Abstract
Introduction
Antidotes are available to treat some specific poisonings; however, the mainstay of treatment for the poisoned patient remains supportive care. Extracorporeal membrane oxygenation (ECMO) is one of the most aggressive supportive measures available to manage poisoned patients.
Objective
To characterize the recommendation and use of ECMO in cases reported to the California Poison Control System (CPCS).
Methods
This retrospective chart review queried the CPCS database from 1997 to 2016 for cases containing the American Association of Poison Control Centers (AAPCC) code for ECMO, and “ECMO” and “ECLS” free-text searches. The collected data included year, age, gender, substances involved, route of exposure, clinical effects, treatments, and medical outcome.
Results
A total of 94 cases discussed ECMO as a supportive option with 16 cases utilizing ECMO. Cases where ECMO was discussed rose from one case in 1997 to 13 cases in 2016. Of the 94 cases where ECMO was discussed, 38 cases (40%) involved toxicity from a cardiovascular agent(s) and 33 cases (35%) involved exposure to hydrocarbons. Of the 16 cases where ECMO was performed, 13 (81%) involved males. The median age was 17 years (range 1 month–54 years). Ten cases (63%) involve patients under the age of 18. In this series, 13 of 16 ECMO-supported patients survived (81%).
Conclusions
ECMO is being recommended more often for treatment of acute poisoning cases by the CPCS. All caregivers involved in the treatment of poisoning should gain a working knowledge of the potentially lifesaving technology of ECMO, its indications for use, adverse effects, and drug or poison interactions.
Keywords: ECMO, ECLS, Poison center, Poisoning
Introduction
The use of extracorporeal membrane oxygenation (ECMO) or extracorporeal life support (ECLS) has gained use in supporting critically ill patients. ECMO is simply defined as an external device that supports the cardiopulmonary system by providing oxygenation, carbon dioxide removal, cardiac function, and insuring adequate perfusion pressures for a patient in cardiac and respiratory failure [1]. The ECMO circuit pumps hypoxic blood out of a patient’s body then runs it through an artificial lung where carbon dioxide is removed and oxygen is added. The oxygenated blood is then warmed to appropriate body temperature and pumped back into the body. This bypass of the heart, lungs, or both allows these organs to recover from toxic insult and still maintain the delivery of nutrients and oxygenated blood to all tissues while allowing continual elimination of toxins [2].
There are two main techniques for ECMO, venovenous (VV) ECMO and venoarterial (VA) ECMO. In VV ECMO, blood is withdrawn peripherally from a large vein (femoral, internal jugular, or subclavian) or centrally from the right atrium, oxygenated and then returned through the same vein or atria. In VA ECMO, blood is withdrawn peripherally from a large vein or centrally from the right atrium and oxygenated, but is instead returned peripherally to a large artery (femoral or carotid) or centrally to the proximal ascending aorta [2]. VV ECMO is used in patients with pulmonary failure who are hemodynamically stable and do not need blood pressure support. The use of VA ECMO is more common, and it is used in patients who have both cardiac and pulmonary failure because it bypasses or augments both the heart and lungs to provide forward blood flow and blood pressure support.
There are no randomized trials of ECMO in poisoned patients with refractory shock or those who have respiratory failure or acute respiratory distress syndrome (ARDS) caused by an intoxication or exposure. Currently, the best evidence is derived from observational cohorts, case series, and case reports. A review by DeLange in 2013 found that typical examples of toxin-induced refractory shock and/or ARDS requiring ECMO included calcium channel antagonists, beta blockers, and hydrocarbons [3]. According to the Extracorporeal Life Support Organization (ELSO), 22 ECMO centers exist in California [4]; though, it is not clear how many can emergently cannulate a critical patient or how many cases involve poisonings. ELSO does publish its approved data requests on their website, and of the 57 approved data requests, only 2 data requests involved analysis of poisoned patients on ECMO [4].
The purpose of this study was to describe the characteristics of poisoning cases where ECMO was recommended by the California Poison Control System (CPCS). The secondary aim was to describe how the specialists in poison information (SPIs) coded the cases involving ECMO.
Methods
This investigation was a retrospective observational case series using data collected by CPCS, a single network of four poison-center answering-site divisions (San Francisco, Sacramento, San Diego, and Madera/Fresno) that are accessible to all 39 million Californians via telephone. The CPCS maintains a free telephone hotline providing advice for poisoning management to both the lay public and medical practitioners. The annual number of incoming calls to CPCS was 247,232 in 2017 [5], with all four CPCS divisions entering patient information into a shared database accessible to all CPCS SPIs. The database software is WBM Software Visual Dotlab Enterprise version 5.0.1, Fresno, CA, USA. Information includes patient demographics, clinical scenario, substances involved, doses, therapies, case notes, medical outcomes, and follow-up information if available. This study did not require institutional review board approval since all researchers received de-identified data. The electronic CPCS database from January 1997 through December 2016 was queried using the free-text search terms “ECMO” and “ECLS” as well as the corresponding therapy code for ECMO. All cases were accessed by the investigators after the patient identifiers were blinded. Inclusion criteria were all patient cases where ECMO or ECLS was discussed as a possible support option.
The query results were reviewed and information including basic demographic and clinical data was recorded into a predefined Microsoft Excel spreadsheet template (Microsoft Corp, Redmond, WA, USA). Two SPI investigators initially abstracted the data and a board-certified clinical toxicologist verified all abstracted data. Any discrepancies were discussed and resolved according to predetermined definitions and consensus. A positive data point was recorded if it was specifically mentioned in the case notes or case coding. The collected data including year, age, gender, reason for exposure, substances reportedly involved, route of exposure, clinical effects reported to CPCS by the health care facility (HCF) as a result of exposure, treatments performed, and medical outcome were abstracted from the chart notes and case coding. Regarding utilization of ECMO, cases were also categorized according to whether ECMO was performed, and if not, why it was not performed. Direct review of the primary hospital medical records was not available in most cases.
All medical outcome categories noted in this study are those used by all AAPCC-certified poison centers in the USA [6]. Medical outcome categories are as follows: No effect, the patient developed no signs or symptoms as a result of the exposure. Minor effect, the patient developed some signs or symptoms as a result of the exposure, but they were minimally bothersome and generally resolved rapidly with no residual disability or disfigurement. Moderate effect, the patient exhibited signs or symptoms as a result of the exposure that were more pronounced, more prolonged, or more systemic in nature than minor symptoms. Usually, some form of treatment is indicated. Major effect, the patient exhibited signs or symptoms as a result of the exposure that were life-threatening or resulted in significant residual disability or disfigurement. Death, the patient died as a result of the exposure or as a direct complication of the exposure [6].
Most reported clinical effects noted in this study are those used by all AAPCC-certified poison centers in the USA [7]. The only clinical effects that do not use the AAPCC standard definitions are aspiration and pneumothorax [8, 9].
Results
One hundred twenty-eight cases were identified. Thirty-four cases were excluded for reasons of case misidentification (4), case miscoding (8), case unrelated to an exposure (11), and case duplication (11). Of the 94 included cases where ECMO was discussed as a support option, 54 (57%) were male. The median age was 2.5 years (range 4 days–80 years). Of the 94 included cases, ECMO was performed only in 16. Of the 16 CPCS cases involving the use of ECMO, only 9 cases had ECMO properly coded as a treatment. All cases where ECMO was performed are listed in Table 1.
Table 1.
CPCS cases reporting the use of ECMO/ECLS
| Year | Age (gender) | Substances | Route of exposure | Adverse clinical effects | Treatments | Survival | Time to ECMO from ED arrival | Duration of ECMO | ECMO-related complications | Did ECMO contribute to death? |
|---|---|---|---|---|---|---|---|---|---|---|
| 1998 | 1 year (male) | Hydrocarbon furniture polish | Oral |
Acidosisa Aspiration Pneumothorax |
Intubation VV ECMO |
Yes | 3 days | 21 days | None reported | Not applicable |
| 2001 | 1 month (male) | Digoxin | IV |
Ventricular-tachycardia (Vtach)/ventricular-fibrillation (Vfib) Asystole |
Digoxin antibodies Cardioversion Cardiopulmonary resuscitation (CPR) VA ECMO |
No | < 1 day | < 1 day | None reported | No |
| 2001 | 9 years (male) | Imipramine | Oral |
Hypotensionb Ventricular-fibrillation |
Transvenous pacer Vasopressors VA ECMO |
Yes | 2 days | 8 days | None reported | Not applicable |
| 2008 | 1 year (male) | Lamp Oil | Oral |
Hypotensionb Hypoxia Respiratory arrest Aspiration |
Intubation Vasopressor VV ECMO |
Yes | < 1 day | 6 days | None reported | Not applicable |
| 2009 | 2 years (male) |
Digoxin Flecainide |
Oral |
Hypotensionb Bradycardiac Vtach/Vfib Seizures Asystole |
Digoxin antibodies Vasopressors Intubation VA ECMO |
Yes | < 1 day | 3 days | None reported | Not applicable |
| 2010 | 17 years (male) |
Atenolol Amlodipine Colchicine |
Oral |
Hypotensionb Pulmonary edema Renal failured Asystole |
Calcium Glucagon Vasopressors VA ECMO |
No | 2 days | < 1 day | None reported | No |
| 2011 | 45 years (male) |
Arsenic trioxide White phosphorous |
Dermal Inhalation |
Hypotensionb Vtach Cyanosis |
Vasopressors Hemodialysis Intubation VA ECMO |
Yes | 2 days | 11 days | None reported | Not applicable |
| 2012 | 22 years (male) | Methylenedioxymethamphetamine | Oral |
Hyperthermiae Acidosisa Ast and/or Alt > 1000 Renal failured Rhabdomyolysisf Multiple seizures Coma |
Vasopressors Hemodialysis Intubation VA ECMO |
Yes | 1 day | 2 days | Compartment syndrome at cannulation site requiring fasciotomy of right leg resulting in infection | Not applicable |
| 2012 | 54 years (female) | Diltiazem | Oral |
Hypotensionb Bradycardiac |
Calcium Glucagon Vasopressors High-dose insulin Intralipid Intubation VA ECMO |
Yes | < 1 day | 2 days | None reported | Not applicable |
| 2013 | 7 months (male) | Flecainide | Oral |
Vtach/Vfib Asystole |
Vasopressors Intralipid Intubation CPR VA ECMO |
Yes | < 1 day | 2 days | None reported | Not applicable |
| 2013 | 26 years (male) | Diltiazem | Oral |
Hypotensionb Bradycardiac Coma Asystole |
Glucagon Vasopressors High-dose insulin Intralipid Transvenous pacing Intubation VA ECMO |
Yes | < 1 day | 2 days | None reported | Not applicable |
| 2014 | 17 years (male) | Amlodipine | Oral |
Hypotensionb Respiratory arrest Asystole |
Vasopressors Intubation VA ECMO |
Yes | 1 day | unknown | None reported | Not applicable |
| 2014 | 1 year (female) | Hydrocarbon furniture polish | Oral |
Hypotensionb Aspiration Asystole |
Vasopressors Intubation CPR VV ECMO |
No | < 1 day | < 1 day | 5 min asystolic arrest during cannulation | Unknown |
| 2015 | 27 years (male) |
Cocaine Ethanol |
Oral Insufflation |
Hypotensionb Respiratory arrest Coma |
Vasopressors Intubation VA ECMO |
Yes | < 1 day | 6 days | None reported | Not applicable |
| 2016 | 17 years (female) |
Diltiazem Metoprolol |
IV |
Hypotensionb Asystole |
Glucagon Calcium Vasopressors Intubation CPR VA ECMO |
Yes | < 1 day | 1 day | None reported | Not applicable |
| 2016 | 24 years (male) | Fentanyl | Oral |
Acidosisa Respiratory arrest |
Intubation VV ECMO |
Yes | < 1 day | 3 days | None reported | Not applicable |
aAcidosis defined as serum bicarbonate < 20 mEq/L (mmol/L), pH < 7.35, or elevated lactic acid levels
bHypotension defined in adults as blood pressure < 90 mmHg systolic or more than 15 mmHg less than patient’s usual systolic blood pressure. In children, systolic blood pressure < (70 + 2(age)) mmHg
cBradycardia defined as heart rate < 60 beats per minute in adults. Age-related standards applied to children
dRenal failure defined as impairment that has produced clinically significant azotemia and loss of renal function
eHyperthermia defined as temperature ≥ 38 °C
fRhabdomyolysis defined as presence of myoglobin in the urine or a creatinine kinase (CK) level > 500 IU/L
Of the 94 cases, 29 cases (31%) involved multiple substances. The most frequently reported class of substances included 51 specific cardiovascular agents followed by 34 hydrocarbons. Other substances reportedly involved can be seen in Table 2. The most common route of exposure was ingestion which occurred in 50 (53%) cases followed by aspiration in 34 (36%) cases. The remaining cases involved routes of inhalation in 7 (7.4%) cases and parenteral in 3 (3.2%) cases.
Table 2.
Substances involved in cases reported to CPCS where ECMO was discussed
| Substances* | N | |
|---|---|---|
| Cardiovascular drugs | 51 | |
| Beta blockers | 14 | |
| Diltiazem | 7 | |
| Verapamil | 7 | |
| Amlodipine | 6 | |
| Flecainide | 6 | |
| ACE/ARB | 4 | |
| Digoxin | 3 | |
| Clonidine | 2 | |
| Diuretics | 2 | |
| Hydrocarbons | 34 | |
| SSRI/SNRI | 9 | |
| Plants | 8 | |
| Analgesics | 7 | |
| Benzodiazepines | 7 | |
| Stimulants | 6 | |
| Opioids | 6 | |
| Caustics | 5 | |
| Ethanol | 5 | |
| Antipsychotics | 4 | |
| Tricyclic Antidepressants | 3 | |
| Anticonvulsants | 2 | |
| Colchicine | 2 | |
| Arsenic | 1 | |
| Baclofen | 1 | |
| Bupivacaine | 1 | |
| Carbon monoxide | 1 | |
| Cyanide | 1 | |
| Glyburide | 1 | |
| Pentobarbital | 1 | |
| Quinine | 1 | |
| Warfarin | 1 | |
| White phosphorous | 1 | |
| Total** | 159 | |
*Most substances were by report and not laboratory confirmation
**Total is greater than 94 since multiple substances can be reported in each case
The discussion and/or recommendation regarding ECMO use was made by various members of the CPCS team. In 49 cases (52%), the medical toxicologist spoke directly with the treating physician over the telephone regarding ECMO use. In 23 cases (24%), the SPI alone discussed ECMO with the treating physician over the telephone; in these cases, the SPI informed the treating physician that ECMO use had been reported in the medical literature as a support modality, but did not make a recommendation for or against its use. In 12 cases (13%), the medical toxicologist discussed ECMO with the treating physicians at the patients’ bedside; this occurred because the patient started at or was transferred to a HCF where a medical toxicology service was available involving a medical toxicologist who also consulted for CPCS. In the remaining 10 cases, the medical toxicologist gave the recommendations to the SPI who communicated with the treating physician via telephone.
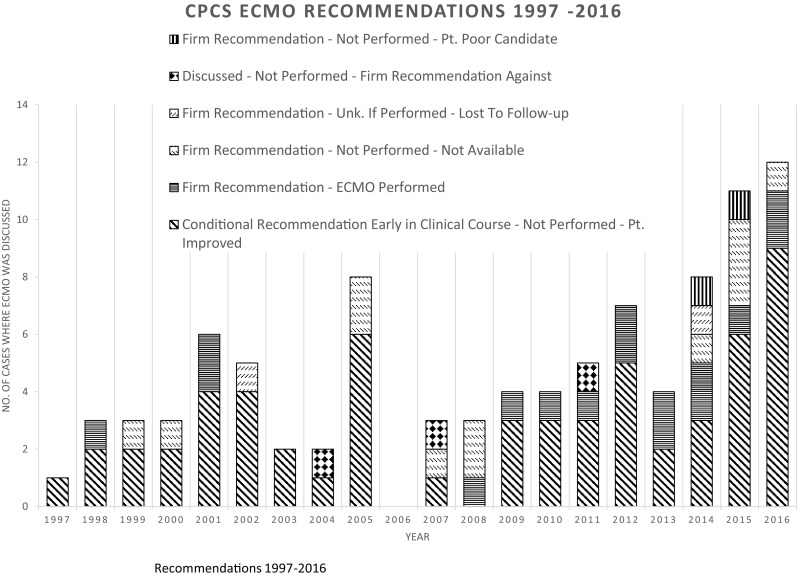
Figure 1 illustrates the annual number of cases reported to CPCS over the study period where ECMO was discussed as a support option. The first case reported to CPCS that discussed ECMO was in 1997 (1 case) which involved aspiration of a hydrocarbon in a 1-year-old female. The total number of cases discussing ECMO was low but did increase over the study period with 13 cases in 2016. In 59 (62%) cases, ECMO was discussed early in the clinical course as a conditional recommendation should other interventions fail and the patient’s clinical status continues to deteriorate. ECMO was not performed in these cases because the patients improved clinically. Of the 59 cases, there were no fatalities, 17 cases had major outcomes, and 42 cases had moderate outcomes.
Fig. 1.
CPCS ECMO Recommendations 1997–2016
In 2 cases, ECMO was initially recommended by the medical toxicologist but the treating physicians felt that the patients were not good ECMO candidates, and 1 of the 2 patients died. The first of these cases involved a female age 62 who in self-harm reportedly ingested amlodipine, metoprolol, risperidone, and venlafaxine. She presented to the emergency department (ED) very lethargic with hypotension, relative bradycardia, and a prolonged QTc of 548 ms. She was intubated for airway protection, and over the next 24 h, her BP continued to decline despite receiving calcium gluconate, glucagon, insulin, multiple vasopressors, and 20% lipid emulsion (ILE) IV. ECMO was recommended at 32 h after arrival, but the cardiothoracic (CT) surgeon declined ECMO for concerns of complications from ILE affecting the ECMO circuit. Over the next 16 days, she recovered and was extubated neurologically intact. Serum toxicology testing confirmed amlodipine at 90 ng/mL drawn 28 h after arrival. The second case involved a female age 22 who was brought to the ED from a botanical garden after she was found apneic and pulseless. The patient was found to have a wide complex rhythm with persistent cardiovascular collapse and expired despite maximal supportive care in the ED. ECMO was initially recommended but decided against by the CT surgeon, as a good neurologic outcome was unlikely to result. Perimortem serum and gastric samples were analyzed to quantify serum and gastric taxane B and 3,5-dimethoxyphenol concentrations [10].
In 3 cases, the medical toxicologist recommended against the use of ECMO. One case involved a male age 37 exposed to cyanide. He remained persistently hypotensive and acidemic despite maximal supportive care, and sodium nitrite and sodium thiosulfate intake. ECMO was not recommended because although it may have helped for blood pressure and oxygenation support, it would unlikely help patients with the uptake and utilization of oxygen by cyanide-poisoned cells. ECMO was not instituted, and the patient died 1 h after the conversation with the medical toxicologist. The second case involved a male age 69 who accidentally ingested 9 mg of his colchicine. He presented to the ED 36 h after ingestion with significant vomiting, abdominal pain, hypotension requiring inotropic support, and severe lactic acidosis. An intra-aortic balloon pump was inserted within 24 h of arriving to the ED. The medical toxicologist thought that with the anticipated course of poisoning, that ECMO was unlikely to be lifesaving. ECMO was not instituted, and the patient died on day 3. The third case involved a female age 44 who ingested acetaminophen, diphenhydramine, and lorazepam in self-harm and presented to the ED lethargic but hemodynamically stable with an elevated anion gap metabolic acidosis and signs of hepatotoxicity (AST 9494, ALT 2084, INR 2.9). The medical toxicologist informed the treating physician that the mainstay of treatment would be acetylcysteine and that ECMO was not currently indicated. The patient did not receive ECMO and recovered after a 7-day hospital stay.
ECMO was firmly recommended in 12 (13%) cases but was unavailable at the HCF, and transport to another ECMO-capable HCF was not considered feasible due to the critically ill status of the patients. Of these 12 cases where ECMO was not available, 9 patients died. In another 2 cases, ECMO was firmly recommended but the patients were lost to follow-up and whether ECMO was instituted or not as well as the patients’ outcomes remain unknown.
Within the 16 cases where ECMO was performed (Table 1), 13 (81%) were male. The median age was 17 years (range 1 month–54 years). Ten cases (63%) involve patients under the age of 18. There was a wide array of adverse clinical effects developed by each patient prior to ECMO (Table 1); the most common being hypotension (69%), asystole (44%), and respiratory arrest (31%). Many treatments were performed on each patient prior to ECMO with the most common being the use of vasopressors (81%) and intubation and mechanical ventilation (75%). Other treatments that were performed are listed in Table 1. Because of the critically ill status of all patients who received ECMO, all of the medical outcomes were either Major or Death. Of the 16 patients that received ECMO, 13 (81%) patients recovered and 3 (19%) patients died.
Patients were placed on ECMO due to cardiovascular collapse (7 cases VA ECMO) or pulmonary failure (4 cases VV ECMO). In the remaining 5 cases, VA ECMO was started due to a combination of both cardiovascular and pulmonary failure. The median time to start ECMO from the time of presentation to the emergency room was 0.5 days (< 1–3 days). The median duration of ECMO therapy was 2.5 days (< 1–21 days).
Discussion
Relatively few cases were reported to CPCS over the study period where ECMO was implemented in poisoned patients. However, it is clear discussion of ECMO use as a support bridge for poisoned patients has increased over time (Fig. 1). In 2016, 64 cases involving the use of ECMO were reported to poison centers nationwide [11]. This increase could be due to the improvements in ECMO techniques, increased availability of ECMO [12], or that more severe and complex cases are being reported to poison centers [11].
Of the 16 cases where ECMO was performed in this study, only 9 cases had properly coded the therapy ECMO in the poison center electronic medical record. Additionally, 8 (6.3%) of the initial 128 identified cases had ECMO coded as a therapy when ECMO was never discussed or performed. This suggests that increased education is needed for poison-center specialists on this increasingly utilized therapy for critically ill poisoned patients. Certainly, national data that relies on the quality of these coding efforts could be in question.
ECMO was recommended but not available in 12 cases, of which 9 patients died. Transport to an ECMO-capable HCF was not thought possible by the treating physicians due to the critically ill status of the patients. Of the 341 acute-care HCFs in California [13], only 22 are listed as ECMO centers on the ELSO registry [4]. Of these 22 ECMO centers, it is not known how many can emergently cannulate a critical patient. The authors are only aware of one ECMO-capable institution in northern California that has an ECMO transport team that can retrieve critically ill patients requiring emergent ECMO from a referring institution. The availability of mobile ECMO is likely different in other states as well as countries; it is possible that if mobile ECMO was more readily available, the rate of survival in these 9 fatal cases could have been improved. For comparison, a recently published abstract by Cole et al. describes a cohort of patients in the USA receiving ECMO for refractory shock due to poisoning. Of 22 cases, 14 cases (64%) were transferred to an ECMO center, and in 5 cases (36%), cannulation occurred prior to interfacility transfer [14].
While there was a wide age range of patients receiving ECMO for life-threatening toxicity in this study (1 month–54 years), 10 cases (63%) involved patients under the age of 18, which is consistent with literature that describes ECMO being used more commonly in pediatric poisoned patients [1, 15–17]. Some reasons for this increased use of ECMO for life-threatening toxicity in pediatric patients include that they are less likely to have other co-morbid conditions and would be expected to recover after ECMO supports their vital organs while the toxin is metabolized and eliminated. The current study also highlights that the duration of ECMO (median 2.5 days) when used in poisoned patients can be shorter than when used for non-poisoned patients [18].
The most common substances involved in the cases receiving ECMO were 8 cases with reported exposures to cardio-toxic prescription medications (Table 2). These findings are consistent with existing case-based literature and support the use of ECMO as a viable support option for severe cases of CV toxicity [19–34]. The second most common substances involved were pediatric hydrocarbon aspiration in three cases which is also consistent with existing case-based literature [35–39].
The majority of clinical adverse effects reported in these patients prior to ECMO were either cardiac or pulmonary in nature. Most patients were started on established treatments for the specific toxicity. However, standard treatments were not effective, and all patients decompensated into either cardiac and/or pulmonary failure, which prompted the initiation of EMCO. In this series, 13 of 16 ECMO-supported patients survived (81%). All cases where ECMO use was firmly recommended for or against involved the medical toxicologist, and most often, the case was discussed directly with the treating physician. Even though ECMO is now a popular treatment recommendation in medical toxicology, it is still not considered a gold-standard treatment option and the appropriate clinical indications for its use in poisoned patients is still being researched.
Limitations
There are several limitations to acknowledge in the current study. Our retrospective design examined exposures documented by voluntary reporting of poisoning by the public or healthcare professionals to CPCS. In most cases, the diagnosis of poisoning was made based on history and clinical course, rather than by documented blood concentrations of the toxic substance, and could not be verified by the CPCS. Information was also limited regarding what type of physician cannulated the patients or what mode of cannulation was performed, which can affect the risk of adverse events. It is also possible that not all the complications of ECMO were reported to CPCS. Because of its retrospective design, this study could not control the confounder that higher general critical care skills and resources might be more available at centers that can provide emergent ECMO which could have affected the survival of patients receiving ECMO. Additional details related to survival is also not known past the acute hospitalization period. The number of patients in this study is small and cannot be generalized to determine the place of ECMO in the treatment of all severe poisonings.
Conclusion
ECMO is being recommended more often as a treatment strategy for acute poisonings reported to the CPCS. Improvements in how ECMO cases are coded by specialists in poison information are needed. All caregivers involved in the treatment of poisoning should gain a working knowledge of the potentially lifesaving technology of ECMO, its indication for use, adverse effects, and drug or poison interactions.
Sources of Funding
None.
Compliance with ethical standards
Conflicts of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang GS, Levitan R, Wiegand TJ, Lowry J, Schult RF, Yin S. Extracorporeal membrane oxygenation (ECMO) for severe toxicological exposures: review of the Toxicology Investigators Consortium (ToxIC) J Med Toxicol. 2016;12:95–99. doi: 10.1007/s13181-015-0486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerns WP, Heffner AC, et al. Extracorporeal membrane oxygenation and cardiopulmonary bypass in the poisoned patient. In: Brent J, et al., editors. Critical Care Toxicology. Berlin: Springer; 2017. [Google Scholar]
- 3.DeLange DW, Sikma MA, Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clin Toxicol. 2013;51(5):385–393. doi: 10.3109/15563650.2013.800876. [DOI] [PubMed] [Google Scholar]
- 4.Extracorporeal Life Support Organization Center Directory. https://www.elso.org/Membership/CenterDirectory.aspx. Accessed 9 Jan 2019.
- 5.California Poison Control System Activity Report 2018. (Internal document).
- 6.Watson WA, Litovitz TL, Rodgers GC, Klein-Schwartz W, Reid N, Youniss J. 2004 annual report of the American Association of Poison Control Centers’ Toxic Exposure Surveillance System. Am J Emerg Med. 2005;23(5):589–666. doi: 10.1016/j.ajem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.National poison data system coding user manual updated 8/10/2017. https://aapcc.org/data-system. Accessed 9 Jan 2019.
- 8.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818–826. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swierzy M, Helmig M, Ismail M, Ruckert J, Walles T, Neudecker J. Pneumothorax. Zentralbl Chir. 2014;139(Suppl 1):S69–S86. doi: 10.1055/s-0034-1383029. [DOI] [PubMed] [Google Scholar]
- 10.Arens AM, Anaebere TC, Horng H, Olson K. Fatal Taxus baccata ingestion with perimortem serum taxine B quantification. Clin Toxicol. 2016;54(9):878–880. doi: 10.1080/15563650.2016.1209765. [DOI] [PubMed] [Google Scholar]
- 11.Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th annual report. Clin Toxicol. 2017;55(10):1072–1254. doi: 10.1080/15563650.2017.1388087. [DOI] [PubMed] [Google Scholar]
- 12.Raleigh L, Ha R, Hill C. Extracorporeal membrane oxygenation applications in cardiac critical care. Semin Cardiothorac Vasc Anesth. 2015;19(4):342–352. doi: 10.1177/1089253215607065. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Hospital compare list. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/hospitalqualityinits/hospitalcompare.html. Accessed 11 Feb 2019.
- 14.Cole J, Olives T, Litell J, Scharber S, Touroutoutoudis M, Singh P, et al. Extracorporeal membrane oxygenation (ECMO) for refractory shock due to poisoning: experience and trends from a Regional Poison Center. Clin Toxicol. 2018;56(10):936–937. [Google Scholar]
- 15.Banner W. Risks of extracorporeal membrane oxygenation: is there a role for use in the management of the acutely poisoned patient? Clin Toxicol. 1996;34(4):365–371. doi: 10.3109/15563659609013805. [DOI] [PubMed] [Google Scholar]
- 16.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu C, Lee W, Wei H, Sung S, Huang C, Shih C, et al. Extracorporeal membrane oxygenation use, expenditure, and outcomes in Taiwan from 2000 to 2010. J Epidemiol. 2015;25(4):321–331. doi: 10.2188/jea.JE20140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan B, Gupta V, Ralhan S, Gupta D, Puri S, Wander GS, Singh B. Role of extracorporeal membrane oxygenation in aluminum phosphide poisoning-induced reversible myocardial dysfunction: a novel therapeutic modality. J Emer Med. 2015;49(5):651–656. doi: 10.1016/j.jemermed.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 19.Johnson NJ, Gaieski DF, Allen SR, Perrone J, DeRoos F. A review of emergency cardiopulmonary bypass for severe poisoning by cardiotoxic drugs. J Med Toxicol. 2013;9:54–60. doi: 10.1007/s13181-012-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masson R, Colas V, Parienti JJ, Lehoux P, Massetti M, Charbonneau P, Saulnier F, Daubin C. A comparison of survival with and without extracorporeal life support treatment for severe poisoning due to drug intoxication. Resuscitation. 2012;83:1413–1417. doi: 10.1016/j.resuscitation.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Haas NA, Wegendt C, Schaffler R, Kirchner G, Welisch E, Kind K, et al. ECMO for cardiac rescue in a neonate with accidental amiodarone overdose. Clin Res Cardiol. 2008;97:878–881. doi: 10.1007/s00392-008-0700-7. [DOI] [PubMed] [Google Scholar]
- 22.Baud FJ, Megarbane B, Deye N, Leprince P. Clinical review: aggressive management and extracorporeal support for drug-induced cardiotoxicity. Crit Care. 2007;11:207. doi: 10.1186/cc5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchard NC, Weinberg RL, Burkart KM, Bacchetta M, Dzierba A, Story D, et al. Prolonged resuscitation for massive amlodipine overdose with maximal vasopressors, intralipids, and veno arterial–extracorporeal membrane oxygenation (VA ECMO). NACCT 2010. Clin Toxicol. 2010;48:604–667. doi: 10.3109/15563650.2010.493290. [DOI] [Google Scholar]
- 24.Hendren WG, Schieber RS, Garrettson LK. Extracorporeal bypass for the treatment of verapamil poisoning. Ann Emerg Med. 1989;18:984–987. doi: 10.1016/S0196-0644(89)80465-2. [DOI] [PubMed] [Google Scholar]
- 25.Babatasi G, Massetti M, Verrier V, Lehoux P, Le Page O, Bruno PG, Khayat A. Severe intoxiciation with cardiotoxic drugs: value of emergency percutaneous cardiocirculatory assistance. Arch Mal Coeur Vaiss. 2001;94:1386–1392. [PubMed] [Google Scholar]
- 26.Durward A, Geurguerian AM, Lefebvre M, Shemie SD. Massive diltiazem overdose treated with extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2003;4:372–376. doi: 10.1097/01.PCC.0000074273.50306.F5. [DOI] [PubMed] [Google Scholar]
- 27.Holzer M, Sterz F, Schoerkhuber W, Behringer W, Domanovits H, Weinmar D, Weinstabl C, Stimpfl T. Successful resuscitation of a verapamil-intoxicated patient with percutaneous cardiopulmonary bypass. Crit Care Med. 1999;27:2818–2823. doi: 10.1097/00003246-199912000-00035. [DOI] [PubMed] [Google Scholar]
- 28.Maclaren G, Butt W, Cameron P, Preovolos A, McEgan R, Marasco S. Treatment of polypharmacy overdose with multimodality extracorporeal life support. Anaesth Intensive Care. 2005;33:120–123. doi: 10.1177/0310057X0503300118. [DOI] [PubMed] [Google Scholar]
- 29.Auzinger GM, Scheinkestel CD. Successful extracorporeal life support in a case of severe flecainide intoxication. Crit Care Med. 2001;29:887–890. doi: 10.1097/00003246-200104000-00041. [DOI] [PubMed] [Google Scholar]
- 30.Corkeron MA, van Heerden PV, Newman SM, Dusci L. Extracorporeal circulatory support in near-fatal flecainide overdose. Anaesth Intensive Care. 1999;27:405–408. doi: 10.1177/0310057X9902700413. [DOI] [PubMed] [Google Scholar]
- 31.Yasui RK, Culclasure TF, Kaufman D, Freed CR. Flecainide overdose; is cardiopulmonary support the treatment? Ann Emerg Med. 1997;29:680–682. doi: 10.1016/S0196-0644(97)70257-9. [DOI] [PubMed] [Google Scholar]
- 32.De Rita F, Barozzi L, Franchi G, Faggian G, Mazzucco A, Luciani GB. Rescue extracorporeal life support for acute verapamil and propranolol toxicity in a neonate. Artif Organs. 2011;35:416–420. doi: 10.1111/j.1525-1594.2010.01134.x. [DOI] [PubMed] [Google Scholar]
- 33.Kolcz J, Pietrzyk J, Januszewska K, Procelewska M, Mroczek T, Malec E. Extracorporeal life support in severe propranolol and verapamil intoxication. J Intensive Care Med. 2007;22:381–385. doi: 10.1177/0885066607307528. [DOI] [PubMed] [Google Scholar]
- 34.McVey FK, Corke CF. Extracorporeal circulation in the management of massive propranolol overdose. Anaesthesia. 1991;46:744–746. doi: 10.1111/j.1365-2044.1991.tb09770.x. [DOI] [PubMed] [Google Scholar]
- 35.Scalzo AJ, Weber TR, Jaeger RW, Conners RH, Thompson MW. Extracorporeal membrane oxygenation for hydrocarbon aspiration. Am J Dis Child. 1990;144:867–871. doi: 10.1001/archpedi.1990.02150320031020. [DOI] [PubMed] [Google Scholar]
- 36.Chyka PA. Benefits of extracorporeal membrane oxygenation for hydrocarbon pneumonitis. J Toxicol Clin Toxicol. 1996;34:357–363. doi: 10.3109/15563659609013804. [DOI] [PubMed] [Google Scholar]
- 37.Moller JC, Vardag AM, Jonas S, Tegtmeyer FK. Poisoning with volatile hydrocarbons. 3 cases and a review. Monatsschr Kinderheilkd. 1992;140:113–116. [PubMed] [Google Scholar]
- 38.Bille AB, Pederson KD, Hertel S. Extracorporeal membrane oxygenation of a child with severe chemical pneumonia. Ugeskr Laeger. 2011;173:3115–3116. [PubMed] [Google Scholar]
- 39.Weber TR, Tracy TF, Connors R, Kountzman B, Pennington DG. Prolonged extracorporeal support for non-neonatal respiratory failure. J Pediatr Surg. 1992;27:1100–1104. doi: 10.1016/0022-3468(92)90568-R. [DOI] [PubMed] [Google Scholar]


