Abstract
Environmental proteases have been widely associated to the pathogenesis of allergic disorders. Der p 1, a cysteine-protease from house dust mite (HDM) Dermatophagoides pteronyssinus, constitutes one of the most clinically relevant indoor aeroallergens worldwide. Der p 1 protease activity depends on the redox status of its catalytic cysteine residue, which has to be in the reduced state to be active. So far, it is unknown whether Der p 1-protease activity could be regulated by host redox microenvironment once it reaches the lung epithelial lining fluid in addition to endogenous mite components. In this sense, Glutathione-S-transferase pi (GSTpi), an enzyme traditionally linked to phase II detoxification, is highly expressed in human lung epithelial cells, which represent the first line of defence against aeroallergens. Moreover, GSTpi is a generalist catalyst of protein S-glutathionylation reactions, and some polymorphic variants of this enzyme has been associated to the development of allergic asthma. Here, we showed that human GSTpi increased the cysteine-protease activity of Der p 1, while GSTmu (the isoenzyme produced by the mite) did not alter it. GSTpi induces the reduction of Cys residues in Der p 1, probably by rearranging its disulphide bridges. Furthermore, GSTpi was detected in the apical medium collected from human bronchial epithelial cell cultures, and more interesting, it increased cysteine-protease activity of Der p 1. Our findings support the role of human GSTpi from airways in modulating of Der p 1 cysteine-protease activity, which may have important clinical implications for immune response to this aeroallergen in genetically susceptible individuals.
Keywords: House dust mite, Cysteine-protease, Der p 1, GSTpi, GSTmu, Allergen
Graphical abstract
Highlights
-
•
Der p 1, the main allergen from house dust mite, acts in the lung epithelial lining.
-
•
Human bronchial Calu-3 epithelial cells secrete GSTpi into the apical medium.
-
•
GSTpi induces a disulphide-bridge rearrangement in Der p 1.
-
•
Human GSTpi, but not mite GSTmu, increases the cysteine-protease activity of Der p 1.
-
•
This can be a mechanism modulating Der p 1 allergenicity.
1. Introduction
Proteases are present in all known sources of airborne allergens from fungi [1], through house dust mites (HDM) [2], cockroaches [3], animal danders [4] to pollens [5], performing diverse functions that are crucial for living organism. Almost all of the allergenic proteases belong to serine protease group (e.g. Der p 3, Per a 10 and Pen ch 13), however aspartic- (e.g. Bla g 2), metallo- (e.g. Asp f 5) and cysteine-proteases (i.e. Der p 1) [6] have been also described.
HDM is the commonest sources of indoor aeroallergens worldwide associated with allergic rhinitis and asthma, and some of them are proteases such as Der p 1 and Der p 3 [7,8]. Der p 1, a 25-kDa polymorphic N-glycoprotein, belongs to group 1 of mite allergens with cysteine-protease activity. Der p 1 is the main allergen of HDM, which is recognized by 80% of mite-sensitized patients [9]. The possible roles of the protease activity of Der p 1 in mite allergenicity have been extensively studied and several mechanisms of action have been proposed. Der p 1 is able to alter bronchial epithelial barrier permeability by disruption of tight junctions, cleaving the extracellular domain of occludins and claudins, and causes intracellular proteolysis of ZO-1, thus facilitating the passage of allergens [10]. Der p 1 induces the secretion of pro-inflammatory mediators, including interleukin (IL)-6, IL-8, eotaxins and GM-CSF (granulocyte/macrophage colony-stimulating factor) by pulmonary epithelial cells, through the activation of membrane receptors, PAR-2 and TLRs [[11], [12], [13]]. In addition, Der p 1 degrades the lung surfactant collectins SP-A and SP-D [14], and cleaves the human protease inhibitors α1-antitrypsin, elafin and SLPI [15,16], thus provoking impairment on lung clearance. Furthermore, Der p 1 cleaves the low-affinity IgE receptor (CD23) on the surface of activated human B lymphocytes, increasing IgE production [17]. Also, Der p 1 can cleave the α-subunit of the IL-2 receptor (IL-2R or CD25) on the surface of human T lymphocytes, which inhibits the production of interferon (IFN)-γ and causes a shift to Th2 response [18,19]. Der p 1 causes in vitro IgE-independent release of IL-4 and IL-13 from mast cells and basophils [20]. Finally, Der p 1 also cleaves CD40 from the surface of human dendritic cells, resulting in decreased production of IL-12 that plays a major role in Th1 skewing [17].
As described for many proteases, Der p 1 is synthetized as an inactive prezymogen -named pre-proDer p 1-, which consists of a signal peptide (18 residues) essential for the secretion, an N-terminal propeptide of 80 residues, which act as chaperone, and the catalytic domain of 222 residues [21]. The zymogen proDer p 1 is self-processed under acidic conditions in the mite digestive tract, then Der p 1 orchestrates the subsequent maturation cascade of its own precursor and other mite protease precursors, including proDer p 3, proDer p 6 and proDer p 9 [22,23]. The three-dimensional (3D)-structure of Der p 1 has been determined by X-ray crystallography at 1.9 Å resolution [7]. Der p 1 exhibits the typical cysteine-protease fold of papain family, consisting of two domains folded together in a “V” configuration. The first domain is composed of three α-helices, while the second one is composed of five β-strands. The catalytic site lies in a cleft formed by juxtaposition of both domains. Catalytic residues consist of Cys34 and His170, together with Gln28 and Asn190. Residues Cys34 and His170, which form a thiolate–imidazolium ion pair, are critical for the enzymatic activity, as this ion pair is conserved in other cysteine-proteases, thus, suggesting a great redox dependence in developing its enzymatic function [24]. Der p 1 has a metal binding site occupied by a magnesium ion, and three disulphide bridges between residues Cys4 and Cys117, Cys31 and Cys71, and Cys65 and Cys103. In the Der p 1 dimer, the catalytic sites face each other, being accessible to polypeptide substrates [25].
Oxidative stress due to either oxidant/antioxidant imbalance or environmental oxidants has been increasingly involved in pathogenesis of various clinical disorders such as allergies and asthma [26,27]. It is known to activate multiple stress kinase pathways and redox-sensitive transcription factors such as nuclear factor NF-κB and AP-1, which differentially regulate the gene expression for pro-inflammatory cytokines as well as protective antioxidant proteins. The tripeptide glutathione (GSH) is the main redox homeostasis orchestrator in the airway epithelial lining fluid (ELF), with a concentration in the lower respiratory tract 140-fold higher (about 429 μM) than in plasma (about 3 μM) [28]. GSH metabolism is tightly regulated and has been implicated in redox signalling and in protection against environmental oxidant-mediated injury.
The protective action of GSH in the lung is usually accompanied by a plethora of enzymatic antioxidants, including Glutathione-S-transferases (GST) family, which are divided into 13 classes. GSTpi is the predominant class in the human lung epithelium, being also described in placenta, breast and prostate [[29], [30], [31]]. In addition to its detoxifying role, other properties have been ascribed to GSTpi, including chaperone function, regulation of nitric oxide pathway, regulation of a variety of kinase signalling pathways, and participation in S-glutathionylation of proteins [32]. GST binds directly to c-Jun N-terminal kinases (JNKs), which are at the end of the mitogen-activated protein kinase (MAPK) pathway, and acts as a negative regulator, thereby modulating proliferation and apoptosis [33]. Moreover, GSTpi modulates NF-κB activation in lung epithelial cells exposed to lipopolysaccharide [34,35]. Polymorphism of GSTP1 locus, which arise from nucleotide transitions that change codon 105 from Ile to Val, and codon 114 from Ala to Val, results in four alleles GSTP1 A-D that differ structurally and functionally [36]. Studies have reported that individuals carrying GSTP1 Val genotype were more susceptible to indoor pollutants such as HDM and cigarette smoke, and showed increased risk of allergic asthma and lung disfunction [37,38]. Interestingly, Der p 8, a clinically relevant allergen from HDM, belongs to a mu class of GST enzymes [39].
The aim of this study was to assess whether human GSTpi regulates the cysteine-protease activity of Der p 1, contributing to its inflammatory potential in comparison to mite GSTmu. Moreover, the secretion of GSTpi by human bronchial epithelial Calu-3 cells cultured at air-liquid interface (ALI) and its effects on protease activity of Der p 1 were also analysed. Our findings indicated that human GSTpi was an elicitor of cysteine-protease activity of Der p 1 in the presence of a reduction agent such as GSH, with a potential role in the allergenicity of Der p 1.
2. Materials & methods
2.1. Protease activity assay using fluorogenic peptide Boc-QAR-AMC
Der p 1 (25 nM, Lot. 38190, Indoor Biotechnologies, Cardiff, UK) was pre-incubated with different concentrations (0–2 mM) of GSH (Sigma-Aldrich, MO, USA) and different amounts of GST (0–2.5 μM) for 15 min at 37 °C. Then, the proteolysis of the substrate Boc-(Glu-Ala-Arg)-7-amino-4-methylcoumarin (Boc-QAR-AMC, 75 μM, Enzo, NY, USA) in PBS was performed, and the formation of AMC was recorded for 90 min at 37 °C. Same molar amounts of l-cysteine (l-Cys, Sigma) were also tested as alternative reduction agents. As for GSTs, two GSTs from different sources were used in this assay: (1) Human GSTpi from placenta (Sigma, Ref. G8642, Lot SLBB8465V), which exhibits a sequence identity over 80% to lung GSTpi (the most abundant GST in human lung), and the same enzymatic activity [40]; and (2) Mite GSTmu from D. pteronyssinus. GSTmu was purified from mite faeces extract as previously described [41], with minor modifications. GST enzymatic activity was checked for both purified proteins using a Glutathione S-Transferase Assay Kit (Sigma), according to the manufacturer's instructions (data not shown).
Apparent kinetic parameters values (Km, Vmax and Kcat/Km) were determined using alternatively the Hanes-Woolf and Eadie-Hoftsee plots (Hyper32 1.0 software).
2.2. Structural analysis by circular dichroism and SDS-PAGE
Circular dichroism (CD) spectra were obtained using a Jasco J-715 spectropolarimeter (Japan Spectroscopic Co., Tokyo, Japan). Der p 1 protease (0.2 mg/mL) was incubated alternatively with 1 mM GSH or l-Cys in 50 mM sodium phosphate buffer, pH 7.4, during 30 min at room temperature. Six wavelength scans were far-UV recorded (200–260 nm) at a constant speed of 50 nm/min. The ellipticity values (mdeg) were corrected with the baseline (buffer), and then transformed into molar ellipticity per residue (ΘMRW) using a Spectra ManagerTM software (JASCO). CDNN CD deconvolution software (Applied Photophysics, Surrey, UK) was used for the theoretical estimation (%) of the secondary structure.
Changes in the electrophoretic mobility were analysed by SDS-PAGE (15%) under non-reducing conditions, after incubating Der p 1 (1 μg) with or without GSTpi (1 μg) in 1 mM GSH for 30 min at 37 °C in PBS. Bands were visualized using the Coomassie blue G250 staining (Sigma). Untreated- and dithiothreitol (DTT)-treated Der p 1 were used as controls.
2.3. Redox fluorescence switch (RFS)
Der p 1 (1 μg) was incubated with 1 mM GSH during 30 min at 37 °C. Then, samples were precipitated with acetone, resuspended in TENS buffer (50 mM Tris pH 7.5, 1 mM EDTA, 100 μM neocuproine, 1% SDS) containing 40 μM BODIPY FL N-(2-aminoethyl)-maleimide (Invitrogen, MA, USA), and incubated for 30 min at 37 °C in darkness. Alternatively, some samples were first treated with 50 mM N-ethylmaleimide (NEM) (Sigma) for 10 min at room temperature, in order to block free cysteine residues, and after resuspending in TENS buffer, with 2.5 mM dithiothreitol (DTT) (Bio-Rad, CA, USA) during 10 min at room temperature to disrupt disulphide bridges. After a new precipitation with acetone, all samples were resuspended in gel loading buffer, and resolved by SDS-PAGE (10%), under non-reducing conditions [42]. Total protein quantification was carried out with SYPRO Ruby (Invitrogen) staining, according to the manufacturer's instructions. Images of the two fluorophores were captured using a Kodak Image Station 4000 MM (λexc = 470 nm/λem = 535 nm for BODIPY-FL-maleimide, and λexc = 430 nm/λem = 600 nm for SYPRO Ruby.
2.4. In-gel digestion & post-translational modification (PTM) peptide analysis
Der p 1 was incubated with GSTpi (1:1, molar ratio) in the absence or presence of 1 mM GSH for 30 min at 37 °C, followed by blocking with 150 mM NEM during 15 min at 37 °C in darkness. Samples were resolved by SDS-PAGE (12%) and after staining with Coomassie blue G250, gel bands were cut and unstained in acetonitrile: water (1:1, v:v).
In-gel digestions either with sequence grade trypsin (Promega, WI, USA) or chymotrypsin (Roche, Basel, Switzerland) were carried out as described by Shevchenko et al. [43], with minor modifications [44]. Briefly, the gel pieces were dried and re-swollen for 1 h in an ice-bath, alternatively with 50 mM ammonium bicarbonate, pH 8.8, containing 12.5 ng/μL trypsin or with 100 mM Tris-HCl, pH 8.5 and 10 mM CaCl2 containing 25 ng/μL chymotrypsin. The digestion buffer was replaced for the corresponding fresh one, and gel pieces were incubated for 12 h at 37 °C or at 25 °C for trypsin or chymotrypsin digestion, respectively. Reaction was stopped by the addition of 1% trifluoroacetic acid and samples were centrifuged. Supernatants were dried down and desalted onto a ZipTip C18 Pipette tip (Millipore, MA, USA) for the mass spectrometric analysis.
Derived proteolytic products were pooled, dried, resuspended in 10 μL of 0.1% formic acid and finally, analysed by RP-LC-MS/MS in an Easy-nLC II system coupled to an ion trap LTQ-Orbitrap-Velos-Pro hybrid mass spectrometer (resolution set at 30.0000, Thermo Scientific, MA, USA). Peptides were concentrated by reverse phase chromatography, using a 0.1 mm × 20 mm C18 RP pre-column (Thermo Scientific). Then, peptides were separated on a 0.075 mm × 250 mm C18 RP column (Thermo Scientific), operating at 0.3 μL/min. The elution was performed using a two-step gradient of 80% acetonitrile containing 0.1% formic acid: (1) from 5 to 25% in 45 min, and (2) from 25 to 40% in 60 min. Water containing 0.1% formic acid was used as solvent A. ESI ionization was done using a Nano-bore emitters Stainless Steel ID 30 μm (Proxeon, Odense, Denmark) interface.
Peptides were detected in survey scans from 400 to 1600 amu (1 μscan), followed by fifteen data dependent MS/MS scans (Top 15), using an isolation width of 2 u (in mass-to-charge ratio units), normalized collision energy of 35%, and dynamic exclusion applied for 30 s periods. Peptide identification from raw data was carried out using the SEQUEST algorithm (Proteome Discoverer 1.4, Thermo Scientific). Database search was performed against local database of Der p 1 using Peaks 8.0 software, according to the following constraints: tryptic cleavage after arginine and lysine, up to two missed cleavage sites, and tolerances of 10 ppm for precursor ions and 0.8 Da for MS/MS fragment ions. Search was performed allowing optional methionine oxidation, cysteine carbamidomethylation, GSH- and NEM-cysteine incorporation [45]. Search against decoy database (integrated decoy approach) using false discovery rate (FDR) < 0.01. Post-translational modification (PTM) assignment was made by taking the maximum absolute value of peptide spectra as comparison with the different experimental conditions assayed. All these experiments were carried out at the Protein Chemistry Facility of Centro de Biología Molecular-Severo Ochoa (CBMSO).
2.5. Analysis of GSTpi epithelial-derived secretion by fluorescent proteolysis assay and Western blot
In order to demonstrate the secretion of GSTpi by the bronchial epithelial cells, apical media of ALI-cultured Calu-3 cells (ATCC No. HTB-55, Lot. 61449062) were assayed. Briefly, cells at passage 23–30 were cultured (3 × 106 cells/cm2) in DMEM nutrient mixture F-12 (Thermo Fisher Scientific), containing 2 mM l-glutamine (Sigma), 100 U/mL penicillin, 100 μg/mL streptomycin (Lonza) and 10% foetal bovine serum (Hyclone, GE Healthcare, IL, USA) at 37 °C and 95% humidified atmosphere of 5% CO2 in air, onto 6.5 mm-Transwell® insert (pore size 0,4 μm, Corning, NY, USA). After 7 days on ALI, cells were apically covered with 100 μL PBS (Lonza, Basel, Switzerland) during 24 h and apical media were collected and keep on ice until use.
Calu-3-derived apical medium (25 μL) was used as a potential source of GSTpi in the proteolysis assays mentioned above. Some samples were preincubated with 0.5 mM of the specific inhibitor of GSTpi TLK199 (beyond this concentration the drug is not soluble, Ezatiostat, Sigma). In addition, the presence of GSTpi in the apical medium was detected by Western blot, using the rabbit polyclonal antibody anti-human GSTpi (Dilution 1:2500, anti-GST3, Abcam, Cambridge, UK).
3. Results
3.1. Human GSTpi upregulated the cysteine-protease activity of Der p 1
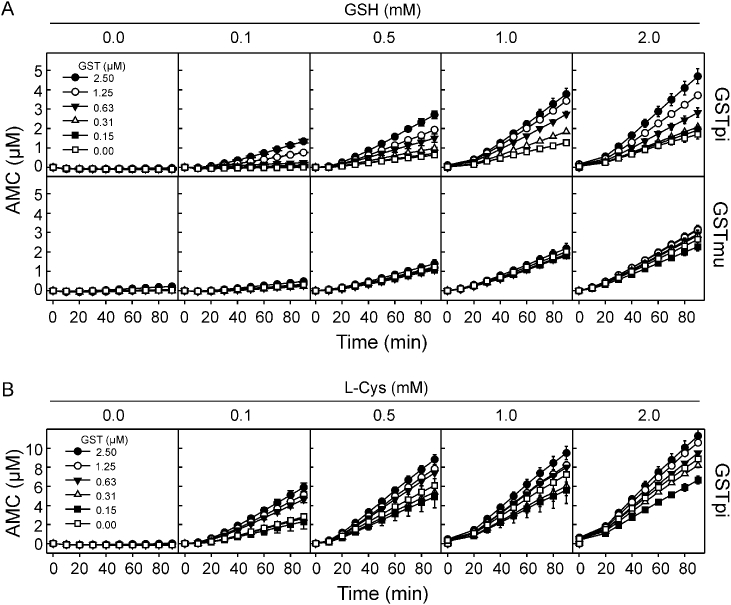
To analyse whether GST affected the cysteine-protease activity of Der p 1, the proteolysis of the fluorogenic peptide Boc-QAR-AMC by Der p 1 was performed in the presence of human increasing amounts of human GSTpi or mite GSTmu, with or without of the thiol reducing agents GSH or l-Cys (from 0.1 to 2 mM) (Fig. 1). The cysteine-protease activity of Der p 1 was upregulated by GSTpi in a dose-dependent manner, after pre-activation with the reducing agent GSH (Fig. 1A). However, no activity was observed without pre-treatment with the reducing agent, required to regenerate the catalytic thiol group of this cysteine-protease. The highest increase on Der p 1-protease activity was observed with 2.5 μΜ GSTpi in the presence of 0.5 mM GSH. Saturating concentrations of GSH (1 and 2 mM) largely reduced GSTpi-upregulatory effect. When l-Cys was used as reducing agent, the same effect was observed (Fig. 1B). Thus, this suggests that upregulation of Der p 1-protease activity by GSTpi does not depend on its glutathione transferase or S-glutathionylation activity, and therefore other mechanism may be involved. In contrast, GSTmu had no effect on the cysteine-protease activity of Der p 1. This shows that GSTpi, but not GSTmu, increases Der p 1 protease activity by GSTpi in the presence of thiol reducing agents.
Fig. 1.
Human GSTpi, but not mite GSTmu, increased the cysteine-protease activity of Der p 1 allergen. The proteolysis of the fluorogenic peptide (Boc-QAR-AMC, 75 μM) by Der p 1 was measured in the presence of different concentration of GSTpi or GSTmu (0–2.5 μM), after preactivation with (A) GSH or (B) l-Cys (range from 0.1 mM to 2 mM), for 90 min. Data are expressed as the μM of 7-amino-4-methylcoumarin (AMC) released by Der p 1 and showed as means ± SD of duplicate measurements. A representative example of two independent experiments is shown.
3.2. GSTpi increased the catalytic efficiency of Der p 1
Since the upregulation of the cysteine-protease activity of Der p 1 by GSTpi required the pre-incubation of the protease with a reducing agent to regenerate the catalytic thiol group of this enzyme, the catalytic efficiencies of Der p 1 with Boc-QAR-AMC in the presence of GSTpi were determined with either GSH or l-Cys as alternative reducing agents. The apparent kinetic parameters Km and Vmax with GSH and l-Cys were calculated using both Hanes-Woolf and Eadie-Hofstee equations, and the data obtained are presented in Table 1. GSTpi produced a clear increase on the affinity of the cysteine-protease enzyme for Boc-QAR-AMC in a dose-dependent way in the presence of GSH, as indicated by a decrease on the apparent Km values (up to 19-fold change). By contrast, with l-Cys, the effect of GSTpi on apparent Km was much lower (2-fold change). In addition, GSTpi increased the apparent Vmax values of Der p 1, showing both GSH and l-Cys similar magnitude effect. This results in a higher apparent catalytic efficiency (Kcat/Km) of Der p 1 in the presence of GSTpi. The increment in this apparent kinetic parameter with GSH was overall higher than with l-Cys, being up to 30-fold and 3-fold for GSH and l-Cys, respectively, with 2.5 mM concentration of the reduction agent. GSTmu did not modify any of the parameters of Der p 1 (data not shown).
Table 1.
Apparent kinetic parameters for the proteolysis of Boc-QAR-AMC by Der p 1 in the presence of GSTpi, using GSH and l-Cys as alternative reducing agents.
| GSTpi (μM) |
0 | 0.15 | 0.3 | 0.6 | 1.3 | 2.5 | |
|---|---|---|---|---|---|---|---|
| (GSH) | |||||||
| Hanes-Woolf | Vmax (nM/min) | 35.8 | 50.3 | 51.4 | 54 | 54.3 | 62.1 |
| Km (mM) | 6.2 | 4.5 | 2.3 | 1.8 | 0.6 | 0.4 | |
| Kcat/Km (min−1) | 5.8 × 10−6 | 1.1 × 10−5 | 2.2 × 10−5 | 3.0 × 10−5 | 9.1 × 10−5 | 1.4 × 10−4 | |
| Eadie-Hofstee | Vmax (nM/min) | 25.4 | 43.8 | 44.5 | 49.8 | 51.6 | 53.8 |
| Km (mM) | 5.7 | 3.4 | 1.8 | 0.8 | 0.5 | 0.3 | |
| Kcat/Km (min−1) | 4.5 × 10−6 | 1.3 × 10−5 | 2.5 × 10−5 | 6.2 × 10−5 | 1.0 × 10−4 | 1.8 × 10−4 | |
|
| |||||||
| GSTpi (μM) | 0 | 0.15 | 0.3 | 0.6 | 1.3 | 2.5 | |
| (l-Cys) | |||||||
| Hanes-Woolf | Vmax (nM/min) | 72.1 | 82.2 | 103 | 112.2 | 125.4 | 132.6 |
| Km (mM) | 0.4 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | |
| Kcat/Km (min−1) | 1.8 × 10−4 | 2.7 × 10−4 | 3.3 × 10−4 | 5.6 × 10−4 | 6.3 × 10−4 | 6.6 × 10−4 | |
| Eadie-Hofstee | Vmax (nM/min) | 66.7 | 78.2 | 89.4 | 103 | 109.7 | 120.7 |
| Km (mM) | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | |
| Kcat/Km (min−1) | 3.3 × 10−4 | 3.9 × 10−4 | 4.5 × 10−4 | 1.0 × 10−3 | 1.1 × 10−3 | 1.2 × 10−3 | |
The apparent Km, Vmax and Kcat/Km values were determined using both Hanes-Woolf and Eadie-Hofstee plots. GSTpi (0–2.5 μM) and substrate (75 μM) concentrations were kept constant for each scanning of the reducing agent (from 0.15 to 2.5 mM).
3.3. GSH modifies the redox state of cysteine residues in Der p 1
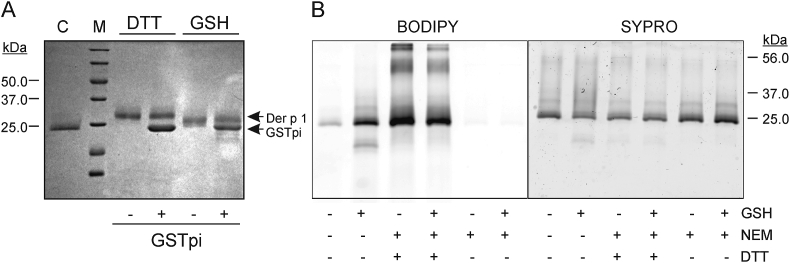
We analysed if GSTpi and GSH could induce a change in the redox state of the Cys residues of Der p 1. SDS-PAGE mobility assays showed that showed that DTT-treated Der p 1 has a retardation on its mobility, coherent with the full reduction of its disulphide bridges; GSH alone caused a relatively minor retardation, slightly increased in the presence of GSTpi (Fig. 2A). This indicates that GSH, together with GSTpi, could be producing a limited rearrangement of Der p 1 disulphide bonds.
Fig. 2.
Effects of GSH on the folding of Der p 1. (A) SDS-PAGE analysis (stained with Coomassie blue) of the effect of GSH (1 mM) on the relative migration distance of Der p 1, with and without GSTpi. Untreated- and DTT-treated proteins were used as controls. Arrows indicate the positions of Der p 1 and the GSTpi. C, untreated Der p 1 used as control; M, molecular masses marker. (B) Redox fluorescence switch (RFS) assay of Der p 1. Der p 1 was incubated with 1 mM GSH for 30 min and then successively with 50 mM NEM, 2.5 mM DTT or fluorescent maleimide (maleimide with BODIPY-FL) as indicated. BODIPY-FL labelling after NEM addition without DTT measures non-specific labelling (negative control). The amount of loaded-protein was analysed with SYPRO staining. A representative example of three independent experiments is shown.
Finally, we used a modification of the RFS assay to determine the relative number of reduced and oxidized Cys residues in Der p 1 without or with GSH (Fig. 2B) [46]. There is a faint labelling of reduced Cys residues (without the addition or NEM or DTT), which clearly increases with GSH treatment. There is a higher signal of reversibly oxidized Cys residues (after addition of NEM and DTT), in corresponding to the high proportion of Cys residues involved in disulphide bridges, which is slightly decreased with GSH treatment. Thus, GSH treatment is altering the redox state of some of the Cys residues, increasing the proportion of Cys in the free thiol form.
3.4. GSH induced a change on the global folding of Der p 1
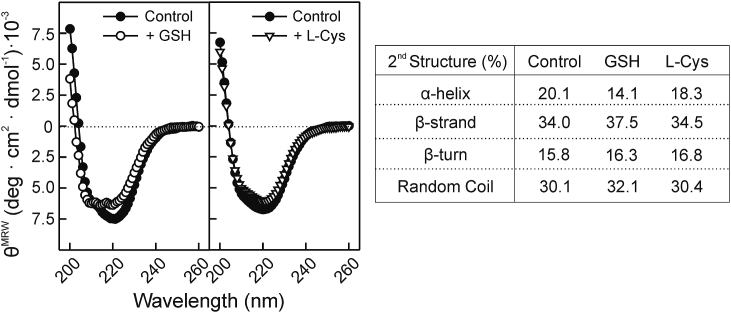
The effect of the reducing agents (GSH or l-Cys) on the secondary structure of Der p 1 was determined by obtaining its far-UV CD spectra (Fig. 3). GSH altered the shape of Der p 1 spectrum, indicating a change in the secondary structure of the protein. Deconvolution of the ellipticity data allowed quantifying the changes in the secondary structure composition. In the absence of the reducing agent, the secondary structure determined of Der p 1 showed 20.1% α-helix, 34.0% β-strand, 15.8% β-turn and 30.1% random coil, in accordance to previously published [47]. GSH induced a reduction in α-helix content, and an increase in both β-strand and random coil components. In contrast, l-Cys did not induce significant changes in the CD spectrum of Der p 1.
Fig. 3.
GSH, but no cysteine, induces changes on the secondary structure of Der p 1. Far-UV circular dichroism spectra of Der p 1 in absence or presence of a reducing agent: GSH or l-Cys. Secondary structure composition of Der p 1 determined from CD spectra by CDNN.
3.5. Identification of the modified-cysteine residues of Der p 1
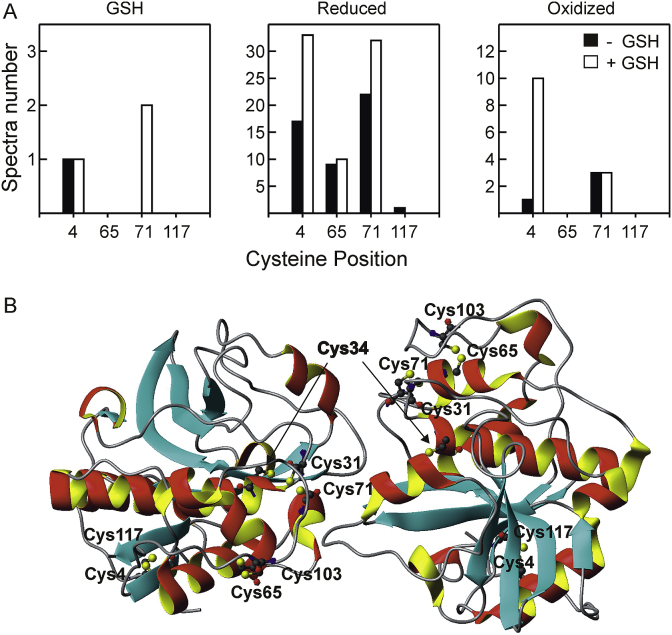
To identify which cysteine residues were modified by GSH, Der p 1 was digested alternatively with trypsin or chymotrypsin after incubation with GSTpi in the absence or presence of the reducing agent. Peptide analysis was carried out by RP-LC-MS/MS. PTM assignment was done considering the absolute number of spectra for each modified peptide (Fig. 4A). Neither Cys31, Cys34 nor Cys103 were detected in any of the peptides, and Cys117 was found in only one spectrum, so we could not draw conclusions regarding their redox state. Both Cys4 and Cys71 increased the number of spectra in the reduced form after treatment with GSH and GSTpi (Fig. 4A; see Tables S1 and S2 in Supplementary data), which correlates with the increase in reduced Cys residues observed with the modified RFS assay (Fig. 2). We also observed appearance of S-glutathionylation in Cys71 after GSH treatment in the presence of GSTpi but no increase in Cys4 S-glutathionylation. Increase of oxidized Cys4 is due to the increase in sulfenic, sulfinic and sulfonic forms (Tables S1 and S2), which can be related to an increased exposure of the reduced thiol after GSH treatment. Fig. 4B shows the reported 3D-structure of Der p 1 dimer, where each monomer faces each other covering the catalytic cysteine residue (Cys34) [7,25]. The three disulphide bridges formed between cysteine residues (Cys4- Cys117, Cys31-Cys71, and Cys65-Cys103) that stabilized the protein folding are indicated. Although we have not obtained a detailed map of the redox state of all the Cys residues, our data and the model suggest that addition of GSH with GSTpi produces a disulphide-bridge rearrangement in Der p1, which may involve at least Cys4 and Cys71.
Fig. 4.
Analysis of modified cysteines of Der p 1 by in-gel digestion, followed by RP-LC-MS/MS, after incubation with GSTpi in the absence or presence of GSH. (A) Summary of peptide spectra number containing unmodified and modified cysteine residues: GSH conjugation, reduced and oxidized. (B) 3D-structure of Der p 1 dimer. Ribbon representation was generated using PyMOL by taking known structure as template (PDB Ref. 3F5V). Cysteine residues are indicated in the structure. Mature enzyme has three disulphide bridges: Cys4-Cys117, Cys31-Cys71, and Cys65-Cys103. The catalytic cysteine residue is Cys34.
3.6. Human bronchial epithelial cells secreted GSTpi into the apical medium
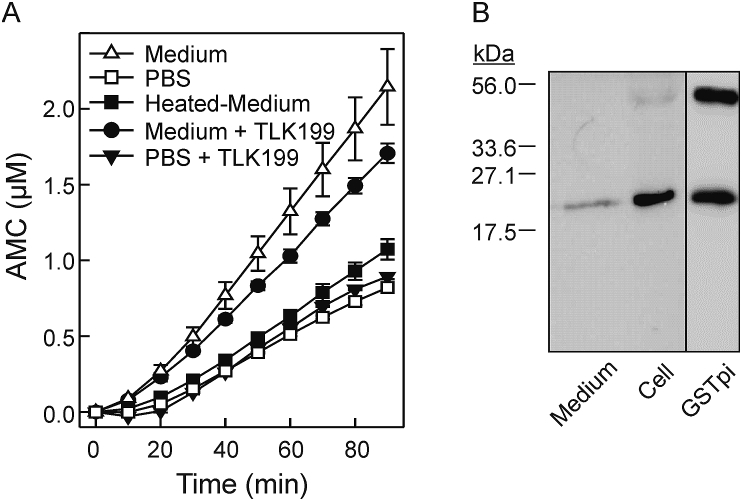
To determine whether human bronchial epithelial cells secreted GSTpi, the apical medium from Calu-3 cells cultured at ALI was used for the proteolytic assay of the fluorogenic peptide by Der p 1. The apical medium increased the proteolytic activity of the cysteine-protease compared to PBS as control (Fig. 5A). When the assay was performed in the presence of 0.5 mM TLK199, a specific inhibitor of GSTpi, the upregulatory effect of the apical medium was partially inhibited, whereas heat-treatment of the medium carried out at 95 °C completely abolished its upregulatory effect. Moreover, the presence of GSTpi in the apical medium was demonstrated by means of immunoblotting with an anti-human GSTpi antibody (Fig. 5B). Overall, our data indicated that GSTpi is one of the components secreted into the medium by cells that could be involved in the upregulation of the cysteine-protease activity of Der p 1.
Fig. 5.
GSTpi is secreted by human bronchial epithelial cells into the apical medium. (A) Effect of apical medium secreted by Calu-3 cells on the proteolysis of the fluorogenic peptide by Der p 1 in the presence of 0.1 mM GSH. Data are expressed as the μM of 7-amino-4-methylcoumarin (AMC) released by Der p 1 and showed as means ± SD of duplicate measurements. Medium, apical medium added to Der p 1 before reaction with the substrate; PBS, assay performed in the presence of the buffer alone; Heated-Medium, medium heated at 95 °C for 5 min before the assay; Medium + TLK199; medium treated with the inhibitor of GSTpi before the assay; PBS + TLK199; PBS treated with the inhibitor before the assay. (B) Detection of human GSTpi on apical medium by immunoblotting using an anti-GSTpi antibody. Medium, apical medium from Calu-3 cells cultured at ALI; Cell, lysate from Calu-3 cells; GSTpi, human GSTpi from placenta as control. Marker molecular masses in kDa are indicated.
4. Discussion
Der p 1 from HDM, one of the most clinically relevant indoor aeroallergens worldwide, is a cysteine-protease which catalytic activity has been linked to its allergenicity [10,[14], [15], [16], [17],19]. However, it is unknown whether Der p 1-protease activity could be regulated by host redox microenvironment once it reaches the lung ELF. In this study, we described a novel mechanism by which cysteine-protease activity of Der p 1 is upregulated. Our data showed that human GSTpi, but not mite GSTmu, upregulated the cysteine-protease activity of Der p 1 in a dose-dependent manner, in the presence of either GSH or l-Cys as reducing agents. The fact that GSTpi upregulates cysteine-protease activity of Der p 1 but not GSTmu can be ascribed to the functional differences reported among GST isoenzymes [32]. An example is provided by the observations that GSTpi, GSTmu and GSTalpha bind and inhibit JNK activity, although with different efficiencies, being GSTpi the most potent inhibitor [48]. Moreover, the upregulation of Der p 1 protease activity by GSTpi may involve a mechanism different of S-glutathionylation and glutathione transferase activity. In support, several reports have demonstrated that GSTpi acts as a regulator of protein function through direct interaction with these proteins [32]. GSTpi binds to NF-κB, c-JNK or p53, thus regulating processes of inflammation, apoptosis or cell proliferation [31,49,50]. In addition, GSTpi modulates NF-κB activation in lung epithelial cells [34,35].
GSTpi was shown to produce a significant increase on the catalytic efficiency of the Der p 1 for the substrate Boc-QAR-AMC in a dose-dependent way, in the presence of GSH. Despite of a direct reducing action over the catalytic site, GSH induced a conformational change in the global folding of Der p 1, as indicated by CD spectra, SDS-PAGE and RFS assays, that could be explained in part by the increase observed in the apparent kinetic constants (Km, Vmax and Kcat/Km). Mass spectrometry data and the RFS assay supported that GSH could induce a disulphide-bridge rearrangement, accounting for the increase in the catalytic efficiency of Der p 1 by GSTpi. Cys4 and Cys71 could be involved in this rearrangement, since are the cysteine residues that display the highest number of modifications. A disulphide-bridge rearrangement has been previously described as a regulatory mechanism of enzymes [24,51,52]. Campos et al. showed that citrus (Citrus sinensis) cyclophilin activity is inhibited by the formation of a disulphide bridges between Cys40 and Cys168, which promotes a conformational change [53]. This redox 2-Cys mechanism control of catalytic activity seems to be conserved in peroxiredoxin family [54].
Finally, our data have shown that GSTpi is secreted into the apical medium by human bronchial epithelial Calu-3 cells. Although widely described as a cytosolic and cellular organelle protein, the presence of GSTpi has been reported in plasma and other body fluids. Elevated levels of GSTpi were detected in plasma and biopsies of patients with oesophagus and gastric cancer -compared to normal subjects-by Mohammadzadeh et al. [55]. Howie et al. [56] found high levels of GSTpi in (and purified from) bronchoalveolar lavage fluid of patients with neoplastic and non-neoplastic lung diseases. The presence of GSTpi in human bile has also been reported [57]. Moreover, our results suggest that GSTpi secreted by human bronchial epithelial cells might be involved in the upregulation of the cysteine-protease activity of Der p 1, since the upregulation effect of the apical medium was reduced by TLK199, a specific inhibitor of GSTpi. In this sense, cystatin A and divalent metal ions (i.e., Ca2+, Mg2+) have already been described as regulators of papain and other papain-like proteases activity such as cathepsin B and Der p 1 [[58], [59], [60]]. Moreover, Herbert et al. [61] have shown that protease activity from several allergenic sources were increased by means of cell lysates as well by cell-secreted medium. The fact that human GSTpi and GSH are present in the lung ELF supported this proposed mechanism by which cysteine-protease activity of Der p 1 could be upregulated once it reaches airway mucosa, thus increasing its allergenicity and contributing to the patient sensitization. Since allelic GSTpi variants are functionally different [36], additional studies will be required to determine their contribution on the cysteine-protease activity of Der p 1. A systematic review demonstrating an association among GSTP1 polymorphism, indoor air pollutant exposure and risk of respiratory and allergic diseases, support our findings [37].
In conclusion, human GSTpi was shown to be an elicitor of the cysteine-protease activity of Der p 1, the main allergen from HDM, upon addition of a reducing agent such as GSH or l-Cys. In combination with GSH, GSTpi could induce a disulphide-bridge rearrangement, accounting for the increase in the activity of Der p 1 by a redox mechanism. The potential clinical implication for these findings in allergy is supported by the fact that GSTpi occurs in the apical secretion of human bronchial epithelial Calu-3 cells cultured at ALI. Further studies are needed to determine the contribution of human GSTpi on the allergenicity of Der p 1.
Acknowledgement
We thank Anabel Marina and Esperanza Morato for performing advanced proteomic analysis. We thank to Athel Cornish-Bowden from CNRS (Centre national de la Recherche Scientifique, Paris, France) for expert advice on enzyme kinetics. This work was supported by the Spanish Government, cofunded by the European Union ERDF [grant numbers SAF2014-53209-R (M.V. and R.B.), PI15/00107 (A.M.-R.), PI15/02256 (D.B.), PI16/00249 (D.B.), RD16/0006/0014 (M.V.) and RD16/0006/0015 (D.B.)]. J.C.L.-R. is supported by an FPU fellowship (FPU13/02393) from the Spanish Government. The Proteomic Service of CBMSO belongs to ProteoRed (PRB3-ISCIII), supported by grants PT13/0001/0024 and PT17/0019/0018 of Spanish Government (cofunded by the European Union ERDF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101256.
Contributor Information
Antonio Martínez-Ruiz, Email: amartinezruiz@salud.madrid.org.
Eva Batanero, Email: ebataner@ucm.es.
Conflicts of interest
The authors have no financial conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Leino M.S. Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reithofer M., Jahn-Schmid B. Allergens with protease activity from house dust mites. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kale S.L., Agrawal K., Gaur S.N., Arora N. Cockroach protease allergen induces allergic airway inflammation via epithelial cell activation. Sci. Rep. 2017;7:42341. doi: 10.1038/srep42341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattsson L., Lundgren T., Everberg H., Larsson H., Lidholm J. Prostatic kallikrein: a new major dog allergen. J. Allergy Clin. Immunol. 2009;123:362–368. doi: 10.1016/j.jaci.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Vinhas R. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy. 2011;66:1088–1098. doi: 10.1111/j.1398-9995.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacquet A. Interactions of airway epithelium with protease allergens in the allergic response. Clin. Exp. Allergy. 2011;41:305–311. doi: 10.1111/j.1365-2222.2010.03661.x. [DOI] [PubMed] [Google Scholar]
- 7.Chruszcz M. Crystal structures of mite allergens Der f 1 and Der p 1 reveal differences in surface-exposed residues that may influence antibody binding. J. Mol. Biol. 2009;386:520–530. doi: 10.1016/j.jmb.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevigne A., Jacquet A. Emerging roles of the protease allergen Der p 1 in house dust mite-induced airway inflammation. J. Allergy Clin. Immunol. 2018;142:398–400. doi: 10.1016/j.jaci.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Dumez M.E. Orchestration of an uncommon maturation cascade of the house dust mite protease allergen quartet. Front. Immunol. 2014;5:138. doi: 10.3389/fimmu.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan H. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin. Exp. Allergy. 2001;31:279–294. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 11.Asokananthan N. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J. Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- 12.King C., Brennan S., Thompson P.J., Stewart G.A. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J. Immunol. 1998;161:3645–3651. [PubMed] [Google Scholar]
- 13.Adam E. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J. Biol. Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 14.Deb R., Shakib F., Reid K., Clark H. Major house dust mite allergens Dermatophagoides pteronyssinus 1 and Dermatophagoides farinae 1 degrade and inactivate lung surfactant proteins A and D. J. Biol. Chem. 2007;282:36808–36819. doi: 10.1074/jbc.M702336200. [DOI] [PubMed] [Google Scholar]
- 15.Kalsheker N.A. The house dust mite allergen Der p1 catalytically inactivates alpha 1-antitrypsin by specific reactive centre loop cleavage: a mechanism that promotes airway inflammation and asthma. Biochem. Biophys. Res. Commun. 1996;221:59–61. doi: 10.1006/bbrc.1996.0544. [DOI] [PubMed] [Google Scholar]
- 16.Brown A. House dust mite Der p 1 downregulates defenses of the lung by inactivating elastase inhibitors. Am. J. Respir. Cell Mol. Biol. 2003;29:381–389. doi: 10.1165/rcmb.2003-0060OC. [DOI] [PubMed] [Google Scholar]
- 17.Hammad H. Th2 polarization by Der p 1--pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood. 2001;98:1135–1141. doi: 10.1182/blood.v98.4.1135. [DOI] [PubMed] [Google Scholar]
- 18.Schulz O., Sewell H.F., Shakib F. A sensitive fluorescent assay for measuring the cysteine protease activity of Der p 1, a major allergen from the dust mite Dermatophagoides pteronyssinus. Mol. Pathol. 1998;51:222–224. doi: 10.1136/mp.51.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaemmaghami A.M., Gough L., Sewell H.F., Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin. Exp. Allergy. 2002;32:1468–1475. doi: 10.1046/j.1365-2745.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- 20.Machado D.C., Horton D., Harrop R., Peachell P.T., Helm B.A. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur. J. Immunol. 1996;26:2972–2980. doi: 10.1002/eji.1830261224. [DOI] [PubMed] [Google Scholar]
- 21.Verma S., Dixit R., Pandey K.C. Cysteine proteases: modes of activation and future prospects as pharmacological targets. Front. Pharmacol. 2016;7:107. doi: 10.3389/fphar.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman J. Der p 1 is the primary activator of Der p 3, Der p 6 and Der p 9 the proteolytic allergens produced by the house dust mite Dermatophagoides pteronyssinus. Biochim. Biophys. Acta. 2014;1840:1117–1124. doi: 10.1016/j.bbagen.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Thomas W.R. Hierarchy and molecular properties of house dust mite allergens. Allergol. Int. 2015;64:304–311. doi: 10.1016/j.alit.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Klomsiri C., Karplus P.A., Poole L.B. Cysteine-based redox switches in enzymes. Antioxidants Redox Signal. 2011;14:1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Halleux S. Three-dimensional structure and IgE-binding properties of mature fully active Der p 1, a clinically relevant major allergen. J. Allergy Clin. Immunol. 2006;117:571–576. doi: 10.1016/j.jaci.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 26.van Rijt L.S., Utsch L., Lutter R., van Ree R. Oxidative stress: promoter of allergic sensitization to protease allergens? Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18061112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzpatrick A.M., Jones D.P., Brown L.A. Glutathione redox control of asthma: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2012;17:375–408. doi: 10.1089/ars.2011.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantin A.M., North S.L., Hubbard R.C., Crystal R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1985;63(1987):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 29.Wilce M.C., Parker M.W. Structure and function of glutathione S-transferases. Biochim. Biophys. Acta. 1994;1205:1–18. doi: 10.1016/0167-4838(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 30.Allocati N., Masulli M., Di Ilio C., Federici L. Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7:8. doi: 10.1038/s41389-017-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasieva O. The many faces of glutathione transferase pi. Curr. Mol. Med. 2011;11:129–139. doi: 10.2174/156652411794859278. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J. Pleiotropic functions of glutathione S-transferase P. Adv. Cancer Res. 2014;122:143–175. doi: 10.1016/B978-0-12-420117-0.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta S. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 34.Reynaert N.L. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones J.T. Glutathione S-transferase pi modulates NF-kappaB activation and pro-inflammatory responses in lung epithelial cells. Redox Biol. 2016;8:375–382. doi: 10.1016/j.redox.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo H.W., Ali-Osman F. Structure of the human allelic glutathione S-transferase-pi gene variant, hGSTP1 C, cloned from a glioblastoma multiforme cell line. Chem. Biol. Interact. 1998;111–112:91–102. doi: 10.1016/s0009-2797(97)00153-1. [DOI] [PubMed] [Google Scholar]
- 37.Dai X. Do glutathione S-transferase genes modify the link between indoor air pollution and asthma, allergies, and lung function? A systematic review. Curr. Allergy Asthma Rep. 2018;18:20. doi: 10.1007/s11882-018-0771-0. [DOI] [PubMed] [Google Scholar]
- 38.Spiteri M.A., Bianco A., Strange R.C., Fryer A.A. Polymorphisms at the glutathione S-transferase, GSTP1 locus: a novel mechanism for susceptibility and development of atopic airway inflammation. Allergy. 2000;55(Suppl 61):15–20. doi: 10.1034/j.1398-9995.2000.00502.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang C.H. Characterization of glutathione S-transferase from dust mite, Der p 8 and its immunoglobulin E cross-reactivity with cockroach glutathione S-transferase. Clin. Exp. Allergy. 2006;36:369–376. doi: 10.1111/j.1365-2222.2006.02447.x. [DOI] [PubMed] [Google Scholar]
- 40.Dao D.D., Partridge C.A., Kurosky A., Awasthi Y.C. Human glutathione S-transferases. Characterization of the anionic forms from lung and placenta. Biochem. J. 1984;221:33–41. doi: 10.1042/bj2210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simons P.C., Vander Jagt D.L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal. Biochem. 1977;82:334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- 42.Izquierdo-Alvarez A., Tello D., Cabrera-Garcia J.D., Martinez-Ruiz A. Identification of S-nitrosylated and reversibly oxidized proteins by fluorescence switch and complementary techniques. Methods Mol. Biol. 2018;1747:73–87. doi: 10.1007/978-1-4939-7695-9_7. [DOI] [PubMed] [Google Scholar]
- 43.Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 44.Perez M. Mutual regulation between SIAH2 and DYRK2 controls hypoxic and genotoxic signaling pathways. J. Mol. Cell Biol. 2012;4:316–330. doi: 10.1093/jmcb/mjs047. [DOI] [PubMed] [Google Scholar]
- 45.Alonso R. Evidence for fungal infection in cerebrospinal fluid and brain tissue from patients with amyotrophic lateral sclerosis. Int. J. Biol. Sci. 2015;11:546–558. doi: 10.7150/ijbs.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izquierdo-Alvarez A. Differential redox proteomics allows identification of proteins reversibly oxidized at cysteine residues in endothelial cells in response to acute hypoxia. J. Proteom. 2012;75:5449–5462. doi: 10.1016/j.jprot.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 47.Stewart G.A., Simpson R.J., Thomas W.R., Turner K.J. Physicochemical characterization of a major protein allergen, Der p I, from the house dust mite, Dermatophagoides pteronyssinus. Amino acid analysis and circular dichroism studies. Int. Arch. Allergy Appl. Immunol. 1987;82:444–446. doi: 10.1159/000234249. [DOI] [PubMed] [Google Scholar]
- 48.Adler V. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tew K.D., Townsend D.M. Regulatory functions of glutathione S-transferase P1-1 unrelated to detoxification. Drug Metab. Rev. 2011;43:179–193. doi: 10.3109/03602532.2011.552912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartolini D., Galli F. The functional interactome of GSTP: a regulatory biomolecular network at the interface with the Nrf2 adaption response to oxidative stress. J. Chromatogr. B. 2016;1019:29–44. doi: 10.1016/j.jchromb.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Burgoyne J.R. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 52.Caselli A. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J. Biol. Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 53.Campos B.M. A redox 2-Cys mechanism regulates the catalytic activity of divergent cyclophilins. Plant Physiol. 2013;162:1311–1323. doi: 10.1104/pp.113.218339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall A., Nelson K., Poole L.B., Karplus P.A. Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxidants Redox Signal. 2011;15:795–815. doi: 10.1089/ars.2010.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohammadzadeh G.S. Measurement of glutathione S-transferase and its class-pi in plasma and tissue biopsies obtained after laparoscopy and endoscopy from subjects with esophagus and gastric cancer. Clin. Biochem. 2003;36:283–288. doi: 10.1016/s0009-9120(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 56.Howie A.F., Bell D., Hayes P.C., Hayes J.D., Beckett G.J. Glutathione S-transferase isoenzymes in human bronchoalveolar lavage: a possible early marker for the detection of lung cancer. Carcinogenesis. 1990;11:295–300. doi: 10.1093/carcin/11.2.295. [DOI] [PubMed] [Google Scholar]
- 57.Howie A.F., Hayes P.C., Bouchier I.A., Hayes J.D., Beckett G.J. Glutathione S-transferase in human bile. Clin. Chim. Acta. 1989;184:269–278. doi: 10.1016/0009-8981(89)90060-0. [DOI] [PubMed] [Google Scholar]
- 58.Giles N.M. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003;10:677–693. doi: 10.1016/s1074-5521(03)00174-1. [DOI] [PubMed] [Google Scholar]
- 59.Kaul P., Sathish H.A., Prakash V. Effect of metal ions on structure and activity of papain from Carica papaya. Nahrung. 2002;46:2–6. doi: 10.1002/1521-3803(20020101)46:1<2::AID-FOOD2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 60.Takai T. Modulation of allergenicity of major house dust mite allergens Der f 1 and Der p 1 by interaction with an endogenous ligand. J. Immunol. 2009;183:7958–7965. doi: 10.4049/jimmunol.0713276. [DOI] [PubMed] [Google Scholar]
- 61.Herbert C.A. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am. J. Respir. Cell Mol. Biol. 1995;12:369–378. doi: 10.1165/ajrcmb.12.4.7695916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.