soc. B 373, 20170431 ( 10.1098/rstb.2017.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duneau D, Ebert D. 2012. Host sexual dimorphism and parasite adaptation. PLoS Biol. 10, e1001271 ( 10.1371/journal.pbio.1001271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebert D, Duneau D, Hall MD, Luijckx P, Andras JP, Du Pasquier L Ben-Ami F. 2016. A population biology perspective on the stepwise infection process of the bacterial pathogen Pasteuria ramosa in Daphnia. Adv. Parasitol. 91, 265–310. ( 10.1016/bs.apar.2015.10.001) [DOI] [PubMed] [Google Scholar]
- 22.Gipson SAY, Hall MD. 2018. Interactions between host sex and age of exposure modify the virulence-transmission trade-off. J. Evol. Biol. 31, 428–437. ( 10.1111/jeb.13237) [DOI] [PubMed] [Google Scholar]
- 23.Thompson O, Gipson SAY, Hall MD. 2017. The impact of host sex on the outcome of co-infection. Sci. Rep. 7, 910 ( 10.1038/s41598-017-00835-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winsor GL, Innes DJ. 2002. Sexual reproduction in Daphnia pulex (Crustacea: Cladocera): observations on male mating behaviour and avoidance of inbreeding. Freshw. Biol. 47, 441–450. ( 10.1046/j.1365-2427.2002.00817.x) [DOI] [Google Scholar]
- 25.Clerc M, Ebert D, Hall MD. 2015. Expression of parasite genetic variation changes over the course of infection: implications of within-host dynamics for the evolution of virulence. Proc. R. Soc. B 282, 20142820 ( 10.1098/rspb.2014.2820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erm P, Hall MD, Phillips BL. 2019. Anywhere but here: local conditions alone drive dispersal in Daphnia. PeerJ 7, e6599 ( 10.7717/peerj.6599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel J, Ebert D, Hall MD. 2016. The trans-generational impact of population density on host–parasite interactions. BMC Evol. Biol. 16, 1–12. ( 10.1186/s12862-016-0828-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roulin AC, Routtu J, Hall MD, Janicke T, Colson I, Haag CR, Ebert D. 2013. Local adaptation of sex induction in a facultative sexual crustacean: insights from QTL mapping and natural populations of Daphnia magna. Mol. Ecol. 22, 3567–3579. ( 10.1111/mec.12308) [DOI] [PubMed] [Google Scholar]
- 29.Thomas F, Adamo S, Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Processes. 68, 185–199. ( 10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 30.Duneau D, Luijckx P, Ruder LF, Ebert D. 2012. Sex-specific effects of a parasite evolving in a female-biased host population. BMC Biol. 10, 104 ( 10.1186/1741-7007-10-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nørgaard SL, Ben LP, Matthew DH. 2019. Data from: Can pathogens optimise both proliferation and dispersal by leveraging the sex of their infected hosts? Biol. Lett. ( 10.26180/5c8045f3e97dd) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nørgaard SL, Ben LP, Matthew DH. 2019. Data from: Can pathogens optimise both proliferation and dispersal by leveraging the sex of their infected hosts? Biol. Lett. ( 10.26180/5c8045f3e97dd) [DOI]
Supplementary Materials
Data Availability Statement
Datasets supporting the conclusions of this article are available via a figshare repository: https://dx.doi.org/10.26180/5c8045f3e97dd [31].