Abstract
Purpose
The l’Actuel PrEP Cohort was established to monitor the uptake, effectiveness, safety and changes in sexual risk behaviours among individuals receiving pre-exposure prophylaxis (PrEP) for the prevention of HIV. This prospective dynamic cohort is based at Clinique médicale l’Actuel, a large sexual health clinic located in Montreal, Canada.
Participants
Since the cohort inception in January of 2013 through June 2018, 2156 individuals consulted for PrEP as participants in the l’Actuel PrEP Cohort. Median age was 35 years (IQR: 29–44 years) and the majority (96%) were men who have sex with men. Among 1551 individuals who initiated PrEP care, the median duration of follow-up was 9.2 months (IQR: 3.7–19.6), with substantial variation based on year of cohort entry. The l’Actuel PrEP Cohort contains both daily and intermittent ‘on-demand’ PrEP users and has the largest reported population of intermittent PrEP users (n=406) in North America.
Findings to date
No incident HIV infections have occurred among individuals using PrEP over 1637 person-years of follow-up. However, retention in PrEP care is essential as three individuals who discontinued PrEP subsequently acquired HIV, translating to an HIV incidence of 3.9 cases per 100 person-years (95% CI: 1.3 to 12.1). Among a sample of participants with 1 year of follow-up before and after PrEP initiation (n=109), a moderate increase in sexually transmitted infections was observed following PrEP start.
Future plans
The l’Actuel PrEP Cohort continues to grow with new participants starting PrEP monthly and extended follow-up for existing users. The cohort data will be used for ongoing monitoring of PrEP and for population-level modelling of the impact of PrEP on HIV incidence in Montreal.
Keywords: HIV & AIDS, sexual medicine, pre-exposure prophylaxis, men who have sex with men, HIV prevention, sexually transmitted infections
Strengths and limitations of this study.
The l’Actuel PrEP Cohort is currently the largest prospective ongoing cohort of pre-exposure prophylaxis (PrEP) for HIV prevention in Canada, and provides essential data documenting the real-world effectiveness of PrEP among men who have sex with men and other populations at-risk for HIV in Montreal.
Strengths include a large sample size and the prospective collection of detailed clinical, behavioural and laboratory data spanning over 5 years.
Data collection occurs as an integrated part of PrEP care provided at a community clinic in a universal healthcare setting; therefore the cohort is believed to be broadly representative of local PrEP users. However, several data-items are self-reported and may be subject to reporting bias, in particular for sexual behaviour variables.
The l’Actuel PrEP Cohort is based at a single site in Montreal and does not track participants who changed their PrEP service provider. PrEP use is dynamic and it is often difficult to distinguish between loss to follow-up and voluntary PrEP discontinuation.
Introduction
Pre-Exposure Prophylaxis (PrEP) is a relatively new biomedical prevention method for individuals at risk of HIV acquisition. Oral PrEP is composed of two antiretroviral drugs, tenofovir disoproxil fumarate and emtricitabine (TDF-FTC), which when combined are highly effective at preventing HIV infection—as shown in several randomised-controlled trials documenting efficacy of daily PrEP among men who have sex with men (MSM),1 2 transgender women,1 heterosexual discordant couples,3 4 and people who inject drugs.5 An intermittent ‘on-demand’ PrEP dosing regimen consisting of two pills taken before sex, followed by one pill every day until 2 days after the last sexual event, has also been shown to be efficacious among MSM.6 7
Despite early evidence of PrEP effectiveness since 2010,1 PrEP uptake in Canada has been relatively slow. The province of Quebec issued a brief set of interim guidelines recommending PrEP use for at-risk MSM in 2013,8 the first Canadian province to do so. Health Canada approved daily PrEP using TDF-FTC in February 2016.9 The Canadian guidelines on PrEP were released in late 2017, recommending daily PrEP as the regimen of choice for adults at ongoing high risk of HIV infection (strong recommendation), and on-demand PrEP as an alternative regimen among MSM (weak recommendation).10 As Health Canada issued approval for daily PrEP only, current use of on-demand PrEP is considered ‘off label’.11 The 2017 Canadian guidelines recommend PrEP for MSM who report condomless anal sex within the last 6 months and have one or more additional risks such as: having syphilis or a rectal sexually transmitted infection (STI); recurrent non-occupational post-exposure prophylaxis (PEP) episodes; having a current HIV-positive sexual partner with significant risk of transmissible HIV; or receiving a high score on the HIV Incidence Risk Index (HIRI)-MSM evaluation tool (score ≥11).10 The updated 2019 Quebec PrEP guidelines include the same PrEP indications among MSM as the Canadian guidlelines, with the exception that the HIRI-MSM criteria is replaced with two additional indications: use of psychoactive substances during sex and/or having had two or more sex partners within the last 6 months.12
In most settings in Canada, PrEP is prescribed primarily by HIV or sexual health specialists, however there are no restrictions for general practitioners to prescribe PrEP.13 Public reimbursement of PrEP varies provincially across Canada. In Quebec, TDF-FTC is reimbursable through the provincial drug plan without restriction by HIV status, thereby permitting off-label dispensing to PrEP users since 2013 with a monthly copayment ($80–90 CAD) or via private insurance. Elsewhere in Canada, TDF-FTC for use as PrEP was not reimbursable until after 2017,13 and therefore was largely inaccessible due to prohibitive out-of-pocket costs ranging from $800 to 1000 CAD for a monthly course of daily PrEP.14 This issue of cost as a barrier to PrEP access was addressed in a 2016 report by the Canadian Drug Expert Committee of the Canadian Agency for Drugs and Technologies in Health recommending that the cost of PrEP be subsidised by public drug plans in Canada.15
In 2013, following Quebec’s interim PrEP guidelines, Clinique médicale l’Actuel became the first centre in Canada to open a PrEP clinic and to promote PrEP uptake directly to individuals at risk for HIV acquisition. L’Actuel is a major sexual health centre located in Montreal’s Gay Village. Since 1984, l’Actuel has served as a top referral site in Montreal for HIV and STI testing and treatment; and since 2000, for medical HIV prevention services using PEP.16
During the early implementation phase of the PrEP clinic (July 2012–November 2013), predictors of interest in taking PrEP were assessed by administering a survey to 1179 MSM clients attending a rapid HIV-testing site in Montreal’s Gay Village by outreach workers. Over half (55%) of respondents expressed interest in PrEP.17 This encouraged the development of the l’Actuel PrEP Cohort with the view that offering PrEP at a community-based sexual health centre could be the optimal delivery model for individuals seeking PrEP, as opposed to offering PrEP in a hospital setting which often poses challenges to patients in terms of navigating complicated tertiary care systems. In contrast to earlier trials that specifically targeted individuals at the highest risk of HIV acquisition, uptake of PrEP in real-world programmes, such as the l’Actuel PrEP Cohort, could therefore result in more diverse risk profiles.
Cohort description
The l’Actuel PrEP Cohort was established in 2013 to measure the effectiveness of PrEP for HIV prevention and to study the clinical, demographic and behavioural characteristics of the population accessing PrEP in Montreal, Canada. The l’Actuel PrEP Cohort prospectively collects data on PrEP care from the initial consultation and throughout PrEP utilisation to provide an evidence base for research areas including: clinical monitoring of side effects related to TDF-FTC, STI and HIV risk, change in sexual behaviour s and access to other HIV prevention services. By providing real-world data to characterise PrEP use and practices, evidence generated from the l’Actuel PrEP Cohort can serve to inform clinical practice and assist public health decision-making. The l’Actuel PrEP Cohort is integrated into, and supported through, routine clinical care. Data collection is carried out by clinicians, nurses and researchers. Data collection is ongoing with no planned end date.
Participants
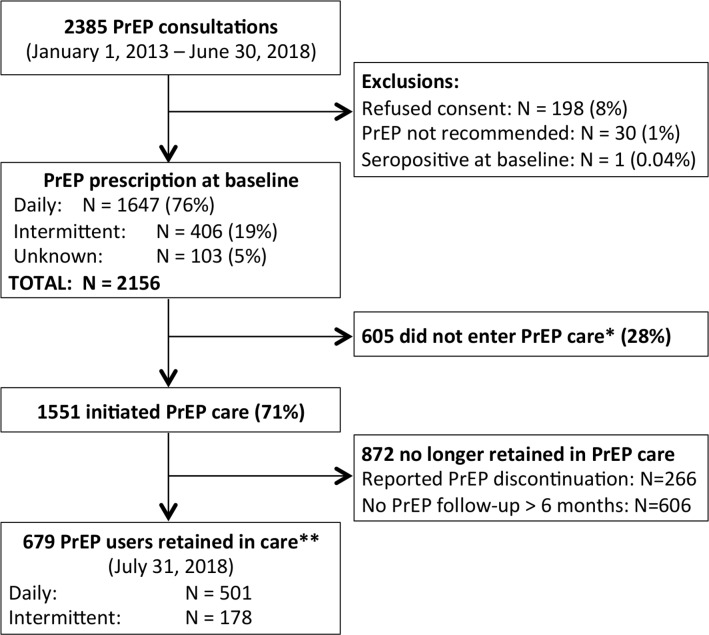
The study is a prospective dynamic cohort. Recruitment of participants into the PrEP cohort has been ongoing since January 2013. Eight individuals initiated PrEP at l’Actuel from 2011 to 2013, outside of the context of the research cohort, and are therefore not included in the present cohort description. Recruitment methods include in-person (active) enrolment whereby healthcare providers recommended PrEP initiation to individuals at risk for HIV acquisition (ie, condomless anal sex, repeated episodes of PEP or recent rectal STI). Additionally, community-directed (passive) messages are delivered via promotion of l’Actuel’s PrEP clinic in local gay magazines, social media platforms and word-of-mouth. All adults (≥18 years) seeking PrEP at l’Actuel are invited to participate. Written informed consent is obtained from all participants entering the cohort. Participation is non-incentivised and entirely voluntary. Research cohort inclusion criteria include: seronegative for HIV at baseline, being recommended or prescribed PrEP and providing consent (figure 1). All analyses are performed on de-identified data.
Figure 1.
Flow chart of l’Actuel PrEP Cohort participants. *Individuals were considered to not have started PrEP care if they did not return for any PrEP follow-up visits after baseline, or if they returned for their PrEP follow-up visit and reported that they did not start PrEP. **Retention in care is measured as an active PrEP prescription at a follow-up visit within the past 6 months. PrEP, pre- exposure prophylaxis.
From January 1, 2013–June 30, 2018, 2385 individuals consulted for PrEP and completed baseline questionnaires (figure 1). Of this number, 229 individuals were excluded: those who did not provide consent for the use of their data for observational research (n=198, 8%), those without substantial risk factors for HIV acquisition among whom PrEP was not recommended (n=30, 1%), and one individual who tested seropositive for HIV at the baseline consultation (n=1, <1%). Among 2156 individuals included in the l’Actuel PrEP Cohort, baseline prescriptions consisted of daily PrEP (n=1647, 76%) or on-demand PrEP (n=406, 19%); baseline prescription was undocumented in 103 (5%) of cases. A total of 1551 individuals (72%) attended the first PrEP follow-up visit (by July 31, 2018) and reported PrEP initiation and 605 did not initiate PrEP care. Follow-up time was lagged 1 month later than the end of reported PrEP baseline consults included in this analysis to allow measurement of PrEP initiation (ie, we included follow-up visits up to July 31, 2018 to measure PrEP initiation for all those consulting up to June 30, 2018). Among those who did not initiate PrEP care, the majority never returned for visits after baseline (n=504, 83%) and others came for a follow-up and reported not initiating PrEP (n=101, 17%). Table 1 outlines characteristics of participants by PrEP initiation status.
Table 1.
Baseline characteristics at PrEP consultation by initiation status in the l’Actuel PrEP Cohort (2013–2018)
| Initiated PrEP | Did not initiate PrEP | Total | ||||
| n (or median) | % (or IQR) | n (or median) | % (or IQR) | n (or median) | % (or IQR) | |
| Gender identity | ||||||
| Cis-male | 1528 | 98.5 | 588 | 97.2 | 2116 | 98.1 |
| Cis-female | 20 | 1.3 | 11 | 1.8 | 31 | 1.4 |
| Trans-male | 2 | 0.1 | 3 | 0.5 | 5 | 0.2 |
| Trans-female | 1 | 0.1 | 3 | 0.5 | 4 | 0.2 |
| Age, median (IQR) | 36 | (29–45) | 33 | (27–41) | 35 | (29–44) |
| Number of partners within past 12 months, median (IQR) | ||||||
| Regular partners | 2 | (1–3) | 2 | (1–3) | 2 | (1–3) |
| Occasional partners | 12 | (5–20) | 10 | (4–20) | 10 | (5–20) |
| Missing data, regular partners, n (%) | 311 | 20.4 | 114 | 18.1 | 425 | 19.7 |
| Missing data, occasional partners, n (%) | 296 | 19.4 | 121 | 19.2 | 417 | 19.4 |
| Sexual orientation, n (%) | ||||||
| Homosexual | 1459 | 94.1 | 534 | 88.3 | 1993 | 92.4 |
| Heterosexual | 21 | 1.4 | 17 | 2.8 | 38 | 1.8 |
| Bisexual | 61 | 3.9 | 44 | 7.3 | 105 | 4.9 |
| Missing data | 10 | 0.6 | 10 | 1.7 | 20 | 0.9 |
| Education, n (%) | ||||||
| Primary | 12 | 0.8 | 3 | 0.5 | 15 | 0.7 |
| Secondary | 166 | 10.7 | 85 | 14.0 | 251 | 11.6 |
| College | 260 | 16.8 | 119 | 19.7 | 379 | 17.6 |
| University | 813 | 52.4 | 283 | 46.8 | 1096 | 50.8 |
| Missing data | 300 | 19.3 | 115 | 19.0 | 415 | 19.2 |
| Annual income ($CAD), n (%) | ||||||
| <10 000 | 107 | 6.9 | 46 | 7.6 | 153 | 7.1 |
| 10 001–20 000 | 120 | 7.7 | 69 | 11.4 | 189 | 8.8 |
| 20 001–35 000 | 154 | 9.9 | 75 | 12.4 | 229 | 10.6 |
| 35 001–55 000 | 311 | 20.1 | 132 | 21.8 | 443 | 20.5 |
| 55 001–75 000 | 245 | 15.8 | 93 | 15.4 | 338 | 15.7 |
| >75 000 | 360 | 23.2 | 106 | 17.5 | 466 | 21.6 |
| Missing data | 254 | 16.4 | 84 | 13.9 | 338 | 15.7 |
| Total, n (%) | 1551 | 71.9 | 605 | 28.1 | 2156 | 100.0 |
PrEP, pre-exposure prophylaxis.
PrEP cohort participants had a median age of 35 years (IQR: 29–44), were mostly cis-male (98%), and identified as either homosexual or bisexual (97%). At baseline, participants reported having had a median of 2 regular and 10 occasional sexual partners within the past 12 months. Regular sexual partners are defined as those who are seen recurrently whereas occasional partners are seen only once or twice (ie, casual hookups). Over two-thirds of participants had post-secondary education (college or university) and 58% had an annual income above $35 000 CAD. For comparison, the median income in Quebec ranged from $32 500 to 33 500 CAD during the study period (2013–2017).18 The majority of participants (87%) had received some form of care at l’Actuel prior to their PrEP consult; whereas 13% of patients came to l’Actuel uniquely for PrEP.
Clinical visits
Participants seeking PrEP complete a baseline PrEP consultation and, if indicated, they receive a prescription for PrEP.8 10 Next, a first follow-up visit is scheduled 30 days later. Subsequent follow-up visits are scheduled at 3-month intervals—at which time the PrEP prescriptions are renewed. Clinical management of individuals on PrEP follows a specific protocol, summarised in figure 2, and is supported by a specialised team of doctors, nurses and pharmacists.
Figure 2.
Protocol for baseline and follow-up pre-exposure (PrEP) visits at l’Actuel PrEP Cohort. ARV, antiretroviral medication; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Overall, 6407 PrEP follow-up visits occurred among 1551 individuals; totalling 1637 person-years of follow-up. Trends in PrEP follow-up visits have been summarised in table 2. The median duration of PrEP use per individual in the cohort is 9.2 months (IQR: 3.7–19.6). Using the definition of active PrEP users as those who have presented for at least one PrEP visit from February 1 to July 31, 2018; there are 679 active participants (32%) in the PrEP cohort. Currently, 606 individuals have not returned for follow-up visits in over 6 months. These individuals may have decided to temporarily or permanently discontinue PrEP or may have changed care providers; however their PrEP use status is unknown. 266 individuals have reported to the clinic that they have discontinued PrEP. PrEP discontinuation among individuals who would continue to benefit from this HIV prevention method is an area of concern. We observed that the rate of individuals not initiating PrEP increased over the study period, from 15% in 2013 to 41% in 2018. We hypothesise that this may be related to different patient characteristics between ‘early initiators’ who sought PrEP when it was a newly available prevention measure (2013–2015) and may have been more motivated to start PrEP than those who consulted for PrEP after approval by Health Canada (2016–2018). Further research is needed to better understand patterns of PrEP initiation/discontinuation and their underlying determinants in our cohort and elsewhere.
Table 2.
L’Actuel PrEP Cohort follow-up status and median duration of PrEP use based on year of cohort entry (2013–2018)
| Follow-up status on July 31, 2018 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018* | Total | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Active PrEP use† | 4 | 14.8 | 21 | 22.3 | 107 | 23.5 | 184 | 25.5 | 224 | 37.9 | 139 | 52.1 | 679 | 31.5 |
| Reported PrEP discontinuation | 8 | 29.6 | 15 | 16.0 | 68 | 14.9 | 93 | 12.9 | 66 | 11.2 | 16 | 6.0 | 266 | 12.3 |
| No PrEP follow-up ≥6 months | 11 | 40.7 | 44 | 46.8 | 212 | 46.6 | 221 | 30.6 | 116 | 19.6 | 2 | 0.7 | 606 | 28.1 |
| Did not initiate PrEP | 4 | 14.8 | 14 | 14.9 | 68 | 14.9 | 224 | 31.0 | 185 | 31.3 | 110 | 41.2 | 605 | 28.1 |
| Duration of PrEP use in months, median (IQR)‡ | 32.2 | (14.8–41.2) | 23.2 | (14.2–40.0) | 13.8 | (6.2–29.9) | 14.7 | (4.9–21.6) | 6.9 | (3.6–10.8) | 1.4 | (1.0–3.4) | 9.2 | (3.7–19.6) |
| Total PrEP consults | 27 | 1.3 | 94 | 4.4 | 455 | 21.1 | 722 | 33.5 | 591 | 27.4 | 267 | 12.4 | 2156 | 100 |
*Includes baseline consults from January 1 to June 30, 2018 only.
†Active PrEP use is defined as an open PrEP prescription at a PrEP follow-up visit within the past 6 months (February 1–July 31, 2018).
‡Duration of follow-up is measured among those who initiated PrEP.
PrEP, pre-exposure prophylaxis.
Measurements
Individuals consulting for PrEP receive a baseline medical exam consisting of HIV tests (fourth generation antibody/antigen test, HIV rapid tests when necessary, and if last potential HIV exposure occurred within window period for seroconversion, additional viral load tests may be performed), syphilis serology, PCR detection of chlamydia and gonorrhoea in three sites (pharynx, urethra and anus), hepatitis C and A virus antibody tests, hepatitis B antigen tests and serum creatinine measurement (table 3). Additionally, a baseline questionnaire is completed to collect demographic and behavioural data. The first component of this questionniare is self-administered by participants and collects demographics and information on behavioural risk factors, the second component collecting data on medical history is filled by the nurse who also completes a blood draw and STI testing, finally, a doctor meets the participant to review the questionnaire and prescribe (or not) PrEP as either a daily or on-demand regimen. The United States Centres for Disease Control risk-evaluation tool, HIRI-MSM,19 has been integrated into baseline PrEP consultations since June 2016 as a self-administered component.
Table 3.
List of variables collected at baseline and follow-up visits in the l’Actuel PrEP Cohort in Montreal (2013–2018)
| Phase | Measurements |
| Baseline measurements |
|
| Follow-up measurements: |
|
ARV, antiretroviral medication; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
The decision to initiate PrEP and, if so, which PrEP regimen to adopt are based on a discussion between patient and clinician which reviews a patient’s indication for PrEP, and addresses, but is not limited to, criteria defined in the Quebec and Canadian PrEP guidelines.8 10 PrEP regimens were only prescribed as daily TDF-FTC up to March 2015. From March 2015 onwards, PrEP was also prescribed as an on-demand regimen consisting of two doses taken 2–24 hours prior to sex, a third dose taken 24 hours after the first doses and a fourth dose 48 hours after the first doses (or one time per day until 48 hours after last sexual exposure). All PrEP users receive in-depth counselling on PrEP use as well as other strategies for reducing risk. This approach encourages the use of combined prevention strategies such as condoms and discussions of the importance of frequent HIV/STI testing for themselves and their partners.
The follow-up visits include repeat HIV/STI tests, monitoring of side effects including gastrointestinal symptoms, fatigue and changes in creatinine levels. The nurse/clinician administered follow-up questionnaire assesses behavioural changes, adherence to PrEP and side effects (table 3). Counselling is provided regarding PrEP adherence and use of combined prevention strategies.
Missing data
Data collection occurs during baseline and follow-up PrEP visits using a combination of paper and electronic forms. The l’Actuel PrEP Clinic conducts approximately 50 PrEP initiations and 200 PrEP follow-up visits per month. PrEP visits are scheduled in 30 minute sessions for PrEP initiations and 15 minute sessions for PrEP follow-up visits. Questionnaires are filled by patients, nurses and doctors and the documentation process can be time-consuming. Reflecting the real-practice high-volume setting of this cohort, some questionnaire responses may not be fully or properly documented on the forms. Additionally there may be reporting biases whereby individuals do not wish to document sensitive personal information regarding sociodemographic status and/or sexual behaviours. For example, baseline data are missing for educational attainment and annual income among 19% and 16% of the sample, respectively. The indication for receiving PrEP is missing in 19% of cases and 20% of cases have missing data for the numbers of sexual partners within the past 12 months.
Patient and public involvement
The initial implementation of the l’Actuel PrEP Cohort and research focus was informed by a survey of Montreal’s MSM community members conducted at a rapid HIV testing site (l’Actuel sur Rue).17 In addition to PrEP-related questions such as, ‘If findings proved that PrEP was effective in protecting you against HIV infection, would you take it?’, participants were asked a series of demographic and behavioural questions aimed at understanding predictors of interest in PrEP. Results showed that 55% of the 1179 gay community members surveyed were interested in taking PrEP and that interest was highest among individuals in serodiscordant couples, those having over 10 sexual partners within the past 3 months, or responding to the survey after Quebec’s interim PrEP guidelines were published. L’Actuel PrEP Cohort’s participants were not formally involved in the study design, recruitment, or conduct of the study. However, word-of-mouth among individuals receiving PrEP was a source of referral for new patients seeking PrEP at l’Actuel. Study results have been disseminated to PrEP users and the public via articles in local news sources and gay media.20–22
Findings to date
Incident HIV infections
To date, no new HIV infections have been detected among individuals using PrEP over 1637 person-years of follow-up. However, we have observed seroconversions following PrEP discontinuation, as explored in a study that included 1258 PrEP users who initiated prophylaxis and returned for ≥1 PrEP visit from January 2011 to September 2017.23 Among those who reported PrEP discontinuation and remained in primary care at l’Actuel (n=203, 75.4 person-years following PrEP discontinuation), three individuals subsequently acquired HIV, translating to an HIV incidence following PrEP discontinuation of 3.9 cases per 100 person-years (95% CI: 1.3 to 12.1).23 A limitation of this study was that we were unable to identify if any incident HIV infections were diagnosed outside of l’Actuel. The most commonly reported reasons for PrEP discontinuation include side effects (14%), changes in sexual behaviours such as entry into a stable relationship with a seronegative (13%) or undetectable partners (5%), periods of sexual abstinence (10%), cost (7%) or health insurance issues (2%), and a preference for condoms over PrEP (3%).
Risk compensation
The phenomenon of ‘risk compensation’ describes a theory whereby individuals may increase their sexual activity or alter their behaviour due to the perceived protective effects conferred by PrEP.24 Changes in sexual behaviour among PrEP users in the cohort is heterogenous and, given the observational nature of the cohort, difficult to causally attribute to PrEP use alone. Among 355 individuals taking PrEP from January 2011 to August 2015, we previously investigated changes in behaviours following PrEP initiation, measured as increases in sexual partners and/or condomless sex acts within the first 3 months of PrEP use. Overall, only 25% of participants reported increases in those behaviours following PrEP initiation, while 43% reported no change and 32% reported decreases.25 Results from this study also indicated that participants were engaging in high-risk sex prior to starting PrEP, with 73% of participants having more than 10 partners within the past year, 80% having a history of STIs and nearly half of participants reporting inconsisent condom use at baseline. Findings from this study indicated that PrEP was being accessed by MSM with ongoing risks for HIV.
Incident STI risk
With respect to STI risk, we previously measured STI incidence in the PrEP cohort among a subgroup of participants who received lab-based STI screening for 12 months prior to PrEP start. This study covered the period from 2010 to 2015 and included 109 PrEP users. We found moderate increases in STI rates among individuals in the 12 months following PrEP initiation versus the 12 months prior (Incidence Rate Ratio (IRR): 1.72, (95% CI 1.22 to 2.41)).26 Participants in this study attended a greater number of STI screening visits in the year on PrEP (median 5 visits, IQR: 4–5) relative to the year prior to initiating PrEP (median 3 visits, IQR: 2–5). When the relative risk was adjusted for differential rates of screening, we found a moderate increased STI risk in the year following PrEP initiation (adjusted IRR: 1.39, (95% CI 0.98 to 1.96)), although negligible increases in STI risk could not be ruled out. Furthermore, we highlight that the majority of PrEP users did not acquire any STIs (53%) in the 12 months following PrEP initiation, and the overall rates of increased STI risk were likely driven by subset of individuals engaging in more condomless sex. Findings demonstrate once more that PrEP users in our cohort have heterogeneous behaviours and levels of risk. Our results fall in line with other observational studies showing increases in rates of STI detection after initiating PrEP.27 28 STI incidence rates among PrEP users in our cohort are similar to those measured among MSM using PrEP in other settings internationally.29 As noted by others, investigating the relationship between PrEP and potential increases in STI risk is challenging and subjected to several limitations inherent to real world studies when experimental designs cannot be used.30 Specifically, these results are mainly limited by a relatively small sample size, precluding our ability to calculate precise estimates of changes in STI rates by site (ie, anal, oral, urethral) or implement analytic methods to control for temporal change in STI rates.
Patterns of PrEP use
We have investigated PrEP prescription patterns and compared differences between individuals who initiate PrEP as a daily or intermittent ‘on-demand’ regimen.21 Findings indicated that individuals opting to initiate PrEP as an intermittent regimen tend to be older and to have reported fewer sexual partners in the 12 months prior to PrEP initiation. An updated version of this analysis is presented in table 4. Switch rates between regimens during follow-up in the l’Actuel PrEP Cohort were equal; 14% of individuals who initiated daily PrEP switched to intermittent PrEP and 14% of individuals who initiated intermittent PrEP switched to daily PrEP. Similar profiles of on-demand as compared with daily PrEP users have also been observed in Amsterdam’s AMPrEP cohort.31
Table 4.
Baseline sociodemographic and behavioural profiles of individuals prescribed daily versus on-demand PrEP in the l’Actuel PrEP Cohort (2013–2018)
| Baseline variables | Daily PrEP | On-demand PrEP | Total | |
| Age, mean (SD) | 36.1 (10.3) | 39.7 (11.2) | 36.9 (10.6) | |
| Education, n (%) |
Primary | 11 (0.8) | 2 (0.5) | 13 (0.7) |
| Secondary | 173 (12) | 36 (9.0) | 209 (11.3) | |
| College | 252 (17.5) | 71 (17.7) | 323 (17.5) | |
| University | 739 (51.2) | 225 (56.1) | 964 (52.2) | |
| Missing data | 269 (18.6) | 67 (16.7) | 336 (18.2) | |
| Income ($CAD) n (%) |
≤10 000 | 112 (7.8) | 18 (4.5) | 130 (7.0) |
| 10 001–20 000 | 122 (8.4) | 28 (7) | 150 (8.1) | |
| 20 001–35 000 | 165 (11.4) | 43 (10.7) | 208 (11.3) | |
| 35 001–55 000 | 311 (21.5) | 84 (20.9) | 395 (21.4) | |
| 55 001–75 000 | 228 (15.8) | 63 (15.7) | 291 (15.8) | |
| >75 000 | 297 (20.6) | 112 (27.9) | 409 (22.2) | |
| Missing data | 209 (14.5) | 53 (13.2) | 262 (14.2) | |
| Indication for PrEP prescription*
n (%) |
Condomless anal sex | 968 (67.0) | 250 (62.3) | 1218 (66.0) |
| Multiple PEPs | 68 (4.7) | 17 (4.2) | 85 (4.6) | |
| Serodifferent partner | 122 (8.4) | 20 (5.0) | 142 (7.7) | |
| Other reason | 140 (9.7) | 61 (15.2) | 201 (10.9) | |
| Missing data | 274 (19) | 78 (19.5) | 352 (19.1) | |
| Total number of sexual partners within past 12 months, n (%) | <5 partners | 148 (10.2) | 50 (12.5) | 198 (10.7) |
| 5–9 partners | 223 (15.4) | 74 (18.5) | 297 (16.1) | |
| 10–19 partners | 342 (23.7) | 108 (26.9) | 450 (24.4) | |
| 20–29 partners | 176 (12.2) | 56 (14.0) | 232 (12.6) | |
| ≥30 partners | 224 (15.5) | 43 (10.7) | 267 (14.5) | |
| Missing data | 331 (22.9) | 70 (17.5) | 401 (21.7) | |
| Year of PrEP initiation, n (%) | 2015 | 312 (21.6) | 90 (22.4) | 402 (21.8) |
| 2016 | 510 (35.3) | 146 (36.4) | 656 (35.6) | |
| 2017 | 427 (29.6) | 117 (29.2) | 544 (29.5) | |
| 2018 (Jan 1–June 30) | 195 (13.5) | 48 (12.0) | 243 (13.2) | |
| Total, n (%) | 1444 (78.3) | 401 (21.7) | 1845 (100) | |
Note: analysis is restricted to cis-MSM who consulted for PrEP during the time that intermittent PrEP was available at l’Actuel (March 1, 2015–June 30, 2018).
*Indications for PrEP are not mutually exclusive.
MSM, men who have sex with men; PEP, post exposure prophylaxis; PrEP, pre-exposure prophylaxis.
With respect to compliance to the protocol of quarterly PrEP visits, we have found no difference between daily and on-demand PrEP users in a sub-study including individuals who initiated PrEP up to May 1, 2016 (followed up to May 1, 2017).32 Overall, 58% of PrEP users demonstrated optimal protocol compliance (at least one visit per 3 months), 25% had near-optimal compliance (one visit per 3–4 months), 11% had suboptimal compliance (1 visit per 4–6 months) and 5% had inadequate compliance (6 months or more between visits).
Using data from the l’Actuel PrEP Cohort to measure patterns of PrEP discontinuation from January 1, 2011 to September 1, 2017 (n=1258), it was observed that 36% of participants were consistent in their PrEP use and attended routine follow-up visits, whereas 9% of participants used PrEP episodically, defined as temporary discontinuations in their PrEP use of 6 months or longer.23 Additionally 17% of participants reported PrEP discontinuation at a PrEP visit, and 38% of participants ceased returning for PrEP follow-up visits and are considered to have been lost to follow-up. These patterns of PrEP discontinuation observed in our cohort may be reflective of the ‘seasons of risk’ theory, which posits that some individuals may be inclined to seek PrEP during short periods of anticipated risk (such as vacations), and discontinue PrEP after those periods pass.33
We have investigated whether clinic access may be a predictor of being lost-to-follow-up from PrEP care in an exploratory study seeking to evaluate geospatial elements of retention in PrEP care.34 In this study, which included data from 1472 individuals initiating PrEP from January 2011 to April 2018, we found that greater distance from an individual’s residence to the l’Actuel clinic and younger age were associated with lower retention in PrEP care.
Finally, we highlight challenges in delivering PrEP within the l’Actuel PrEP Cohort. First, while the cost of PrEP is subsidised via the Quebec public drug plan, over the course of the study period, users paid a copayment $80–90 CAD for 30 pills of PrEP if not covered by supplemental private insurance or if they do not qualify for exemptions. There are no fees for accessing medical visits to prescribe PrEP or follow-up visits and lab testing is free of charge unless patients opt to pay $15 CAD per visit for an expedited process. Costs may be a barrier to accessing PrEP for lower-income individuals. Our sociodemographic data indicates that the annual income of individuals in the cohort is relatively high, which suggests lower income individuals may not be accessing PrEP as needed. Further efforts to reduce out-of-pocket expenditures for PrEP in Quebec are important to maximise population-level reductions in HIV transmission. National efforts are also underway to make PrEP reimbursable at low-cost in other Canadian provinces.15
Strengths and limitations
A limitation of the l’Actuel PrEP Cohort is that these results represent the experience of a single-site clinic and may not be generalisable to other sites in Quebec or Canada. Unfortunately, there is no data available at present to quantify the total number of PrEP users in Montreal or the proportion of PrEP users accessing care at Clinique l’Actuel. However, we feel that the l’Actuel PrEP Cohort is broadly representative of PrEP users in Montreal given that Clinique l’Actuel is the largest PrEP provider in the city and was the first sexual health clinic to offer this prevention method in Montreal. Following the official approval of PrEP by Health Canada in February 2016,35 more options for accessing PrEP became available (at other sexual health clinics and through individual providers), which may slightly increase the proportion of users that are lost-to-follow-up and continuing PrEP care elsewhere. The Engage study, a cross-sectional respondent-driven sampling survey of MSM in Montreal, found that 78% of PrEP users accessed PrEP through a clinic specialised in sexual health in 2017–2018, such as Actuel.36 A further limitation of the cohort is the use of routinely collected data, leading to missing observations (up to 20% for certain variables), which may present a risk of bias in findings. Finally, as in other cohorts, sexual behaviours are self-reported and subject to reporting bias.
The cohort’s strengths include its large sample size and collection of detailed clinical, behavioural and laboratory data spanning over 5 years. The l’Actuel PrEP Cohort is currently the largest ongoing prospective PrEP cohort in Canada. The cohort is unique due to its substantial number of on-demand PrEP users, making it among the largest cohorts of on-demand PrEP users internationally. Data collection occurred as an integrated part of PrEP care; therefore the results are representative of local PrEP users.
In 2017, Montreal joined the Fast-Track City Initiative towards ending the HIV epidemic. PrEP is a key part of the combined prevention measures towards reducing HIV incidence to meet 95-95-95 targets by 2030. The l’Actuel PrEP Cohort will provide an evidence base to contribute towards public health monitoring of programme implementation. This work fits into international efforts to provide high-quality observational data on PrEP implementation among MSM In high-income countries.
Acknowledgments
The authors thank the l’Actuel PrEP Cohort participants, clinicians, nurses and research staff without whom this work would not have been possible.
Footnotes
Contributors: Study concept and design: ZRG, RT. Data acquisition: JS, JABR, MB, V-KN, RT. Data analysis: ZRG. Data interpretation: ZRG, MM-G, V-KN, RT. Drafting of the manuscript: ZRG, MM-G, V-KN. Critical revision of the manuscript for important intellectual content and final approval: ZRG, MM-G, JS, JABR, MB, V-KN, RT.
Funding: There is no independent funding source for this study. MM-G’s research program is funded by a career award from the Fonds de recherche du Québec—Santé, and grants from the Canadian Foundation for AIDS Research and the Canadian Institutes of Health Research. VKN is supported through the European Research Council Consolidator Grant 617930.
Competing interests: MM-G reports an investigator-sponsored research grant from Gilead Sciences Inc., contractual arrangements from both the World Health Organization and the Joint United Nations Programme on HIV/AIDS (UNAIDS), all outside of the submitted work. JS has served as a consultant and member of a scientific advisory board for ViiV, Gilead, Merck and Teva; and received speakers fees from Gilead, Merck and Theratechnologies. RT is a member of advisory boards for AbbVie, Gilead, Merck and ViiV; has received grants/honoraria from AbbVie, Gilead, Merck and ViiV; and has participated in clinical trials with AbbVie, Gilead, Merck, GSK/ViiV and Janssen. VKN has received honoraria from Gilead.
Ethics approval: Ethics approval was granted by the Veritas Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
Collaborators: Clinique l’Actuel welcomes collaborations. Data from the l’Actuel PrEP Cohort on collected measures outlined in Table 3 may be available for research use. Further information about the data can be obtained by contacting the study principal investigator: rejean.thomas@lactuel.ca. For all collaborative work, relevant ethical approval will need to be sought.
Patient consent for publication: Not required.
References
- 1. Grant RM, Lama JR, Anderson PL, et al. iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–99. 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387:53–60. 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thigpen MC, Rose CE, Paxton LA. Antiretroviral preexposure prophylaxis for HIV prevention. N Engl J Med 2013;368:82–3. 10.1056/NEJMc1210464 [DOI] [PubMed] [Google Scholar]
- 5. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381:2083–90. 10.1016/S0140-6736(13)61127-7 [DOI] [PubMed] [Google Scholar]
- 6. Molina JM, Capitant C, Spire B, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med 2015;373:2237–46. 10.1056/NEJMoa1506273 [DOI] [PubMed] [Google Scholar]
- 7. Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017;4:e402–e410. 10.1016/S2352-3018(17)30089-9 [DOI] [PubMed] [Google Scholar]
- 8. La Direction des communications du ministère de la Santé et des Services sociaux. Avis intérimaire sur la prophylaxie préexposition au virus de l’immunodéficience humaine. 2013.
- 9. PrTruvada® [Canadian Product Monograph] Gilead Sciences Canada, Inc. Missisauga, 2016. [Google Scholar]
- 10. Tan DHS, Hull MW, Yoong D, et al. Canadian guideline on HIV pre-exposure prophylaxis and nonoccupational postexposure prophylaxis. CMAJ 2017;189:E1448–E58. 10.1503/cmaj.170494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh AE, Tan D, Hull M, et al. Canadian guidelines on HIV pre-exposure prophylaxis (PrEP) and non-occupational post-exposure prophylaxis (nPEP): Discussion beyond the guidelines and commentary on the role of infectious diseases specialists. Official Journal of the Association of Medical Microbiology and Infectious Disease Canada 2018;3:165–77. 10.3138/jammi.2018-0024 [DOI] [Google Scholar]
- 12. La Direction des communications du ministère de la Santé et des Services sociaux. La prophylaxie préexposition au virus de l’immunodéficience humaine: Guide pour les professionnels de la santé du Québec. 2019.
- 13. Hull M, Tan D. Setting the stage for expanding HIV pre-exposure prophylaxis use in Canada. Can Commun Dis Rep 2017;43:272–8. 10.14745/ccdr.v43i12a05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arkell C. PrEP in Canada: What do we know about awareness, acceptability and use? CATIE: Prevention in Focus. 2017. Available: https://www.catie.ca/en/pif/spring-2017/prep-canada-what-do-we-know-about-awareness-acceptability-and-use [Accessed 20 Mar 2019].
- 15. Canadian Agency for Drugs and Technologies in Health. CADTH Canadian Drug Expert Committee Final Recommendation: Emtricitbaine/Tenofovir disoproxil fumarate. Common Drug Review; Notice of Final Recommendation, 2016. https://www.cadth.ca/sites/default/files/cdr/complete/SR0479_complete_Truvada_Aug-26-16.pdf
- 16. Thomas R, Galanakis C, Vézina S, et al. Adherence to Post-Exposure Prophylaxis (PEP) and Incidence of HIV Seroconversion in a Major North American Cohort. PLoS One 2015;10:e0142534 10.1371/journal.pone.0142534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lebouché B, Engler K, Machouf N, et al. Predictors of interest in taking pre-exposure prophylaxis among men who have sex with men who used a rapid HIV-testing site in Montreal (Actuel sur Rue). HIV Med 2016;17:152–8. 10.1111/hiv.12286 [DOI] [PubMed] [Google Scholar]
- 18. Statistics Canada. Table 11-10-0239-01 Income of individuals by age group, sex and income source, Canada, provinces and selected census metropolitan areas. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1110023901&pickMembers%5B0%5D=1.7&pickMembers%5B1%5D=2.1&pickMembers%5B2%5D=3.1&pickMembers%5B3%5D=4.1 [Accessed 20 Mar 2019].
- 19. Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline. 2018. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
- 20. Daoust-Boivert A. Inquiétudes devant la montée du «chemsex». Le Devoir 2018. https://www.ledevoir.com/societe/sante/531229/chemsex-sexe-drogue-et-vies-brisees (Cited 2018-12-26). [Google Scholar]
- 21. Samson F. Une pilule contre le VIH, l’autre révolution sexuelle. Radio-Canada 2016. https://ici.radio-canada.ca/nouvelle/800200/vih-truvada-prevention-sida-revolution-sexuelle (Cited 2018-12-26). [Google Scholar]
- 22. Paré E. Québec refuse de rendre gratuit un médicament essentiel à la prévention du sida. Le Journal de Montréal 2018. https://www.journaldemontreal.com/2018/02/05/quebec-refuse-de-rendre-gratuit-un-medicament-essentiel-a-la-prevention-du-sida (Cited 2018-12-26). [Google Scholar]
- 23. Greenwald Z, Beauchemin M, Benomar K, et al. High Seroconversion Rates Following PrEP Discontinuance in a Montreal Clinic. Conference on Retroviruses and Opportunistic Infections. 2018.
- 24. Blumenthal J, Haubrich RH. Will risk compensation accompany pre-exposure prophylaxis for HIV? Virtual Mentor 2014;16:909–15. 10.1001/virtualmentor.2014.16.11.stas1-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas R, Galanakis C, Vézina S, et al. P-01-066 PrEP in Montreal: good adherence, no seroconversion and no evidence of risk compensation. J Sex Med 2016;13:S165 10.1016/j.jsxm.2016.03.217 [DOI] [Google Scholar]
- 26. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS 2018;32:523–30. 10.1097/QAD.0000000000001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of Pre-exposure Prophylaxis for the Prevention of Human Immunodeficiency Virus Infection on Sexual Risk Behavior in Men Who Have Sex With Men: A Systematic Review and Meta-analysis. Clin Infect Dis 2018;67:676–86. 10.1093/cid/ciy182 [DOI] [PubMed] [Google Scholar]
- 28. Traeger MW, Cornelisse VJ, Asselin J, et al. PrEPX Study Team. Association of HIV Preexposure Prophylaxis With Incidence of Sexually Transmitted Infections Among Individuals at High Risk of HIV Infection. JAMA 2019;321:1380–90. 10.1001/jama.2019.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Werner RN, Gaskins M, Nast A, et al. Incidence of sexually transmitted infections in men who have sex with men and who are at substantial risk of HIV infection - A meta-analysis of data from trials and observational studies of HIV pre-exposure prophylaxis. PLoS One 2018;13:e0208107 10.1371/journal.pone.0208107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marcus JL, Volk JE, Snowden JM. Concerns about a study on sexually transmitted infections after initiation of HIV preexposure prophylaxis. AIDS 2018;32:955–6. 10.1097/QAD.0000000000001769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoornenborg E, Achterbergh RC, van der Loeff MFS, et al. Men who have sex with men more often chose daily than event-driven use of pre-exposure prophylaxis: baseline analysis of a demonstration study in Amsterdam. J Int AIDS Soc 2018;21:e25105 10.1002/jia2.25105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenwald Z, Beauchemin M, Girard G, et al. From PrEP Prescription to Usage, a Strategy which Adapts to Patients' Realities. Poster presented at the Adherence 2017 Conference. Miami, 2017. [Google Scholar]
- 33. Elsesser SA, Oldenburg CE, Biello KB, et al. Seasons of Risk: Anticipated Behavior on Vacation and Interest in Episodic Antiretroviral Pre-exposure Prophylaxis (PrEP) Among a Large National Sample of U.S. Men Who have Sex with Men (MSM). AIDS Behav 2016;20:1400–7. 10.1007/s10461-015-1238-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greenwald Z, Card K, Niaki N, et al. Geographic barriers result in HIV pre-exposure prophylaxis discontinuation: how to improve retention in care. JIAS 2018;21. [Google Scholar]
- 35. Health Canada. Notice of Compliance: Truvada, 2016. Available: https://health-products.canada.ca/noc-ac/info.do?lang=en&no=17808 [Google Scholar]
- 36. Apelian H, Messier-Peet M, Cox J, et al. KP4.05 PrEP-use Experience Among Gay, Bisexual, and Other Men Who Have Sex With Men (gbMSM) in Montreal. 27th Annual Canadian Conference on HIV/AIDS Research. Vancouver, 2018. Available: http://www.cahr-acrv.ca/wpcontent/uploads/2018/04/CAHR2018-Abstract-Book.pdf [Google Scholar]