Abstract
Non-iridescent structural colour in avian feathers is produced by coherent light scattering through quasi-ordered nanocavities in the keratin cortex of the barbs. To absorb unscattered light, melanosomes form a basal layer underneath the nanocavities. It has been shown that throughout Aves, melanosome morphology reflects broad categories of melanin-based coloration, as well as iridescence, allowing identification of palaeocolours in exceptionally preserved fossils. However, no studies have yet investigated the morphology of melanosomes in non-iridescent structural colour. Here, we analyse a wide sample of melanosomes from feathers that express non-iridescent structural colour from a phylogenetically broad range of extant avians to describe their morphology and compare them with other avian melanosome categories. We find that investigated melanosomes are typically wide (approx. 300 nm) and long (approx. 1400 nm), distinct from melanosomes found in black, brown and iridescent feathers, but overlapping significantly with melanosomes from grey feathers. This may suggest a developmental, and perhaps evolutionary, relationship between grey coloration and non-iridescent structural colours. We show that through analyses of fossil melanosomes, melanosomes indicative of non-iridescent structural colour can be predicted in an Eocene stem group roller (Eocoracias: Coraciiformes) and with phylogenetic comparative methods the likely hue can be surmised. The overlap between melanosomes from grey and non-iridescent structurally coloured feathers complicates their distinction in fossil samples where keratin does not preserve. However, the abundance of grey coloration relative to non-iridescent structural coloration makes the former a more likely occurrence except in phylogenetically bracketed specimens like the specimen of Eocoracias studied here.
Keywords: palaeocolour, structural colour, melanosomes
1. Introduction
Melanosomes produce colours in avian feathers through the selective absorption and reflection of specific wavelengths of light by the melanin pigment molecules they contain [1]. Structural colours, on the other hand, are produced by light scattering through ordered nanostructural arrangements of keratin, melanosomes and/or air within the feather [2]. The differences in refractive index between these contrasting phases generate some of the most vivid colours known in nature. Iridescent structural colours are produced through angle-dependent, coherent light scattering by layers of melanosomes and keratin in the feather barbules [2]. Another, distinct mechanism of structural colour production is generated by coherent scattering of light by a medullary or spongy layer underneath a keratin cortex, and above a basal melanosome layer [2–4] which serves to absorb unscattered light. Unlike iridescence, this type of structural colour is consistent from different viewing angles and is located in feather barbs. It is generally referred to as non-iridescent structural colour [5] and produces a range of colours that is perceived by human observers in the spectrum between blue, violet and turquoise [4]. When combined with yellow pigments (for example carotenoids), it can also produce green hues [4]. The basal melanosome layer is important in the production of non-iridescent structural colour as it absorbs incoherently scattered white light, preventing it from being reflected back to an observer. In the case of amelanism, a genetic disorder causing a lack of melanin biosynthesis in birds, the resulting colour of birds that would normally exhibit non-iridescent structural colours is pale, or ‘washed-out’ [5].
Melanin has been shown to have high preservation potential in fossils, both at a chemical and macrostructural level, often preserving the three-dimensional morphology of the lysosome-derived organelles containing the melanin (melanosomes) as well as the spherical granules of cephalopod ink [6–11]. Melanosomes are responsible for the exceptional fossilization of many vertebrate soft tissues, such as skin, hair and feathers as well as eyes and some internal organs, such as the liver [9]. Work investigating the morphologies of melanosomes from extant avian taxa has shown a clear link between their shape, colours produced and the mechanism of their production [11]. Reddish-brown phaeomelanin is contained within ovoid melanosomes that are on average approximately 500 nm long in birds and mammals [11,12], while black eumelanin is contained within a more oblong melanosome, averaging 800–1000 nm in length [11,12]. Melanosomes may have a mixed eumelanin/phaeomelanin composition and their morphology seems to form a spectrum between these end members. Eumelanin-rich melanosomes can vary further in size and shape which correspond to a further spectrum of colours and coloration mechanisms, such as iridescent and grey [11,12]. Furthermore, penguins have been observed to display melanosomes of a unique morphology which requires further investigation [10].
Inferences of colour in extinct avian and non-avian taxa have been done in the past using melanosome morphology and statistical methods, such as quadratic discriminant analysis [7,9–11,13]. These analyses have shown that plumage colours can be predicted through melanosome morphology alone with 82% accuracy. These predictions are currently based on broad colour categories and mechanisms of colour production involving melanosomes (black, brown, grey and iridescent—after the colours of feathers from which melanosomes were extracted) [11] as well as a category for extant penguin melanosomes [10]. Predicting the coloration of fossil birds and dinosaurs has allowed aspects of their ecology and lifestyle (such as signalling strategies, behaviour and habitat preference) to be inferred [10,11,13,14]. To date, non-iridescent structural colour has not been investigated in the fossil record. It has been shown that keratin degrades during the process of fossilization [15,16]. Hence, the reliability of reconstructing non-iridescent structural colours, which are generated by keratin nanocavities in feather barbs would be impossible. However, since melanosomes associated with non-iridescent structural colours will preserve, these could potentially be conflated with other melanosomes involved in other colour production mechanisms.
Here we explore the morphology of melanosomes from feathers that produce non-iridescent structural colour to determine whether they are distinct from previously identified melanosome categories used to predict palaeocolour in quadratic discriminant analyses. We also investigate the early Eocene stem group coraciiform Eocoracias brachyptera with preserved feathering from Messel Formation in Germany [17]. The modern relatives of this taxon ostensibly display non-iridescent structural colours. Furthermore, to corroborate the phylogenetic bracketing of Eocoracias and its likelihood of displaying non-iridescent structural colour, we conducted an ancestral state reconstruction and evaluated the likely ancestral hues of non-iridescent structural colour.
2. Material and methods
2.1. Sampling of feathers and melanosome extraction
A total of 72 feather samples expressing non-iridescent structural colour were collected at the Zoological Museum, Natural History Museum of Denmark, University of Copenhagen (see electronic supplementary material for details of samples). The presence of non-iridescent structural colour in each feather sample was confirmed based on the phylogenetic sample used in Saranathan et al. [18].
Feathers were cleaned with ethanol, and the parts of the feather expressing non-iridescent structural colour were cut for sampling. These coloured sections were then subjected to the enzymatic extraction method described in Colleary et al. [8] to remove the keratin and extract melanosomes. The resulting pellets of melanin were mounted on scanning electron microscopy (SEM) stubs and gold coated prior to investigation with a SEM instrument.
2.2. Fossil specimen
A new specimen of the stem group roller E. brachyptera (figure 1) from the Early Eocene Messel Formation in Germany [17,19], housed in the collections of the Senckenberg Research Institute, Frankfurt (SMF) was investigated in this study (collection number: SMF-ME 1450A). This fossil was chosen due to its position as sister to the clade containing the extant Coraciidae (true rollers), a clade in which non-iridescent structural colour is common, and the Brachypteraciidae (ground rollers), which show a more restricted occurrence [20]. This allowed for determination of whether non-iridescent structural colour was present in the stem group representative of this colourful clade. It also provides an ideal test case for ascertaining whether these colours can be detected in the fossil record. A total of 12 samples were removed from the plumage of the fossil by gently scraping small sections of organic material (generally under 2 mm2) off the surface of the preserved feathers using a clean scalpel (figure 1). These samples were mounted on SEM stubs and sputter coated with gold for SEM imaging.
Figure 1.
The sampled fossil of Eocoracias brachyptera (SMF-ME 1450A) with SEM images of preserved melanosomes. Numbers indicate where sampling was done and correspond to SEM images. All scale bars for SEM images, 2 µm. (Online version in colour.)
2.3. Investigation of melanosome morphology
The gold coated fossil feather samples and melanosome extracts were investigated using a Zeiss Sigma HD VP field emission SEM under high vacuum mode at an accelerating voltage of 12–20 keV and a working distance of 10 mm. Enough images were produced to be able to measure 100 fully exposed (non-overlapping) melanosomes per sample. To assess the morphology of melanosomes, the width (nm), length (nm) and aspect ratio of 100 melanosomes were measured for each sample using ImageJ [21]. Additional calculations were carried out for each sample: mean, standard deviation, coefficient of variance (CV) and skew for length, width and aspect ratio of each sample. Results were added to a previously constructed dataset from Li et al. [11].
One-way ANOVA and Tukey's post hoc test were carried out on data in R [22,23] to determine whether melanosomes extracted from feather samples exhibiting non-iridescent structural colour differed significantly in their morphology from other defined melanosome morphologies (from black, brown, grey, iridescent and penguin feathers). Quadratic discriminant analyses (QDA) were used to investigate the accuracy of colour predictions when non-iridescent structural colour was added as a separate colour category [11]. This method uses extant feather colour categories as the independent variable, and melanosome morphology measurements as the dependent variable. The model fitted to a training subset was applied to the test subset to determine its accuracy. This process was performed 100 times, to account for random sample selection of training and test subsets. Forward stepwise regression was performed to test which of the eight dependent variables (length, length CV, length skew, width, width CV, width skew, aspect ratio and aspect ratio skew) explained most of the variance in the model. Finally, the values of the first two variables (those that explained the variation best) obtained by forward stepwise regression were plotted to provide a visual assessment of melanosome categories.
2.4. Ancestral state reconstruction in the Coraciidae
Phylogenetic comparative methods were used to investigate the likely colour of E. brachyptera. A database of bird plumage coloration for Coraciiformes was constructed based on visual assessment and plumage descriptions from the Handbook of the Birds of the World [20]. Colour states were coded based on the presence and absence of black colour, grey colour, non-iridescent structural colour, and carotenoids across the plumage (electronic supplementary material). We chose grey and non-iridescent structural colour as categories because the melanosomes that produce these colours are similar in morphology (result of this study). Carotenoids were chosen as a colour category due to their combined effect with non-iridescent structural colour on the overall coloration of bird plumage. Presence of carotenoids that produce specific colours was confirmed based on Thomas et al. for selected bird species [24]. The black colour category was chosen as several samples from E. brachyptera feathers were predicted as black in the QDA (see below). Each colour was treated as a discrete character state with absence coded as 0 and presence as 1. The same process was repeated for both males and females. The posterior probability of the tip state for E. brachyptera was estimated, given an equal prior probability of 0.5 for each state. A stochastic character mapping approach was implemented using the function make.simmap in the R package phytools [25], while the phylogenetic backbone was taken from 1000 randomly sampled trees from Jetz et al. [26]. The fossil taxon E. brachyptera was added to each of the 1000 trees from Jetz et al. as outgroup to crown Coraciiformes; the age of the tip for E. brachyptera was set at 48 Ma and the branching time that E. brachyptera diverged from the Coraciiformes was randomly sampled from a uniform distribution bounded by the total-group Coraciiformes age for that tree and 48 Ma using motmot [27]. Using these trees, a consensus tree was built using maxCladeCred in the R package phangorn [28].
3. Results
3.1. Melanosome morphology in feathers with non-iridescent structural colour
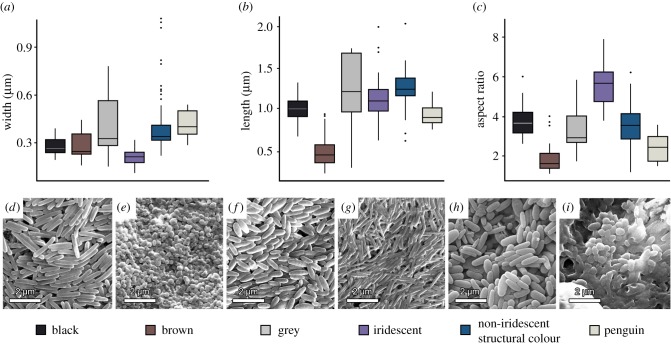
Melanosomes extracted from feathers that exhibit non-iridescent structural colour are all ellipsoidal in morphology. Their mean length, width and aspect ratio are 1249.2 nm ± 230.6 nm, 408.5 nm ± 186 nm, and 3.4 ± 0.9, respectively. The most significant variables that determine the shape of melanosomes (length, width and aspect ratio), were compared among groups divided by colour categories. Results of the one-way ANOVA indicated that there are groups that are not significantly different. The Tukey's post hoc test revealed that the melanosomes extracted from feathers exhibiting non-iridescent structural colours were significantly different (p < 0.05) in their morphology from all other colour categories except grey (see electronic supplementary material for details). No significant difference was found in any variable, i.e. length, width and aspect ratio, with melanosomes from grey feathers (p = 1.0, p = 0.99, p = 0.99 respectively). There was also no significant difference between the length of melanosomes from feathers expressing non-iridescent structural colours and melanosomes from iridescent feathers (p = 0.35), the width of melanosomes from non-iridescent structural colour feathers and ‘penguin-type’ melanosomes (p = 0.99) and the aspect ratio of melanosomes from feathers that express non-iridescent structural colour and melanosomes from black feathers (p = 0.53) (figure 2a–c), highlighting the importance of including multiple shape variables in statistical analyses of melanosome morphology–colour relationships.
Figure 2.
Comparison of melanosome shape (in extant bird feathers) for different colour categories. Box plots of mean width (a), length (b), and aspect ratio (c) of measured melanosomes from 232 birds of various feather types, based on the dataset of Li et al. [11] with non-iridescent structural colour added as a new colour category. SEM images of melanosomes from six colour categories in feathers of the extant taxa: (d) black feathers of wrinkled hornbill (Rhabdotorrhinus corrugatus), (e) brown feathers of the great jacamar (Jacamerops aureus), (f) grey feather of the toucan barbet (Semnornis ramphastinus), (g) iridescent feather of the great jacamar (Jacamerops aureus), (h) feathers expressing non-iridescent structural colour of the blue paradise flycatcher (Terpsiphone cyanescens), and (i) black feather of an African penguin (Spheniscus demersus). (Online version in colour.)
3.2. Accuracy of melanosome prediction
As the two variables that explain most of the variation in the data based on the forward stepwise addition, length and width were plotted separately for each colour category with 95% confidence intervals (electronic supplementary material). The 100 simulations demonstrate the accuracy of the QDA. Samples were predicted accurately in 61.8% of the cases, which is a drop from 82% before the introduction of the non-iridescent structural colour category. Percentages of accurate prediction within each colour category are outlined in figure 3. Melanosome morphology (length and width) is the strongest predictor for category of non-iridescent structural colour (75.5%), followed by decreasing percentage of accurate prediction: brown (71.8%), iridescent (63.6%), ‘penguin-type’ (62.2%), grey (54.7%) and black (45.4%). The accuracy of melanosome categorization in the Li et al. database was reported as 82% [11]. The same methodology of accurate prediction was also performed on just the Li et al. database [11]. The ‘penguin-type’ melanosome category showed the highest percentage of accurate prediction (81.2%) followed by iridescent (72.2%), brown (73%), grey (63.5%) and black (55.6%). When melanosomes from feathers expressing non-iridescent structural colours were included in the Li et al. database and treated as ‘unknown’ in terms of colour category, 29 samples were predicted as grey, 28 as black, seven as ‘penguin-type’, six as iridescent, and two as brown.
Figure 3.
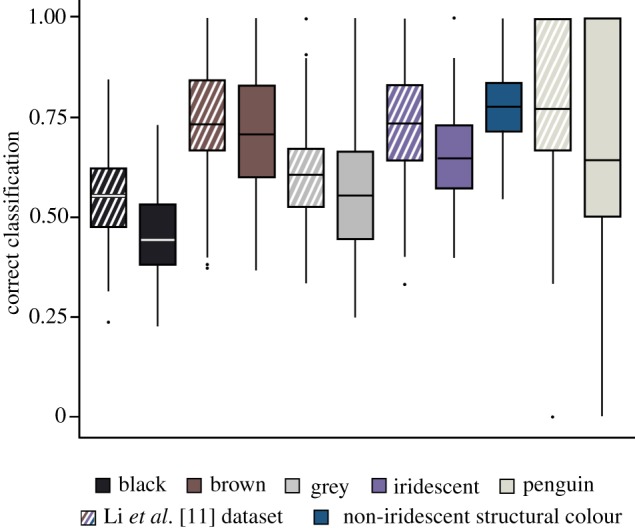
Percentage of correctly classified cases for each colour category based on melanosome morphology in a simulation within a quadratic discriminant analysis (100 repeats). The horizontal line on each box plot indicates the mean value. Cross-hatched boxes indicate correct classifications based on just the Li et al. dataset [11], while solid boxes indicate accurate prediction when non-iridescent structural colour was introduced into the same database. (Online version in colour.)
3.3. Reconstruction of the plumage colour of Eocoracias brachyptera
Of the 12 samples taken from E. brachyptera 10 contained melanosomes visible using SEM. These melanosomes were all notably large, conforming to the ones found in non-iridescent structural colours and those characteristic for grey colour (with average length: 1462.02 nm, width: 483.09 nm and aspect ratio: 3.1). From the QDA, seven samples were predicted as belonging to grey colour category (abdomen, ventral side of the neck, dorsal side of the neck, caudal region of the skull, lower part of the neck, sternum, dorsal region in front of synsacrum), while three samples were predicted as black colour category (the dorsal side of the neck, rump and the tail).
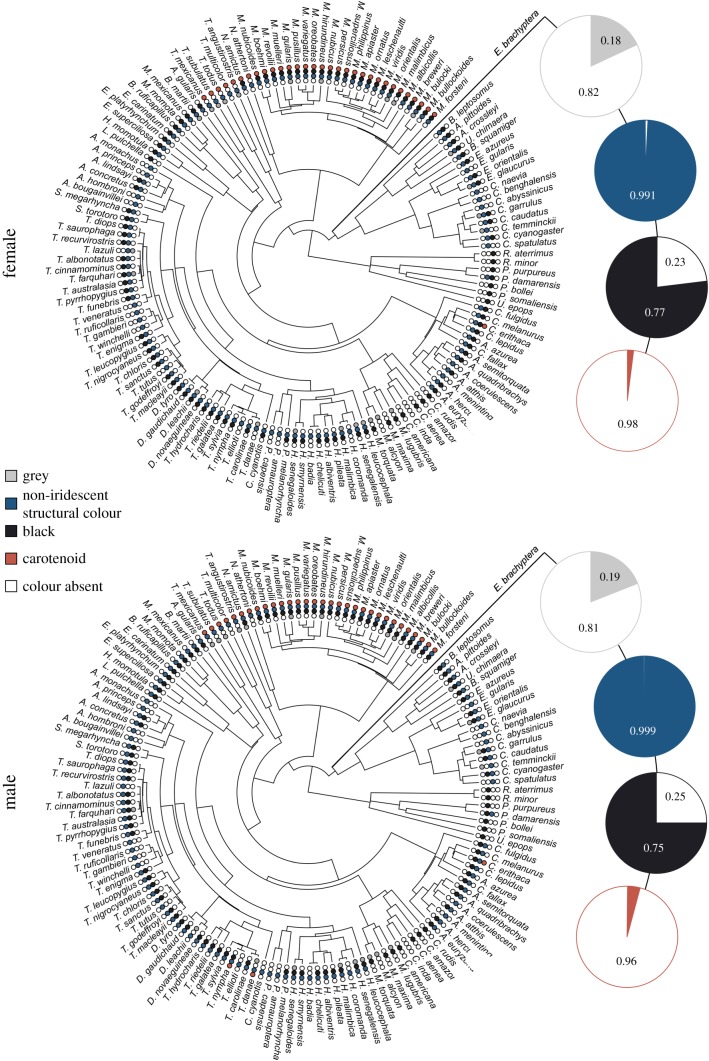
The ancestral state reconstruction of the plumage coloration in E. brachyptera predicted a high probability of non-iridescent structural colour being present (0.99 posterior probability for males and females). Black colour was also likely to have been present (posterior probability 0.75 for males and 0.77 females). However, both grey (posterior probability 0.19 for males, and 0.17 for females) and carotenoids (0.04 for males, and 0.02 for females) were not likely to have been present (figure 4).
Figure 4.
Ancestral state reconstructions of colour presence or absence for the Eocoracias brachyptera fossil for both female and male. Small circles indicate presence or absence of each colour category as indicated in the colour legend. Posterior probabilities of colour presence from stochastic character mapping are represented by large pie charts. (Online version in colour.)
Figure 5 shows a reconstruction of E. brachyptera with a hypothetical plumage coloration based on the results of our study. We did not recover melanosomes in the primary feathers. This is likely due to the sampling method rather than a lack of pigmentation, as these feathers are preserved as dark organics in the same way as the areas with melanosomes present. Due to the preparation methods used for Messel fossils, using resin and often coating them with lacquer, removing organics suitable for imaging under the SEM is difficult because microstructural details are obscured, including melanosomes. Therefore, melanosomes will only likely be exposed if the organics from between the resin and lacquer are removed and imaged. Parts of the neck, tail and rump of E. brachyptera were reconstructed as black due to the support from results of the study of melanosome morphology and ancestral state reconstruction. Based on the same combination of methods we reconstructed the rest of the plumage as blue. The wing feathers were reconstructed as black and blue, following the ancestral state reconstruction and the melanosome morphology detected in the other parts of the bird's plumage. Our results show, however, that melanosome shape alone does not allow for a distinction between grey and non-iridescent structural colour for E. brachyptera.
Figure 5.

Reconstruction of Eocoracias brachyptera with hypothesized plumage coloration. Our reconstruction of the external appearance of the species is based on Mayr and Mourer-Chauviré's description of the fossils [19]. (Online version in colour.)
4. Discussion
4.1. Reconstruction of non-iridescent structural colour in the fossil record
Distinct melanosome shapes have been determined for black, brown, grey, and iridescent colours, and for special ‘penguin-type’ melanosomes [11]. The introduction of a melanosome type associated with non-iridescent structural colour reduced the accuracy of melanosome categorization from 82% to 61.9%. Investigated melanosomes are generally distinct to most other melanosome categories but overlap significantly with those significant for grey feather coloration. Given the clear correlation between broad colour categories however, melanosome morphology is currently the best way to determine palaeocolour, particularly for non-iridescent structural colour, grey and iridescent, which can only be determined based on melanosome shape. In this study, the QDA have high accuracy when determining non-iridescent structural colour, iridescence and brown colour while black and grey dropped in accuracy (figure 2). Some of these issues could be surmised to be alleviated by coupling melanosome analysis with chemical analyses, such as time-of-flight secondary ion mass spectrometry, which can distinguish between relative amounts of eumelanin and phaeomelanin in fossil and extant melanosomes [8], and through further probing of the relatively broad colour categories, which in reality form a spectrum of hues. However, chemical analyses will likely not be able to distinguish melanin from melanosomes involved in the production of grey and non-iridescent structural colour and black and iridescent.
The colours of the studied avian taxa included in the ancestral state reconstruction revealed a high probability for the existence of non-iridescent structural colour, and black, while there was a low probability of the occurrence of carotenoid-based and grey colours in the plumage of E. brachyptera. By contrast, the morphology of most melanosomes obtained from E. brachyptera predicted grey rather than non-iridescent structural colour in the QDA. In the case of the Eocoracias fossil, it therefore appears that incorporating ancestral state reconstructions with the aim of discriminating between overlapping melanosome morphologies (e.g. grey colour and non-iridescent structural colour) is an essential method for reconstructing the likely plumage coloration in fossil taxa.
Due to the poor preservation potential of keratin [15,16], the ‘spongy’ barb cortex responsible for selective light scattering is lost in fossil feathers, leaving only melanosomes. The lack of significant morphological differences between melanosomes characteristic of grey and those associated with non-iridescent structural colour makes them hard to distinguish from one another in fossils. Overall, non-iridescent structural colour occurs in about 10 extant avian lineages (Anseriformes, Galliformes, Columbiformes, Musophagiformes, Procellariiformes, Gruiformes, Charadriiformes, Coliiformes, Coraciiformes, Piciformes, Psittaciformes and Passeriformes) out of 61 lineages according to Stoddard & Prum [29] and is usually even highly restricted within each. Hence, the most conservative inference would be that an avian fossil outside these clades would likely exhibit grey coloration rather than non-iridescent structural colours.
4.2. Developmental pathways in non-iridescent structural and grey colours—a possible evolutionary link?
Our observed overlap between melanosomes involved in the production of grey colour and non-iridescent structural colour is of interest for understanding the role of melanosome morphology in plumage development. Initially, melanosomes are transported into keratinocytes during the early stages of feather development [30]. The melanosome-laden keratinocytes are arranged to form the major feather structures—the calamus, the barb and the barbule [30,31]. After death of the keratinocytes, melanosomes are observed to arrange themselves while the cell hardens by polymerizing keratin. This self-assembly process is best known from studies of iridescent feathers, in which melanosomes are arranged to create photonic nanostructures [31]. Maia et al. [31] have suggested that this self-assembly takes place due to depletion–attraction forces between the melanosomes and the polymerizing keratin. It has been shown that the size, shape and concentration of melanosomes may be important factors in facilitating this process. The developmental processes that involve self-assembly mechanisms have been described in feathers with black [31], iridescent [2,31,32] and non-iridescent structural colours [2,33]. So far however, the process of producing melanin-based colours has received little attention and a better understanding of the relative role of the various potential factors in melanosome arrangement (e.g. shape and concentration) is needed. The overlap in melanosome shape between those found in grey feathers and those in feathers expressing non-iridescent structural colour could be indicative of similar developmental mechanisms during the growth of the feather that uses these two colours. Iridescent and melanized feathers have melanosomes concentrated toward the outer region of the feather cortex [31,32,34]. Feathers expressing non-iridescent structural colours differ from this arrangement as melanosomes are concentrated towards the barb core [2,33]. Grey coloration is generated by a more diffuse arrangement of melanosomes and a greater concentration of melanosomes in the core than the cortex. We hypothesize that a transition from grey colour to non-iridescent structural colours would be a potential pathway for the evolution of non-iridescent structural colour. The larger melanosomes would be driven to concentrate in the feather core, leaving a space for keratin nanocavities to form. We surmise that the generally larger and wider melanosomes facilitate this process, while narrower melanosomes, those involved in black and iridescent colours, are pulled to the cortex of the feather [31,34]. Corroborating the notion that there could be an evolutionary relationship between grey and non-iridescent structural colours, some birds are intermediate in coloration between these two colour categories, such as the Victoria crowned pigeon (Goura victoria). Future studies should focus on the link between grey and non-iridescent structural coloration during feather development and how melanosome morphology influences nanostructural feather assembly outside of well-studied iridescent feathers.
5. Conclusion
Melanosomes in barbs arranged near the keratin cortex producing non-iridescent structural colour are wider and longer than melanosomes in most other feather colour categories. However, melanosomes isolated from grey feathers overlap significantly with the shape of melanosomes from feathers expressing non-iridescent structural colour. Introducing melanosomes from non-iridescent structural colour as a colour category in the QDA reduced the accuracy of melanosome colour categorization from 82% to 61.9%. This increases in particular the uncertainty when distinguishing grey from non-iridescent structural colour in palaeocolour inference. As non-iridescent structural colours are relatively phylogenetically constrained in modern birds, their prevalence in extinct taxa would have been similarly limited. Therefore, grey is a more likely occurrence unless phylogenetic or ecological data suggest otherwise. It has been argued that melanosome shape is important in facilitating their position inside the keratin matrix during feather ontogeny and the resultant colour. The similarity of melanosome shape involved in the production of grey and non-iridescent structural colours highlights a possible common dependence on melanosome shape during the development of feather coloration and is potentially linked evolutionarily.
Supplementary Material
Acknowledgements
The authors would like to thank Jon Fjeldså from Zoological Museum, University of Copenhagen, for providing access to the collections at the SNM in Denmark. Maja Fabijanic, University of Zagreb, Croatia, is thanked for comments and suggestions to our statistical analyses. Klara Norden is thanked for insightful suggestions to the project and reading an earlier version of the manuscript. F.B. would like to thank Lady Gaga for creating music that provided continuous support and encouragement throughout the process of making this publication. We thank two anonymous reviewers for their helpful comments.
Ethics
All feathers were picked from museum skin samples (in accordance with museum staff).
Data accessibility
All data are available in electronic supplementary material: (1) Melanosome measurements—1.1. Measurements of melanosomes isolated from fresh feathers that express non-iridescent structural colour are included as a separate category in the previously existing database from Li. et al. (sheet number 7 in our electronic supplementary material). 1.2. Measurements of melanosomes from the surface of the fossil are under sheet number 2 in the electronic supplementary material. (2) Other important data that we have collected for our research are colour presence/absence matrix that is included in sheet number 3 of the electronic supplementary material.
Authors' contributions
F.B. and J.V. designed the experiment. J.V. and G.M. sampled the fossil. F.B. extracted melanosomes and performed the electron microscopy with assistance from E.-J.G., E.L. and F.M.S. E.-J.G. and E.L. analysed a preliminary dataset for their undergraduate practical project, supervised by F.B. and J.V. F.B. collated the database for ancestral state reconstruction and statistical analysis. M.N.P. performed the discrete character reconstruction. M.Z., G.M. and F.B. performed the anatomical description of the fossil. M.Z. made the artistic reconstruction of Eocoracias. F.B. and M.Z. produced figures. All authors contributed to the manuscript.
Competing interests
We declare we have no competing interests.
Funding
F.B. was financially supported by the Erasmus + Traineeship Program and F.M.S. was supported by the Natural Environment Research Council (PhD grant no. NE/L002434/1).
References
- 1.McGraw KJ. 2006. Mechanics of melanin-bases coloration. In Bird coloration: mechanisms and measurements (eds Hill GE, McGraw KJ), pp. 243–294. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Prum RO. 2006. Anatomy, physics, and evolution of structural colors. In Bird coloration: mechanisms and measurements (eds Hill GE, McGraw KJ), pp. 177–242. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Prum RO, Torres RH, Williamson S, Dyck J. 1998. Coherent light scattering by blue feather barbs. Nature 396, 28–29. ( 10.1038/23838) [DOI] [Google Scholar]
- 4.Shawkey MD, D'Alba L. 2017. Interactions between colour-producing mechanisms and their effects on the integumentary colour palette. Phil. Trans. R. Soc. B 372, 20160536 ( 10.1098/rstb.2016.0536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shawkey MD, Hill GE. 2006. Significance of a basal melanin layer to production of non-iridescent structural plumage color: evidence from an amelanotic Steller's jay (Cyanocitta stelleri). J. Exp. Biol. 209, 1245–1250. ( 10.1242/jeb.02115) [DOI] [PubMed] [Google Scholar]
- 6.Doguzhaeva LA, Mapes RH, Mutvei H. 2004. Occurrence of ink in Paleozoic and Mesozoic coleoids (Cephalopoda). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 88, 145–156. [Google Scholar]
- 7.Vinther J, Briggs DE, Prum RO, Saranathan V. 2008. The colour of fossil feathers. Biol. Lett. 4, 522–525. ( 10.1098/rsbl.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colleary C, et al. 2015. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proc. Natl Acad. Sci. USA 112, 12 592–12 597. ( 10.1073/pnas.1509831112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinther J. 2015. A guide to the field of palaeo colour. Bioessays 37, 643–656. ( 10.1002/bies.201500018) [DOI] [PubMed] [Google Scholar]
- 10.Clarke JA, Ksepka DT, Salas-Gismondi R, Altamirano AJ, Shawkey MD, D'Alba L, Vinther J, DeVries TJ, Baby P. 2010. Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957. ( 10.1126/science.1193604) [DOI] [PubMed] [Google Scholar]
- 11.Li Q, et al. 2012. Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335, 1215–1219. ( 10.1126/science.1213780) [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Hong L, Wakamatsu K, Ito S, Adhyaru B, Cheng CY, Bowers CR, Simon JD. 2005. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem. Photobiol. 81, 135–144. ( 10.1562/2004-08-03-RA-259.1) [DOI] [PubMed] [Google Scholar]
- 13.Vinther J, Nicholls R, Lautenschlager S, Michael P, Kaye TG, Rayfield E, Mayr G, Cuthill IC. 2016. 3D camouflage in an ornithischian dinosaur. Curr. Biol. 26, 2456–2462. ( 10.1016/j.cub.2016.06.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smithwick FM, Nicholls R, Cuthill IC, Vinther J. 2017. Countershading and stripes in the theropod dinosaur Sinosauropteryx reveal heterogeneous habitats in the Early Cretaceous Jehol Biota. Curr. Biol. 27, 3337–3343. ( 10.1016/j.cub.2017.09.032) [DOI] [PubMed] [Google Scholar]
- 15.Saitta ET, et al. 2017. Low fossilization potential of keratin protein revealed by experimental taphonomy. Palaeontology 60, 547–556. ( 10.1111/pala.12299) [DOI] [Google Scholar]
- 16.Parry LA, et al. 2018. Soft-bodied fossils are not simply rotten carcasses—toward a holistic understanding of exceptional fossil preservation. Bioessays 40, 1700167 ( 10.1002/bies.201700167) [DOI] [PubMed] [Google Scholar]
- 17.Mayr G, Mourer-Chauviré C. 2000. Rollers (Aves: Coraciiformes s.s.) from the Middle Eocene of Messel (Germany) and the Upper Eocene of the Quercy (France). J. Vertebr. Paleontol. 30, 533–546. ( 10.1671/0272-4634(2000)020[0533:RACSSF]2.0.CO;2) [DOI] [Google Scholar]
- 18.Saranathan V, Forster JD, Noh H, Liew SF, Mochrie SGJ, Cao H, Dufresne ER, Prum RO. 2012. Structure and optical function of amorphous photonic nanostructures from avian feather barbs: a comparative small angle X-ray scattering (SAXS) analysis of 230 bird species. J. R. Soc. Interface 9, 2563–2580. ( 10.1098/rsif.2012.0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayr G. 2017. The early Eocene birds of the Messel fossil site: a 48 million-year-old bird community adds a temporal perspective to the evolution of tropical avifaunas. Biol. Rev. 92, 1174–1188. ( 10.1111/brv.12274) [DOI] [PubMed] [Google Scholar]
- 20.del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E. Handbook of the birds of the world alive. [Online]. See https://www.hbw.com/. [Google Scholar]
- 21.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 23.Chambers JM, Freeny AE, Heiberger RM.. 1992. Analysis of variance; designed experiments. In Statistical models in S (eds Chambers JM, Hastie TJ). Pacific Grove, CA: Wadsworth & Brooks/Cole. [Google Scholar]
- 24.Thomas DB, McGraw KJ, Butler MW, Carrano MT, Madden O, James HF. 2014. Ancient origins and multiple appearances of carotenoid-pigmented feathers in birds. Proc. R. Soc. B 281, 20140806 ( 10.1098/rspb.2014.0806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 26.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 27.Thomas GH, Freckleton RP. 2012. MOTMOT: models of trait macroevolution on trees. Methods Ecol. Evol. 3, 145–151. ( 10.1111/j.2041-210X.2011.00132.x) [DOI] [Google Scholar]
- 28.Schliep KP. 2011. Phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593 ( 10.1093/bioinformatics/btq706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoddard MC, Prum RO. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052. ( 10.1093/beheco/arr088) [DOI] [Google Scholar]
- 30.Yu M, Yue Z, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM. 2004. The developmental biology of feather follicles. Int. J. Dev. Biol. 48, 181–191. ( 10.1387/ijdb.031776my) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maia R, Macedo RHF, Shawkey MD. 2012. Nanostructural self-assembly of iridescent feather barbules through depletion attraction of melanosomes during keratinization. J. R. Soc. Interface 9, 734–743. ( 10.1098/rsif.2011.0456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durrer H. 1977. Schillerfarben der Vogelfeder als Evolutionsproblem. Denkschr. Schweiz. Naturforsch. Ges. 91, 1–127. [Google Scholar]
- 33.Prum RO, Dufresne ER, Quinn T, Waters K. 2009. Development of colour-producing β-keratin nanostructures in avian feather barbs. J. R. Soc. Interface 6, S253–S265. ( 10.1098/rsif.2008.0466.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maia R, D'Alba L, Shawkey MD. 2010. What makes a feather shine? A nanostructural basis for glossy black colours in feathers. Proc. R. Soc. B 278, 1973–1980. ( 10.1098/rspb.2010.1637) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in electronic supplementary material: (1) Melanosome measurements—1.1. Measurements of melanosomes isolated from fresh feathers that express non-iridescent structural colour are included as a separate category in the previously existing database from Li. et al. (sheet number 7 in our electronic supplementary material). 1.2. Measurements of melanosomes from the surface of the fossil are under sheet number 2 in the electronic supplementary material. (2) Other important data that we have collected for our research are colour presence/absence matrix that is included in sheet number 3 of the electronic supplementary material.