Abstract
Objectives
Many studies have explored the association between Helicobacter pylori infection and osteoporosis. However, the results remain controversial. Therefore, we performed this systematic review and meta-analysis to evaluate the association between H. pylori infection and osteoporosis.
Design
Systematic review and meta-analysis of case–control studies.
Data sources
Databases, including PubMed, Embase, Web of Science and Chinese Biomedical Literature Database, were screened from inception to 30 April 2018.
Eligibility criteria
Case–control studies aimed at assessing the association between H. pylori infection and osteoporosis.
Data extraction and analysis
Study characteristics and study quality sections were reviewed. Studies were selected, and data were extracted by two reviewers. Pooled ORs and 95% CIs were calculated using random effects model if heterogeneity existed; otherwise, fixed effects model was used. Subgroup analyses were performed to explore the source of heterogeneity. Publication bias and sensitivity analyses were also tested.
Results
A total of 21 studies with 9655 participants were included in our analyses. Taking together, we found that H. pylori infection was associated with increased odds of osteoporosis (OR (95% CI): 1.39 (1.13 to 1.71)); there was no significant difference between osteoporosis and osteopaenia; the association between osteoporosis and H. pylori infection was relatively higher in men than women but did not reach significant level. However, the decrease of bone mineral density in H. pylori-positive patients was not significant when compared with H. pylori negative controls, which may due to the sample size.
Conclusions
Our meta-analysis suggests an association between osteoporosis and H. pylori infection. The clinicians should pay more attention to the patients infected with H. pylori. Further studies were still needed to exploring the confounding factors among studies and to elucidate the underlying biological mechanisms.
Keywords: osteoporosis, bone mineral density, helicobacter pylori, meta-analysis
Strengths and limitations of this study.
Twenty-one studies with conflicting results were included for testing the association between osteoporosis and Helicobacter pylori infection.
This is the third and most comprehensive meta-analysis, bringing the overall results of statistical significance and increased odds.
The results of the meta-analysis should be interpreted with caution due to the number and quality of studies included and obvious heterogeneity.
Causality cannot be established in observational study as the chronological order between H. pylori infection and osteoporosis cannot be confirmed.
Introduction
Helicobacter pylori, a Gram-negative and spiral-shaped bacterium dwelling on the gastric epithelium, has an influence on approximately 50% of the global population, especially those living in developing countries.1 The prevalence of H. pylori infection is approximately 30% in developed countries and up to 80% in developing countries2 3 and up to 90% in patients with dyspepsia.4 In North Europe and North America, about one-third of adults are infected, and in South and East Europe, South America and Asia, the prevalence of H. pylori is often higher than 50%.5 Moreover, infected subjects born abroad (first-generation immigrants) had a higher risk of H. pylori infection than second-generation immigrants in a multiethnic European city.6 H. pylori has been well known to be associated with gastrointestinal diseases, such as gastritis, gastric ulcer, stomach cancer and so on.7 Furthermore, some non-gastrointestinal diseases have also been proven to be associated with H. pylori by large-scale population researches or meta-analysis, such as pre-eclampsia,8 autoimmune thyroid diseases,9 myocardial infarction,10 hepatic encephalopathy11 and prostatitis.12
Osteoporosis is one of the most common metabolic bone diseases, characterised by decreased bone mineral density (BMD), increased bone fragility and then increased susceptibility to fracture,13 especially in spine and hip. Osteoporosis has become a major health concern for both individuals and societies. Osteoporosis has huge adverse impacts on life quality and is associated with increased morbidity rates. The in-hospital mortality rate is between 0.85% and 2.26%.14 In Europe, about half of women and one-fifth of men aged over 50 years develop pathological fractures in hip, spine, forearm or humerus due to osteoporosis during their remaining lifetime.15 The same situation happens in other countries or districts, such as Japan and Taiwan.16 17
There are well-established evidence regarding the risk factors for osteoporosis,17 such as age, sex, body mass index, alcohol and smoking. H. pylori infection can induce inflammatory and immune responses, such as increasing the level of interleukin (IL)-1 and tumour necrosis factor (TNF)-α, which could trigger bone resorption and regulate bone regeneration.18 Recently, many studies about the association between osteoporosis and H. pylori have been performed. However, the role of H. pylori in osteoporosis remains controversial. This issue has been discussed in previous meta-analysis,19 20 but no significant association was found. As more studies evaluating the association between H. pylori infection and osteoporosis have been published since then,2 21–27 we carried out this updated meta-analysis to further evaluate the association between H. pylori infection and osteoporosis qualitatively and the quantitative alterations of BMD in H. pylori-infected patients compared with those in healthy controls.
Materials and methods
This study was reported based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).28 Study searching and selection, quality assessment and data extraction were done by two researchers (TW and XL) independently to avoid bias, and disagreements were discussed by the two reviewers and by seeking the opinion of the third author (YZ) if necessary.
Search strategy
We searched through the databases of PubMed, Embase, Web of Science and Chinese Biomedical Literature Database (CBM). The last search for all databases was updated to 30 April 2018. We used the combined method of Medical Subject Headings (MeSH) Term and free words by applying the following terms: Helicobacter pylori, campylobacter pylori, H. pylori, hp, helicobacter, helicobacter bill, helicobacter hepaticus, helicobacter pullorum, helicobacter species, helicobacter sp, helicobacter genus, campylobacter, campylobacter infection, campylobacteriosis, Helicobacter pylori infection, Helicobacter infection, pylori, enterohepatic helicobacter spp, campylobacter sp and fragility fracture, bone density, bone mass density, osteocalcin, bone loss and osteoporosis. The search strategy is presented in the online supplementary appendix 1. Two authors evaluated potential publications by checking their titles and abstracts and then procured the most relevant publications for further examination. Bibliographies section of retrieved articles were also reviewed for additional pertinent studies that were possibly missed in the initial search.
bmjopen-2018-027356supp001.pdf (95.2KB, pdf)
Studies selection and data extraction
Studies were included if they met the following criteria: (1) it is an observational study; (2) its objective is to assess the association between H. pylori infection and osteoporosis or compare the alteration of BMD between H. pylori-positive and H. pylori-negative participants; (3) they either provided ORs and 95% CIs, or sufficient information was available to calculate the ORs and 95%CIs, or BMD in both H. pylori-positive and H. pylori-negative participants. Articles were excluded if they were duplicate publications, reviews, animal studies, editorials or case reports. The papers were also excluded if no effect estimate was reported or not enough raw data for ORs and 95% CIs calculation was available. In the case of multiple studies with the same or overlapping data published by the same researchers, we selected the most recent study with the largest number of participants. All papers meeting the criteria defined above were included for further analysis.
The literatures included were carefully reviewed for information about the first author, publication year, country, population, sample size, sex, age, detection methods of H. pylori and osteoporosis, diagnosis location, diagnosis and adjusted covariates.
If data could be acquired from the tabulated literature search results, they would be extracted carefully into 2×2 tables from all eligible publications by two independent reviewers. If data were not directly available, they would be calculated from published positive predictive values and/or negative predictive values if appropriate. The adjusted OR (95% CI), if existed, was adopted instead of rude OR (95% CI).29 In addition, for the studies comparing the BMD of participants with and without H. pylori infection, the data on BMD were also extracted.
Quality assessment
Quality assessment was performed using the Newcastle-Ottawa Quality Assessment Scale(NOS).30 Two researchers conducted blinded quality assessment of the included literatures. The NOS assigns a maximum of 9 points to studies of highest quality according to three quality parameters: selection, comparability and outcome.
Statistical analyses
The primary measures were ORs and 95% CIs for the association between H. pylori infection and osteoporosis, and standardised mean difference (SMD) for BMD alterations between H. pylori-positive and H. pylori-negative participants. To assess heterogeneity among the studies, we calculated the Cochran’s χ2 test (with p<0.10 indicating statistically significant heterogeneity) and the statistic I 2 (the heterogeneity might not be important with I 2 of 0%–40%, while moderate heterogeneity with I 2 of 30%–60%, substantial heterogeneity with I 2 of 50%–90% and considerable heterogeneity with I 2 of 75%–100%).31 The pooled results were calculated using fixed effects model (inverse variance) if no obvious heterogeneity existed; otherwise, random effects model (I–V heterogeneity) was used (p<0.10 was considered indicative of obvious heterogeneity). The cumulative meta-analysis was conducted for the extracted data using a pooled random effects model with the publication year to confirm whether the effect size was affected by sample size or not. In the event of obvious heterogeneity, subgroup analysis was performed according to sex, postmenopausal or not, country, Asian or not, detection methods of H. pylori, detection methods of osteoporosis and detection location of dual-energy X-ray absorptiometry (DEXA). Meta regression (using ReML methods) was also performed to explore the potential heterogeneity. Publication bias was assessed by funnel plot and Egger’s test.32 A sensitivity analysis was completed by converting the pooled results from random effects model into fixed effects model or from fixed effects model into random effects model. All statistical analyses were performed using Stata V.12.0.
Patient and public involvement
There was no patient and public involvement as this was a database research study.
Results
Search results
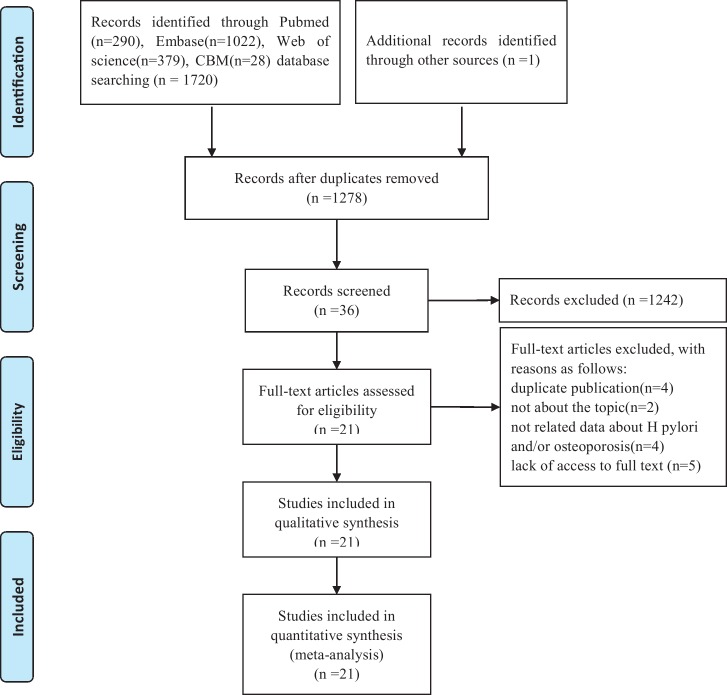
Using our search strategy, a total of 1720 articles were identified through PubMed, Web of Science, Embase and CBM, and one additional record was identified through other sources. Then, 443 duplicate papers were removed first, and 1242 papers were excluded after scanning their titles and abstracts. After screening the full texts of the included articles, 15 studies were excluded for the following reasons: duplicate publication (n=4), not about the topic (n=2), no related data about H. pylori and/or osteoporosis (n=4) and lack of access to full text (n=5). A total of 21 studies2 16 21–27 33–44 were included for further analysis (figure 1).
Figure 1.
Flow diagram of the article selection for systematic review.
Study characteristics
A total of 21 articles were included in this study. Of the 21 articles included, 20 provided data for association between H. pylori and osteoporosis,2 16 21–27 33–41 43 44 4 for the BMD alterations in H. pylori positive participants compared with negative controls23 34 35 42 and 3 provided both23 34 35. All these studies were published from 2005 to 2018. Four studies were conducted in China, three in Iran, one in Italy, nine in Japan, two in Brazil, one in Korea and one in Turkey. As to the sex of participants, four were postmenopausal women, four were women, four were men, nine involved both men and women. The detection methods of H. pylori were mainly ELISA and 13C-urea breath test, while the detection methods of osteoporosis were DEXA and quantitative ultrasound. As to the diagnosis, 5 were osteopaenia, 13 were osteoporosis and 2 provided decreased BMD (treated as osteopaenia for analysis) (table 1). In addition, 12 studies showed no significant associations of H. pylori infection and osteoporosis (or osteopaenia), while 8 showed significant associations.
Table 1.
Characteristics and quality assessment of the studies included
| Author (reference) |
Year | Country | Sex (M/F) | Age (mean age±SD or (range age) years) | Detection method of H. pylori | Detection methods of osteoporosis | Diagnosis locations | Diagnosis | Cases /controls /total |
Scores of NOS | Main adjusted factors (the methods used for adjusting) |
| Figura et al 44 | 2005 | Italy | Males | 65 (55–82) for patients; 64.5 (55–80) for controls. |
ELISA | DEXA | Lumbar and femur bone. | Osteoporosis. | 80/160/240 | 7 | Age, socioeconomic background and smoking habits. |
| Kakehasi et al 43 | 2007 | Brazil | Postmenopausal women | 61.6±7 (50–79). | Non-ELISA | DEXA | Lumbar spine. | Osteoporosis. | 18/32/50 | 6 | Mean age, body mass index, age at menarche and postmenopausal period. |
| Kakehasi et al 42 | 2009 | Brazil | Postmenopausal women | 63.7±7.3 for Hp(+). 62.5±7.0 for Hp(−). |
Non-ELISA | DEXA | Lumbar spine and hip. | Not fitted.* | 34/27/61 | - | Age, postmenopausal time and BMI. |
| Akkaya et al 41 | 2011 | Turkey | Postmenopausal women | 65.29±6.09 patients; 63.57±6.53 controls. |
ELISA | DEXA | Lumbar and femur neck. | Osteoporosis. | 58/47/105 | 6 | Age, education level, occupation, age of menarche or menopause, duration of postmenopausal, period or daily consumption of tea, coffee, alcohol or dairy products. |
| Chinda et al 40 | 2013 | Japan | 379/631 | Not mentioned. | ELISA | QU | Calcaneal osteo. | Osteopaenia. | –/–/1010 | 7 | Age, BMI, smoking, alcohol consumption, periodical exercise and latest educational level (logistic regression analysis). |
| Asaoka et al 39 | 2014 | Japan | 95/105 | 63.1±8.8 years. | Both | DEXA | Lumbar vertebrae. | Osteoporosis. | 41/159/200 | 6 | Age, gender, BMI, alcohol consumption, smoking, BAP, PUD and EGA (multivariate logistic regression analysis). |
| Asaoka et al 38 | 2014 | Japan | 131/26 | 71.1±7.5 patients. 61.6±8.9 controls. |
Not mentioned | DEXA | Lumbar. | Osteoporosis. | 24/133/157 | 6 | Age, sex, BMI, Brinkman idex (BI) and accmulated amount of alcohol (multivariate analysis). |
| Lin et al 37 | 2014 | China | Female | 77 (65–97). | Non-ELISA | DEXA | Not mentioned. | Osteoporosis. | 101/264/365 | 5 | Age group, body mass index group and use of proton-pump inhibitor (multivariate logistic regression analyses). |
| Asaoka et al 36 | 2015 | Japan | 130/134 | 69.8±6.8 for patients. 61.9±8.2 for controls. |
Not mentioned | DEXA | Not mentioned. | Osteoporosis. | 45/219/264 | 7 | Age, sex, BMI and so on (multivariate analysis). |
| Asaoka et al 16 | 2015 | Japan | 120/135 | 63.2±8.5. | Both | DEXA | Lumbar vertebrae. | Osteoporosis. | 43/212/255 | 6 | Age, sex, BMI, cumulative alcohol intake, BI, type 2 diabetes mellitus, calcium channel blocker, PPI, haemoglobin, calcium, gamma glutamyl transpeptidase, bone-specifi alkaline phosphatase, NTX, hiatal hernia and EGA (multivariate logistic regression analysis). |
| Chung et al 35* | 2015 | Korea | Men | 54.4±10.7 for Hp+. 51.9±12.1 for Hp−. |
ELISA | DEXA | Lumbar (L1–L4). | Osteopaenia. | –/–/1126 | 7 | Height, weight, BMI, alcohol and exercise. |
| Fotouk-Kiai et al 34* | 2015 | Iran | 575/392 | 68.3±6.8 for Hp+. 69.3±7.4 for Hp−. |
ELISA | DEXA | Lumbar vertebra and femur. | Osteoporosis. | 314/653/967 | 5 | Age, sex, smoking, alcohol consumption and BMI. |
| Mizuno et al 33 | 2015 | Japanese | Men | 62.1±5.0 for low TBD. 58.4±5.7 for normal. |
ELISA | QU | Not mentioned. | Decreased BMD. | 116/114/230 | 8 | Age, BMI and smoking habit (logistic regression analysis). |
| Chinda et al 21 | 2016 | Japan | Men | 50.2±15.4 years. | ELISA | QU | Not mentioned. | Osteopaenia. | –/–/295 | 7 | Age, BMI, serum level of oestradiol, the intake of calcium per day, smoking, drinking, periodical exercise and last educational background (logistic regression). |
| Chinda et al 22 | 2016 | Japan | Females | 52.2±15.2. | ELISA | QU | Not mentioned. | Osteopaenia. | –/–/473 | 6 | Age, BMI, smoking, alcohol consumption, periodical exercise, last educational level, serum level of oestradiol and calcium intake per day (multiple logistic regression). |
| Kalantarhormozi et al 23* | 2016 | Iran | Postmenopausal women | 58.87±8.02. | ELISA | DEXA | Lumbar spine and femur. | Osteoporosis. | 16/234/250 | 6 | Age and BMI (multiple linear regression). |
| Zhan et al 24 | 2016 | China | 194/126 | 38.32±10.64 for patients. 38.27±7.46 for controls. |
Non-ELISA | DEXA | Not mentioned. | Osteoporosis. | 160/160/320 | 5 | Age, gender and gene (multiple logistic regression). |
| Abdolahi et al 25 | 2017 | Iran | Postmenopausal women | Not mentioned. | ELISA | Not mentioned | Not mentioned. | Osteoporosis. | 73/34/107 | 8 | Not mentioned. |
| Chinda et al 26 | 2017 | Japan | Females | 62.5±8.6 for patients. 44.9±10.9 for controls. |
ELISA | QU | Calcaneus. | Osteopaenia. | 197/276/473 | 4 | Age, smoking and drinking habit, schooling duration, estradiol levels, menopause, birth history (multiple logistic regression analysis). |
| Lu et al 2 | 2018 | China | 1474/393 | 54.0±9.6. | Non-ELISA | QU | Calcaneus. | Osteoporosis. | 900/967/1867 | 6 | Gender and age. |
| Pan et al 27 | 2018 | China | 568/299 | 55.9±11.3. | Non-ELISA | DEXA | Not mentioned. | Decreased BMD. | 311/556/867 | 5 | Sex, age, BMI, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and peptic ulcer disease (multiple stepwise logistic regression analyses). |
Not fitted*: this study only explored the alteration of BMD in patients with H. pylori infection.
*This study also reported the BMD in patients with H. pylori infection.
BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; DEXA, dual-energy X-ray absorptiometry scan; EGA, endoscopic gastric mucosal atrophy; H. pylori, Helicobacter pylori; NOS, Newcastle-Ottawa S cale; NTX, collagen type I cross-linked N telopeptide; PPI, proton pump inhibitor; PUD, peptic ulcer disease; TBD, trabecular bone density; QU, quantitative ultrasound.
Quality evaluation
The NOS was adopted to evaluate the quality of these case–control studies. Among the selection items, the evaluation results ranged from 4 to 8, with the median NOS score was 6, indicating a medium quality of the studies included. The most common source of bias came from selection and comparability (table 1)
Synthesis of the results
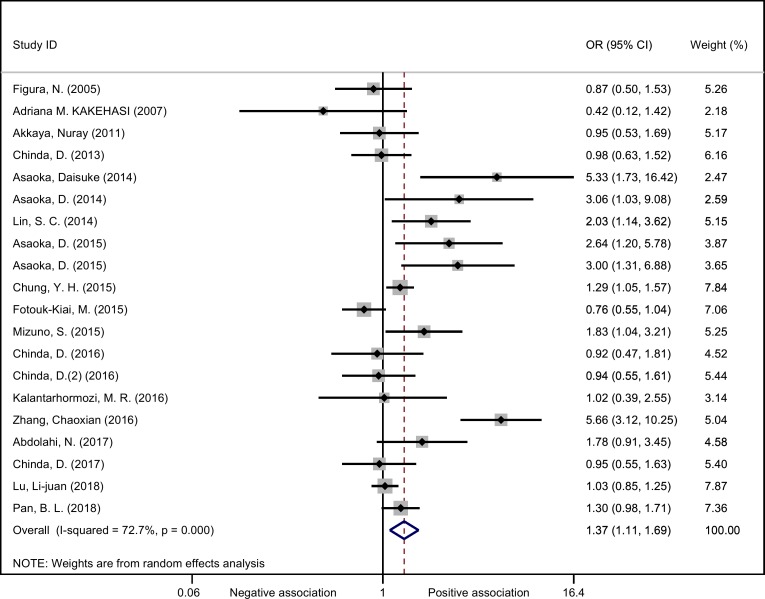
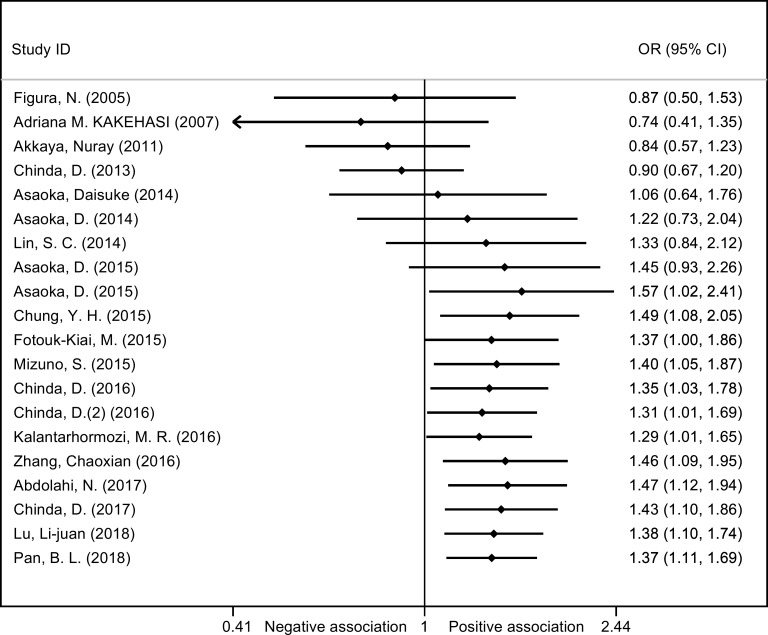
As shown in figure 2, the overall OR was obtained based on the 20 studies involving the H. pylori and osteoporosis (including osteopaenia) (a total of 8788 patients and healthy controls). As the existence of obvious heterogeneity (χ2=69.60, I2=72.7%, p<0.01), random effect model was used and the pooled results of OR and its 95% CI were 1.37 (1.11 to 1.69), indicating H. pylori infection was significantly associated with increased odds of osteoporosis/osteopaenia. A cumulative meta-analysis was conducted with publication year in ascending order, and the results indicated that the pooled OR (95% CI) started to show statistical significance at 1.57 (95% CI 1.02 to 2.41) from the ninth analysed study, with gradually stabilising results afterwards (figure 3).
Figure 2.
Forest plot of the included studies assessing the association between Helicobacter pylori and osteoporosis (random effects models).
Figure 3.
Forest plot of cumulative meta-analysis.
Subgroup analyses
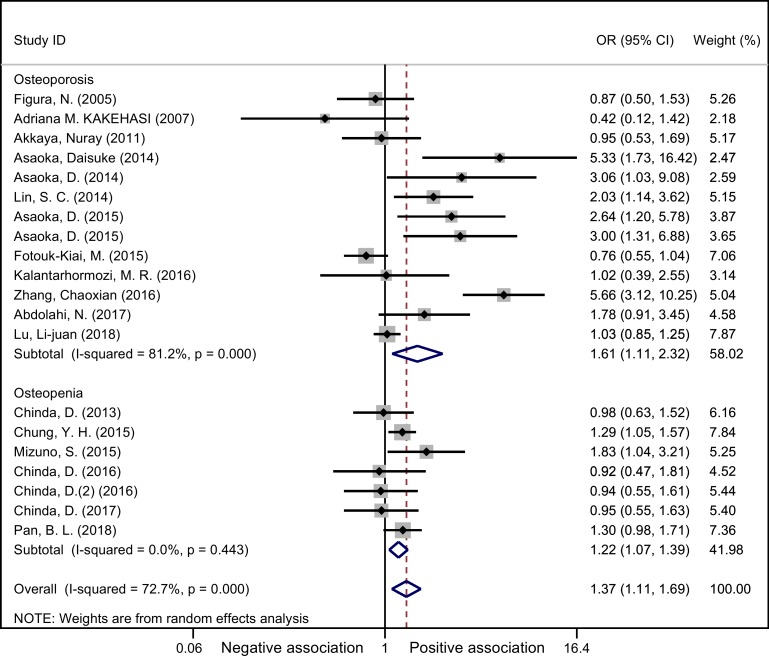
Given that obvious heterogeneity existed, subgroup analyses were performed based on the potential confounding factors. All 20 studies were involved in these subgroup analyses. Figure 4 showed that both osteoporosis and osteopaenia were significantly associated with H. pylori infection with OR (95% CI) of 1.61 (1.11 to 2.32) and 1.22 (1.07 to 1.39), respectively. Although the OR was a little higher in osteoporosis group, the meta regression analysis showed no significant difference between these two groups (t=1.18, p=0.26). Therefore, we pooled osteoporosis and osteopaenia together to analyse other confounding factors.
Figure 4.
Forest plot of subgroup meta-analysis according to diagnosis (random effects models).
Results of subgroup analyses by other factors were shown in table 2. We found that the association between H. pylori infection and osteoporosis was significant in men and both sexes, but not in women. However, meta-regression analysis showed no significant difference between these two groups. Moreover, no significant associations between H. pylori infection and osteoporosis were observed in either the postmenopausal women or non-postmenopausal women subgroup. When stratified by countries, we found significant associations between H. pylori infection and osteoporosis in China, Japan and Korea (three East Asian countries). Other factors that may affect the results were presented in table 2.
Table 2.
Overall effect estimates for Helicobacter pylori infection and osteoporosis according to study characteristics
| Factors | Categories | No. of studies | OR (95% CI) | Model used | Heterogeneity | Meta-regression | ||
| I2 | P value | t | P value | |||||
| Sex* | ||||||||
| Women | 8 | 1.09 (0.87 to 1.35) | Fixed | 33.0% | 0.17 | – | – | |
| Men | 5 | 1.27 (1.07 to 1.50) | Fixed | 14.6% | 0.32 | 0.47 | 0.64 | |
| Both | 9 | 1.21 (1.07 to 1.37) | Random | 85.6% | 0.00 | 1.78 | 0.09 | |
| Postmenopausal or not | ||||||||
| Non-postmenopausal women | 4 | 1.08 (0.83 to 1.41) | Fixed | 48.0% | 0.12 | – | – | |
| Postmenopausal women | 4 | 1.09 (0.75 to 1.58) | Fixed | 35.8% | 0.20 | −0.13 | 0.90 | |
| Country | ||||||||
| China | 4 | 1.86 (1.06 to 3.28) | Random | 90.4% | 0.00 | – | – | |
| Japan | 9 | 1.57 (1.08 to 2.28) | Random | 63.7% | 0.005 | −0.39 | 0.70 | |
| Italy | 1 | 0.87 (0.50 to 1.53) | ≈ | – | −1.11 | 0.29 | ||
| Brazil | 1 | 0.42 (0.12 to 1.42) | – | – | −1.69 | 0.11 | ||
| Korea | 1 | 1.29 (1.05 to 1.57) | – | – | −0.59 | 0.57 | ||
| Iran | 3 | 1.06 (0.60 to 1.86) | Random | 61.3% | 0.075 | −1.16 | 0.27 | |
| Turkey | 1 | 0.95 (0.53 to 1.69) | – | – | −0.98 | 0.34 | ||
| Asian country or not | ||||||||
| Non-Asian country | 2 | 0.77 (0.46 to 1.28) | Fixed | 12.8% | 0.28 | – | – | |
| Asian country | 18 | 1.44 (1.16 to 1.79) | Random | 73.9% | 0.00 | 1.60 | 0.13 | |
| Detection methods of H. pylori | ||||||||
| ELISA | 11 | 1.09 (0.96 to 1.24) | Fixed | 32.1% | 0.14 | – | – | |
| Non-ELISA | 5 | 1.62 (0.96 to 2.72) | Random | 88.4% | 0.00 | 1.52 | 0.15 | |
| Both | 2 | 3.67 (1.88 to 7.16) | Fixed | 0% | 0.42 | 2.65 | 0.02 | |
| Detection methods of osteoporosis | ||||||||
| DEXA | 13 | 1.58 (1.14 to 2.18) | Random | 79.5% | 0.00 | – | – | |
| QU | 6 | 1.05 (0.90 to 1.22) | Fixed | 0% | 0.51 | −1.33 | 0.20 | |
| Detection location of DEXA | ||||||||
| Lumbar | 6 | 1.75 (0.99 to 3.07) | Random | 65.4% | 0.013 | – | – | |
| Femur | 3 | 1.56 (1.17 to 2.08) | Fixed | 0% | 0.90 | −0.17 | 0.87 | |
* One study reported males, females and overall results, therefore this study was used in all subgroups analysis (males, females and both).
DEXA, dual-energy X-ray absorptiometry scan; QU, quantitative ultrasound
Publication bias and sensitivity analyses
Funnel plot was used to examine the publication bias of this meta-analysis. As shown in figure 5, the funnel plot indicated no publication bias, which was also confirmed by Egger’s test, with t of 1.57 and p of 0.13 in figure 6. Sensitivity analysis was also performed by converting the pooled model from the random effects model to the fixed effects model. The result of fixed effects model was 1.21 (1.10–1.33), which showed no obvious differences compared with the result of random effects model, indicating the pooled results was relatively stable.
Figure 5.
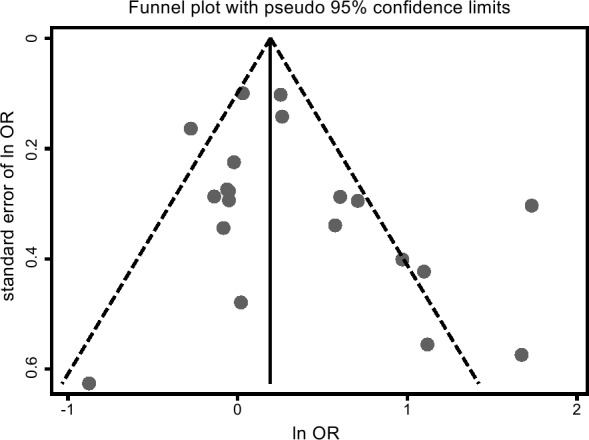
Funnel plot of publication bias for the association between Helicobacter pylori and osteoporosis.
Figure 6.
The Egger’s test for publication bias.
Alterations of BMD in H. pylori-infected population
Four studies were involved in this meta-analysis.23 34 35 42 As each study included for this analysis has two different DEXA detection locations, we carried out the subgroup analysis based on detection locations, and the patients are not being counted twice in each subgroup. In addition, we chose SMD as pooled outcome for the detection methods varied with studies. As shown in online supplementary appendix 2, the BMD (g/cm2) alterations between H. pylori-positive and H. pylori-negative participants were −0.01 (−0.45 to 0.42) for hip, −0.94 (−3.15 to 1.28) for lumber and −0.04 (-0.40 to 0.31) for femur using random effects model as obvious heterogeneity existed. No significant associations were observed so far.
Discussion
Although osteoporosis is not a deadly disease, it causes huge burden to individuals and society owing to its high morbidity. Here, we got a comprehensive result by meta-analysis, indicating that H. pylori infection may be a risk factor for osteoporosis. However, the mechanism is still unclear. Several possible mechanisms may explain this result. First, H. pylori infection may lead to systemic inflammation, and release of cytokines, such as TNF-α, IL-1 and IL-6,45 which may cause bone turnover indirectly. Second, many studies have shown that low vitamin B12 may be associated with H. pylori infection.46 If the serum vitamin B12 levels are decreased, the folate becomes trapped as methyltetrahydrofolate and interrupts for folate-related DNA synthesis, which is an important factor for bone remodelling. Therefore, the decrease of vitamin B12 may lead to decreased BMD and osteoporosis.47 Third, H. pylori infection may decrease the calcium absorption by causing the gastric mucosal atrophy and decreasing acid secretion. Thus, eradication of H. pylori may increase calcium absorption and stop the process of osteoporosis through decreasing the levels of inflammatory cytokines and improving gastric mucosal atrophy.
The present meta-analysis of 20 studies indicated that patients with H. pylori infection were associated with an estimated 1.37 times higher ORs of developing osteoporosis as compared with those without H. pylori infection, while no associations were found in previous meta-analysis19 20 (one had five studies involving 1321 participants, and one had four studies involving 520 participants). As the previous meta-analysis studies had no quality assessment and our analysis included more studies and participants, the results in our study might be more reliable than the previous meta-analysis studies.
Despite the significant association between H. pylori infection and osteoporosis, obvious heterogeneity existed between the included studies. We found that sex of participates may affect the results. As known to all, women and postmenopausal women are independent risk factors of osteoporosis. Here, we explored the relationship between osteoporosis and H. pylori infection and found that the relationship was significant in men, but not in women (whether postmenopausal or not), which was not paradoxical with the fact that women with postmenopausal should have a higher risk of osteoporosis than men. In the group of both sexes, the results showed statistic difference and obvious heterogeneity, which may be due to the ratio of M/F and other confounding factors. Therefore, we might suggest that more attention should be paid to men than women in H. pylori-positive patients. However, only seven studies (four were about postmenopausal women and three were about non-postmenopausal women) were conducted in women, the results may be not that reliable due to the small sample size. Another reason may also be possible that the different degree of osteoporosis may affect the diagnosis, and some early patients may be regarded as healthy controls. Further studies with dose–response relationship of different severity of osteoporosis and prevalence may help to confirm this hypothesis. In the subgroup analysis by criteria (osteoporosis and osteopaenia), the OR in osteoporosis was a little higher than that in osteopaenia, which may also help to prove our hypothesis. In the subgroup analysis based on countries, significant association was evidenced in three East-Asian countries (China, Japan and Korea are from East Asia), indicating many other factors associated with geography may affect the results. As only two studies were non-Asian countries, the reason of this phenomenon may be due to the sample size, the same situations also happened in other Asian countries with only one study included. In addition, as most studies included in our studies were Asian countries, especially East Asia, whether our findings can be applied to other populations around the world needs further exploring.
In our research, we also explored the heterogeneity from diagnosis methods factors. We found that the detection methods of osteoporosis (DEXA and quantitative ultrasound) affected the pooled results, and the detection locations of DEXA also contributed to the heterogeneity. From our results, we thought that DEXA might be a better tool to diagnose osteoporosis in assessing the association between H. pylori and osteoporosis. The same situation also happened in the detection methods of H. pylori. We found ELISA and multimethod strategy may provide more homogeneous results. In total, in despite of the significant association between H. pylori and osteoporosis, as the heterogeneity still existed obviously, further studies were still needed to address its potential confounding factors.
In a previous meta-analysis study, Wijarnpreecha et al 48 found increased odds of non-alcoholic fatty liver disease (NAFLD) among patients infected with H. pylori. However, Upala et al 49 found that no significant difference in BMD between patients with fatty liver disease and controls.49 Combine the two meta-analysis and our results, we may guess that H. pylori may be an independent risk factor of NAFLD and osteoporosis, and/or H. pylori infection may be an important confounding factor in exploring the relationship between NAFLD and osteoporosis, or no actual relationship between NAFLD and osteoporosis exists. However, as the authors stated,49 the review was a preliminary result because of limited amount of literature, it might be too early to have definite conclusion.
We also compared the quantitative alterations of BMD in H. pylori infected subjects. However, no significant difference was found. The reason may be that: (1) the sample size was relatively small, (2) the severities of H. pylori infection were not serious, or the infection of H. pylori did not last long enough to cause alterations, (3) though the basic characteristics of included studies were comparable, many other confounding factors that might affect BMD have not been adjusted. Therefore, more studies with large sample size were still needed to verify the alterations of BMD in H. pylori infection.
The strength of the present meta-analysis lies in inclusion of 21 observational studies reporting data on H. pylori infection and osteoporosis and the alterations of BMD by H. pylori. However, our meta-analysis has several limitations that should be recognised when interpreting the results. First, most of the included studies were hospital based or health centre based, which were not affected by detection bias but might be subjected to selection bias. However, the prevalence of H. pylori infection in most studies that we selected was consistent with the incidence rate in the general population. Second, our analysis had an ascertainment bias that might be present because progression of osteoporosis is continuous, and some patients may be classified as controls. However, this may lead to a more conservative result, which may help to indicate that our overall result is reliable. Third, the heterogeneity is still obvious. However, we performed subgroup analyses based on study characteristics and found that some factors may affect the association. In addition, when available, adjusted estimates were used in preference to unadjusted estimates. Even though the adjusted estimates may be closer to the true effect for adjusted results could control confounding factors,50 51 the different adjusted factors in different studies may also contribute to the heterogeneity. Four, the qualities of included studies were medium, and some studies were published informally. We also included all these studies based on inclusion and exclusion criteria to avoid publication bias. Nevertheless, our study is still the most comprehensive about the association between H. pylori infection and osteoporosis.
In summary, our results suggest significant increased odds of osteoporosis in patients with H. pylori infection. The clinicians should pay more attentions to the patients infected with H. pylori by using DEXA scan, especially those patients with chronic gastritis. However, the results should be cautiously interpreted considering the heterogeneity and the fact that all studies are non-randomised and retrospective. Further studies are needed to explore the mechanism and confounding factors between H. pylori and osteoporosis.
bmjopen-2018-027356supp002.pdf (174KB, pdf)
Supplementary Material
Footnotes
TW and XL contributed equally.
Contributors: YZ and HX led the study by designing, interpreting results and revising manuscript critically for important intellectual content, jointly supervised this work; TW and XL contributed to data analysis, result interpretation and drafting of the manuscript; QZ, BG and JZ participated in study data collection and revising manuscript; TW, XL, TC and LY participated in study conduct and results interpretation. All authors read and approved the final manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81202220 to YZ; No. 81673211 and 81372952 to TC), by the Natural Science Foundation of Jiangsu Province, China (No. BK20150094 to QZ) and by the Chongqing Health and Family Planning Commission (No. 20141027 to HX).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Patient consent for publication: Not required.
References
- 1. Zeng MD, Fan JG, Lu LG, et al. Chinese National Consensus Workshop on Nonalcoholic Fatty Liver Disease. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis 2008;9:108–12. 10.1111/j.1751-2980.2008.00331.x [DOI] [PubMed] [Google Scholar]
- 2. Lu LJ, Hao NB, Liu JJ, et al. Correlation between Helicobacter pylori Infection and Metabolic Abnormality in General Population: A Cross-Sectional Study. Gastroenterol Res Pract 2018;2018 10.1155/2018/7410801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozaydin N, Turkyilmaz SA, Cali S. Prevalence and risk factors of Helicobacter pylori in Turkey: a nationally-representative, cross-sectional, screening with the ¹³C-Urea breath test. BMC Public Health 2013;13:1215 10.1186/1471-2458-13-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorji D, Dendup T, Malaty HM, et al. Epidemiology of Helicobacter pylori in Bhutan: the role of environment and Geographic location. Helicobacter 2014;19:69–73. 10.1111/hel.12088 [DOI] [PubMed] [Google Scholar]
- 5. Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter 2014;19(Suppl 1):1–5. 10.1111/hel.12165 [DOI] [PubMed] [Google Scholar]
- 6. den Hollander WJ, Holster IL, den Hoed CM, et al. Ethnicity is a strong predictor for Helicobacter pylori infection in young women in a multi-ethnic European city. J Gastroenterol Hepatol 2013;28:1705–11. 10.1111/jgh.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pereira MI, Medeiros JA. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J Gastroenterol 2014;20:684–98. 10.3748/wjg.v20.i3.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellos I, Daskalakis G, Pergialiotis V. Helicobacter pylori infection increases the risk of developing preeclampsia: A meta-analysis of observational studies. Int J Clin Pract 2018;72(2):e13064 10.1111/ijcp.13064 [DOI] [PubMed] [Google Scholar]
- 9. Hou Y, Sun W, Zhang C, et al. Meta-analysis of the correlation between Helicobacter pylori infection and autoimmune thyroid diseases. Oncotarget 2017;8(70):115691–700. 10.18632/oncotarget.22929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahmani Y, Mohammadi S, Babanejad M, et al. Association of Helicobacter Pylori with Presence of Myocardial Infarction in Iran: A Systematic Review and Meta-Analysis. Ethiop J Health Sci 2017;27:433–40. 10.4314/ejhs.v27i4.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wijarnpreecha K, Chesdachai S, Thongprayoon C, et al. Association of Helicobacter pylori with the Risk of Hepatic Encephalopathy. Dig Dis Sci 2017;62:3614–21. 10.1007/s10620-017-4834-1 [DOI] [PubMed] [Google Scholar]
- 12. Abdollahi A, Etemadian M, Shoar S, et al. Is Helicobacter pylori Infection a Risk Factor for Prostatitis? A Case-Control Study in a Referring Tertiary Care Center. Iran J Pathol 2016;11:323–7. [PMC free article] [PubMed] [Google Scholar]
- 13. Consensus development conference. diagnosis, prophylaxis, and treatment of osteoporosis. The American journal of medicine 1993;94:646–50. [DOI] [PubMed] [Google Scholar]
- 14. Wu TY, Hu HY, Lin SY, et al. Trends in hip fracture rates in Taiwan: a nationwide study from 1996 to 2010. Osteoporos Int 2017;28:653–65. 10.1007/s00198-016-3783-4 [DOI] [PubMed] [Google Scholar]
- 15. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013;8:136 10.1007/s11657-013-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asaoka D, Nagahara A, Shimada Y, et al. Risk factors for osteoporosis in Japan: is it associated with Helicobacter pylori? Ther Clin Risk Manag 2015;11:381–91. 10.2147/TCRM.S80647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshimura N, Suzuki T, Hosoi T, et al. Epidemiology of hip fracture in Japan: incidence and risk factors. J Bone Miner Metab 2005;23(Suppl):78–80. 10.1007/BF03026328 [DOI] [PubMed] [Google Scholar]
- 18. Liu K, Liu P, Liu R, et al. Relationship between serum leptin levels and bone mineral density: A systematic review and meta-analysis. Clinica Chimica Acta 2015;444:260–3. 10.1016/j.cca.2015.02.040 [DOI] [PubMed] [Google Scholar]
- 19. Upala S, Sanguankeo A, Wijarnpreecha K, et al. Association between Helicobacter pylori infection and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab 2016;34:482–3. 10.1007/s00774-015-0703-1 [DOI] [PubMed] [Google Scholar]
- 20. Upala S, Sanguankeo A, Wijarnpreecha K, et al. Association between Helicobacter pylori infection and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab 2016;34:S1021 10.1007/s00774-015-0703-1 [DOI] [PubMed] [Google Scholar]
- 21. Chinda D, Shimoyama T, Matsuzaka M, et al. Helicobacter pylori infection is not a risk for osteopenia in Japanese healthy males. Am J Gastroenterol 2016;111:S1289.27685280 [Google Scholar]
- 22. Chinda D, Shimoyama T, Matsuzaka M, et al. Decrease of estradiol and several life style factors, but not helicobacter pylori infection, are significant risks for osteopenia in Japanese females. Am J Gastroenterol 2016;111:S499. [DOI] [PubMed] [Google Scholar]
- 23. Kalantarhormozi MR, Assadi M, Vahdat K, et al. Chlamydia pneumoniae and Helicobacter pylori IgG seropositivities are not predictors of osteoporosis-associated bone loss: a prospective cohort study. J Bone Miner Metab 2016;34:422–8. 10.1007/s00774-015-0688-9 [DOI] [PubMed] [Google Scholar]
- 24. Zhang C, Guo L, Hou W, et al. Relationship between the interaction of gastric helicobacter pylori infection and polymorphism of TLR4 gene G11367C and NADPH oxidase gene His72Tyr and the idiopathic osteoporosis in adults. Chin J Traumatol 2016;22:515–23. [Google Scholar]
- 25. Abdolahi N, Aghaei M, Naghdi M. Helicobacter pylori infection and osteoporosis in post monopausal women. Ann Rheum Dis 2017;76:1354. [Google Scholar]
- 26. Chinda D, Shimoyama T, Iino C, et al. Decrease of Estradiol and Several Lifestyle Factors, but Not Helicobacter pylori Infection, Are Significant Risks for Osteopenia in Japanese Females. Digestion 2017;96:103–9. 10.1159/000479317 [DOI] [PubMed] [Google Scholar]
- 27. Pan B-L, Huang C-F, Chuah S-K, et al. Relationship between Helicobacter pylori infection and bone mineral density: a retrospective cross-sectional study. BMC Gastroenterol 2018;18:54 10.1186/s12876-018-0780-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wright E, Schofield PT, Molokhia M. Bisphosphonates and evidence for association with esophageal and gastric cancer: a systematic review and meta-analysis. BMJ Open 2015;5:e007133 10.1136/bmjopen-2014-007133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ottawa Hospital Research Institute,. “The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2011. http://www.ohri.ca/programs/clinical epidemiology/oxford.asp.
- 31. Deeks JJ, Higgins JP, Altman DG, Higgins JP, et al. Analysing data and undertaking meta-analysis Cochrane Handbook for systematic Reviews of Interventions. Chichester: The Cochrane Collaboration, 2008:243–96. [Google Scholar]
- 32. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mizuno S, Matsui D, Watanabe I, et al. Serologically Determined Gastric Mucosal Condition Is a Predictive Factor for Osteoporosis in Japanese Men. Dig Dis Sci 2015;60:2063–9. 10.1007/s10620-015-3576-1 [DOI] [PubMed] [Google Scholar]
- 34. Fotouk-Kiai M, Hoseini SR, Meftah N, et al. Relationship between Helicobacter pylori infection (HP) and bone mineral density (BMD) in elderly people. Caspian journal of internal medicine 2015;6:62–6. [PMC free article] [PubMed] [Google Scholar]
- 35. Chung YH, Gwak JS, Hong SW, et al. Helicobacter pylori : A Possible Risk Factor for Bone Health. Korean J Fam Med 2015;36:239–44. 10.4082/kjfm.2015.36.5.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asaoka D, Nagahara A, Shimada Y, et al. Sa1806 Risk Factors for Osteoporosis - Are Infection or Eradication of Helicobacter pylori Associated? Gastroenterology 2015;148:S-337–0. 10.1016/S0016-5085(15)31122-7 [DOI] [Google Scholar]
- 37. Lin SC, Koo M, Tsai KW. Association between Helicobacter pylori Infection and Risk of Osteoporosis in Elderly Taiwanese Women with Upper Gastrointestinal Diseases: A Retrospective Patient Record Review. Gastroenterol Res Pract 2014;2014:1–5. 10.1155/2014/814756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Asaoka D, Nagahara A, Shimada Y, et al. Su1941 H.Pylori Infection Is a Risk Factor of Osteoporosis in Japan. Gastroenterology 2014;146(5):S-504 10.1016/S0016-5085(14)61821-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Asaoka D, Nagahara A, Hojo M, et al. The Relationship between H. pylori Infection and Osteoporosis in Japan. Gastroenterol Res Pract 2014;2014:1–9. 10.1155/2014/340765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chinda D, Shimoyama T, Matsuzaka M, et al. Assessment of the association between helicobacter pylori infection and osteopenia in japanese healthy adults. Helicobacter 2013;18. [Google Scholar]
- 41. Akkaya N, Akkaya S, Polat Y, et al. Helicobacter Pylori Seropositivity in Patients with Postmenopausal Osteoporosis. J Phy Ther Sci 2011;23:61–4. 10.1589/jpts.23.61 [DOI] [Google Scholar]
- 42. Kakehasi AM, Rodrigues CB, Carvalho AV, et al. Chronic Gastritis and Bone Mineral Density in Women. Dig Dis Sci 2009;54:819–24. 10.1007/s10620-008-0417-5 [DOI] [PubMed] [Google Scholar]
- 43. Kakehasi AM, Mendes CMC, Coelho LGV, et al. The presence of Helicobacter Pylori in postmenopausal women is not a factor to the decrease of bone mineral density. Arq Gastroenterol 2007;44:266–70. 10.1590/S0004-28032007000300016 [DOI] [PubMed] [Google Scholar]
- 44. Figura N, Gennari L, Merlotti D, et al. Prevalence of Helicobacter pylori Infection in Male Patients with Osteoporosis and Controls. Dig Dis Sci 2005;50:847–52. 10.1007/s10620-005-2651-4 [DOI] [PubMed] [Google Scholar]
- 45. Noach LA, Bosma NB, Jansen J, et al. Mucosal Tumor Necrosis Factor-or, Interleukin-1/3, and Interleukin-8 Production in Patients with Helicobacter pylori Infection. Scand J Gastroenterol 1994;29:425–9. 10.3109/00365529409096833 [DOI] [PubMed] [Google Scholar]
- 46. Kalkan Çağdaş, Karakaya F, Tüzün A, et al. Factors related to low serum vitamin B12 levels in elderly patients with non-atrophic gastritis in contrast to patients with normal vitamin B12 levels. Geriatr Gerontol Int 2016;16:686–92. 10.1111/ggi.12537 [DOI] [PubMed] [Google Scholar]
- 47. Tucker KL, Hannan MT, Qiao N, et al. Low plasma vitamin B12 is associated with lower BMD: the Framingham Osteoporosis Study. J Bone Miner Res 2005;20:152–8. 10.1359/jbmr.2005.20.1.152 [DOI] [PubMed] [Google Scholar]
- 48. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, et al. Helicobacter pylori and Risk of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. J Clin Gastroenterol 2018;52:386–91. 10.1097/MCG.0000000000000784 [DOI] [PubMed] [Google Scholar]
- 49. Upala S, Jaruvongvanich V, Wijarnpreecha K, et al. Nonalcoholic fatty liver disease and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab 2017;35:685–93. 10.1007/s00774-016-0807-2 [DOI] [PubMed] [Google Scholar]
- 50. Luo Y, Bao X, Zheng S, et al. A potential risk factor of essential hypertension in case-control study: MicroRNAs miR-10a-5p. Clin Exp Hypertens 2019;172:1–7. 10.1080/10641963.2019.1571597 [DOI] [PubMed] [Google Scholar]
- 51. Yan S, Sun R, Wu S, et al. Single nucleotide polymorphism in the 3′ untranslated region of LPP is a risk factor for lung cancer: a case-control study. BMC Cancer 2019;19:35 10.1186/s12885-018-5241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027356supp001.pdf (95.2KB, pdf)
bmjopen-2018-027356supp002.pdf (174KB, pdf)