Abstract
Invasive lobular carcinoma (ILC) is an understudied subtype of breast cancer that requires novel therapies in the advanced setting. To study acquired resistance to endocrine therapy in ILC, we have recently performed RNA-Sequencing on long-term estrogen deprived cell lines and identified FGFR4 overexpression as a top druggable target. Here, we show that FGFR4 expression also increases dramatically in endocrine-treated distant metastases, with an average fold change of 4.8 relative to the paired primary breast tumor for ILC, and 2.4-fold for invasive ductal carcinoma (IDC). In addition, we now report that FGFR4 hotspot mutations are enriched in metastatic breast cancer, with an additional enrichment for ILC, suggesting a multimodal selection of FGFR4 activation. These data collectively support the notion that FGFR4 is an important mediator of endocrine resistance in ILC, warranting future mechanistic studies on downstream signaling of overexpressed wild-type and mutant FGFR4.
Subject terms: Breast cancer, Metastasis
Introduction
Invasive lobular carcinoma (ILC) is a common histological subtype, accounting for 10–15% of all breast cancer diagnoses. Since most of these tumors are estrogen receptor positive (ER+), patients with ILC are often treated with endocrine therapy. Although these treatments are highly efficacious for most patients initially, long-term recurrences remain a major clinical problem for ILC.1,2 We have recently performed RNA-Sequencing on paired, metachronous primary, and metastatic tumors to the brain and bone.3,4 Here, we perform a subset analysis on the previously published clinical data, focusing only on ER+ patients treated with endocrine therapy prior to their recurrence, as well as report additional FGFR4 expression data from paired gastrointestinal (GI) and ovarian metastases.5
Results
FGFR4 overexpression in endocrine-treated cell lines
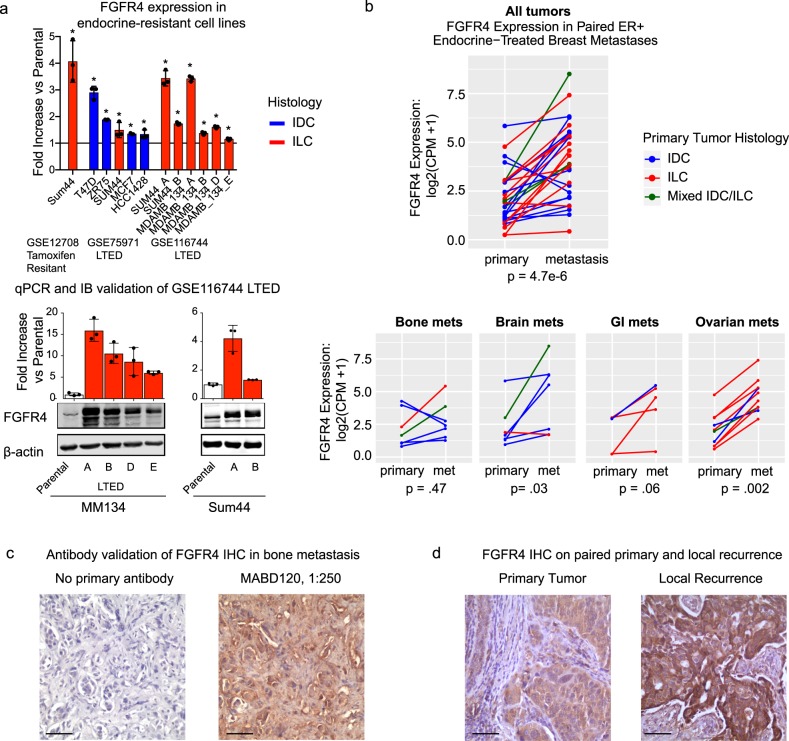
To model acquired resistance to endocrine therapy in the laboratory, we and others have recently performed RNA-Sequencing on long-term estrogen deprived (LTED) cell lines (GSE1167446 and GSE759717) or microarray analysis on tamoxifen resistant cell lines (GSE127088). FGFR4 is overexpressed in 8/8 ILC cell line models and 4/4 invasive ductal carcinoma (IDC) cell line models at the RNA level relative to parental cells subjected to short-term estrogen deprivation (Fig. 1a, top panel). Importantly, the FGFR4 overexpression in our ILC LTED cells was also observed relative to parental cells in full serum, at the RNA and protein level (Fig. 1a, bottom panel).
Fig. 1.
FGFR4 expression is elevated in cell lines and patient samples treated with endocrine therapy. a Top, FGFR4 RNA expression fold-change in long-term endocrine-resistant cell line models of ER+ breast cancer, relative to parental cell lines with short-term estrogen deprivation. From left to right, tamoxifen-resistant cells from GSE12708, long-term estrogen deprived (LTED) cells from GSE75971, LTED cells from GSE116744. *p < 0.05 for differential expression versus parental, corrected for multiple comparisons with Benjamini–Hochberg. Bottom, qRT-PCR and immunoblot comparison of FGFR4 expression in MM134 and SUM44 LTED cells, relative to parental cells grown in FBS. Error bars represent ± SD for three biological replicates. Red bars represent ILC and blue bars represent IDC. b Top, FGFR4 expression gain in 29 ER+ paired tumors. Bottom, FGFR4 expression gain in the tumors separated by site of metastasis (met). Red lines represent primary tumor histology of ILC, blue lines represent IDC, and green lines represent mixed IDC/ILC tumors. Two-sided paired Wilcoxon rank tests were used to calculate p values for FGFR4 gain. c IHC staining of an orphan bone metastasis (left, no primary antibody. right, FGFR4 (MABD120, 1:250 dilution)). d IHC staining of FGFR4 (MABD120, 1:250 dilution) in a paired primary breast tumor and endocrine-treated local recurrence. Scale bars represent 100 µm. See Supplementary Material for additional antibody validation
FGFR4 overexpression in endocrine-treated metastases
To investigate the clinical relevance of this finding, FGFR4 RNA expression was next examined in our previous and ongoing studies of paired primary and metastatic tumors. In this subset analysis, we focus on the patients with ER+ primary tumors who received endocrine therapy prior to the recurrence of bone,3 brain,4 or GI/ovarian metastases.5 From a total of 26 patients, we collected treatment-naïve primary tumors and 29 endocrine-treated metastases, consisting of 7 bone, 7 brain, 5 GI, and 10 ovarian metastases. The average time to recurrence from primary to matched metastasis was 59 months. Our study cohorts were enriched for ILC, and this subset analysis consists of a histological distribution of 13 IDCs, 13 ILCs, and 3 cases of mixed IDC/ILC. Overall, 26/29 (90%) metastases have an increase in FGFR4 RNA relative to their matched primary tumor (p = 4.7e−6), including 19/29 (66%) with a fold change >2 (Fig. 1b, top panel). Of note, there were patients with large gains in FGFR4 across all four distant metastatic sites studied, with significant gains in brain and ovarian metastases (Fig. 1b, bottom panel). Because of the small sample size for each metastatic site, there is no significant difference for FGFR4 expression gain by tumor site or histological type, nor is there an interaction effect (p > 0.05 for all three tests by two-way ANOVA). However, there is a trend for increased FGFR4 gain in ILC, with a mean increase of 4.8-fold for the ILCs versus 2.4-fold for the IDCs. We have validated an antibody for immunohistochemistry (IHC) detection of FGFR4 protein expression and have preliminary data for staining primary and recurrent breast tissue (Supplementary Material, Fig. 1c, d).
FGFR4 hotspot mutations are enriched in metastatic ER+ ILC
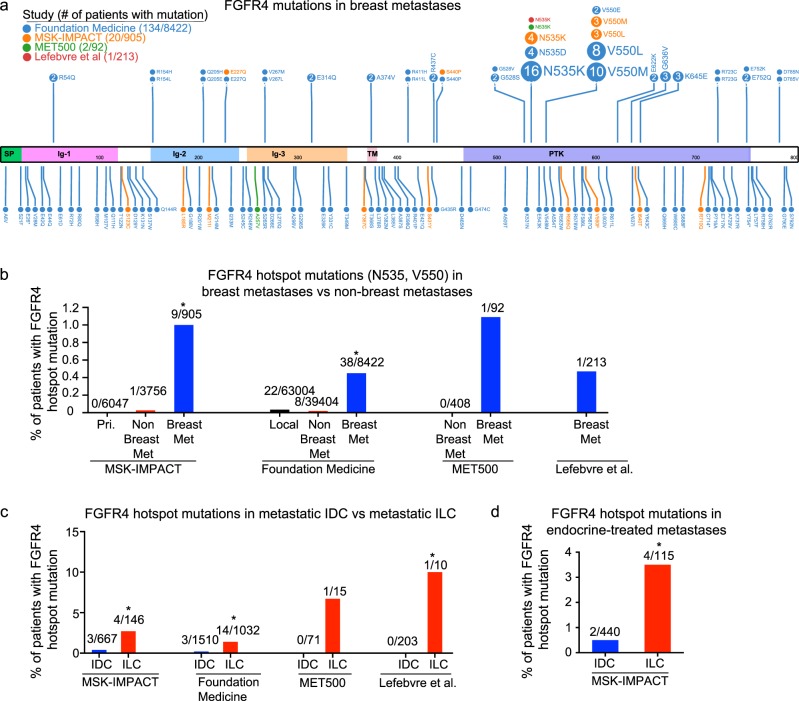
Next, the rate of FGFR4 mutations in metastatic cancer was examined in all patients from three recent sequencing studies: MSK-IMPACT,9,10 MET500,11 and Lefebvre et al.,12 as well as from sequencing data from Foundation Medicine. Figure 2a shows the distribution of FGFR4 mutations in these studies, with the most frequently mutated sites being the FGFR4 hotspot mutations previously identified in rhabdomyosarcomas (N535 and V550).13–15 Although FGFR4 hotspot mutations are rarely detected in primary tumors (<0.05%), they are present in ~0.5–1% of breast metastases, significantly enriched relative to nonbreast metastases (~0.02%) (Fig. 2b). In addition, these hotspot mutations are enriched significantly in metastatic ILC relative to metastatic IDC (Fig. 2c). Treatment data is only available for the MSK-IMPACT data, which shows that 8/9 patients with FGFR4 hotspot mutations were previously treated with endocrine therapy (Supplementary Data 2). The total rate of FGFR4 hotspot mutations in patients with endocrine-treated metastases is 3.5% for ILC versus 0.5% for IDC (Fig. 2d). There are no mutations or copy-number alterations that significantly co-occur or are mutually exclusive with the FGFR4 hotspot mutations in patients with endocrine-treated metastases (q > 0.1).
Fig. 2.
FGFR4 hotspot (N535 and V550) mutations are enriched in metastatic ILC. a Lollipop plot of FGFR4 mutations generated using ProteinPaint.31 Top: all mutations appearing at least twice. b FGFR4 hotspot (N535 and V550) mutations in MSK-IMPACT primary (pri.), nonbreast metastatic (met), and breast metastatic tumors, Foundation Medicine local, nonbreast metastatic, and breast metastatic tumors, MET500 nonbreast and breast metastatic, and Lefebvre et al. breast metastatic tumors. *FGFR4 hotspot mutations are enriched in breast metastatic tumors versus nonbreast metastatic tumors (MSK-IMPACT OR: 38.7, Fisher exact p = 5.8e-6, Foundation Medicine OR = 22.3, Fisher exact p < 2.2e−16). c FGFR4 hotspot mutations in metastatic ILC versus metastatic IDC. *FGFR4 hotspot mutations are enriched in ILC (MSK-IMPACT: OR = 6.2, p = 0.02, Foundation Medicine: OR = 6.9, p < 0.0007, Lefebvre et al.: OR = Inf., p = 0.05). d FGFR4 hotspot mutations in endocrine-treated metastatic ILC versus metastatic IDC. *FGFR4 hotspot mutations are enriched in ILC (MSK-IMPACT: OR = 7.9, p = 0.02)
Discussion
In our analyses of cell line models of acquired endocrine resistance, as well as clinical samples from pre- and post-endocrine treatment, we find that FGFR4 overexpression is a remarkably common phenomenon. FGFR4 overexpression in endocrine-resistant cell lines is seen relative to parental cells subjected to short-term estrogen deprivation, suggesting that the FGFR4 gains are not an artifact of estrogen loss. FGFR4 overexpression is also seen relative to parental cells growing in full serum, suggesting that FGFR4 is not simply a marker of proliferation. Instead, FGFR4 overexpression may represent a long-term signaling adaption in tumor cells following endocrine therapy. Studies are currently ongoing to assess possible causes of FGFR4 overexpression, the functional impact of FGFR4 inhibition, and the signaling mechanisms downstream of FGFR4. Recently, FGFR4 has been shown to activate AKT16 and inhibit MST1/217 signaling in breast cancer cells, but the role of FGFR4 in ILC remains uncertain. Given that recent studies show that loss of E-cadherin expression can directly drive increased growth-factor receptor signaling in ILC, future studies will examine the interaction between E-cadherin and FGFR4.18,19
In our analyses of clinical specimens, we observed that the large gains in FGFR4 spanned all four distant metastatic sites. This data, as well as the fact that the brain, GI, and ovarian metastases underwent macrodissection prior to RNA extraction, suggest that the gains in FGFR4 are a result of overexpression within tumor cells. Tumor cell expression of FGFR4 is confirmed in our preliminary IHC analysis, but more samples are needed to fully assess the correlation between RNA and protein levels in metastatic tissue and the contribution of FGFR4 RNA from stromal cells.
The MSK-IMPACT data include copy-number analysis, which finds a low rate of FGFR4 DNA amplification (2/596, 0.3%) in endocrine-treated metastases, suggesting that copy-number gains are unlikely to account for the high rate of overexpression seen in our paired samples. Additional studies that contain both DNA and RNA analysis will be needed to assess if mutated FGFR4 is overexpressed.
Because of the low rate of mutations, copy number amplifications, and fusions of FGFR4 identified in previous studies, FGFR4 has been understudied in clinical trials relative to the other FGFR family members.20 However, there are clinical trials with novel pan-FGFR inhibitors that have high potency for wild-type and/or mutated FGFR421,22 (NCT03238196), as well as at least four ongoing clinical trials with FGFR4-specific small molecules (NCT02325739, NCT02834780, NCT03144661, and NCT02508467). These FGFR4-specific inhibitors exhibit their specificity by interacting with a cysteine residue near the hotspot mutations, suggesting that although they are appropriate for wild-type overexpression of FGFR4, modifications would likely be needed to treat patients with hotspot mutations.23 Recent studies show that FGFR1 amplification may play a role in endocrine resistance, and that combined FGFR1 and CDK4/6 inhibition can reverse this phenotype.24,25 Future studies of resistance to combined endocrine therapy and CDK4/6 inhibition would benefit from evaluating FGFR4 overexpression and mutations as potential resistance factors, particularly for patients with lobular carcinoma.
Methods
RNA-Sequencing
RNA extraction and sequencing for the GI and ovarian metastases was performed as previously described for our brain and bone metastases cohorts.3,4 Briefly, biospecimens were reviewed by a trained molecular pathologist to confirm pathology, quantify tumor cellularity, and to highlight regions of relatively high tumor cellularity for macrodissection. RNA was extracted from FFPE tissue using Qiagen’s All-Prep Kit, and library preparation performed using Illumina’s TruSeq RNA Access Library Preparation protocol. Transcript counts from all samples were quantified with Salmon26 v.0.8.2 and converted to gene-level counts with tximport.27 The gene-level counts from all studies were then normalized together using TMM with edgeR.28 Log2 transformed TMM-normalized counts per million: log2 (TMM-CPM+1) expression values were used for the analysis. Collection and analysis of specimens was approved under the University of Pittsburgh (distant metastases) and Charite Universitaetsmedizin Berlin IRB (paired local recurrence) guidelines. Requirement for informed consent was waived, considering all samples were de-identified, there was no more than minimal risk to human subjects, and all tissue was obtained as part of routine clinical care.
Cell culture and reagents
MDA-MB-134VI (MM134) (American Type Culture Collection [ATCC], Manassas, VA, USA) and SUM44/F (Asterand Bioscience, Detroit, MI, USA) cells were maintained in 1:1 DMEM (11965; Life Technologies, Carlsbad, CA, USA): L-15 (11415, Life Technologies) +10% fetal bovine serum (FBS) (26140; Life Technologies).6 LTED cell lines were maintained in IMEM (A10488; Life Technologies. Richter’s modification, no Phenol Red, no Gentamycin) +10% charcoal-stripped FBS. The following primers were used for qRT-PCR: FGFR4: 5′-tgcagaatctcaccttgattaca-3′, 5′-ggggtaactgtgcctattcg-3′, RPLP0: 5′-taaaccctgcgtggcaatc-3′, 5′-ttgtctgctcccacaatgaaa-3′. FGFR4 expression was normalized to RPLP0 for each of three biological replicates, before calculating fold-change relative to parental cell lines in full serum conditions. For IB, FGFR4 antibody sc-124 (Santa Cruz) was used at a 1:1000 dilution, and beta-actin (Sigma) at 1:10,000. Blots were imaged on the Olympus LI-COR system.
FGFR4 IHC
For IHC, FGFR4 antibody MABD120 (Millipore Sigma) was used at a 1:250 dilution after antigen retrieval using heated citrate buffer, pH 6.0. Staining was detected using Envision Dual Link+ HRP Polymer and DAB (Dako). FGFR4 IHC was performed on an ER+ IDC bone metastasis collected from the University of Pittsburgh, and a paired ER+ IDC primary tumor and metachronous local recurrence collected from Charite Universitaetsmedizin Berlin. The bone metastasis was detected 41 months following primary tumor diagnosis and treatment with chemotherapy, trastuzamab, and anastrozole. The local recurrence was detected 37 months following primary tumor diagnosis and treatment with chemotherapy, trastuzamab, and tamoxifen. Additional antibody validation of MABD120 is described in the Supplementary Material, using cell lines and 22 primary ER+ ILCs.
FGFR4 hotspot mutation rates
The FGFR4 hotspots (N535 and V550) were queried in MSK-IMPACT and the Lefebvre et al. study using the cBio portal,29 and MET500 using the MET500 portal (https://met500.path.med.umich.edu). MSK-IMPACT contains designations for primary and metastatic tumors, whereas Foundation Medicine contains designations for local (including local recurrences) and metastatic tumors. In all cases, lymph node metastases and distant recurrences were grouped together. For analysis of mutation rate in the Foundation Medicine and Lefebvre et al. studies, tumors of unspecified histology with a CDH1 mutation or homozygous deletion in CDH1 were classified as ILC. Approval for use of the Foundation Medicine data was obtained from the Western Institutional Review Board (Protocol no. 20152817).
Statistical considerations
GraphPad Prism software version 7, and R version 3.4.1 were used for statistical analysis. All tests were two-tailed, with p < 0.05 considered statistically significant. Paired Wilcoxon rank signed tests were used for expression gains in metastases. Fisher’s exact tests were used to quantify odds-ratios and significance for enrichment of FGFR4 hotspot mutations.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This project benefited from resources provided by the University of Pittsburgh HSCRF Genomics Research Core and Health Sciences Tissue Bank, the UPMC Hillman Cancer Center Tissue and Research Pathology Services supported in part by National Institutes of Health award number P30CA047904, the University of Pittsburgh Center for Research Computing and the University of Pittsburgh Center for Simulation and Modeling. The authors would like to thank the patients who contributed samples to this study, and Jian Chen for outstanding support of the study. This work was supported by the Breast Cancer Research Foundation [A.V.L. and S.O.]; Susan G. Komen Scholar awards [SAC110021 to A.V.L., SAC160073 to S.O.]; the Metastatic Breast Cancer Network [S.O.]; the National Cancer Institute [R01CA224909 to S.O., 5F30CA203154 to K.M.L., 5F30CA203095 to N.P.]; and the Fashion Footwear Association of New York, Magee-Women’s Research Institute and Foundation, Nicole Meloche Foundation, Penguins Alumni Foundation, and the Shear Family Foundation. S.O. and A.V.L. are Hillman Fellows. Research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM008208 (K.M.L. and N.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
Study concept and design (K.M.L., S.O.); acquisition, analysis, or interpretation of data (all authors); drafting of the paper (K.M.L., S.O.); critical revision of the manuscript for important intellectual content (all authors); administrative, technical, or material support (N.P., A.B., N.T., M.J.S., E.S.S., R.J.H., K.D., N.Z.A., R.J.W., K.R.W., J.U.B., C.D., A.M., M.M.K., M.M.B., E.E. and P.C.L.).
Data availability
The data generated and analyzed during this study are described in the following data record30: 10.6084/m9.figshare.7704371. Clinicopathologic data and FGFR4 expression for matched primary:metastatic tumors studied are available in Supplementary Data 1. Clinicopathologic data and FGFR4 hotspot mutation allele frequencies from MSK-IMPACT are available in Supplementary Data 2. Additional validation for IHC antibody and additional data comparing RNA and protein expression are available in the Supplementary Material. Raw RNA-Seq data for the paired primary and metastatic samples are not published openly in order to protect participant identities, but will be made available upon request and under regulatory compliance via a data usage agreement (DUA). For all RNA-Seq samples, the transcript counts processed via Salmon are available at https://github.com/leeoesterreich.
Competing interests
R.J.H. and E.S.S. were employees of and had ownership interest in Foundation Medicine Inc at the time of data generation. Remaining authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies the paper on the npj Breast Cancer website (10.1038/s41523-019-0114-x).
References
- 1.Pestalozzi BC, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J. Clin. Oncol. 2008;26:3006–3014. doi: 10.1200/JCO.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 2.Metzger Filho O, et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 Trial. J. Clin. Oncol. 2015;33:2772–2779. doi: 10.1200/JCO.2015.60.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priedigkeit N, et al. Exome-capture RNA sequencing of decade-old breast cancers and matched decalcified bone metastases. JCI Insight. 2017;2:95703. doi: 10.1172/jci.insight.95703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varešlija Damir, Priedigkeit Nolan, Fagan Ailís, Purcell Siobhan, Cosgrove Nicola, O’Halloran Philip J, Ward Elspeth, Cocchiglia Sinéad, Hartmaier Ryan, Castro Carlos A, Zhu Li, Tseng George C, Lucas Peter C, Puhalla Shannon L, Brufsky Adam M, Hamilton Ronald L, Mathew Aju, Leone Jose P, Basudan Ahmed, Hudson Lance, Dwyer Róisín, Das Sudipto, O’Connor Darran P, Buckley Patrick G, Farrell Michael, Hill Arnold D K, Oesterreich Steffi, Lee Adrian V, Young Leonie S. Transcriptome Characterization of Matched Primary Breast and Brain Metastatic Tumors to Detect Novel Actionable Targets. JNCI: Journal of the National Cancer Institute. 2018;111(4):388–398. doi: 10.1093/jnci/djy110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basudan A, et al. Frequent ESR1 and CDK pathway copy-number alterations in metastatic breast cancer. Mol. Cancer Res. 2019;17:457–468. doi: 10.1158/1541-7786.MCR-18-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikora MJ, et al. WNT4 mediates estrogen receptor signaling and endocrine resistance in invasive lobular carcinoma cell lines. Breast Cancer Res. 2016;18:92. doi: 10.1186/s13058-016-0748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simigdala N, et al. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016;18:58. doi: 10.1186/s13058-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riggins RB, et al. ERRgamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res. 2008;68:8908–8917. doi: 10.1158/0008-5472.CAN-08-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zehir A, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razavi P, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–438.e6. doi: 10.1016/j.ccell.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson DR, et al. Integrative clinical genomics of metastatic cancer. Nature. 2017;548:297–303. doi: 10.1038/nature23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefebvre C, et al. Mutational profile of metastatic breast cancers: a retrospective analysis. PLoS Med. 2016;13:e1002201. doi: 10.1371/journal.pmed.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor JG, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J. Clin. Invest. 2009;119:3395–3407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li SQ, et al. Targeting wild-type and mutationally activated FGFR4 in rhabdomyosarcoma with the inhibitor ponatinib (AP24534) PLoS ONE. 2013;8:e76551. doi: 10.1371/journal.pone.0076551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shern JF, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, et al. FGFR4 provides the conduit to facilitate FGF19 signaling in breast cancer progression. Mol. Carcinog. 2018;57:1616–1625. doi: 10.1002/mc.22884. [DOI] [PubMed] [Google Scholar]
- 17.Turunen, S. P. et al. FGFR4 phosphorylates MST1 to confer breast cancer cells resistance to MST1/2-dependent apoptosis. Cell Death Differ. 10.1038/s41418-019-0321-x (2019). [DOI] [PMC free article] [PubMed]
- 18.Nagle, A. M. et al. Loss of E-cadherin enhances IGF1-IGF1R pathway activation and sensitizes breast cancers to anti-IGF1R/InsR inhibitors. Clin. Cancer Res. 10.1158/1078-0432.CCR-18-0279 (2018). [DOI] [PMC free article] [PubMed]
- 19.Teo K, et al. E-cadherin loss induces targetable autocrine activation of growth factor signalling in lobular breast cancer. Sci. Rep. 2018;8:15454. doi: 10.1038/s41598-018-33525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 21.Perera TPS, et al. Discovery and pharmacological characterization of JNJ-42756493 (Erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol. Cancer Ther. 2017;16:1010–1020. doi: 10.1158/1535-7163.MCT-16-0589. [DOI] [PubMed] [Google Scholar]
- 22.Wu Daichao, Guo Ming, Min Xiaoli, Dai Shuyan, Li Meixiang, Tan Sijie, Li Guoqing, Chen Xiaojuan, Ma Yao, Li Jun, Jiang Longying, Qu Lingzhi, Zhou Zhan, Chen Zhuchu, Chen Lin, Xu Guangyu, Chen Yongheng. LY2874455 potently inhibits FGFR gatekeeper mutants and overcomes mutation-based resistance. Chemical Communications. 2018;54(85):12089–12092. doi: 10.1039/C8CC07546H. [DOI] [PubMed] [Google Scholar]
- 23.Hagel M, et al. First selective small molecule inhibitor of FGFR4 for the treatment of hepatocellular carcinomas with an activated FGFR4 signaling pathway. Cancer Discov. 2015;5:424–437. doi: 10.1158/2159-8290.CD-14-1029. [DOI] [PubMed] [Google Scholar]
- 24.Giltnane JM, et al. Genomic profiling of ER+breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci. Transl. Med. 2017;9:eaai7993. doi: 10.1126/scitranslmed.aai7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Formisano L, et al. Association of FGFR1 with ERα maintains ligand-independent ER transcription and mediates resistance to estrogen deprivation in ER+breast cancer. Clin. Cancer Res. 2017;23:6138–6150. doi: 10.1158/1078-0432.CCR-17-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. [version 2; peer review: 2 approved] F1000Res. 2015;4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine, K. M. et al. Metadata and data files supporting “FGFR4 overexpression and hotspot mutations in metastatic ER+breast cancer are enriched in the lobular subtype”. figshare. at 10.6084/m9.figshare.7704371 (2019). [DOI] [PMC free article] [PubMed]
- 31.Zhou X, et al. Exploring genomic alteration in pediatric cancer using ProteinPaint. Nat. Genet. 2016;48:4–6. doi: 10.1038/ng.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analyzed during this study are described in the following data record30: 10.6084/m9.figshare.7704371. Clinicopathologic data and FGFR4 expression for matched primary:metastatic tumors studied are available in Supplementary Data 1. Clinicopathologic data and FGFR4 hotspot mutation allele frequencies from MSK-IMPACT are available in Supplementary Data 2. Additional validation for IHC antibody and additional data comparing RNA and protein expression are available in the Supplementary Material. Raw RNA-Seq data for the paired primary and metastatic samples are not published openly in order to protect participant identities, but will be made available upon request and under regulatory compliance via a data usage agreement (DUA). For all RNA-Seq samples, the transcript counts processed via Salmon are available at https://github.com/leeoesterreich.