Abstract
Stress urinary incontinence (SUI) in women is strongly associated with childbirth which injures the pudendal nerve (PN) and the external urethral sphincter (EUS) during delivery. Electrical stimulation (ES) can increase brain-derived neurotrophic factor (BDNF) expression in injured neurons, activate Schwann cells and promote neuroregeneration after nerve injury. The aim of this study was to determine if more frequent ES would increase recovery from SUI in a rat model. Forty female Sprague–Dawley rats underwent either sham injury or pudendal nerve crush (PNC) and vaginal distention (VD) to establish SUI. Immediately after injury, electrodes were implanted at the pudendal nerve bilaterally. Each injured animal underwent sham ES, twice per week ES (2/week), or daily ES of 1 h duration for two weeks. Urethral and nerve function were assessed with leak point pressure (LPP), EUS electromyography and pudendal nerve sensory branch potential (PNSBP) recordings two weeks after injury. LPP was significantly increased after daily ES compared to 2/week ES. EUS neuromuscular junction innervation was decreased after injury with sham ES, but improved after 2/week or daily ES. This study demonstrates that daily bilateral ES to the pudendal nerve can accelerate recovery from SUI. Daily ES improved urethral function more than 2/week ES.
Keywords: urodynamics, external urethral sphincter, leak point pressure, neurourology, nerve regeneration
1. Introduction
Pudendal nerve (PN) injury and denervation of the external urethral sphincter (EUS) are strongly correlated with childbirth. With insufficient neuroregeneration and reinnervation of the EUS, they contribute to both postpartum incontinence and stress urinary incontinence (SUI) later in life since these injuries are strongly correlated with development of SUI [1,2].
Brain-derived neurotrophic factor (BDNF) is expressed in striated muscle and motor neurons after peripheral nerve injury and facilitates regeneration and myelination of the injured peripheral nerve [3]. However, when muscles are injured, BDNF is downregulated since it inhibits neuromuscular junction (NMJ) reformation [4,5]. When both a nerve and the muscle it innervates are injured, as occurs in childbirth, BDNF has contradictory effects by promoting neuroregeneration but inhibiting muscle recovery [5,6].
We have developed a dual muscle and nerve injury SUI animal model consisting of vaginal distention (VD) and PN crush (PNC), and have demonstrated that a neurotrophin-mediated mechanism could be responsible for delayed and incomplete recovery [4,6]. Treatment with BDNF improves recovery after simulated vaginal delivery and decreases BDNF expression in the EUS [6]. However, treatment with BDNF has high potential for significant side effects [6].
Electrical stimulation (ES) can increase BDNF expression in injured neurons, activate Schwann cells and promote neuroregeneration after nerve injury [7]. ES could therefore facilitate recovery after a dual nerve and muscle injury by promoting neuroregeneration and reinnervation without inhibiting NMJ repair and muscle recovery. We have previously demonstrated feasibility of ES for improving recovery in the dual injury model of SUI [8,9]. The goal of this study was to test the hypothesis that more frequent stimulation would provide greater improvement in functional and anatomic recovery in the dual injury model of SUI.
2. Material and methods
2.1. Study design
This research was approved by the Institutional Animal Care and Use Committee of the Cleveland VA Medical Center. Age-matched female Sprague–Dawley rats (250–300 g) underwent sham injury with no electrode implantation (sham + no implant, n = 5), sham injury with sham ES (sham + sham ES, n = 5), PNC&VD with sham ES (PNC&VD + sham ES, n = 10), PNC&VD with twice weekly ES (PNC&VD + 2/week ES, n = 10) or PNC&VD with daily ES (PNC&VD + daily ES, n = 10). The last four groups received 1 h duration bilateral PN stimulation or sham stimulation for two weeks after the injury when each rat underwent functional testing including leak point pressure (LPP), external urethral sphincter (EUS) electromyography (EMG) and PN sensory branch potential (PNSBP) recordings. The urethra and PN were then harvested for anatomical assessment by histology and immunofluorescence.
2.2. Determining stimulation parameters
Five additional female age-matched rats were used in an initial study to determine the amplitude of PN stimulation, as we have done previously [8,9]. Bilateral ES of the PN (20 Hz, 0.1 ms pulse duration, square wave) was tested by increasing current from 0.1 to 1 mA. The subthreshold stimulation intensity used in the full experiment was defined as the current amplitude just below that causing visible anal contraction.
2.3. Pudendal nerve crush, wire electrode implantation and vaginal distension
PNC was performed, as previously described [8]. In brief, rats were anaesthetized with isoflurane and the PN was isolated bilaterally and crushed for 30 s. For sham PNC, the PN was isolated bilaterally, but not crushed. Each rat then had two stainless steel wire electrodes (A-M Systems, Carlsborg, WA) implanted on each side at the PN, approximately 1 cm apart, and hooked around the PN using 5-0 silk to stabilize the proximal electrode. The wire electrodes were implanted at the pudendal nerve approximately 1 cm apart, across the injury site. The other end of each wire was tunnelled subcutaneously to the dorsal neck where it exited and was secured to the skin. Immediately after PNC and electrode implantation, a modified 10F Foley catheter was inserted into the vagina and the balloon was inflated with 3 ml of water for 4 h for VD as done previously [5]. Sham VD was done by inserting the modified Foley catheter for 4 h without balloon inflation.
2.4. Pudendal nerve electrical stimulation
Immediately after PNC&VD and while maintaining anaesthesia, the electrodes were connected to a stimulator (Grass model S88, Astro-Med Inc., West Warwick, RI) and the PN was stimulated bilaterally for 1 h (20 Hz, 0.3 mA, 0.1 ms). According to their groups, rats were anaesthetized with isoflurane and received daily sham ES, ES for 1 h two times per week (2/week), daily ES or no ES (sham ES) until functional testing two weeks after injury.
2.5. Functional testing
Two weeks after injury and treatment or sham treatment, animals underwent in vivo functional recordings including leak point pressure (LPP), external urethral sphincter electromyography (EUS EMG) and pudendal nerve sensory branch potential (PNSBP), as described previously [10]. Rats were anaesthetized with urethane and a transvesical catheter was inserted into the bladder dome and secured with a suture. The catheter was connected to both a pressure transducer (Model PT300; Astro-Med) and syringe pump. The pubic symphysis was opened to expose the urethra, and a pair of bipolar parallel platinum iridium electrodes (250 µm diameter, 1 mm apart; FHC, Inc., Bowdoin, ME) were placed on the mid urethra and were connected to an amplifier (Model P511; AC Amplifier, Astro-Med; band pass frequencies: 3 Hz–3 kHz) and recording system (PowerLab 8/35, ADInstruments, Inc., Colorado Springs, CO; 10 kHz sampling rate). The bladder was filled at 5 ml h−1, and at approximately half capacity a cotton swab was pressed gently on the rat's abdomen above the bladder to increase intravesical pressure until leakage was observed when the external pressure was rapidly removed. LPP and EUS EMG were recorded simultaneously and repeated four to six times in each rat.
After LPP and EUS EMG testing, the PN sensory branch was separated 0.5 cm apart from the distal wire on the ventral side and was suspended over platinum iridium recording electrodes (250 µm diameter wire, 0.8 mm apart, 1.0 mm hook diameter; FHC) connected to the amplifier and recording system as above. The clitoris was brushed gently with a cotton swab or gauze to trigger a response in the PN sensory branch. This test was repeated six to nine times in each rat.
2.6. Histology
After the rats were euthanized with an intracardiac overdose of pentobarbital (390 mg ml−1), the urethra and PN were dissected, flash frozen in OCT, and stored at −20°C. Tissues were sectioned transversely (14 µm) and stored at −80°C. PN sections underwent immunofluorescence to identify axons with the primary antibodies anti-neurofilament 200 and 68 (1 : 400 dilution; Item # N0142 and N5139, respectively, Sigma-Aldrich) and secondary antibody, Alexa Fluor 488 conjugated donkey anti-mouse IgG (1 : 400; Item # R37114; ThermoFisher, Waltham, MA, USA).
Immunofluorescence was also used to assess innervation of neuromuscular junctions (NMJs) of the EUS using the primary anti-neurofilament antibodies and secondary Alexa Fluor 488 antibodies to identify axons, and 4 µg ml−1 of tetramethylrhodamine conjugated alpha-bungarotoxin (Rh-α-BTX; 1 : 400 dilution, Item # T1175, ThermoFisher). Alexa Fluor 350 conjugated phalloidin (1 : 40 dilution, Item # A22281, ThermoFisher) was used to identify striated muscle of the EUS. Additional near sections of the urethra were stained with Masson's trichrome stain to assess muscle and collagen in the EUS and Hart's stain for elastin.
2.7. Data analysis
LPP was defined as baseline bladder pressure just prior to the increase in external abdominal pressure subtracted from peak bladder pressure at leakage during LPP testing. Quantitative assessment of EUS EMG and PNSBP signals was performed by selecting four to six 1 s segments for each rat at baseline and peak activity (figure 1) and determining the mean amplitude and firing rate of muscle and nerve activity, as done previously [10]. Increase in amplitude and firing rate at peak activity were calculated by subtracting baseline from peak activity values. A custom threshold was created for each baseline-peak pair to remove background noise by calculating interquartile range (Matlab, V. R2012b, MathWorks, Natick, MA). Mean values of each quantitative variable were calculated for each animal and were used to calculate a mean and standard error for each experimental group. One-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test was used to compare LPP, EUS EMG and PNSBP results when data passed normality testing. If normality failed, ANOVA on ranks followed by a Dunn's test was used. p < 0.05 indicated a statistically significant difference between groups in all cases.
Figure 1.
Examples of functional testing results including leak point pressure (LPP) with simultaneous external urethral sphincter (EUS) electromyography (EMG) and pudendal nerve sensory branch potential (PNSBP). Examples demonstrate the 1 s segments selected from baseline (B) and peak activity (P) that were used to calculate amplitude increase (µV) and firing rate increase (Hz) from baseline to peak pressure in EUS EMG or during PNSBP recordings. PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation.
2.8. Semi-quantitative assessments
Representational histology and immunofluorescence images of each specimen were evaluated semi-quantitatively by two blinded observers according to the following criteria. EUS striated muscle morphology was assessed using Masson's staining based on whether the EUS was intact (yes or no), if collagen had infiltrated between the muscle fibres (yes or no), and if the EUS muscle was disrupted or atrophied (yes or no). Urethral elastin was assessed using Hart's stained specimens based on whether elastin fibres were long or short, elastin fibre orientation (radial or circumferential) and elastin organization (organized or disorganized). EUS NMJ assessments were evaluated using immunofluorescence on whether NMJs were compact with well-defined edges (yes or no), innervated (yes or no) and if there was a single innervating axon (yes or no). PN sensory branch morphology was assessed using immunofluorescence to determine extent of PN recovery by evaluating axon density (1: low density, 2: moderated density, or 3: high density) and organization of axons (1: disorganized alignment, 2: moderate alignment, or 3: highly aligned organized axons). Scores are presented as the percentage with each criteria in each group, except for nerve fascicle scoring, in which each specimen's density and organization scores were added together and the group average is displayed. A one-way ANOVA followed by a Student–Newman–Keuls post hoc test was used to indicate a statistically significant difference between groups (p < 0.05) for nerve fascicle scoring. The Fisher exact test followed by a Bonferroni correction was used to indicate a statistically significant difference between groups (p < 0.05) for all other histological outcomes.
3. Results
3.1. Determination of threshold for pudendal nerve electrical stimulation
In all five rats used to determine amplitude of ES to be used in the full study, anal contraction was visualized when the stimulation current reached 0.4 mA. This threshold for induction of a visible anal contraction remained at 0.4 mA during the two week testing period. We therefore chose 0.3 mA as the ES amplitude for the full experiment.
3.2. Electrical stimulation treatment improves urethral function after simulated childbirth injury
Example LPP traces show a decrease in LPP in PNC&VD + sham ES compared to sham injured rats (figure 1). Two weeks after injury, LPP was significantly increased with either 2/week ES (28.2 ± 0.6 cm H2O) or daily ES (31.3 ± 1.0 cm H2O) compared to PNC&VD + sham ES (figure 2), demonstrating that ES accelerates functional recovery of the continence mechanism after PNC&VD. LPP after daily ES was also significantly increased compared to 2/week ES, indicating that more frequent stimulation promotes greater recovery. However, all injured animals had significantly decreased LPP compared to either the sham + no implant or sham + sham ES groups (figure 2), suggesting that ES promotes partial recovery two weeks after PNC&VD. Comparison of the control groups demonstrated that LPP was not significantly different between the sham + no implant (38.7 ± 0.8 cm H2O) and sham + sham ES (37.1 ± 1.0 cm H2O) groups, indicating that implantation of the electrode did not affect LPP. In contrast, LPP of PNC&VD + sham ES rats (20.2 ± 1.0 cm H2O) was significantly decreased compared to both sham injury groups (figure 2), indicating that urethral dysfunction created by the model remained with electrode implantation.
Figure 2.
Leak point pressure (LPP) results two weeks after injury. Each box represents the 25th and 75th confidence intervals with whiskers representing 10th and 90th confidence intervals and the horizontal line representing the median of data from 5 to 10 animals for each group. Boxes without whiskers did not have a high enough number of animals in the group to calculate the 10th and 90th percentiles. *Significant difference compared to sham + no implant (p < 0.001). #Significant difference compared to sham + sham ES (p < 0.001). †Significant difference compared to PNC&VD + sham ES (p < 0.001). ‡Significant difference compared to PNC&VD + 2/week ES (p < 0.001). PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation.
EUS EMG firing rate after 2/week (444 ± 48 Hz) or daily ES (422.5 ± 94.8 Hz) was significantly increased compared to the PNC&VD + sham ES group (p < 0.05) and was not significantly different from the sham injured groups (figure 3), indicating that ES of the PN accelerated functional recovery of the EUS. However, EUS EMG amplitude was significantly decreased after 2/week ES (4.4 ± 1.5 µV) or daily ES (4.5 ± 61.8 µV) compared to the sham + no implant group, suggesting partial recovery. Comparison of the controls groups demonstrated that EUS EMG amplitude and firing rate were not significantly different between sham + no implant (15.3 ± 3.9 µV, 420 ± 78 Hz) and sham + sham ES (8.1 ± 3.8 µV, 549 ± 273 Hz) rats (figure 3), indicating that there was not a significant effect of electrode implantation on EUS function. However, EUS EMG amplitude (0.5 ± 0.1 µV) and firing rate (84 ± 111 Hz) after PNC&VD + sham ES were significantly decreased compared to both sham injury groups (p < 0.05), demonstrating that the injury model decreases EUS function.
Figure 3.
External urethral sphincter electromyography (EUS EMG) results two weeks after injury and treatment. Increase of amplitude (a) and firing rate (b) of EUS EMG results in each group two weeks after injury and treatment. Each box represents the 25th and 75th confidence intervals with whiskers representing 10th and 90th confidence intervals and the horizontal line representing the median of data from 5 to 10 animals for each group. Boxes without whiskers did not have a high enough number of animals in the group to calculate the 10th and 90th percentiles. *Significant difference compared to sham + no implant (p < 0.001). †Significant difference compared to PNC&VD + sham ES (p < 0.001). PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation.
PNSBP amplitude (0.04 ± 0.01 µV, 0.1 ± 0.0 µV, 0.2 ± 0.1 µV) and firing rate (50 ± 34 Hz, 169.3 ± 35.0 Hz, 294.9 ± 135.3 Hz) in all injured groups, regardless of treatment, were significantly decreased compared to that of both sham injury groups (p < 0.05, figure 4). In addition, comparison of the control groups showed that amplitude (1.0 ± 0.2 µV) and firing rate (1336 ± 521 Hz) of PNSBP in the sham + no implant group were significantly higher than in the sham + sham ES group, indicating a strong deleterious effect of electrode implantation on PNSBP (p < 0.05, figure 4) that was not overcome with ES.
Figure 4.
Pudendal nerve sensory branch potential (PNSBP) results two weeks after injury and treatment. Increase of amplitude (a) and firing rate (b) of PNSBP results in each group two weeks after injury and treatment. Each box represents the 25th and 75th confidence intervals with whiskers representing 10th and 90th confidence intervals and the horizontal line representing the median of data from 5 to 10 animals for each group. Boxes without whiskers did not have a high enough number of animals in the group to calculate the 10th and 90th percentiles. *Significant difference compared to sham + no implant. #Significant difference compared to sham + sham stim ES. PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation.
3.3. Electrical stimulation treatment promotes neuromuscular junction and external urethral sphincter regeneration
Example EUS NMJ staining showed good innervation and compact NMJ in the sham injured groups (figure 5). In contrast, no PNC&VD + sham ES specimen had compact or innervated NMJs, a significant difference compared to the sham + no implant group (figure 5), indicating that EUS NMJs are denervated by PNC&VD injury. After two weeks of daily ES, 50% of EUS specimen had compact NMJs and 33% had innervated NMJs, demonstrating that ES facilitated reinnervation of EUS NMJs. Only 17% of 2/week ES specimens had compact NMJs and 50% had innervated NMJs. In contrast, there were no specimens with individual neurons innervating the NMJ in any of the injury groups (figure 5), indicating only partial recovery with ES. Comparing the control groups, 100% of sham + no implant specimen had innervated and compact NMJs, while only 60% of sham + sham ES specimens had compact NMJs and 80% had innervated NMJs (figure 5), suggesting an impact of electrode implantation on reinnervation of the EUS by the pudendal nerve.
Figure 5.
EUS NMJ immunostaining results. EUS NMJ assessments were assessed by two blinded investigators based on whether the NMJ was compact with well-defined edges (yes or no), the NMJ was innervated (yes or no), and whether there was a single innervating axon (yes or no). Green colour indicates neurofilament, red colour indicates NMJ, blue colour indicates striated muscle. A representative image was chosen for each group (a–e). Data presented in the graph is shown as percentage of specimen that present with criteria as follows: compact NMJ (f), innervated NMJ (g), individual nerves (h). Each bar represents data from five or six animals. *Significant difference compared to sham + no implant (p < 0.05). Scale bar is 20 µm. EUS, external urethral sphincter; NMJ, neuromuscular junction; PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation. (Online version in colour.)
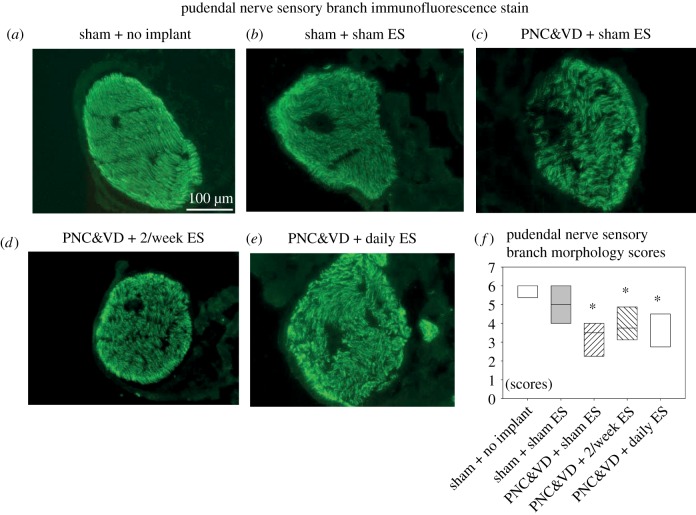
Example PN specimens from the PNC&VD + sham ES group showed less dense and more disorganized axons than from all the other groups (figure 6), confirming that PNC&VD injures the pudendal nerve. Summed density and orientation scores of the PNC&VD + sham ES (3.0 ± 0.5), 2/week ES (3.9 ± 0.5) and daily ES (3.8 ± 0.5) groups were all significantly decreased compared to sham + no implant but not sham + sham ES, suggesting a denervation effect of electrode implantation on the PN. However, there was no difference in blinded scoring between sham + no implant (5.8 ± 0.2) and sham + sham ES (5.0 ± 1.0; figure 6).
Figure 6.
Pudendal nerve sensory branch immunofluorescence results with cross-sections of axons stained in green. A representative image was chosen for each group (a–e). A semi-quantitative method was used to analyse pudendal nerve morphology including density from low to high (1–3) and orientation from disorganized to aligned (1–3). Two blinded investigators separately scored each animal using the above criteria. Each score was summed to give a total score for each animal (f). Each box in (f) represents the 25th and 75th confidence intervals with the horizontal line representing the median of data from five to six animals for each group. *Significant difference compared to sham + no implant with p < 0.05. Scale bar is 100 µm. PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation. (Online version in colour.)
3.4. Electrical stimulation treatment promotes urethral structure and elastogenesis
Masson's trichrome staining of the urethra in the two sham injured groups showed normal intact EUS with little infiltration of collagen and no significant differences between the two groups (figure 7). In contrast, no PNC&VD + sham ES specimens had intact EUS muscle without collagen or EUS disruption, demonstrating significant damage to the urethra by PNC&VD. However, 60% of daily ES specimens had intact EUS muscle, 20% had no collagen infiltration and 60% had EUS disruption (figure 7), indicating improved recovery of the EUS with daily ES. In contrast, only 14% of 2/week ES specimens had intact EUS muscle without collagen infiltration and EUS disruption. The percentage of specimens without collagen infiltration and EUS disruption in the PNC&VD + sham ES and PNC&VD + 2/week ES group was significantly decreased compared to the sham + no implant group (figure 7). In contrast, outcomes of the EUS and urethral collagen in the PNC&VD + daily ES group were not significantly different from other groups, indicating partial recovery of the EUS after daily ES.
Figure 7.
Urethral Masson's trichrome stain results. EUS striated muscle morphology was assessed by two blinded investigators based on whether the EUS was intact (yes or no), if collagen had not infiltrated between the muscle fibres (yes or no), and if the EUS muscle was not disrupted or atrophied (yes or no). A representative image was chosen for each group (a–e). Data presented in the graph are shown as percentage of specimens that present with criteria as follows: intact EUS muscle (f), without EUS collagen infiltration (g), without EUS disruption (h). Each bar represents data from five or six animals. *Significant difference compared to sham + no implant (p < 0.05). Scale bar is 50 µm. PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation. (Online version in colour.)
There were no significant differences in elastin fibres as shown by Hart staining between the groups (figure 5). Nonetheless, there are some striking differences between the groups. Only 25% of PNC&VD + sham ES specimens had short elastin fibres and 0% of had parallel or circumferential fibres, demonstrating a detrimental effect of PNC&VD on elastin fibres in the urethra. In contrast 43% of 2/week ES specimens had long elastin fibres, 14% had circumferential and 43% had parallel fibres after two weeks of treatment. Sixty-seven per cent of daily ES specimens had long elastin fibres, 50% of were circumferential and 67% had parallel fibres, suggesting greater improvement in elastin repair with ES. Comparison of the control groups demonstrated that Hart staining showed 67% of sham + no implant specimens had long elastin fibres, 83% had circumferential fibres and 83% parallel fibres, not significantly different from the sham + sham ES group (figure 8). Eighty per cent of sham + sham ES specimens had long elastin fibres, 80% had circumferential fibres and 80% had parallel fibres.
Figure 8.
Urethral HART's elastic stain results. Two blinded investigators used a semi-quantitative method to assess urethral elastin fibre length (long or short), orientation (radial or circumferential), and organization (organized or disorganized). A representative image was chosen for each group (a–e). Data presented in the graph are shown as percentage of specimens that present with criteria as follows: long elastin fibres (f), circumferential fibres (g), organized fibres (h). Each bar represents data from five or six animals. Black arrows indicates elastic fibres. Scale bar is 50 µm. PNC, pudendal nerve crush; VD, vaginal distention; ES, electrical stimulation. (Online version in colour.)
4. Discussion
SUI decreases quality of life in 30% of women over the age of 40 [11]. Sling procedures have gained wide popularity while the success rates remain good but controversial [12,13]. Nonetheless, while the sling procedure can be used to treat intrinsic sphincter deficiency, it does not address the underlying neuromuscular pathology. Hence, a new therapeutic approach is needed to address the underlying pathophysiology of the disorder [2]. It is known that while the baby's head is passing through the birth canal it injures both the PN and the EUS, which is innervated by the PN [14–16]. Clinical studies have found that post-partum incontinent women have increased PN motor latency compared to continent women, indicating that PN function and regeneration is important for maintaining continence [14].
BDNF is necessary for nerve regeneration and can be dysregulated when both a nerve and the muscle it innervates are injured [5,17,18], suggesting that upregulation of BDNF could aid in PN regeneration following childbirth injury [6,17]. However, treatment with BDNF can have significant side effects [6,19]. On the other hand, ES can upregulate BDNF and accelerate nerve regeneration and functional recovery with few side effects [20]. The aim of this study was to test the hypothesis that more frequent stimulation would provide greater functional and anatomic recovery after PNC&VD.
The ES current just below that triggering an anal contraction was determined to be 0.3 mA, consistent with our previous work [8,9]. LPP was significantly increased after PNC&VD and 2/week or daily ES compared to PNC&VD + sham ES, demonstrating that ES accelerates functional recovery of the continence mechanism after PNC&VD. Daily ES resulted in significantly greater LPP than 2/week ES, indicating that more frequent stimulation promotes greater recovery, although the results clearly demonstrate partial recovery two weeks after injury with daily stimulation. Longer time points of study may demonstrate greater recovery with stimulation.
EUS EMG firing rate after 2/week or daily ES was significantly increased compared to PNC&VD + sham ES, but not significantly different from the sham injury groups, indicating that ES of the PN accelerated functional recovery of the EUS. As with LPP, EUS EMG amplitude after PNC&VD with 2/week or daily ES was significantly decreased compared to the sham injury groups, indicating they had not yet fully recovered two weeks after injury, consistent with our prior work demonstrating slow recovery of reinnervation of the EUS in this injury model [21].
The guarding reflex during LPP testing plays a key role in maintaining continence and is represented by the response in EUS EMG. Both amplitude and firing rate in EUS EMG signals are critical for generating contractile activity [21]. EMG amplitude correlates with the volume of muscle fibres while the firing rate is affected by the number of recruited motor units [22]. They were both significantly decreased two weeks after PNC+VD, but only the EMG firing rate was significantly increased with ES compared to sham ES, indicating that more EUS motor units were re-innervated with ES. This is confirmed by immunofluorescence of EUS NMJ in the same animals which, while not a statistically significant change, showed a trend to greater innervation of EUS NMJ with ES. A longer time point would allow time for amplitude to recover as well as firing rate [23].
In contrast to LPP and EUS EMG, PNSBP amplitude and firing rate in all injured groups, regardless of treatment, were significantly decreased compared to that of both sham + no implant and sham + sham ES groups indicating that electrode implantation decreased PN function. Thus, less invasive methods are needed for clinical translation. If shown to be effective for PN regeneration, vaginal or perigenital stimulation would be a method translatable to clinical practice [26,27].
ES is known to have a greater effect on neuroregeneration of motor axons than sensory axons [28]. Thus, although PNSBP did not recover significantly with ES, the PN motor branch is likely to regenerate and reinnervate the EUS, contributing to the increased EUS EMG firing rate with ES [8]. We did not measure the PN motor branch in this study as it is small and more difficult to measure after fibrosis from electrode implantation, a limitation that could be corrected in future studies using ES methods that do not require electrode implantation.
PN stimulation has been used successfully in patients with voiding dysfunction; however, few, if any, clinical trials are under investigation for SUI patients [24]. In that research, the frequency of stimulation was weekly with a duration of 30 min or 1 h for up to 12 weeks [25]. We tested daily ES in an animal model to assess a case of high frequency of repeat treatments although daily treatment would be difficult to implement clinically. Nonetheless, the concept of increasing stimulation sessions provides valuable evidence to optimize a stimulation strategy for clinical use.
Implantation of electrodes was chosen in this study over a previous method of blind insertion of electrodes [8,9]. While that approach was more clinically relevant it was difficult to ensure exact placement of electrodes. However, both approaches showed improved recovery two weeks after the injury with ES [8].
Immunofluorescence of the PN showed a significant decrease in morphology scores in all injured groups compared to the sham injured groups, indicating that the sensory branch of the PN had not recovered to a sham injured state, consistent with the PNSBP firing rate measure of PN sensory branch functions. We did not measure PN motor branch function or anatomy, because it was not possible with scarring from electrode implantation since it is much smaller than the sensory branch. Recovery of motor neurons has been shown to be facilitated with ES to a greater extent than sensory neurons [28,29], which may explain the positive EUS EMG response to ES despite little response in PNSBP on nerve morphology.
Masson's trichrome stain showed that the integrity of the EUS in all injured groups was significantly decreased compared to the sham injured groups, suggesting that the status of the EUS was not fully recovered two weeks after injury. However, more intact muscle of the EUS can be found in both PNC&VD with 2/week or daily ES compared to PNC&VD + sham ES, consistent with our previous work [8], demonstrating that ES promotes the recovery of EUS which is likely to facilitate improvement of LPP results.
NMJ staining demonstrated an increase in compact, innervated NMJs compared to PNC&VD + no implant. Consistent with previous studies, this demonstrates that ES accelerates motoneuron axonal growth and promotes innervation of the targeted muscles [8,9]. A longer period of recovery after injury may allow the nerve and innervated muscle to recover more completely particularly since innervation of NMJs of the EUS has been shown to be incomplete even nine weeks after injury [21].
Notably, in this study ES led to an increase in elastogenesis as has been observed previously [30]. Future studies will need to investigate the possible mechanism of accelerated elastogenesis. One possible explanation for this is that accelerated recovery of the EUS resulted in the accelerated recovery of elastic fibres [31,32]. Additionally, it is known that the extracellular matrix and elastin are influenced by the immune system [25]. It is possible that multiple stimulation events modulated the immune system, allowing for accelerated elastogenesis.
The limitations of this study include the use of acute traumatic injuries on nulliparous quadruped animals for the creation of SUI model. However, this dual injury animal model has been well investigated and can represent the neuromuscular and extracellular matrix injuries incurred during childbirth [4,32,33]. In this study, we investigated a two week time point after an injury which best mimics the acute injury women suffer after delivery [34]. A further study is needed for investigation of a longer time point to observe complete nerve and innervated musculature recovery as well as to investigate the mechanisms of accelerated neuroregeneration with ES.
An additional limitation is that the electrodes were placed at the nerve crush site, with the electrodes flanking the crush site. It is possible that the proximal electrode could have shifted distally, resulting in the proximal end of the nerve stump not generating an antidromic action potential, which is required to induce the accelerated regeneration mechanism [7,27]. While this is a possibility, the beneficial effect of ES observed in this study indicates that either this did not happen because the wire was secured in place by a suture or that electrode movement was uniformly distributed among the groups. In addition, the semi-quantitative analysis method used for assessment of histology specimens has the limitation of being subjective, even though we had two independent investigators blindly assess images from each specimen.
In this study we demonstrated that chronic bilateral ES to the PN is feasible and can accelerate recovery in a model of SUI. Daily ES provides better preservation of urethral function than 2/week ES. ES for treatment of postpartum SUI is promising if less invasive stimulation methods can be used.
Ethics
This research was approved by the Institutional Animal Care and Use Committee of the Cleveland VA Medical Center.
Data accessibility
The Department of Veteran's Affairs funded this work and the research was conducted at the Louis Stokes Veteran's Affairs Medical Center and is covered under the Freedom of Information Act. Any request for information must be sent to the Department of Veteran's Affairs and all request will be honoured.
Authors' contributions
K.D. participated in study design, conducting the experiment, statistical analyses and drafting the article; B.M.B. participated in statistical analyses and drafting the article; D.L.L. participated in conducting the experiment, and analysing the data; B.H. participated in conducting the experiment, statistical analyses and drafting the article; Q.-X.S. participated in conducting the experiment, and analysing the data; H.Z. participated in study design; and M.S.D. participated in study design, statistical analyses and drafting the article. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported in part by grants from the Rehabilitation Research and Development Service of the Department of Veterans Affairs, (I01 RX001262A1 and F9261-L to M.S.D.), the Cleveland Clinic, the National Natural Science Foundation of China (grant no. 81700669 to K.D.), the Natural Science Foundation of Hubei Province, People's Republic of China (grant no. 2016CFB217 to K.D.), and the China Scholarship Council.
References
- 1.Morley R, Cumming J, Weller R. 1996. Morphology and neuropathology of the pelvic floor in patients with stress incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 7, 3–12. ( 10.1007/BF01895096) [DOI] [PubMed] [Google Scholar]
- 2.Wood LN, Anger JT. 2014. Urinary incontinence in women. BMJ 349, g4531 ( 10.1136/bmj.g4531) [DOI] [PubMed] [Google Scholar]
- 3.Simon M, Porter R, Brown R, Coulton GR, Terenghi G. 2003. Effect of NT-4 and BDNF delivery to damaged sciatic nerves on phenotypic recovery of fast and slow muscles fibres. Eur. J. Neurosci. 18, 2460–2466. ( 10.1046/j.1460-9568.2003.02978.x) [DOI] [PubMed] [Google Scholar]
- 4.Pan HQ, Kerns JM, Lin DL, Sypert D, Steward J, Hoover CR, Zaszczurynski P, Butler RS, Damaser MS. 2009. Dual simulated childbirth injury delays anatomic recovery. Am. J. Physiol. Renal Physiol. 296, F277–F283. ( 10.1152/ajprenal.90602.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakuma K, et al. 2001. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res. 907, 1–19. ( 10.1016/S0006-8993(01)02288-0) [DOI] [PubMed] [Google Scholar]
- 6.Gill BC, Balog BM, Dissaranan C, Jiang HH, Steward JB, Lin DL, Damaser MS. 2013. Neurotrophin therapy improves recovery of the neuromuscular continence mechanism following simulated birth injury in rats. Neurourol. Urodyn. 32, 82–87. ( 10.1002/nau.22264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Majed AA, Brushart TM, Gordon T. 2000. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 12, 4381–4390. [PubMed] [Google Scholar]
- 8.Jiang HH, Gill BC, Dissaranan C, Zutshi M, Balog BM, Lin D, Damaser MS. 2013. Effects of acute selective pudendal nerve electrical stimulation after simulated childbirth injury. Am. J. Physiol. Renal Physiol. 304, F239–F247. ( 10.1152/ajprenal.00235.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang HH, Song QX, Gill BC, Balog BM, Juarez R, Cruz Y, Damaser MS. 2018. Electrical stimulation of the pudendal nerve promotes neuroregeneration and functional recovery from stress urinary incontinence in a rat model. Am. J. Physiol. Renal Physiol. 315, F1555–F1564. ( 10.1152/ajprenal.00431.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng K, et al. 2015. Mesenchymal stem cells and their secretome partially restore nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. Am. J. Physiol. Renal Physiol. 308, F92–F100. ( 10.1152/ajprenal.00510.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer S, de Grandi P, Kuntzer T, Hurlimann P, Schmidt N. 1993. Birth trauma: its effect on the urine continence mechanisms. Gynakol. Geburtshilfliche Rundsch. 33, 236–242. ( 10.1159/000272115) [DOI] [PubMed] [Google Scholar]
- 12.Fong ED, Nitti VW. 2010. Review article: Mid-urethral synthetic slings for female stress urinary incontinence. BJU Int. 106, 596–608. ( 10.1111/j.1464-410X.2010.09544.x) [DOI] [PubMed] [Google Scholar]
- 13.Oliphant SS, Wang L, Bunker CH, Lowder JL. 2009. Trends in stress urinary incontinence inpatient procedures in the United States, 1979–2004. Am. J. Obstet. Gynecol. 200, 521.e521–526. ( 10.1016/j.ajog.2009.01.007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AR, Hosker GL, Warrell DW. 1989. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. Br. J. Obstet. Gynaecol. 96, 29–32. ( 10.1111/j.1471-0528.1989.tb01572.x) [DOI] [PubMed] [Google Scholar]
- 15.Snooks SJ, Setchell M, Swash M, Henry MM. 1984. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 2, 546–550. ( 10.1016/S0140-6736(84)90766-9) [DOI] [PubMed] [Google Scholar]
- 16.Snooks SJ, Swash M, Henry MM, Setchell M. 1986. Risk factors in childbirth causing damage to the pelvic floor innervation. Br. J. Surg. 72(Suppl), S15–S17. [DOI] [PubMed] [Google Scholar]
- 17.Eberhardt KA, Irintchev A, Al-Majed AA, Simova O, Brushart TM, Gordon T, Schachner M. 2006. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp. Neurol. 198, 500–510. ( 10.1016/j.expneurol.2005.12.018) [DOI] [PubMed] [Google Scholar]
- 18.Song QX, Chermansky CJ, Birder LA, Li L, Damaser MS. 2014. Brain-derived neurotrophic factor in urinary continence and incontinence. Nat. Rev. Urol. 11, 579–588. ( 10.1038/nrurol.2014.244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weishaupt N, Blesch A, Fouad K. 2012. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 238, 254–264. ( 10.1016/j.expneurol.2012.09.001) [DOI] [PubMed] [Google Scholar]
- 20.Gordon T, Amirjani N, Edwards DC, Chan KM. 2010. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 223, 192–202. ( 10.1016/j.expneurol.2009.09.020) [DOI] [PubMed] [Google Scholar]
- 21.Stalberg E, Erdem H. 2002. Quantitative motor unit potential analysis in routine. Electromyogr Clin. Neurophysiol. 42, 433–442. [PubMed] [Google Scholar]
- 22.Raez MB, Hussain MS, Mohd-Yasin F. 2006. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol. Proced. Online 8, 11–35. ( 10.1251/bpo115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song QX, Balog BM, Kerns J, Lin DL, Sun Y, Damaser MS, Jiang HH. 2015. Long-term effects of simulated childbirth injury on function and innervation of the urethra. Neurourol. Urodyn. 34, 381–386. ( 10.1002/nau.22561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon T, Sulaiman OA, Ladak A. 2009. Chapter 24: Electrical stimulation for improving nerve regeneration: where do we stand? Int. Rev. Neurobiol. 87, 433–444. ( 10.1016/S0074-7742(09)87024-4) [DOI] [PubMed] [Google Scholar]
- 25.Schreiner L, Santos TG, Souza AB, Nygaard CC, Silva Filho IG. 2013. Electrical stimulation for urinary incontinence in women: a systematic review. Int. Braz. J. Urol. 39, 454–464. ( 10.1590/S1677-5538.IBJU.2013.04.02) [DOI] [PubMed] [Google Scholar]
- 26.Visco AG, Figuers C. 1998. Nonsurgical management of pelvic floor dysfunction. Obstet. Gynecol. Clin. North Am. 25, 849–865, vii ( 10.1016/S0889-8545(05)70046-2) [DOI] [PubMed] [Google Scholar]
- 27.Friedman B, Kleinfeld D, Ip NY, Verge VM, Moulton R, Boland P, Zlotchenko E, Lindsay RM, Liu L. 1995. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J. Neurosci. 15, 1044–1056. ( 10.1523/JNEUROSCI.15-02-01044.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. 2000. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 20, 2602–2608. ( 10.1523/JNEUROSCI.20-07-02602.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. 2002. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J. Neurosci. 22, 6631–6638. ( 10.1523/JNEUROSCI.22-15-06631.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durigan JL, Peviani SM, Delfino GB, de Souza Jose RJ, Parra T, Salvini TF. 2014. Neuromuscular electrical stimulation induces beneficial adaptations in the extracellular matrix of quadriceps muscle after anterior cruciate ligament transection of rats. Am. J. Phys. Med. Rehabil. 93, 948–961. ( 10.1097/PHM.0000000000000110) [DOI] [PubMed] [Google Scholar]
- 31.McLean NA, Verge VM. 2016. Dynamic impact of brief electrical nerve stimulation on the neural immune axis-polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve. Glia 64, 1546–1561. ( 10.1002/glia.23021) [DOI] [PubMed] [Google Scholar]
- 32.Herrera-Imbroda B, Lara MF, Izeta A, Sievert KD, Hart ML. 2015. Stress urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter. Adv. Drug Deliv. Rev. 82–83, 106–116. ( 10.1016/j.addr.2014.10.018) [DOI] [PubMed] [Google Scholar]
- 33.Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P, Damaser M. 2009. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol. Urodyn. 28, 229–235. ( 10.1002/nau.20632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomason AD, Miller JM, Delancey JO. 2007. Urinary incontinence symptoms during and after pregnancy in continent and incontinent primiparas. Int. Urogynecol. J. Pelvic Floor Dysfunct. 18, 147–151. ( 10.1007/s00192-006-0124-8) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Department of Veteran's Affairs funded this work and the research was conducted at the Louis Stokes Veteran's Affairs Medical Center and is covered under the Freedom of Information Act. Any request for information must be sent to the Department of Veteran's Affairs and all request will be honoured.