Abstract
A cross-sectional study was carried out to determine the prevalence and risk factors of bovine mastitis caused by Streptococcus agalactiae from farms in and around Haramaya district, eastern Ethiopia. A total of 384 lactating cows were selected from small-, medium-, and large-scale production systems. California mastitis test (CMT) was used for screening subclinical mastitis. Out of the total animals examined, 63.02% (n = 242) had mastitis, where 6.77% (n = 26) and 56.25% (n = 216) were clinical and subclinical mastitis respectively. The quarter-level prevalence was 29.04% (n = 446), from which the clinical form was 6.38% (n = 98) and the subclinical was 22.66% (n = 348), and the rest quarters were blind 3.32% (n = 51). Milk samples from clinical as well as CMT positive quarters were cultured for isolation of S. agalactiae, where 10.3% (n = 46) resulted in growth of the bacterium. The prevalence of mastitis was found to be statistically significant among the age groups (p = 0.002), breed (p = 0.000), and parity (p = 0.000). Similar findings were found to the extrinsic risk factors considered; as production type (p = 0.010), teat injury (p = 0.02), and type of floor (p = 0.000). The study confirmed the importance of S. agalactiae as the cause of contagious mastitis and also identified the associated risk factors in the study farms and hence warrants serious attention.
Keywords: CMT, Haramaya district, Risk factors, Streptococcus agalactiae
Introduction
Mastitis is the inflammation of the mammary gland that has over 130 different isolated causative agents from mastitis milk samples but Staphylococcus aureus, Streptococci, and members of the Enterobacteriaceae are among the most common etiological agents in cows and in other animal species (Quinn et al. 1999). It is often classified as subclinical or clinical depending on the severity of the disease or contagious and environmental based on the causative agents (Quinn et al. 2002; Andrews et al. 2003). Mastitis caused by Staphylococcal and streptococcal are the commonest and economically a great concern for dairy farming. Unlike Staphylococcus aureus, Streptococcus agalactiae is one of the mastitis-causing bacteria that can only grow and multiply in the udder (Andersen et al. 2003). However, it can survive for short time periods on hands, milking machine parts and teat skin, leading to its spread from cow to cow during milking. S. agalactiae is most commonly introduced into a clean herd when an infected cow is purchased. Because of the silent nature of infections and highly contagious nature, infections can spread quickly (Sandy 2011). As with most infectious diseases, mastitis risk factors depends upon three components; exposure to microbes, cow defense mechanisms, and environmental and management factors (Mungube et al. 2004).
Mastitis has been contributing to reduced milk production and a major source of economic loss to the dairy industry (Erskine 1992), through reduced milk yield and quality, cost of drugs and veterinary treatment, discarded milk, and forced culling (Quinn et al. 1999). Mungube et al. (2005) estimated the economic losses from urban and peri urban areas of Addis Ababa, to be US$58 and 78.65 per cow and per lactation, respectively. In addition to its economic impact, Streptococcus agalactiae; group B Streptococcus (GBS), is the major etiologic agent of invasive neonatal infections in humans in industrialized countries, causing sepsis, pneumonia, meningitis, Osteomylits, and soft tissue infections (Baker 2000).
In Ethiopia, a few studies have been conducted with the purpose of estimating the prevalence of bovine mastitis (Kifle and Tadele 2008; Almaw et al. 2009; Sori et al. 2011; Dabash et al. 2014). However, mastitis as a disease particularly the subclinical mastitis has received very little attention. Therefore, the study was conducted with the objectives to determine the prevalence and associated risk factors of bovine mastitis caused by Streptococcus agalactiae from farms in and around Haramaya district, Ethiopia.
Materials and methods
Study areas
The study was conducted in selected small holder, medium- and large-scale dairy farms in and around Haramaya district, Ethiopia. It is located 503 km east of Addis Ababa; at 41°59′58″ latitude and 09°10′24″ longitudes with 2000 m a.s.l. The district receives an average annual rain fall approximately 900 mm, and climatically, there are two ecological zones of which 66.5% is midland and 33.5% is lowland (Shimelis 2010).
Study population and husbandry practice
Lactating Holstein-Zebu and local Zebu breeds from 20 dairy farms in and around Haramaya district were categorized into small-scale dairy production (SSDP), medium-scale dairy production (MSDP), and large-scale dairy production (LSDP) based on herd size having 5 or less, 6–30, and 72–171 dairy cattle, respectively (Mureda and Mekuria 2008). The cows in Haramaya University dairy farm were all cross breeds (Holstein Friesian × Zebu) and milked by a milking machine twice a day (morning and afternoon) in a separate milking parlor. The cows were managed under intensive husbandry practice in stall barn made of concrete floor. They were mainly fed hay, brans, and silage. Regular washing of milker’s hand before and after milking of the cows is an established practice at the farm. Age of animals was determined from birth records and categorized as young adults (3–6 years), adults (6 to ≤ 10 years), and old (> 10). Stage of lactation was categorized as early (1–4 month), middle (> 4–8 month), and late (> 8 month to the beginning of dry period). Parity was categorized as few (with ≤ 3calves), moderate (4–7 calves), and many (> 7 calves) (Biffa et al. 2005). The barn floor was grouped into poor (barn which was not well managed and muddy) and good (barn floor which is concrete or well managed).
Sample size determination
The desired sample size for the study was calculated using the formula given by Thursfield (2005) with an expected prevalence rate of 50%, 95% confidence interval, and 5% absolute precision:
where
- n
required sample size
- pexp
expected prevalence
- d2
desired absolute precision
So, a total of 384 lactating cows with about 1485 teat quarters were considered for the study.
Study design and sampling strategy
A cross-sectional study was conducted to determine the prevalence and associated risk factors of bovine mastitis caused by S. agalactiae. Cows were examined directly for clinical and indirectly using CMT for subclinical mastitis. Purposive sampling method was used to select study farms based on their willingness to be part of the study. The study animals were only lactating cows and were selected randomly.
Study methodology
Structured questionnaire
Structured questionnaires were developed to include information on cow attributes such as breed, age, parity number, lactation stage, teat or udder condition (lesion, fibrosis, atrophy), tick infestation of udder or teat, presence of blind teat, milk condition (watery, bloody, pussy). The age, lactation stage, and parity numbers were recorded from farm record documents, farm owners, and milkers. The farm attributes like herd size, production type, and status of barn floor were also considered in the questionnaire.
Clinical inspection and preparation of udder and teat for sample collection
First, udders and teats were physically examined by visualization and then palpation to detect if there is fibrosis, visible injury, tick infestation, atrophy of tissue, and any blindness. The udder and teats were disinfected with alcohol impregnated cotton, and washing is practiced when the udder is full of dung or dirty materials. The teat on the far side of the udder is cleaned first than those on the near side. Scrubbing was continued until the towel remains clean (Moges et al. 2011).
Milk sample collection and CMT
The first two streams of milk were discarded and approximately 2–3 ml of milk samples was collected into the mastitis paddle from individual quarters immediately after the udder is dry. Teats towards sample collection were taken first and then far once (Christos 2011). CMT was carried out on all sample collected in the mastitis paddle. The CMT reagent is mixed with the quarter milk sample that has been collected in the mastitis paddle in approximately equal proportion of the milk sample. Then, after the mixture was swirled in rotary motion, the result is then read within 10–15 s as negative, trace, + 1,+ 2, and + 3 (Radostitis et al. 2007).
Bacteriological isolation and characterization
Approximately 10 ml of milk from positive quarters collected into sterile test tubes was placed in ice box and transported to the Haramaya University, Veterinary Microbiology Laboratory. The milk samples were bacteriologically examined according to the procedures employed by Quinn et al. (1999). A loopful of milk sample was streaked on blood agar base enriched with 7% sterile sheep blood for each quarter. Blood agar plates were incubated aerobically at 37 °C for 24–48 h. The plates were examined for gross colony morphology, Gram’s stain, and hemolytic characteristics after 24–48 h. Presumptive colonies of Streptococcus species were selected and sub-cultured on nutrient agar and Edward media and incubated aerobically at 37 °C for 24–48 h. The catalase negative cocci were considered as Streptococci (Quinn 2002). The esculin negative colonies were preserved on nutrient agar plates for CAMP (Christie, Atkins, Munch-Petersen) test. S. agalactiae were identified by the hemolysis, not hydrolyzing esculin on Edward media and CAMP test.
Data management and analysis
The data generated during the sample collection and from the questionnaire were entered into the Microsoft Excel spread sheets and was later analyzed by using STATA version 11 software. The effect of risk factors with possible association of the disease was analyzed using chi-square. The associations between dependent and independent variables were tested, and p < 0.05 was taken as statistically significant.
Results
Overall prevalence
Out of 384 lactating cows, 63.02% (n = 242) were affected by mastitis and from the total of 1536 quarters examined, the prevalence to the quarter level was 29.04% (n = 446) and the rest 3.32% (n = 51) were blind. The prevalence of both clinical and subclinical at quarter and cow level is shown (Table 1).
Table 1.
The prevalence of bovine mastitis at cow and quarter levels
| Cow level | Quarter level | ||||
|---|---|---|---|---|---|
| Positives | Prevalence (%) | No. of teats | Positives | Prevalence (%) | |
| Blind | 51 | 13.28 | 1536 | 51 | 3.32 |
| Clinical | 26 | 6.77 | 1536 | 98 | 6.38 |
| Subclinical | 216 | 56.25 | 1536 | 348 | 22.66 |
| Total | 242 | 63.02 | 1536 | 446 | 29.04 |
Isolation of Streptococcus agalactiae
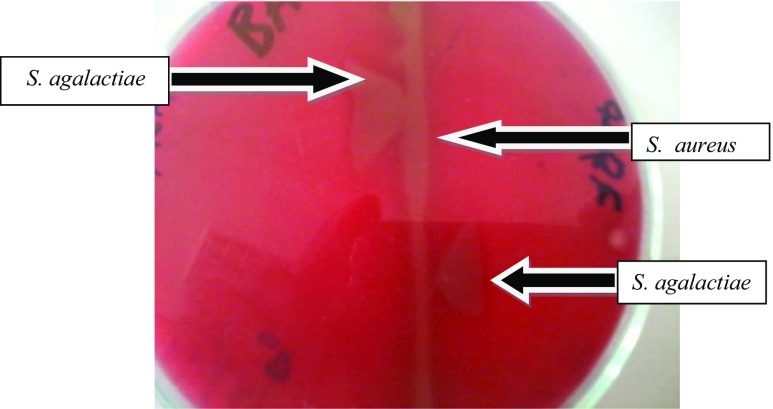
A total of 446 milk samples were collected and cultured from clinically and CMT-positive quarters; the prevalence of the S. agalactiae at the quarter level was found to be 10.3% (Fig. 1).
Fig. 1.
The “arrow head” formation at the junction of Staphylococcus aureus and Streptococcus agalactiae
Prevalence based on risk factors
Intrinsic risk factors
The prevalence of mastitis at the cow level showed statistically significant difference (p < 0.05) among different parity numbers, breeds, and ages considered in the study (Table 2).
Table 2.
The prevalence of mastitis based on the intrinsic risk factors
| Intrinsic risk factors | Total animals examined | Positive animals (%) | prevalence | χ2 | p value |
|---|---|---|---|---|---|
| Breed | |||||
| Local | 74 | 29(39.2) | 7.55 | 23.3 | 0.000 |
| Crossbreed | 310 | 213(68.7) | 55.47 | ||
| Age | |||||
| Young adult | 96 | 50 (52.1) | 13.02 | 17.3 | 0.002 |
| Adult | 137 | 78 (56.9) | 20.31 | ||
| Old | 151 | 114 (75.5) | 29.69 | ||
| Parity | |||||
| Few | 201 | 89 (44.3) | 23.18 | ||
| Moderate | 65 | 40 (61.5) | 10.42 | 88.1 | 0.000 |
| Many | 118 | 113 (95.8) | 29.43 | ||
| lactation stage | |||||
| Early | 206 | 135 (65.5) | 35.16 | 6.2 | 0.185 |
| Middle | 95 | 50 (52.6) | 13.02 | ||
| Late | 83 | 57 (68.7) | 14.84 | ||
| Total | 384 | 242 | 63.02 | ||
Extrinsic risk factors
Management factors such as production type, teat injury, and floor types were also evaluated where all of them had a statistically significant difference (P < 0.05) on the prevalence of mastitis (Table 3).
Table 3.
The prevalence of bovine mastitis based on the extrinsic risk factors
| Extrinsic risk factors | Total animals examined | Positive animals (%) | prevalence | χ2 | p value |
|---|---|---|---|---|---|
| Production types | |||||
| Small scale | 39 | 18 (46.2) | 4.69 | 13.4 | 0.010 |
| Medium scale | 177 | 103 (58.2) | 26.82 | ||
| Large scale | 168 | 121 (72.0) | 31.51 | ||
| Teat injury | |||||
| Present | 11 | 10 (90.9) | 2.60 | 7.9 | 0.02 |
| Absent | 373 | 232 (62.2) | 60.42 | ||
| Tick infestation | |||||
| Absent (negligible) | 363 | 226 (62.3) | 58.85 | 3.85 | 0.427 |
| Moderate | 7 | 4 (57.1) | 1.04 | ||
| Infested | 14 | 12 (85.7) | 3.13 | ||
| Type of floor | |||||
| Concrete | 306 | 209 (68.3) | 54.43 | 18.58 | 0.000 |
| Muddy | 78 | 33 (42.3) | 8.59 | ||
| Milking type | |||||
| Manual | 328 | 201 (61.3) | 52.34 | 3.6 | 0.165 |
| Machine | 56 | 41 (73.2) | 10.68 | ||
| Total | 384 | 242 | 63.02 | ||
Prevalence of bovine mastitis at farms level
The study also revealed the prevalence of mastitis at the different farms (Table 4).
Table 4.
The prevalence of mastitis at farm level
| Farms | Total animal examined | Clinical (%) | Prevalence (%) | Subclinical (%) | Prevalence (%) | Total (%) |
|---|---|---|---|---|---|---|
| 1 | 38 | 4 (10.53) | 1.04 | 23 (60.5) | 5.99 | 27 (7.03) |
| 2 | 14 | 3 (21.43) | 0.78 | 7 (50) | 1.82 | 10 (2.6) |
| 3 | 22 | 1 (4.55) | 0.26 | 8 (36.36) | 2.08 | 9 (2.34) |
| 4 | 5 | 0 | 0 | 4 (80) | 1.04 | 4 (1.04) |
| 5 | 5 | 1 (20) | 0.26 | 3 (60) | 0.78 | 4 (1.04) |
| 6 | 23 | 0 | 0 | 13 (56.5) | 3.39 | 13 (3.39) |
| 7 | 3 | 0 | 0 | 2 (66.7) | 0.52 | 2 (0.52) |
| 8 | 12 | 1(8.33) | 0.26 | 3 (25) | 0.78 | 4 (1.04) |
| 9 | 3 | 1 (33.33) | 0.26 | 0 | 0 | 1 (0.26) |
| 10 | 5 | 1 (20) | 0.26 | 0 | 0 | 1(0.26) |
| 11 | 4 | 0 | 0 | 0 | 0 | 0 |
| 12 | 5 | 0 | 0 | 2 (40) | 0.52 | 2 (0.52) |
| 13 | 23 | 1 (4.35) | 0.26 | 14 (60.9) | 3.65 | 15 (3.91) |
| 14 | 4 | 0 | 0 | 3 (75) | 0.78 | 3 (0.78) |
| 15 | 5 | 0 | 0 | 3 (60) | 0.78 | 3 (0.78) |
| 16 | 56 | 3 (5.36) | 0.78 | 38 (67.9) | 9.9 | 41 (10.68) |
| 17 | 29 | 0 | 0 | 18 (62.1) | 4.69 | 18 (4.69) |
| 18 | 27 | 2 (7.4) | 0.52 | 16(59.25) | 4.17 | 18 (4.69) |
| 19 | 27 | 1 (3.7) | 0.26 | 13 (48.15) | 3.39 | 14 (3.65) |
| 20 | 74 | 7 (9.46) | 1.82 | 46 (62.16) | 11.98 | 53 (13.8) |
| Total | 384 | 26 | 6.77 | 216 | 56.25 | 242 (63.02) |
Discussion
The study showed that the prevalence of bovine mastitis from farms in and around Haramaya district to be 63.02% at cows’ level as determined by the CMT and clinical examinations of the udder. This finding is in agreement with the report of 63.11% by Kassa et al. (2014) in Hawassa and Wando Genet and 61.11% by Tolla (1996) in South Wollo. However, the prevalence was higher than the report of 34.9% by Biffa et al. (2005), 40.40% by Dego and Tareke (2003), 52.9% by G/Michael et al. (2013) in Southern Ethiopia, and 46.7% by Abera et al. (2013) in Adama town and 53.25%; by Biniam et al. (2015) in Dire Dawa town but lower than the report of Mekibib et al. (2010a, b) in Holeta town in Central Ethiopia and Zeryehun et al. (2013) in and around Addis Ababa who reported 71.05 and 74.7% respectively. This variability in the prevalence between different reports could suggest the complexity of the disease, which involves the interaction of several factors mainly of farm management practices, production type and environment, animal risk factors, and causative agent; its prevalence is expected to vary from place to place (Radostitis et al. 2007).
The prevalence of clinical and subclinical mastitis were 6.77 and 56.25%, respectively. The clinical prevalence in this study was comparable to the report of Bishi (1998) who reported the prevalence of 5.3% in Addis Ababa and lower than those reported by Tolosa et al. (2009) who reported the prevalence of 9.5% at Wolayta Sodo and Hundera et al. (2005) with the prevalence of 16.11% in and around Sebeta. In case of subclinical mastitis, the prevalence at cow level (56.25%) in this study was comparable with the finding 54.4% reported by Biffa et al. (2005), 55.1% by Zeryehun et al. (2013), and 55.8% by Bedada and Hiko (2011) but higher than 36.67% reported by Sori et al. (2005) and 44.16% by Biniam et al. (2015). The overall prevalence of subclinical mastitis at both cow and quarter level was found to be higher than clinical mastitis. This could be attributed to the little attention given to subclinical mastitis while treating clinical cases. According to Sori et al. (2005), subclinical mastitis was higher than clinical mastitis owing to the defense mechanism of the udder, which reduces the severity of the disease. Moreover, farmers in Ethiopia are not well informed about the silent cases of mastitis (Zeryehun et al. 2013).
The prevalence of mastitis was higher in older cows (29.69) than young adults (13.02%) and adults (20.31%). The increasing prevalence of mastitis with increasing age is in agreement with the findings by Dego and Tareke (2003) and by Abera et al. (2013) who found that the risk of mastitis increase significantly with the advancing age of the cow. Radostitis et al. (2007) have explained that older cows have largest teats and more relaxed sphincter muscles, which increase the accessibility of infectious agent in the cows’ udder. The increase in prevalence of mastitis with parity reported in the study is comparable with the previous reports (Biffa et al. 2005; Tamirat 2007; Mekibib et al. 2010a, b; Moges et al. 2011; Biniam et al. 2015). This might be due to the increased opportunity of infection with time and the prolonged duration of infection, especially in a herd without mastitis control program and also an increase for teat injuries (Radostitis et al. 2007).
The study also showed that there were significant statistical association between prevalence of mastitis with herd size, floor types, and breeds. This finding is in agreement with Sori et al. (2005); Moges et al. (2011); Kassa et al. (2014). Quinn et al. (1999) have explained that genetic predisposition factors to mastitis such as teat shape, sphincter tone, anatomy of the teat canal, and susceptibility to weakening of the suspensory ligament (“pendulous udder”). In line with this, it was found in this study that the prevalence of mastitis in crossbred cows was statistically higher than that of local cattle.
From 446 milk samples subjected to bacteriological examinations, 10.3% (n = 46) of S. agalactiae was isolated. This finding is comparable with the report of Zeryehun et al. (2013) and Yohannes and Molla (2013) which were 21.2 and 17.78% respectively and much higher than the report of G/Michael et al. (2013) which was 1.6%, but lower than 26.5% by Megersa et al. (2012). A high proportion of S. agalactiae (17.36%) was isolated from CMT-positive cows. This could be because S. agalactiae is a highly contagious obligate parasite of the bovine mammary gland (Meiri-Bendek et al. 2002).
Conclusion
The study showed that the prevalence of mastitis at cow and quarter levels to be high which affects the dairy production. In this study, S. agalactiae was isolated more from subclinically infected cows. This indicates that contagious mastitis was prevailing in the studied farms and could be associated with unhygienic milking practice and poor herd management by the farms.
Acknowledgements
The authors would like to thank the laboratory technicians at the Veterinary Microbiology laboratory for the realization of the research.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abera M, Demie B, Aragaw K, Regassa F, Regassa A. Isolation and identification of Staphylococcus aureus from bovine mastitic milk and their drug resistance patterns in Adama town, Ethiopia. J. Vet. Med. and Anim. Health. 2013;1(2):19–23. [Google Scholar]
- Andersen HJ, Pedersen LH, Aarestrup FM, Chriél M. Evaluation of the surveillance program of Streptococcus agalactiae in Danish dairy herds. J. Dairy Sci. 2003;86:1233–1239. doi: 10.3168/jds.S0022-0302(03)73707-2. [DOI] [PubMed] [Google Scholar]
- Andrews AH, Blowey RW, Boyd H, Eddy RG. Bovine medicine: diseases and husbandry of cattle. Victoria: Blackwell Publishing; 2003. pp. 427–432. [Google Scholar]
- Baker, CJ. (2000). Group B streptococcal infections. In: Stevens D. L, Kaplan E. L, editorseds. Streptococcal infections. Clinical aspects, microbiology, and molecular pathogenesis. New York: Oxford University Press. p. 222–37.
- Bedada BA, Hiko A. Mastitis and antimicrobial susceptibility test at Asella, Oromia Regional state, Ethiopia. J. Microbiol.Antimicrobials. 2011;3(9):228–232. [Google Scholar]
- Biffa D, Debela E, Beyene F. Prevalence and risk factor of mastitis in lactating dairy cows in southern Ethiopia, Int. J. Appl.Res. Vet. Med. 2005;3(3):189–198. [Google Scholar]
- Biniam T, Rediet T, Yonus A. Prevalence and potential risk factors of bovine mastitis in selected dairy farms of dire Dawa town, Eastern Ethiopia. Appl. J. hygiene. 2015;4(1):06–11. [Google Scholar]
- Bishi, A S. (1998). Cross-sectional and longitudinal prospective of bovine clinical and subclinical mastitis in the urban and periurban production sytems in Addis Ababa region, (Msc Thesis, Free university of Berlin, Germany and Addis Ababa University, Ethiopia, Joint Programme).
- Christos, M. (2011). Study on prevalence and risk factors of bovine mastitis in and around Mekelle small scale dairy farm. DVM Thesis. Mekelle University, Mekelle, Ethipia.
- Dego, O. K., and Tareke, F. (2003). Bovine mastitis in selected areas of southern Ethiopia. Trop. Anim. Health Prod., 197–205. [DOI] [PubMed]
- Erskine RJ. Mastitis control in dairy herds with high prevalence of subclinical mastitis. Compend Contin Educ PractVet. 1992;14:969–979. [Google Scholar]
- G/Michael L, Deressa B, Begn F, Mekuria A. Study on prevalence of bovine mastitis in lactating cows and associated risk factors in and around Areka town, Southern of Ethiopia. Afr. J. Microbiol. Res. 2013;7(43):5051–5056. doi: 10.5897/AJMR2013.6202. [DOI] [Google Scholar]
- Hundera S, Ademe Z, Sintayehu A. Dairy cattle mastitis in and around Sebeta, Ethiopia. Intern. J. Appl. Vet. Med. 2005;3(4):1525–1530. [Google Scholar]
- Kassa F, Ayano AA, Abera M, kiros A. Longitudinal study of bovine mastitis in Hawassa and Wendo Genet Small Holder Dairy Farms. Global J. Sci. Frontier Res. 2014;14(2):33–41. [Google Scholar]
- Megersa B, Manedo A, Abera M, Regassa A, Abunna F. Mastitis in lactating cows at Hawassa town: prevalence, risk factors, major bacterial causes and treatment response to routinely used antibiotics. American. J. Sci. Res. 2012;7(2):86–91. [Google Scholar]
- Meiri-Bendek I, Lipkin E, Friedmann A, Leitner G, Saran A, Friedman S, Kashi Y. A PCR-based method for the detection of Streptococcus agalactiae in Milk. J. Dairy Sci. 2002;85:1717–1723. doi: 10.3168/jds.S0022-0302(02)74245-8. [DOI] [PubMed] [Google Scholar]
- Mekibib B, Fergasa M, Abunna F, Megersa B, Regassa A. Bovine mastitis: prevalance, risk factors and major pathogens in dairy farms of Holeta town, Central Ethiopia. Vet. World. 2010;3:397–403. doi: 10.5455/vetworld.2010.397-403. [DOI] [Google Scholar]
- Moges N, Asfaw Y, Belihu K. A cross sectional study on the prevalence of subclinical mastitis and associated risk factors in and around Gondar, Northern Ethiopia. Int. J. Ani. Vet. Adv. 2011;3(6):455–459. [Google Scholar]
- Mungube EO, Tenhagen BA, Regassa F, Kuyle MN, Sheferaw Y, Kassa T, Baumann MPO. Reduced milk production in udder quarters with subclinical mastitis and associated economic losses in crossed breed dairy cows in Ethiopia. Trop. Anim. Health. Prod. 2005;37(6):503–512. doi: 10.1007/s11250-005-7049-y. [DOI] [PubMed] [Google Scholar]
- Mungube EO, Tehagen BA, Kassa T, Regassa F, Kyule MN, Greiner M, Baumann MPO. Risk factors for dairy cow mastitis in the central highlands of Ethiopia. Tropical Animal Health and Production. 2004;36:463–472. doi: 10.1023/B:TROP.0000034999.08368.f3. [DOI] [PubMed] [Google Scholar]
- Mureda E, Mekuria Z. Reproductive performance of crossbreed dairy cows in eastern lowlands of Ethiopia. LSRD. 2008;20(4):0121–3778. [Google Scholar]
- Quinn PJ, Carter ME, Markey B, Carter GR. Clinical veterinary microbiology. London: Mosby; 1999. pp. 21–66. [Google Scholar]
- Quinn PJ, Carter ME, Markey BK, Carter GR. Veterinary microbiology microbial diseases, bacterial causes of bovine mastitis. 8. London: Mosby International Limited; 2002. pp. 465–475. [Google Scholar]
- Radostitis, O. M., Gay, C. C., Hinchcliff, K. W. and Constable, P. D. (2007). Mastitis. In: Veterinary medicine: a text book of disease of cattle, sheep, pigs, goats, and horses 10th edition, Ballier, Tindall, London. Pp 674–762.
- Sandy, C. (2011). Milk Quality Pays:Streptococcus agalactiae (Strep ag) Mastitis. A review can.vet J. pp. 1–5.
- Shimelis A. Prevalence of abomasal nematode in small ruminants slaughtered at Haramaya Municipal Abattoir. Ethiopia: Eastern Hararghe; 2010. [Google Scholar]
- Sori H, Zerihun A, Abdicho S. Dairy cattle mastitis in and around Sebeta. Intern J Appl Res Vet Med. 2005;3:338–341. [Google Scholar]
- Tamirat, T.A. (2007). Comparison of clinical trials of bovine mastitis with the use of honey, MSc thesis, Addis Ababa University, Ethiopia. pp. 14-30.
- Thursfield M. Veterinary Epidemiology. 3. London, UK: Blackwell science, Ltd.; 2005. pp. 228–246. [Google Scholar]
- Tolla, T. (1996). Bovine mastitis in indigenous zebu and Borona Holistein crosses in Southern Wollo. Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia.
- Tolosa T, Geberetsadik Z, Regassa F. Bovine mastitis and its associated risk factor in lactating cow in Wolayta Sodo, Southern Ethiopia. Animal Health Production. 2009;57(4):311–319. [Google Scholar]
- Yohannes M, Molla W. Prevalence, risk factors and major bacterial causes of bovine mastitis in and around Wolaita Sodo, Southern Ethiopia. Global J. Microbiol Res. 2013;1(1):106–111. [Google Scholar]
- Zeryehun T, Aya T, Bayecha R. Study on prevalence, bacterial pathogens and associated risk factors of bovine mastitis in small holder dairy farms in and around Addis Ababa, Ethiopia. The J. Anim & Plant Sci. 2013;23(1):50–55. [Google Scholar]
- Kifle A, Tadele T. Prevalence of sub clinical mastitis in small holder dairy farms in Selale, North Shewa Zone, Central Ethiopia. The Internet Journal of Veterinary Medicine. 2008;5(1):1–4. [Google Scholar]
- Almaw G, Molla W, Melaku A. Prevalence of bovine subclinical mastitis in Gondar town and surrounding areas, Ethiopia. Livestock Research for Rural Development. 2009;21:7. [Google Scholar]
- Mekibib B, Furgasa M, Abunna F, Megersa B, Regassa A. Bovine mastitis: prevalence, risk factors and major pathogens in dairy farms of holeta town central Ethiopia. Veterinary World. 2010;3(9):397–403. doi: 10.5455/vetworld.2010.397-403. [DOI] [Google Scholar]
- Sori T, Hussien J, Bitew M. Prevalence and susceptibility assay of Staphylococcus aureus isolated from bovine mastitis in dairy farms of Jimma town, South West Ethiopia. Journal of Animal and Veterinary Advances. 2011;10(6):745–749. doi: 10.3923/javaa.2011.745.749. [DOI] [Google Scholar]
- Dabash H, Petros A, Fekadu A. Prevalence and identification of bacterial pathogens causing bovine mastitis from crossbred of dairy cows in North Showa Zone of Ethiopia. Global Veterinaria. 2014;13(2):189–195. [Google Scholar]