Abstract
Chronic Obstructive Pulmonary Disease (COPD) is a syndrome that comprises several distinct and overlapping phenotypes. In addition to persistent airflow limitation and respiratory symptoms, COPD is also characterized by chronic systemic inflammation. Epidemiological studies have shown that dietary fibers, fruits and vegetables intake protects against the COPD development, while fructose-loading is associated with increased risk of asthma and chronic bronchitis. Since dietary factors might affect susceptibility to COPD by modulating oxidative stress and inflammatory responses, we evaluated how fructose feeding might affect the smoking-induced emphysema in mice. We found that chronic fructose intake induced destruction and remodeling of lung parenchyma and impairment of respiratory mechanics, which are associated with distinctive cytokine profiles in bronchoalveolar lavage fluid, blood plasma and skeletal muscle. The combined effects of chronic fructose intake and cigarette smoking on destruction of lung parenchyma are more pronounced than the effects of either alone. Excessive intake of fructose might directly cause pulmonary emphysema in mice rather than just altering its natural history by facilitating the installation of a low-grade systemic inflammatory milieu.
Subject terms: Chronic obstructive pulmonary disease, Experimental models of disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide and it may become the third leading cause of death by 2030 according to estimates of World Health Organization1. COPD is a syndrome that comprises several distinct and overlapping phenotypes characterized by airflow limitation and chronic respiratory symptoms2. COPD arises from an interplay between exposure to environmental factors and host factors such as genetics, airway hyperresponsiveness and poor lung development3. The main morphological alterations underlying COPD are emphysema, obstructive bronchiolitis, and, in many cases, chronic bronchitis. The relative participation of each pathological feature varies between patients2,3. Thus, many COPD patients shows a combination, in different proportions, of both “pink puffer” (more emphysema, lung hyperinflation, and dyspnea) and “blue bloater” (more chronic bronchitis, expectoration, higher body mass index, higher frequency of cardiovascular comorbidities) classic phenotypes2.
COPD affects not only the lungs. Muscle dysfunction and cachexia is found in many COPD patients. Also, several morbidities such as malignant neoplasms, coronary artery disease, arrhythmias, hypertension, congestive heart disease, diabetes and metabolic syndrome occur in greater frequency among COPD patients than in general population4,5. For this reason, COPD can be considered as a systemic disease. The coexisting diseases impair the functional status, the quality of life and the survival of COPD patients2,6. Two hypotheses have been proposed to explain the relationship between COPD and its extra-pulmonary manifestations and comorbidities. The first one considers the systemic manifestations and comorbidities as the result of a systemic “spill-over” of the inflammatory and reparatory events occurring in the lungs of patients with COPD, while in the second hypothesis the COPD is viewed as a systemic inflammatory disease that involves multiple organs with the pulmonary manifestations being one of its facets5. Both hypotheses predict that attenuation of persistent inflammation will decrease the severity of COPD and its extra-pulmonary manifestations despite their significant divergence regarding the primary source of the inflammatory events.
Treating COPD patients with statins – a class of cholesterol lowering agents that has anti-inflammatory properties7 – reduces all-cause mortality and mortality due to cancer, COPD, or cardiovascular disease as well as the frequency of COPD exacerbations8,9. Interestingly, high consumption of dietary fiber or fruits and vegetables is associated with lower risk of COPD among individuals that has a (current or former) history of cigarette smoking10,11, while a diet rich in fiber-containing foods is associated with better lung function irrespectively of smoking status12. These findings support the notion that attenuation of inflammatory events by either pharmacological or non-pharmacological approach might be beneficial to COPD patients. They also suggest that dietary factors, due to their effects on oxidative stress and inflammation, might modulate a person’s susceptibility to the known risk factors for COPD.
A possible dietary factor that might affect the lung function and clinical course of COPD is the content of fructose in the food. High-fructose corn syrup (HFCS) is used as a sweetening agent for industrialized food and beverages in several countries13,14. Rodents fed with a high fructose diet develop hallmarks of metabolic syndrome (MS) like elevation of serum triglycerides, increased blood pressure, decreased insulin sensitivity with hyperinsulinemia and altered secretion of very low-density lipoprotein15. As MS is characterized by a persistent low-grade systemic inflammation an important question is whether and how the use of fructose as a food addictive might affect lung function. Moreover, retrospective studies have shown that ingestion of HFCS sweetened beverage or beverages that have excess free fructose (EFF, fructose to glucose ratio larger than 1:1) is associated to the risk of asthma and chronic bronchitis16,17. In the present work, we used a mouse model of cigarette-induced emphysema in the context of fructose-feeding to assess how chronic fructose intake interacts with exposition to cigarette smoke and affects the development of pulmonary emphysema. Our results suggest that chronic fructose ingestion may cause emphysema in mice independently of cigarette smoking.
Results
Body weight gain
After 12 weeks, animals from all the groups showed an increase in the body weight. Ingestion of fructose caused largest increase while smoking was associated with smallest increase in weight gain. Weight gain of animals allocated to Smoke and Smoke + Fructose groups was significantly lower than weight of the littermate assigned to, respectively, Control and Fructose groups (Fig. 1).
Figure 1.
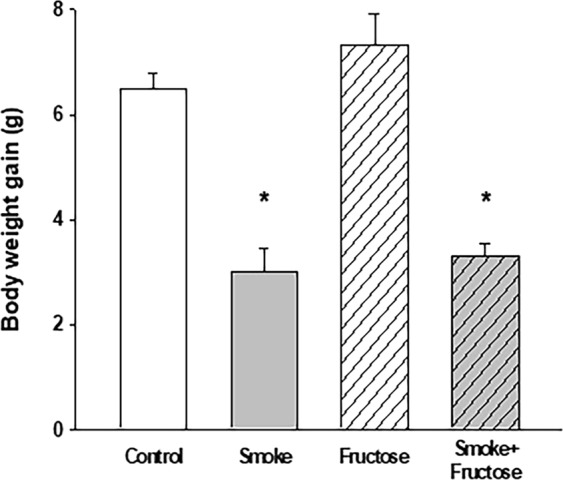
Body weight gain in grams of the mice after 12 weeks of the experimental protocols. Data are presented as mean and standard deviation. *p < 0.001 compared to the Control and Fructose groups.
Food, water and calorie intake
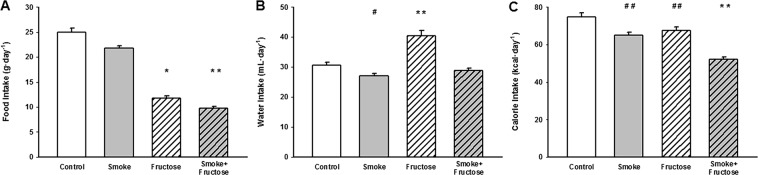
Exposure to either CS or fructose altered intake of food, fluid and calories. The Smoke group ingested less food than the Control group (Fig. 2A) while the Fructose group decreased food intake compared to either the Control or Smoke groups (Fig. 2A). The food intake was lower in the Smoke + Fructose group than either treatment alone (Fig. 2A). The water intake was higher in the Fructose group compared to the Control, Smoke or Smoke + Fructose groups (Fig. 2B). In contrast, the Smoke group ingested less water than the Control group (Fig. 2B). Both the Smoke and the Fructose groups showed a decreased calorie intake compared to the Control group (Fig. 2C). The calorie intake was lower in the Smoke + Fructose group than any treatment alone (Fig. 2C).
Figure 2.
Food intake (A), water intake (B) and calorie intake (C). Data are presented as mean and standard deviation. *p < 0.001 compared to the Control and Fructose groups. **p < 0.001 compared to the other groups. #p < 0.05 compared to the Control group. ##p < 0.01 compared to the Control group.
Respiratory mechanics
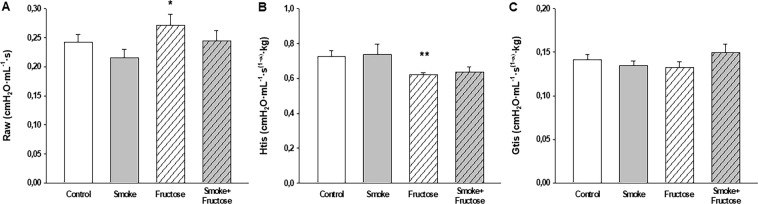
The Fructose group showed an increase in Raw compared to the Smoke group (Fig. 3A) and a decrease in Htis compared to the Control group (Fig. 3B). There were no significant differences in Gtis across the treatment groups (Fig. 3C).
Figure 3.
Airway resistance (Raw) (A), tissue elastance (Htis) (B) and tissue resistance (Gtis) (C) after 12 weeks of the experimental protocols. Data are presented as mean and standard deviation. *p < 0.05 compared to the Smoke group. **p < 0.05 compared to the Control group.
Cytological profile of BALF and lung tissue
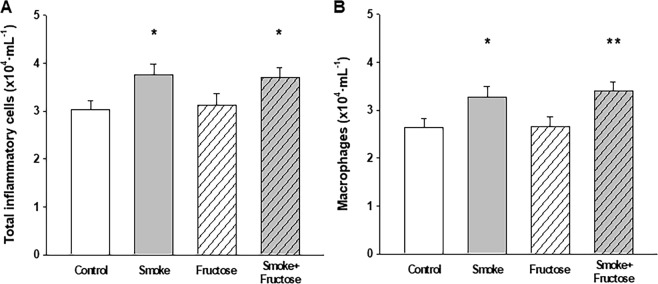
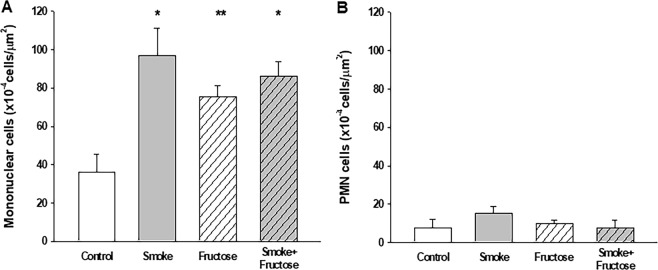
Exposure to CS increased the number of inflammatory cells in BALF. Total number of inflammatory cells was increased in both Smoke and Smoke + Fructose groups compared to the Control group (Fig. 4A). This increase was due the macrophages, which was increased in the Smoke group and in the Smoke + Fructose group (Fig. 4B). There were no significant differences in the number of neutrophils or lymphocytes in BALF across the groups (data not shown). Exposure to fructose did not alter the number of inflammatory cells in BALF. Exposure to either CS or fructose increased the number of mononuclear cells in lung parenchyma (Fig. 5A). There were no significant differences in the number of PMN cells in lung parenchyma among groups (Fig. 5B).
Figure 4.
Total number of inflammatory cells (A) and macrophages (B) in the BALF. Data are presented as mean and standard deviation. *p < 0.05 compared to the Control group. **p < 0.05 compared to the Control and Fructose groups.
Figure 5.
Mononuclear (A) and PMN (B) cells in the lung parenchyma. Data are presented as mean and standard deviation. *p ≤ 0.001 compared to the Control group. **p < 0.05 compared to the Control group.
Lung histology
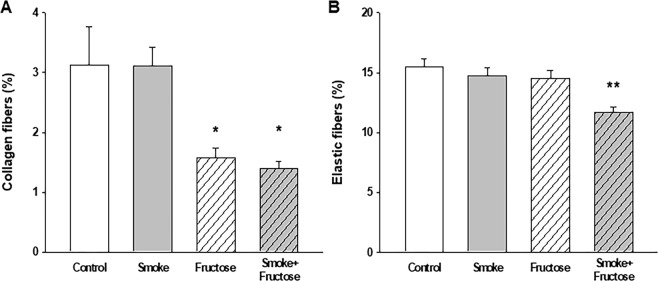
Exposure to either CS or fructose resulted in an increase in the Lm. Both the Smoke and the Fructose groups showed an increase in the Lm compared to the Control group (Fig. 6). Combination of CS and fructose increased even further the Lm (Fig. 6). Exposure to fructose decreased the content of collagen fibers in the lungs. Both the Fructose and Smoke + Fructose groups showed a decrease in the content of collagen fibers compared to the Control and Smoke groups (Fig. 7A). The Smoke + Fructose group also showed a decrease in the content of elastic fibers in the lungs (Fig. 7B).
Figure 6.
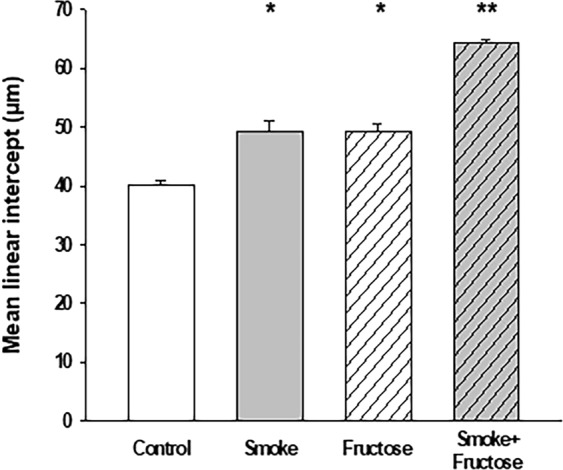
Mean linear intercept (Lm) after 12 weeks of the experimental protocols. Data are presented as mean and standard deviation. *p < 0.001 compared to the Control group. **p < 0.001 compared to the other groups.
Figure 7.
Percentage of collagen (A) and elastic (B) fibers in the lung parenchyma. Data are presented as mean and standard deviation. *p < 0.01 compared to the Control and Smoke groups. **p < 0.01 compared to the other groups.
Cytokines analyses in the BALF
The Smoke and Fructose groups showed an increase in BALF leptin levels compared to the Control group (Table 1). Fructose exposure, alone or in combination with the CS, increased the adiponectin levels (Table 1). The Smoke + Fructose group showed an increase in IL-1ra levels compared to the Control group (Table 1). There were no statistically significant differences in the levels of IL-10, IL-6, IL-1β and TNF-α in the BALF among groups (Table 1).
Table 1.
Cytokines levels in the BALF, blood plasma and muscle homogenate.
| Control | Smoke | Fructose | Smoke + Fructose | |
|---|---|---|---|---|
| BALF | ||||
| IL-10 | 15.131 ± 1.562 | 13.570 ± 1.831 | 18.220 ± 1.638 | 16.492 ± 1.638 |
| IL-6 | 14.492 ± 3,178 | 17.271 ± 3.553 | 15.683 ± 3.178 | 12.542 ± 3.178 |
| IL-1β | 122.571 ± 9.211 | 108,786 ± 10,800 | 119,868 ± 10,183 | 123,419 ± 9,660 |
| IL-1ra | 12,635 ± 0,766 | 14,374 ± 0,898 | 14,416 ± 0,803 | 16,524 ± 0,847a |
| TNF-α | 46,075 ± 2,779 | 50,683 ± 3,483 | 50,948 ± 3,072 | 52,908 ± 2,914 |
| Leptin | 133,994 ± 16,463 | 190,658 ± 20,637a | 184,198 ± 17,266a | 148,479 ± 18,200 |
| Adiponectin | 35,752 ± 55,555 | 32,683 ± 65,144 | 722,633 ± 61,418j | 375,217 ± 58,267b,f |
| Blood plasma | ||||
| IL-10 | 33,949 ± 28,212 | 71,319 ± 26,119 | 149,976 ± 24,432b | 197,309 ± 26,119c,e |
| IL-6 | 21,272 ± 6,483 | 10,365 ± 6,002 | 31,726 ± 5,614d | 32,079 ± 6,002d |
| IL-1β | 295,596 ± 30,458 | 270,963 ± 28,198 | 397,272 ± 26,377i | 310,096 ± 30,458 |
| IL-1ra | 274,438 ± 25,455 | 261,376 ± 23,567 | 190,470 ± 22,045a | 218,477 ± 23,567 |
| TNF-α | 24,409 ± 3,823 | 21,996 ± 3,539 | 24,230 ± 3,310 | 25,897 ± 3,539 |
| Leptin | 2149,409 ± 554,284 | 1596,220 ± 554,284 | 2615,494 ± 554,284 | 373,860 ± 592,555c,g |
| Adiponectin | 2013,072 ± 714,101 | 2262,935 ± 661,129 | 1819,221 ± 618,430 | 3388,850 ± 661,129 |
| Muscle homogenate | ||||
| IL-10 | 30,221 ± 11,010 | 29,343 ± 11,010 | 72,362 ± 11,010a,e | 99,504 ± 11,771b,f |
| IL-6 | 19,802 ± 4,106 | 22,779 ± 4,106 | 34,775 ± 4,106a | 38,319 ± 4,389b,d |
| IL-1β | 136,936 ± 7,387 | 133,055 ± 7,387 | 175,210 ± 7,387c,d | 182,130 ± 7,897c,f |
| IL-1ra | 257,697 ± 6,969 | 261,974 ± 6,969 | 252,811 ± 6,969 | 261,289 ± 7,451 |
| TNF-α | 20,020 ± 1,403 | 17,836 ± 1,403 | 23,786 ± 1,403d | 27,888 ± 1,499 b, f |
| Leptin | 1715,085 ± 700,797 | 1346,756 ± 700,797 | 6482,549 ± 749,183c,f | 4747,476 ± 749,183a,e |
| Adiponectin | 91,846 ± 84,009 | 82,094 ± 84,009 | 1148,719 ± 84,009c,f,h | 820,123 ± 89,809c,f |
Data are presented as mean and standard deviation. ap < 0.05 compared to the Control group; bp < 0.01 compared to the Control group; cp ≤ 0.001 compared to the Control group; dp < 0.05 compared to the Smoke group; ep < 0.01 compared to the Smoke group; fp < 0.001 compared to the Smoke group; gp ≤ 0.01 compared to the Fructose group; hp < 0.05 compared to the Smoke + Fructose group; ip < 0.05 compared to the other groups; jp < 0.001 compared to the other groups.
Cytokines analyses in the blood plasma
The plasma leptin levels in Smoke + Fructose group was decreased compared to the Control and Fructose groups (Table 1). Ingestion of fructose, with CS exposure or not, was associated with increased IL-10 levels (Table 1). Fructose exposure also increased the IL-6 levels (Table 1). The Fructose group showed an increase in IL-1β levels compared to all other groups (Table 1) and a decrease in IL-1ra levels compared to the Control group (Table 1). There were no statistically significant differences in the levels of adiponectin and TNF-α in the blood plasma among groups (Table 1).
Cytokines analyses in the muscle homogenate
Exposure to fructose increased leptin and adiponectin levels in muscle homogenate regardless of CS exposure (Table 1). Levels of IL-10 were increased in both Fructose and Smoke + Fructose groups compared to the Control and Smoke groups (Table 1). Fructose feeding, independently of CS exposure, elevated the levels of IL-6 in muscle homogenate (Table 1). Both Fructose and Smoke + Fructose groups showed an increase in IL-1β levels compared to the Control or the Smoke groups (Table 1). The Fructose group showed an increase in TNF-α levels compared to the Smoke group (Table 1). The Smoke + Fructose group showed an increase in TNF-α levels compared to the Control and Smoke groups (Table 1). There were no statistically significant differences in the levels of IL-1ra in the muscle homogenate among groups (Table 1).
Discussion
In the present study, we showed that chronic fructose loading promotes destruction and remodeling of lung parenchyma and impaired respiratory mechanics. Such alterations in the structure and function of lung parenchyma are associated with distinctive cytokine profiles in BALF, blood plasma and skeletal muscle. Furthermore, the combined effects of chronic fructose intake and CS on destruction of lung parenchyma are more pronounced than the effects of either treatment alone. These results suggest that excessive intake of fructose might be a cause of alveolar destruction and pulmonary emphysema in C57BL/6 mice rather than just playing an accessory role of creating a systemic inflammatory milieu, which might alter the natural history of emphysema induced by inhaled noxious stimuli. This is an unexpected finding given the previously reported relationship between excessive body weight or adiposity, insulin resistance and airflow limitation18–20. Our results complement reports from other authors16,17,21,22 and suggest that, in addition to airway hyperresponsiveness and chronic bronchitis, alveolar remodeling and emphysema might also be a deleterious consequence of chronic fructose exposure. The accumulating evidences of negative health effects of fructose, HFCS, and certain fruit drinks illustrate the importance of a deeper understanding on the relationship between dietary habits and noncommunicable disease.
The lung injury presented by our fructose-loaded animals bears similarities with the emphysema caused by CS exposure as both are characterized by increased Lm and Htis. However, unlike the CS-exposed mice in this study, our fructose-exposed mice present increased airway resistance and decreased content of collagen fiber in their alveolar septa. Previous work from our group have shown that C57BL/6 mice exposed to CS develop pulmonary emphysema without showing significant alterations in airway resistance23. Also, a study using specimens of human lung tissue24 and our group’s observations using mouse models of experimentally induced emphysema23,25 have shown that CS-induced emphysema is accompanied by an increase in the collagen content in alveolar wall. These specific features of fructose-associated pulmonary lesion suggest that fructose load and CS exposure rely on different pathophysiological mechanisms to produce alveolar lesion.
Besides the difference in airway resistance and collagen content of alveolar septa, our fructose-loaded mice present a distinctive profile of leukocytes in BALF and lung parenchyma when compared to CS-exposed littermates. Exposure to CS increases the number of inflammatory cells in BALF due to increased number of macrophages with no significant change in the number of neutrophils and lymphocytes. In contrast, fructose loading does not alter the number or composition of cells in BALF. It is noteworthy that both CS exposure and fructose loading causes an increase in the number of mononuclear cells that infiltrate the lung parenchyma. The difference between CS and fructose exposure in cytological pattern of BALF might be explained by differences in how CS and fructose exposure cause the inflammation in lung parenchyma. In the former the irritation caused by CS on airway epithelium and alveolar surface recruits inflammatory cells to lung parenchyma and alveolar space, while in the latter fructose ingestion causes inflammatory response elsewhere which is propagated to lung parenchyma causing the accumulation of mononuclear cells only in this tissue.
Except for an increased concentration of leptin in BALF mice exposed to CS have no significant change in cytokine profile. In contrast, the fructose-fed mice exhibit multiple alterations in cytokine profile. The increased concentrations of IL-10, IL-6, IL-1β and TNFα in muscle homogenate are probably markers of skeletal muscle inflammation caused by chronic fructose overload while the elevated accumulation of both leptin and adiponectin in this material can be viewed as a protective response to limit the deleterious effects of fructose-induced metabolic overload26. Similarly, elevated concentration of IL-10, IL-6, IL-1β and decreased concentration of IL-1ra in blood plasma might be markers of a fructose-induced systemic inflammatory state. Finally, high concentration of leptin and adiponectin in BALF of fructose-exposed mice might have a connection with accumulation of collagen in the alveolar septa as both have been reported to modulate the remodeling of extracellular matrix in cardiovascular tissues27,28.
One possible explanation for the link between fructose consumption and pulmonary emphysema is the involvement of the lungs by an inflammatory response initiated in an extra-pulmonary site by chronic fructose consumption. The liver is a natural candidate for the initiation site of fructose-triggered inflammatory state, since it is the major site for metabolism of orally ingested fructose29 and ingestion of fructose has been reported to cause hepatic stress response, activation of c-Jun N-terminal kinases and inflammatory response in rodents30. A competing explanation is the “intestinal enFruAGE hypothesis”31,32. According to this hypothesis, EFF in the ingested food or beverages promotes, due to fructose malabsorption, the formation of advanced glycation endproducts in the intestinal lumen which engage receptor for advanced glycation endproducts (RAGE) after being translocated into circulation. Inflammation elicited by activation of RAGE would be the mechanism underlying the association between the ingestion of foods with EFF and chronic inflammatory diseases. In support of this hypothesis, RAGE is highly expressed in lung and has been shown to play a central role in the pathogenesis of asthma33,34. Secondly, regular consumption of EFF beverages has been reported to be associated with childhood or adult asthma17,21, chronic bronchitis16, and arthritis35 whereas beverages that do not have EFF show no such associations. However, a study comparing mice fed with high-fat diet and mice fed with high-fructose diet seems to challenge the “intestinal enFruAGE hypothesis”, as airway hyperresponsiveness was observed in either model of MS, irrespectively of fructose ingestion22. Furthermore, fructose malabsorption - a phenomenon that is central to the “intestinal enFruAGE hypothesis” - may not occur in rodents as fructose malabsorption protects against accumulation of fat in liver36 and it is known that both mouse and rat develop liver steatosis after receiving fructose in drinking water for 8 weeks37. Finally, none of our fructose-fed mice displayed enlarged intestines – a gross morphological alteration presented by mice that have fructose malabsorption38 – on autopsy. Thus, it is unlikely that significant fructose malabsorption had occurred in our fructose-exposed mice.
Our study has some limitations; we did not evaluate the effects of fructose load and CS exposure on the oxidant-antioxidant balance and protease-antiprotease balance on the emphysema development. Therefore, our results do not allow us to assess the involvement of these mechanisms in the destruction of alveoli caused by fructose exposure. In addition, we did not assess how different dosages of fructose might alter the severity of lung emphysema and missed an opportunity to test the “intestinal enFruAGE hypothesis”. Furthermore, we cannot conclude whether the alveolar destruction is a specific effect of fructose ingestion or it can be produced by excessive loading of other monosaccharides as we did not include groups of animals to address this question. A sucrose consuming group should have also been included to assess similarities/differences in response to fructose fed group, since, according to “intestinal enFruAGE hypothesis”, the deleterious effects of fructose on the respiratory system would be related to the high fructose to glucose ratios, and not with the amount of ingested fructose. It would have been of interest if we included a group that mimic fructose malabsorption, i.e. GLUT5 knockout group. Future studies should be performed to clarify these points.
In summary, we have shown that chronic fructose intake promotes destruction and remodeling of lung parenchyma and impairment of respiratory mechanics, which is associated with distinctive cytokine profiles in BALF, blood plasma and skeletal muscle. The direct connection between chronic intake of a macronutrient and chronic respiratory disease reported in this study illustrates the importance of a deeper understanding on the relationship between dietary habits and noncommunicable disease.
Methods
Animal handling and experimental protocol
Six to eight weeks old male C57BL/6J mice were provided by the University of Sao Paulo School of Medicine’s Animal Facility. The animals were randomly allocated into one of four experimental groups (n = 8–10 per group): Control; Smoke; Fructose and Smoke + Fructose and treated accordingly for 12 weeks. The animals were kept in cages with four to five animals under a 12-h light/dark cycle and were given ad libitum access to food and water. Standard chow (2990 kcal/kg) was given as a solid diet. The mice assigned to fructose treatment were given a 20% (wt/vol) fructose (Lowçucar®, PR, Brazil) as drinking solution. Fructose or cigarette smoke (CS) exposure began on the same day.
Body weight (g) was recorded for each mouse once a month. Food (g·day−1) and water intake (ml·day−1) were assessed weekly. Total calorie intake (kcal·day−1) was calculated from the weight of food and the volume of fluid ingested.
This study was approved by the Ethics Committee of the School of Medicine of the University of Sao Paulo (Sao Paulo, Brazil, protocol 001/14) and all animal handling and experiments were performed according to the procedures approved at our institution.
Cigarette smoke exposure
Animals assigned to Smoke or Smoke + Fructose groups were exposed to CS for 30 minutes, twice·daily, 5 days·a week, for 12 weeks by using a custom-made smoking machine following previously published protocol23. The animals were exposed to 11 (±1) commercially filtered cigarettes (0.8 mg of nicotine, 10 mg of CO and 10 mg of tar per cigarette) per session in this study. Control and Fructose groups were exposed to room air.
Respiratory mechanics
Twenty-four hours after the end of the exposure protocols, animals were anaesthetized (50 mg·kg−1 intraperitoneal thiopental) tracheostomized and mechanically ventilated (FlexiVent, Scireq, Montreal, QC, Canada). Breathing efforts were abolished by pancuronium (0.2 mg·kg−1 intraperitoneal). The forced oscillatory technique and a constant phase model were used to obtain airway resistance (Raw), tissue damping (Gtis) and tissue elastance (Htis) parameters39. The values of Gtis and Htis were normalized by body weight40.
Blood plasma collection
The animals were exsanguinated via puncture of the abdominal aorta immediately after respiratory mechanics measurements. The blood was collected and centrifuged at 3000 g for 10 minutes at 5 °C. The plasma was stored at −80 °C for further analysis.
Bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) samples were collected after washing the lungs with 3 × 0.5 ml of sterile 0.9% saline and were centrifuged at 900 g for 10 minutes at 5 °C. The supernatant was collected and stored at −80 °C for further analysis and the cell pellet was resuspended in 300 μL of 0.9% saline. The cells were counted by using a Neubauer haemocytometer chamber (Carl Roth, Karlsruhe, Germany) and cytological examination was performed by examining 300 cells per slide under 1000X magnification after preparing BALF samples in cytocentrifuge slides and stained with Diff Quick (Medion Diagnostics, Dündingen, Switzerland)41.
Lung histology
For histological studies, lungs were removed and fixed at a constant pressure of 20 cm H2O for 24 h. Five-micrometers thick sections of lung tissue were stained with haematoxylin and eosin to evaluate the density of the polymorphonuclear (PMN) and mononuclear cells in the lung parenchyma42 and to measure the mean linear intercept (Lm), as described previously43. The content of collagen and elastic fibers were evaluated after staining, respectively, with Sirius-Red or Oxidate Weigert’s Resorcin-Fuchsin. The areas that were positive to the stains of collagen or elastic fibers was measured as described previously and expressed as a percentage of the total parenchyma area44.
Enzyme-linked immunosorbent assay (ELISA)
Tumour necrosis factor alpha (TNF-α), interleukin (IL)-6), IL-10, IL-1β, IL-1ra, leptin and adiponectin were assayed in BALF supernatant, blood plasma and skeletal muscle (quadriceps femori) homogenate using ELISA kits (eBioscience, San Diego, CA, USA), according to the manufacturer’s instructions as described previously. The intensity of colorimetric reaction was measured by absorbance at 450 nm and the results are expressed in pg/mL45.
Statistical analysis
Two-way analysis of variance was used to assess the effects of CS or fructose and Method of Holm-Sidak was used for pairwise multiple comparison. The analyses were performed using Sigma Stat 11 software (Systat Software, Inc., San Jose, CA, USA). A value of p < 0.05 was considered significant.
Acknowledgements
This work was supported by the following Brazilian Scientific Agencies: São Paulo Research Foundation (FAPESP) grant 2009/53904-9, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Laboratórios de Investigação Médica da Faculdade de Medicina da Universidade de São Paulo (LIM-HC-FMUSP). The opinions, hypothesis, conclusions and recommendations expressed in this manuscript are the responsibility of the authors and do not necessarily reflect the vision of FAPESP, CAPES and LIM-HC-FMUSP.
Author Contributions
C.L.S., A.C.T., R.P.V., M.A.M. and C.J.L. conception and design of research; C.L.S., A.C.T., C.R.O., F.M.A. and C.J.L. performed experiments, analyzed data and interpreted results of experiments; C.L.S., A.C.T. and C.J.L. prepared figures; C.L.S., A.C.T. and C.J.L. drafted the manuscript; critical revision and approved final version of manuscript - all authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chronic Obstructive Pulmonary Disease, http://www.who.int/respiratory/copd/burden/en/ (2018).
- 2.Barnes PJ, et al. Chronic obstructive pulmonary disease. Nature reviews. Disease primers. 2015;1:15076. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- 3.The Global Initiative for Chronic Obstructive Lung Disease (GOLD), http://goldcopd.org/ (2018).
- 4.Yin HL, et al. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: A meta-analysis. Medicine. 2017;96:e6836. doi: 10.1097/MD.0000000000006836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 6.Divo M, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 7.Diamantis E, Kyriakos G, Quiles-Sanchez LV, Farmaki P, Troupis T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Current cardiology reviews. 2017;13:209–216. doi: 10.2174/1573403X13666170426104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janda S, Park K, FitzGerald JM, Etminan M, Swiston J. Statins in COPD: a systematic review. Chest. 2009;136:734–743. doi: 10.1378/chest.09-0194. [DOI] [PubMed] [Google Scholar]
- 9.Cao C, et al. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: a systematic review and meta-analysis of observational research. Scientific reports. 2015;5:16461. doi: 10.1038/srep16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaluza J, Larsson SC, Orsini N, Linden A, Wolk A. Fruit and vegetable consumption and risk of COPD: a prospective cohort study of men. Thorax. 2017;72:500–509. doi: 10.1136/thoraxjnl-2015-207851. [DOI] [PubMed] [Google Scholar]
- 11.Kaluza J, Harris H, Wallin A, Linden A, Wolk A. Dietary Fiber Intake and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Epidemiology. 2018;29:254–260. doi: 10.1097/EDE.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 12.Hanson C, et al. The Relationship between Dietary Fiber Intake and Lung Function in the National Health and Nutrition Examination Surveys. Ann Am Thorac Soc. 2016;13:643–650. doi: 10.1513/AnnalsATS.201509-609OC. [DOI] [PubMed] [Google Scholar]
- 13.Malik VS, Hu FB. Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. Journal of the American College of Cardiology. 2015;66:1615–1624. doi: 10.1016/j.jacc.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Akinbami LJ, McGuire LC, Blanck HM. Association of sugar-sweetened beverage intake frequency and asthma among U.S. adults, 2013. Preventive medicine. 2016;91:58–61. doi: 10.1016/j.ypmed.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley GL, Allan G, Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology. 2004;145:548–555. doi: 10.1210/en.2003-1167. [DOI] [PubMed] [Google Scholar]
- 16.DeChristopher LR, Uribarri J, Tucker KL. Intake of high fructose corn syrup sweetened soft drinks is associated with prevalent chronic bronchitis in USAdults, ages 20-55 y. Nutrition journal. 2015;14:107. doi: 10.1186/s12937-015-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeChristopher LR, Uribarri J, Tucker KL. Intakes of apple juice, fruit drinks and soda are associated with prevalent asthma in US children aged 2-9 years. Public health nutrition. 2016;19:123–130. doi: 10.1017/S1368980015000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rastogi D, Bhalani K, Hall CB, Isasi CR. Association of pulmonary function with adiposity and metabolic abnormalities in urban minority adolescents. Ann Am Thorac Soc. 2014;11:744–752. doi: 10.1513/AnnalsATS.201311-403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Huisstede A, et al. Systemic inflammation and lung function impairment in morbidly obese subjects with the metabolic syndrome. J Obes. 2013;2013:131349. doi: 10.1155/2013/131349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136:304–311.e308. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeChristopher LR, Tucker KL. Excess free fructose, high-fructose corn syrup and adult asthma: the Framingham Offspring Cohort. The British journal of nutrition. 2018;119:1157–1167. doi: 10.1017/S0007114518000417. [DOI] [PubMed] [Google Scholar]
- 22.Singh VP, et al. Metabolic Syndrome Is Associated with Increased Oxo-Nitrative Stress and Asthma-Like Changes in Lungs. PLoS One. 2015;10:e0129850. doi: 10.1371/journal.pone.0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toledo AC, et al. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. European Respiratory Journal. 2012;39:254–264. doi: 10.1183/09031936.00003411. [DOI] [PubMed] [Google Scholar]
- 24.Lang MR, et al. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994;49:319–326. doi: 10.1136/thx.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopes FD, et al. A comparative study of extracellular matrix remodeling in two murine models of emphysema. Histol Histopathol. 2013;28:269–276. doi: 10.14670/HH-28.269. [DOI] [PubMed] [Google Scholar]
- 26.Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes (Lond) 2005;29:1175–1183. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 27.Jenke Alexander, Schur Robert, Röger Carsten, Karadeniz Zehra, Grüger Mathias, Holzhauser Luise, Savvatis Kostas, Poller Wolfgang, Schultheiss Heinz-Peter, Landmesser Ulf, Skurk Carsten. Adiponectin attenuates profibrotic extracellular matrix remodeling following cardiac injury by up-regulating matrix metalloproteinase 9 expression in mice. Physiological Reports. 2017;5(24):e13523. doi: 10.14814/phy2.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Martínez E, et al. The lysyl oxidase inhibitor (β-aminopropionitrile) reduces leptin profibrotic effects and ameliorates cardiovascular remodeling in diet-induced obesity in rats. J Mol Cell Cardiol. 2016;92:96–104. doi: 10.1016/j.yjmcc.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 30.Basaranoglu M, Basaranoglu G, Sabuncu T, Sentürk H. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol. 2013;19:1166–1172. doi: 10.3748/wjg.v19.i8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeChristopher LR. Excess free fructose and childhood asthma. Eur J Clin Nutr. 2015;69:1371. doi: 10.1038/ejcn.2015.101. [DOI] [PubMed] [Google Scholar]
- 32.Gugliucci A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv Nutr. 2017;8:54–62. doi: 10.3945/an.116.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brett J, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 34.Milutinovic PS, Alcorn JF, Englert JM, Crum LT, Oury TD. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis. The American journal of pathology. 2012;181:1215–1225. doi: 10.1016/j.ajpath.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeChristopher LR, Uribarri J, Tucker KL. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20-30 years. Nutr Diabetes. 2016;6:e199. doi: 10.1038/nutd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker RW, et al. High rates of fructose malabsorption are associated with reduced liver fat in obese African Americans. J Am Coll Nutr. 2012;31:369–374. doi: 10.1080/07315724.2012.10720445. [DOI] [PubMed] [Google Scholar]
- 37.Van Herck Mikhaïl, Vonghia Luisa, Francque Sven. Animal Models of Nonalcoholic Fatty Liver Disease—A Starter’s Guide. Nutrients. 2017;9(10):1072. doi: 10.3390/nu9101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barone S, et al. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem. 2009;284:5056–5066. doi: 10.1074/jbc.M808128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol (1985. 1992;72:168–178. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 40.Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol (1985) 2000;89:908–916. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- 41.Ramos DS, et al. Low-intensity swimming training partially inhibits lipopolysaccharide-induced acute lung injury. Med Sci Sports Exerc. 2010;42:113–119. doi: 10.1249/MSS.0b013e3181ad1c72. [DOI] [PubMed] [Google Scholar]
- 42.Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- 43.Takubo Y, et al. Alpha1-antitrypsin determines the pattern of emphysema and function in tobacco smoke-exposed mice: parallels with human disease. Am J Respir Crit Care Med. 2002;166:1596–1603. doi: 10.1164/rccm.2202001. [DOI] [PubMed] [Google Scholar]
- 44.Prado CM, et al. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2006;35:457–465. doi: 10.1165/rcmb.2005-0391OC. [DOI] [PubMed] [Google Scholar]
- 45.Mackenzie B, et al. Dendritic Cells Are Involved in the Effects of Exercise in a Model of Asthma. Med Sci Sports Exerc. 2016;48:1459–1467. doi: 10.1249/MSS.0000000000000927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.