GALGT2 (also B4GALNT2) encodes a glycosyltransferase that is normally confined to the neuromuscular and myotendinous junction in adult skeletal muscle. GALGT2 overexpression in muscle can inhibit muscular dystrophy in mouse models of the disease by inducing the overexpression of surrogate muscle proteins, including utrophin, agrin, laminins, and integrins. Despite its well-documented biological properties, little is known about the endogenous regulation of muscle GALGT2 expression.
KEYWORDS: AAV, EGFR, GALGT2, HB-EGF, agrin, laminin, muscular dystrophy, neuromuscular junction, skeletal muscle
ABSTRACT
GALGT2 (also B4GALNT2) encodes a glycosyltransferase that is normally confined to the neuromuscular and myotendinous junction in adult skeletal muscle. GALGT2 overexpression in muscle can inhibit muscular dystrophy in mouse models of the disease by inducing the overexpression of surrogate muscle proteins, including utrophin, agrin, laminins, and integrins. Despite its well-documented biological properties, little is known about the endogenous regulation of muscle GALGT2 expression. Here, we demonstrate that epidermal growth factor receptor (EGFR) ligands can activate the human GALGT2 promoter. Overexpression of one such ligand, soluble heparin-binding EGF-like growth factor (sHB-EGF), also stimulated mouse muscle Galgt2 gene expression and expression of GALGT2-inducible surrogate muscle genes. Deletion analysis of the GALGT2 promoter identified a 45-bp region containing a TFAP4-binding site that was required for sHB-EGF activation. sHB-EGF increased TFAP4 binding to this site in muscle cells and increased endogenous Tfap4 gene expression. sHB-EGF also increased muscle EGFR protein expression and activated EGFR-Akt signaling. sHB-EGF expression was concentrated at the neuromuscular junction, and Hbegf deletion reduced Galgt2-dependent synaptic glycosylation. Hbegf deletion also mimicked Galgt2-dependent neuromuscular and muscular dystrophy phenotypes. These data demonstrate that sHB-EGF is an endogenous regulator of muscle Galgt2 gene expression and can mimic Galgt2-dependent muscle phenotypes.
INTRODUCTION
In a variety of neuromuscular disorders, the primary genetic defect can be overcome not only by gene replacement but also by overexpression of surrogate genes that encode proteins homologous in structure or function to the mutated gene. For example, overexpression of utrophin or integrin α7 can inhibit muscular dystrophy (MD) in the dystrophin-deficient mdx model of Duchenne muscular dystrophy (1–5), and overexpression of laminin α1, agrin, or laminin α4 can inhibit MD in the laminin α2-deficient dyW model of congenital muscular dystrophy 1A (6–8). We have studied a surrogate gene therapy called GALGT2 (also called B4GALNT2). When overexpressed in skeletal muscle, GALGT2 induces the ectopic overexpression of many surrogate genes and proteins, including utrophin, plectin 1, agrin, laminin α2, laminin α4, laminin α5, integrin α7, and integrin β1 (9, 10). The GALGT2 gene encodes β1,4-N-acetylgalacosaminyltransferase that creates the cytotoxic T cell (CT) glycan Neu5Acα2-3[GalNAcβ1-4]Galβ1-4GlcNAcβ-R on glycoproteins, including α dystroglycan (11–14). Dystroglycan, which comprises α and β chains, is a muscle membrane protein that serves as an essential transmembrane link between the extracellular matrix (ECM) and the F-actin cytoskeleton (15, 16). GALGT2 overexpression increases expression of ECM proteins, including laminin α4, laminin α5, and agrin, increases ECM protein binding to α dystroglycan, and increases expression of cytoplasmic F-actin binding proteins that link the cytoskeleton to β dystroglycan, including dystrophin, utrophin, and plectin1 (9, 10, 17–20). In changing the molecular nature of the muscle membrane, GALGT2 overexpression protects both wild-type (WT) and dystrophin-deficient (mdx) skeletal myofibers from eccentric contraction-induced injury and can inhibit the development of muscular dystrophy in four different animal models of the disease (mdx, dyW, Sgca, and FKRPP448L) (14, 18, 19, 21, 22). Many of the surrogate genes and proteins induced by GALGT2 are, like GALGT2, normally confined in expression to the neuromuscular junction (NMJ) and myotendinous junction (MTJ) in adult muscle (23–27). Similarly, like GALGT2, many of these genes and proteins are highly expressed in extrasynaptic muscle regions in the early postnatal period (23, 25, 26, 28–34). Thus, by drawing such proteins into an extrasynaptic expression pattern, GALGT2 is essentially recreating the molecular environment normally seen in a young muscle. As such, GALGT2 overexpression provides a means of understanding mechanisms controlling the expression of these therapeutic genes and proteins, with regard to not only muscle therapy but also normal muscle development.
Although the potential of GALGT2 gene therapy has been appreciated and successfully applied in neuromuscular disease models, regulation of endogenous Galgt2 gene expression in mouse muscle remains poorly understood. Given its potential as a therapeutic, a better understanding of the endogenous mechanisms controlling muscle Galgt2 expression, as well as the expression of GALGT2-inducible muscle surrogate genes and GALGT2-dependent muscle phenotypes, is highly warranted, and this is the goal of our current study.
RESULTS
EGFR ligands stimulate the human GALGT2 promoter in muscle cells.
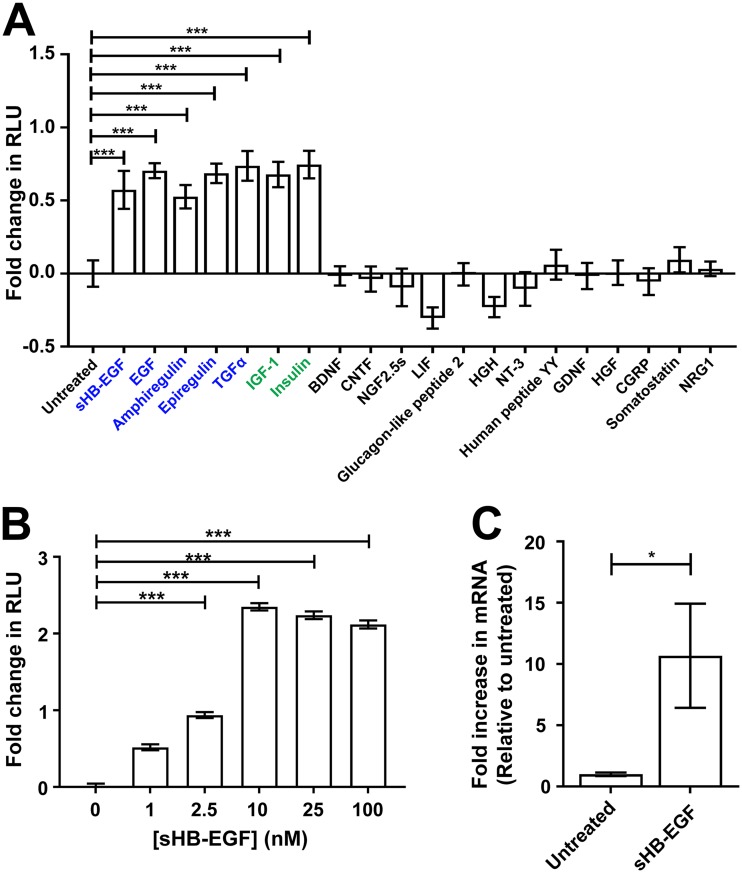
Using C2C12 myotube cultures stably expressing a bp −5870 GALGT2 promoter firefly luciferase (Luc2) reporter, we performed a screen of trophic factors to assess their ability to induce the GALGT2 promoter (Fig. 1A). Positives were also validated using C2C12 myotubes stably expressing a GALGT2 promoter Renilla luciferase (hRluc) reporter (not shown). Many trophic factors, including brain-derived neurotrophic factor (BDNF), glucagon-like peptide 2, ciliary neurotrophic factor (CNTF), nerve growth factor 2.5S (NGF 2.5S), leukemia inhibitory factor (LIF), human growth hormone (HGH), neurotrophin 3 (NT3), peptide YY, glial cell line-derived neurotrophic factor (GDNF), hepatocyte growth factor (HGF), calcitonin gene-related peptide (CGRP), somatostatin, and neuregulin 1 (NRG1) did not increase luciferase signal when added for 24 h. Concentrations chosen are described in Materials and Methods and exceed or equal the binding affinity of each ligand for their known signaling receptor. Ligands known to activate the epidermal growth factor receptor (EGFR), including soluble heparin-binding EGF-like growth factor (sHB-EGF), epidermal growth factor (EGF), amphiregulin, epiregulin, and transforming growth factor alpha (TGFα), all induced GALGT2 promoter activity, as did insulin and insulin-like growth factor 1 (IGF1) (Fig. 1A). We specifically tested sHB-EGF, as the full-length transmembrane form of HB-EGF does not activate EGFR until it is cleaved to generate sHB-EGF by ADAM proteases (35–38). Of the EGFR agonists, only HB-EGF and TGFα are appreciably expressed in skeletal muscle, with HB-EGF showing more specificity for muscle than TGFα, which is expressed in almost all tissues (39). sHB-EGF was able to activate the GALGT2 promoter in a dose-dependent manner, with saturation occurring at 10 nM (Fig. 1B). sHB-EGF only activated the GALGT2 promoter luciferase reporter in C2C12 cells if they were first differentiated into myotubes (not shown). Furthermore, in differentiated C2C12 cells, sHB-EGF increased the expression of endogenous Galgt2 mRNA levels by 10-fold relative to levels for untreated cells (Fig. 1C).
FIG 1.
Muscle trophic factor screen of GALGT2 promoter activity. (A) Trophic factors were added for 1 day to C2C12 myotube cultures containing a GALGT2 promoter firefly luciferase reporter. Factors in blue indicate ligands that activate the epidermal growth factor receptor (EGFR). Factors in green indicate factors that activate the insulin or insulin-like growth factor receptor. (B) sHB-EGF was added at different concentrations to GALGT2 promoter firefly luciferase reporter cells. Data are reported relative to those of untreated C2C12 myotube cultures in panels A and B (set to 0). (C) qRT-PCR measures of gene expression for endogenous Galgt2 following treatment with sHB-EGF. Fold change is reported relative to the level for saline-injected contralateral muscles (set to 1). NGF, mouse nerve growth factor 2.5S. Errors are standard errors of the means (SEM) for n = 12 (A and B) and n = 9 (C) measures. *, P < 0.05; ***, P < 0.001.
sHB-EGF activates the human GALGT2 promoter via a TFAP4-responsive element.
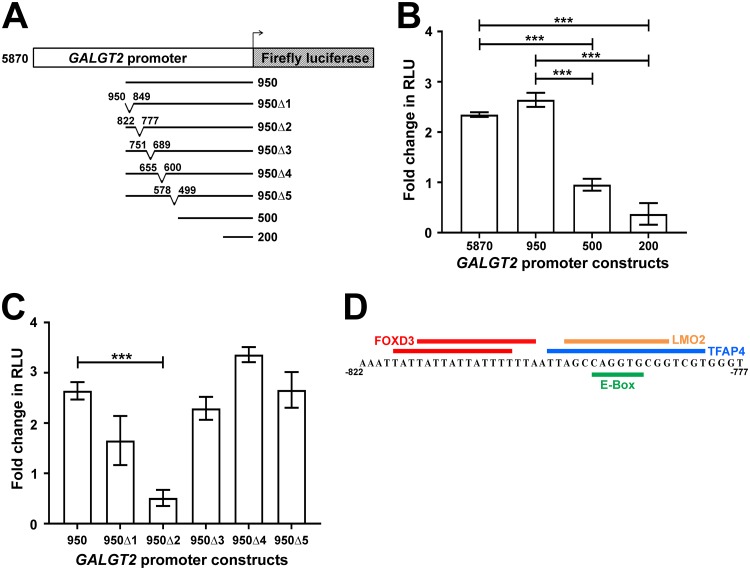
We next wanted to define the DNA element or elements in the GALGT2 promoter responsible for driving elevated luciferase reporter expression in response to sHB-EGF. To do this, we created a series of deletions in the GALGT2 promoter (Fig. 2A). Deletions up to the last bp, bp −950, of the 5′ end of the promoter had no impact on the ability of sHB-EGF to activate luciferase. Deletions leaving only bp −500 or bp −200, however, significantly diminished promoter reporter activity (Fig. 2B), suggesting the 450-bp sequence between bp −950 and bp −500 responded to sHB-EGF. We next created five smaller deletions, each approximately 50 bp, within this 450-bp sequence. Of these, only the second deletion (950Δ2; deletion of bp −822 to −777) showed a significant decrease in luciferase signal (Fig. 2C). A motif analysis identified only two major transcription factor binding sites in the bp −822 to −777 region (Fig. 2D) (40). One site included two overlapping FOXD3 binding sites at bp −818 to −804 and bp −815 to −801, while the second site contained overlapping binding sites for TFAP4, LMO2, and myogenin (MYOG) (which binds at E boxes) at bp −798 to −781, bp −796 to −785, and bp −793 to −788, respectively.
FIG 2.
Mapping of an sHB-EGF-responsive element on the human GALGT2 promoter. (A) C2C12 myotube cultures were made bearing a human GALGT2 promoter firefly luciferase reporter construct with promoter elements ranging from bp −5870 to −200 relative to the GALGT2 transcriptional start site. Five smaller deletions were also made within the bp −950 GALGT2 promoter as indicated. (B) bp −5870, −950, −500, and −200 GALGT2 promoter constructs were assayed for luciferase activity in relative light units (RLU) after the addition of 10 nM sHB-EGF. (C) Each bp −950 deletion construct was compared to unaltered bp −950. Data are reported relative to untreated C2C12 myotube cultures in panels B and C (set to 0). (D) Predicted transcription factor binding domains in the bp −822 to −777 region of the GALGT2 promoter. Errors are SEM for n = 12 measures per condition in panels B and C. ***, P < 0.001.
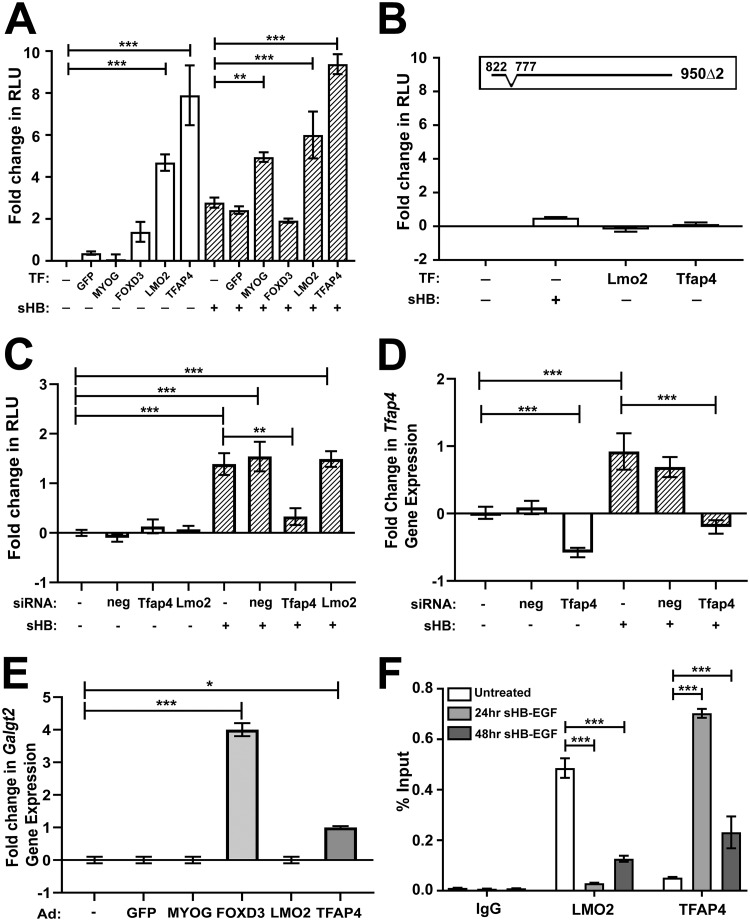
We next assessed whether the transcription factors predicted to bind to the bp −822 to −777 region could activate the GALGT2 promoter when overexpressed. To do this, we obtained adenovirus (Ad) constructs that overexpress MYOG, FOXD3, LMO2, or TFAP4. Ad-GFP (green fluorescent protein) was used as a negative control. C2C12 myotube cultures stably expressing the bp −950 GALGT2 promoter firefly luciferase reporter were treated with each Ad vector either in the presence or absence of sHB-EGF. Neither Ad-GFP, Ad-MYOG, nor Ad-FOXD3 significantly increased promoter activity (relative to untreated cells), while Ad-LMO2 and Ad-TFAP4 did, with Ad-TFAP4 showing the greatest activation (Fig. 3A). Ad-LMO2 and Ad-TFAP4 also significantly activated the bp −950 GALGT2 promoter in the presence of sHB-EGF relative to sHB-EGF alone (Fig. 3A). In contrast, Ad-LMO2 or Ad-TFAP4 was unable to significantly increase expression in the 950Δ2 construct (Fig. 3B).
FIG 3.
Characterization of LMO2 and TFAP4 activation of and binding to the GALGT2 promoter. (A) Adenovirus vectors were used to infect and overexpress GFP, MYOG, FOXD3, LMO2, or TFAP4 in C2C12 myotubes expressing the bp −950 GALGT2 promoter luciferase reporter in the absence (open bars) or presence (hatched bars) of sHB-EGF. Luciferase reporter activity was measured in relative light units (RLU). (B) C2C12 myotubes stably expressing the −950Δ2 GALGT2 promoter luciferase reporter were assayed as described for panel A after addition of sHB-EGF or adenovirus (Ad) containing LMO2 or TFAP4. (C) C2C12 myotubes expressing the GALGT2 promoter were treated with siRNA against TFAP4, LMO2, or a scrambled negative (neg) control in the presence or absence of sHB-EGF. Luciferase reporter activity was measured in RLU. (D) Tfap4 gene expression was measured in C2C12 myotube cultures treated with siRNAs for scrambled negative (neg) control or against Tfap4, with or without sHB-EGF. (E) Mouse Galgt2 mRNA was measured in C2C12 cells treated with adenovirus (Ad) containing GFP, MYOG, TFAP4, LMO2, or FOXD3. (F) Chromatin immunoprecipitation measuring binding of LMO2 or TFAP4 to the bp −951 to −726 region of the GALGT2 promoter in C2C12 myotubes at 24 and 48 h after sHB-EGF addition relative to those of cells not treated with sHB-EGF. Fold change data in panels A to E are reported relative to the level of the untreated control, set to 0. Errors are SEM for n = 4 to 8 (A to E) and standard deviations for n = 3 (F) measures per condition. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next used short interfering RNAs (siRNAs) targeting mouse Tfap4 or Lmo2 to knock down mRNA expression and then retest sHB-EGF induction (Fig. 3C). siRNA targeting Tfap4 significantly reduced GALGT2 promoter activity in sHB-EGF-treated cells (P < 0.01), while siRNA targeting Lmo2 and a scrambled Tfap4 control did not (Fig. 3C). The siRNA targeting Tfap4 knocked down endogenous Tfap4 gene expression in both resting and sHB-EGF-treated cells by 58% relative to the relevant control (Fig. 3D). Interestingly, sHB-EGF treatment alone induced Tfap4 gene expression 1.9- ± 0.3-fold (P < 0.001). siRNA targeting Lmo2 reduced Lmo2 gene expression by only 30%, and sHB-EGF did not induce endogenous Lmo2 gene expression (not shown). Interestingly, both Ad-TFAP4 and Ad-FOXD3 increased endogenous Galgt2 gene expression in C2C12 myotubes, while Ad-MYOG, Ad-GFP, and Ad-LMO2 did not (Fig. 3E). Ad-TFAP4, Ad-LMO2, Ad-MYOG, and Ad-FOXD3 all induced expression for their respective (human) genes by 15- to 20-fold relative to levels for the same endogenous mouse gene, but Ad-FOXD3 also increased endogenous mouse Tfap4 expression by 2.1- ± 0.1-fold and decreased endogenous mouse Lmo2 expression to 0.3 ± 0.1 of normal levels. Thus, FOXD3 overexpression also led to increased expression of Tfap4 in C2C12 cells. As we could not detect any expression of Foxd3 in mouse muscle (not shown), we did not pursue this result further.
Lastly, we determined if sHB-EGF would induce binding of LMO2 or TFAP4 to the bp −951 to −726 region by performing chromatin immunoprecipitation (ChIP) of nuclear lysates from C2C12 myotubes before or after sHB-EGF addition (Fig. 3F). sHB-EGF increased TFAP4 occupancy to this site at 24 and 48 h after sHB-EGF addition. LMO2 binding, in contrast, showed the opposite pattern. Thus, sHB-EGF appears to induce a switch in occupancy of transcription factors in the bp −951 to −726 region of the GALGT2 promoter, increasing bound TFAP4 at the expense of bound LMO2.
sHB-EGF activates an EGFR phosphorylation cascade in muscle cells.
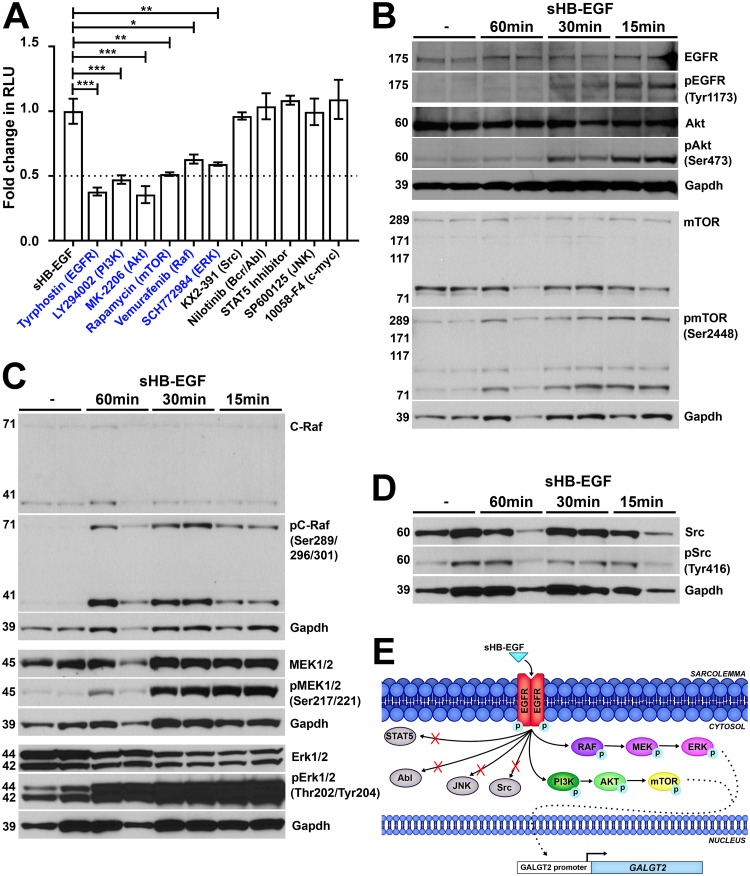
To better characterize the sHB-EGF signaling pathway, we added inhibitors of various signaling pathways to the bp −5870 GALGT2 promoter reporter muscle cells along with sHB-EGF. All inhibitors were tested in a dose-response curve, and concentrations shown reflect maximal levels of inhibition. Inhibitors of EGFR (tyrophostin; AG-1478), a known sHB-EGF receptor, phosphatidyl inositol 3 (PI3) kinase (LY294002), Akt kinase (MK-2206), mTOR (rapamycin), Raf kinase (vamurafenib), and extracellular signal-regulated kinase (ERK; SCH772984) all maximally inhibited sHB-EGF activation by more than 50%, while inhibitors of Src (KX2-391), Bcr/Abl (nilotinib), STAT5, c-Jun N-terminal kinase (JNK; SP600125), and c-myc (10058-F4) did not (Fig. 4A).
FIG 4.
sHB-EGF stimulates phosphorylation signaling cascades in skeletal muscle cells. (A) sHB-EGF was added to C2C12 myotube cultures in the presence of drugs that block specific signal transduction pathways. Drugs in blue showed significant decreases in sHB-EGF activation of the GALGT2 promoter relative to sHB-EGF alone. GALGT2 promoter luciferase reporter activity was measured in relative light units (RLU). Errors are SEM for n = 12 measures per condition. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Fold changes are reported relative to the level of sHB-EGF alone, set to 1. (B to D) Western blots of C2C12 myotube protein lysates before or after sHB-EGF addition for 15, 30, or 60 min using antibodies to GAPDH protein (a protein loading and transfer control). Western blots in panel B were additionally probed for members of the Akt signaling pathway, including antibodies to EGFR, Akt, and mTOR protein or antibodies specific to phosphorylated EGFR (at Tyr1173), Akt (at Ser473), and mTOR (at Ser2448). Western blots in panel C were probed for members of the RAF signaling pathway, including antibodies to C-Raf, MEK1/2, and Erk1/2 protein or antibodies specific to phosphorylated C-Raf (at Ser289/296/301), MEK1/2 (at Ser217/221), and Erk1/2 (at Thr202/Tyr204). Western blots in panel D were probed for Src protein or phosphorylated Src (at Tyr416). (E) Diagram illustrating the pathways utilized by sHB-EGF to activate the GALGT2 promoter in skeletal muscle.
We next assessed sHB-EGF induction of EGFR, Akt, Erk1/2, MEK1/2, and C-Raf activity by comparing signals on Western blots using phosphospecific and protein-specific antibodies to each component (Fig. 4B to D). sHB-EGF addition caused a 20-fold increase in phosphorylation of EGFR (pEGFR [Tyr1173]/EGFR, 23.9 ± 1.3; P < 0.05) within 15 min compared to levels for untreated cells (Fig. 4B). Similarly, phosphorylation of Akt (pAkt [Ser473]/Akt, 19.7 ± 1.6; P < 0.001) and mTOR (pmTOR [Ser2448]/mTOR, 7.6 ± 1.7; P < 0.05) was increased (Fig. 4B), as was that of C-Raf (pC-Raf [Ser289/296/301]/C-Raf, 34.3 ± 3.8; P < 0.001), MEK1/2 (pMEK1/2 [Ser217/221]/MEK1/2, 6.9 ± 0.1; P < 0.001), and Erk1/2 (pErk1/2 [Thr202/Tyr204]/Erk1/2, 4.0 ± 0.3; P < 0.01) (Fig. 4C). Activation of all proteins was transient, with most protein phosphorylation reverting to near baseline levels by 60 min after treatment (Fig. 4B and C). Phosphorylation of c-Src was not significantly increased following sHB-EGF treatment (pSrc [Tyr416]/Src, 1.8 ± 0.4; P = 0.37) (Fig. 4D), consistent with the results of our inhibitor screen (Fig. 4A). These studies support the notion that sHB-EGF activates an EGFR-Akt/mTOR-Mek/Erk kinase signaling cascade in muscle cells (Fig. 4E).
sHB-EGF induces endogenous expression of Galgt2 and Galgt2-inducible surrogate genes in skeletal muscle.
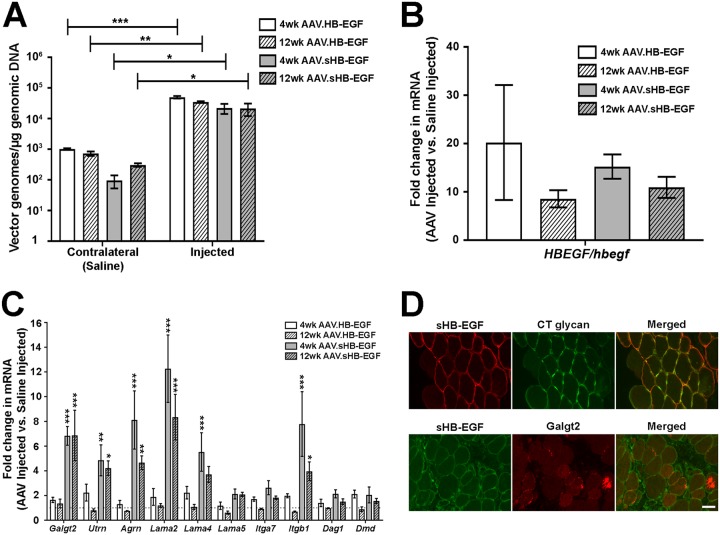
We next assessed the ability of sHB-EGF to induce Galgt2 transcription in mouse skeletal muscle tissue. To do this, we created a recombinant, self-complementary [r(sc)] adeno-associated virus (AAV) vector that would express either full-length human HB-EGF or active sHB-EGF. The AAV9 serotype of viral capsid was used, which allows for robust muscle transgene expression (41, 42). r(sc)AAV9.CMV.HB-EGF or r(sc)AAV9.CMV.sHB-EGF (5 × 1010 vector genomes [vg]) was injected into the gastrocnemius muscles of 8-week-old C57BL/6J mice, with the contralateral gastrocnemius receiving an equal volume of phosphate-buffered saline (PBS) as a control. Injected muscles showed 20,000 to 50,000 vg per μg of genomic DNA, while contralateral control muscles showed two or more logarithms less transduction (Fig. 5A). Because the rAAV9 serotype efficiently crosses the vascular barrier to transduce muscles throughout the body (43, 44), this modest amount of AAV vector was expected in the contralateral muscles even after a focal intramuscular injection, but the extent of transduction was extremely low (no more than 1 vg per 150 nuclei).
FIG 5.
sHB-EGF induces muscle expression of Galgt2 and some Galgt2-inducible therapeutic genes. (A) qPCR measures of AAV vector genomes in muscles 4 and 12 weeks after treatment with r(sc)AAV9.CMV.sHB-EGF or r(sc)AAV9.CMV.HB-EGF compared to levels for saline-injected contralateral muscles from the same animals. (B) qRT-PCR measures of human HBEGF gene expression in r(sc)AAV9.CMV.sHB-EGF- or r(sc)AAV9.CMV.HB-EGF-injected muscles relative to endogenous mouse Hbegf gene expression levels in saline-injected muscles at 4 and 12 weeks after treatment. (C) qRT-PCR measures of gene expression for Galgt2 and other therapeutic surrogate genes (and controls) in muscles 4 and 12 weeks after treatment with r(sc)AAV9.CMV.sHB-EGF or r(sc)AAV9.CMV.HB-EGF. Fold changes are relative to levels for saline-injected contralateral muscles (set to 1). (D) Coimmunostaining of sHB-EGF (red) and CT glycan (green) or of sHB-EGF (green) and Galgt2 protein (red). Merged images on the right show overlapping staining in orange and/or yellow. Scale bar is 25 μm. Errors are SEM for n = 4 (A to C) measures per condition. *, P < 0.05; **, P < 0.01; *** P < 0.001.
Expression of sHB-EGF and HB-EGF was increased between 10- and 20-fold over endogenous mouse Hbegf expression at both 4 and 12 weeks after AAV injection (Fig. 5B). Overexpression of full-length, uncleaved HB-EGF was unable to significantly increase Galgt2 gene expression, while sHB-EGF increased Galgt2 expression more than 6-fold compared to that of mock-injected contralateral muscles (Fig. 5C). In addition to elevating Galgt2, sHB-EGF increased the expression of surrogate muscle genes that can have a therapeutic impact on muscular dystrophy, including the utrophin (Utrn), agrin (Agrn), laminin α2 (Lama2), laminin α4 (Lama4), and integrin β1 (Itgb1) genes (9, 10), while full-length HB-EGF did not (Fig. 5C). Interestingly, neither sHB-EGF nor HB-EGF significantly upregulated expression of the dystroglycan (Dag1), dystrophin (Dmd), laminin α5 (Lama5), or integrin α7 (Itga7) gene (Fig. 5C), while GALGT2 overexpression can increase expression of these genes in muscle (10). Thus, HB-EGF must be cleaved to its active soluble form in skeletal muscle to increase endogenous Galgt2 transcription and can induce the expression of some, but not all, Galgt2-inducible therapeutic surrogate genes.
We next analyzed Galgt2 protein and CT glycan expression in sHB-EGF-injected muscles (Fig. 5D). sHB-EGF was expressed along the sarcolemmal membrane of skeletal myofibers in muscles analyzed at 4 weeks postinjection. CT glycan was highly upregulated along the sarcolemmal membrane in such fibers, as shown by costaining with sHB-EGF protein, while myofibers not overexpressing sHB-EGF did not have elevated CT glycan. As Galgt2 is a trans-Golgi protein (12), it would not be expected to be colocalized with sHB-EGF at the sarcolemmal membrane. Galgt2 protein staining was, however, highly elevated in many sHB-EGF-overexpressing myofibers (Fig. 5D), consistent with increased Galgt2 mRNA expression (Fig. 5C). As in cultured myotubes, sHB-EGF overexpression in skeletal muscle also increased Tfap4 gene expression (1.9- ± 0.1-fold).
sHB-EGF activates an EGFR-Akt pathway in skeletal muscle.
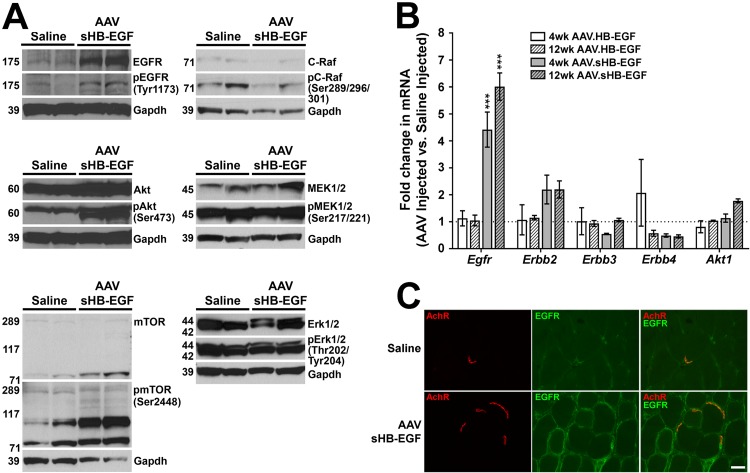
Skeletal muscles treated with sHB-EGF for 4 weeks showed a significant increase in Akt phosphorylation (pAkt [Ser473]/Akt, 2.6 ± 0.2; P < 0.05) but showed no significant change in phosphorylation for EGFR (pEGFR [Tyr1173]/EGFR, 0.5 ± 0.1; P = 0.501), mTOR (pmTOR [Ser2448]/mTOR, 0.5 ± 0.1; P = 0.074), C-Raf (pC-Raf [Ser289/296/301]/C-Raf, 1.5 ± 0.4; P = 0.457), MEK1/2 (pMEK1/2 [Ser217/221]/MEK1/2, 0.7 ± 0.1; P = 0.251), or Erk1/2 (pErk1/2 [Thr202/Tyr204]/Erk1/2, 0.9 ± 0.1; P = 0.669) (Fig. 6A). Expression of EGFR protein, however, was increased almost 5-fold after sHB-EGF treatment (EGFR/GAPDH, 5.7 ± 0.6; P < 0.05) (Fig. 6A), as was mTOR protein (9- ± 5-fold). Thus, while the pEGFR/EGFR and pmTOR/mTOR ratios did not significantly change, elevated EGFR and mTOR protein expression meant pEGFR and pTOR levels also increased. Mouse Egfr gene expression was also significantly increased at both 4 and 12 weeks by sHB-EGF compared to that of the PBS-injected control (P < 0.001 for each) (Fig. 6B). Overexpression of full-length transmembrane HB-EGF, in contrast, did not significantly increase Egfr mRNA (Fig. 6B). sHB-EGF overexpression also caused a 2-fold increase in Erbb2 mRNA expression at 4 and 12 weeks postinjection, although this did not reach statistical significance (4 weeks, 2.2 ± 0.5; P = 0.13; 12 weeks, 2.2 ± 0.3; P = 0.13), while expression of Erbb3, Erbb4, and Akt1 did not change (Fig. 6B). We also immunostained untreated and sHB-EGF-treated muscles with antibodies to EGFR protein. In control muscle, EGFR staining was concentrated at the neuromuscular junction (NMJ), but staining was increased along extrasynaptic regions of the myofiber membrane after sHB-EGF treatment (Fig. 6C). Thus, some facets of EGFR activation and Akt signaling found in muscle cells were present after sHB-EGF overexpression in muscle tissue, but others (MEK1/2, Erk1/2, and C-Raf) were not.
FIG 6.
sHB-EGF stimulates an EGFR-Akt signaling pathway in skeletal muscle tissue. (A) Western blots of protein lysates from muscles treated for 4 weeks with r(sc)AAV9.CMV.sHB-EGF and saline-treated contralateral control muscles probed using antibodies to GAPDH protein (a protein loading and transfer control), antibodies to EGFR, Akt, mTOR, C-Raf, MEK1/2, or Erk1/2 protein, or antibodies specific to phosphorylated EGFR (at Tyr1173), Akt (at Ser473), mTOR (at Ser2448), C-Raf (at Ser289/296/301), MEK1/2 (at Ser217/221), or Erk1/2 (at Thr202/Tyr204). (B) qRT-PCR measures of Egfr, Erbb2, Erbb3, Erbb4, and Akt1 gene expression in muscles from mice treated for 4 or 12 weeks with r(sc)AAV9.CMV.sHB-EGF or r(sc)AAV9.CMV.HB-EGF relative to levels for saline-injected control muscles (set to 1). Errors are SEM for n = 4 measures per condition. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Wild-type saline-injected or r(sc)AAV9.CMV.sHB-EGF-injected muscles were stained with rhodamine-conjugated α-bugarotoxin to identify nicotinic acetylcholine receptors (AChRs) (red) at the neuromuscular junction and with an antibody to EGFR (green). Merged images on the right show overlapping staining in orange and/or yellow. Scale bar is 25 μm.
sHB-EGF is required for Galgt2-dependent NMJ glycosylation.
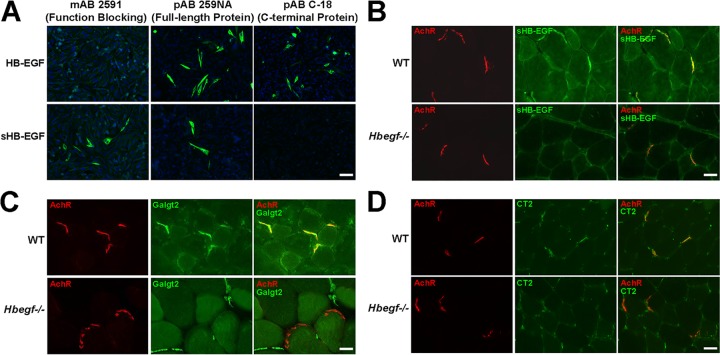
To stain for sHB-EGF in muscle, we used a function-blocking sHB-EGF-specific monoclonal antibody (MAb 2591). We confirmed the specificity of MAb 2591 by staining Chinese hamster ovary (CHO) cells transfected with a cDNA encoding the full-length transmembrane form of HB-EGF (208 amino acids [aa]) or with a cDNA encoding only sHB-EGF (148 aa). MAb 2591 stained sHB-EGF-transfected cells but not HB-EGF-transfected cells, while polyclonal antibody (PAb) 259 NA, an HB-EGF polyclonal antibody, recognized both sHB-EGF and HB-EGF (Fig. 7A). C18, an antibody to the C-terminal domain of HB-EGF (that only exists in the full-length HB-EGF), recognized HB-EGF but not sHB-EGF (Fig. 7A). In adult skeletal muscle, MAb 2591 stained NMJs (Fig. 7B). sHB-EGF staining coincided with α-bungarotoxin staining, which stains nicotinic acetylcholine receptors (AChRs) in the postsynaptic muscle membrane (Fig. 7B) (45). sHB-EGF muscle staining was eliminated in mice lacking HB-EGF (Hbegf−/−) (Fig. 7B). Staining for Galgt2 protein (Fig. 7C) and the CT glycan (Fig. 7D) was reduced, and sometimes eliminated, at NMJs in Hbegf−/− muscles, suggesting a requirement, or partial requirement, of HB-EGF. Staining of Galgt2 protein and CT glycan remained present in intramuscular capillaries in Hbegf−/− mice, providing an internal staining control (Fig. 7C and D).
FIG 7.
Enrichment of sHB-EGF at the neuromuscular synapse. (A) CHO cells were transfected with cDNAs expressing either soluble HB-EGF (sHB-EGF) or full-length transmembrane HB-EGF. Cells were then immunostained with MAb 2591 (an sHB-EGF-specific function blocking antibody), PAb 259 NA (recognizes full-length HB-EGF protein as well as sHB-EGF), or PAb C18 (recognizes the C-terminal intracellular domain of full-length HB-EGF). Nuclear (DAPI) staining is shown in blue. Scale bar is 50 μm. WT and HB-EGF-deficient (Hbegf−/−) muscle sections were stained with the sHB-EGF-specific antibody MAb 2591 (B), a Galgt2 antibody (C), or an antibody to CT glycan (CT2) (D) (all shown in green). Tissue sections were costained with rhodamine-conjugated α-bungarotoxin (red) to label acetylcholine receptors (AChRs) at the neuromuscular junction. Merged images at the right show staining overlap in yellow or orange. Scale bar is 25 μm.
Absence of sHB-EGF mimics Galgt2−/− neuromuscular junction and muscle phenotypes.
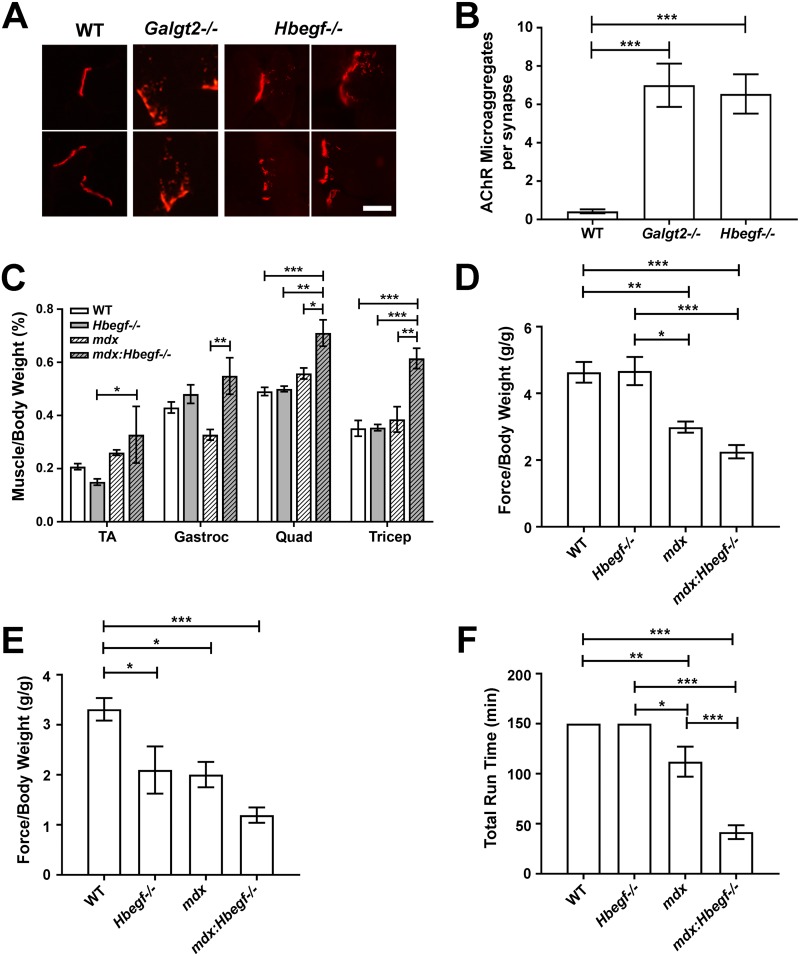
Because Hbegf−/− muscles had reduced Galgt2 and CT glycan expression, we next determined if loss of Hbegf would mimic NMJ or muscle phenotypes found in Galgt2−/− or mdx::Galgt2−/− mice. Galgt2−/− muscles show increased fragmentation of postsynaptic AChRs and an increased localization of AChRs in clathrin-coated endosomes (46). The number of AChR microaggregates in the vicinity of the NMJ was also increased by an order of magnitude in 7-month-old Hbegf−/− muscles (Fig. 8A and B). This was equivalent to the pattern and extent of subsynaptic AChR alterations found in 3-month-old Galgt2−/− muscles (46). Galgt2 is also a modulator of skeletal muscle disease severity in mdx mice (31). mdx::Galgt2−/− muscles show increased size and decreased strength compared to mdx muscles. Eight- to 12-month-old mdx::Hbegf−/− mice had increased skeletal muscle mass relative to mdx mice (Fig. 8C), much like mdxGalgt2−/− muscles (31). Muscles in Hbegf−/− mice, in contrast, were not significantly different from those of the WT. Forelimb (Fig. 8D) and hindlimb (Fig. 8E) grip strength also trended lower in mdx::Hbegf−/− mice relative to that of mdx mice, although these changes did not reach statistical significance. Hbegf−/− forelimb grip strength was unchanged from that of the WT, while hindlimb grip strength was reduced. When run on a treadmill, Hbegf−/− mice were indistinguishable from WT mice (P > 0.999), while mdx mice showed reduced ambulation (P < 0.01 and P < 0.05 versus WT and Hbegf−/−, respectively) and mdx::Hbegf−/− mice were further significantly diminished relative to mdx mice (P < 0.001 versus mdx mice) (Fig. 8F). Thus, deletion of Hbegf in mdx mice mimicked dystrophic phenotypes of Galgt2 deletion. sHB-EGF overexpression, however, was very different than GALGT2 overexpression in mdx muscles under certain circumstances. For example, while GALGT2 overexpression can prevent muscle damage, can reduce fibrosis, can prevent loss of muscle force in mdx skeletal muscles, and has an excellent safety profile at high doses (22, 47–49), systemic overexpression of sHB-EGF at postnatal day 1 in mdx and wild-type mice led to uniform lethality within 3 weeks of treatment (5 out of 5 each).
FIG 8.
Hbegf−/− mice exhibit Galgt2−/− neuromuscular junction and muscle phenotypes. (A) Examples of increased AChR microaggregates at neuromuscular junctions in Galgt2−/− (2 panels) and Hbegf−/− (4 panels) muscles compared to levels for the wild type (2 panels). Tibilais anterior muscle is shown at 3 months of age for Galgt2−/− muscle and at 7 months of age for Hbegf−/− and WT muscle. Scale bar is 10 μm. (B) Quantification of AChR microaggregates in WT, Galgt2−/−, and Hbegf−/− muscle. WT (n = 45) and Hbegf−/− (n = 75) TA muscles were from 7-month-old mice, while Galgt2−/− (n = 37) TA muscles were from 3-month-old mice. Data are means ± SEM. ***, P < 0.001 by one-way ANOVA. (C) Mouse skeletal muscle weights were normalized to total body mass. Abbreviations: TA, tibialis anterior; Gastroc, gastrocnemius; Quad, quadriceps. (D to F) Mice participated in a week-long test of strength and endurance via forelimb grip strength (D), hindlimb grip strength (E), and total run time on a treadmill (F). For panels D and E, force is normalized to body weight in each animal. For panels D to F, data are means ± SEM for n = 6 (WT), n = 4 (mdx and mdx::Hbegf−/−), and n = 3 (Hbegf−/−) measures. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one-way ANOVA.
DISCUSSION
The induction of therapeutic surrogate genes and proteins to inhibit muscle damage associated with muscular dystrophy represents a major opportunity for researchers trying to improve muscle disease outcomes. GALGT2 gene overexpression is an attractive approach to inducing surrogate therapeutic genes and proteins because it can alter expression of multiple muscular dystrophy modifiers. Such genes include those encoding utrophin (9, 10, 21), which can inhibit muscular dystrophy in the mdx mouse model of Duchenne muscular dystrophy (DMD) (1–3), agrin (9, 10, 18), which can inhibit muscular dystrophy in the dyW mouse model of laminin α2-dependent congenital muscular dystrophy (or MDC1A) (8), plectin 1 (9, 19), which when deleted increases the severity of muscular dystrophy in mdx mice (50) and itself causes muscular dystrophy (with epidermolysis bullosa) (51), laminin α4 and α5 (9, 10), which can diminish muscular dystrophy caused by deficits in laminin α2 (7, 52), and integrin α7, which can inhibit muscular dystrophy in mdx mice (4, 53). Endogenous regulation of these therapeutic genes and proteins in skeletal muscle, however, remains poorly understood, which is why we undertook the current studies focused on regulation of endogenous muscle Galgt2 gene expression.
While studies of nerve-derived signals that induce expression of synaptic muscle genes have been fairly extensive, little is known about how muscle-derived factors might induce synaptic gene expression, particularly in the postnatal period when Galgt2 expression becomes confined to the synapse. Neuregulin 1 (NRG1), for example, is a nerve-derived signal that induces synaptic gene expression in embryonic skeletal muscle, including expression of nicotinic acetylcholine receptor genes (54). Galgt2, along with other synaptic proteins, such as utrophin, agrin, laminin α4, and laminin α5, becomes localized to the NMJ in skeletal muscle 2 to 3 weeks after birth (23, 25, 26, 29–31, 34), a later developmental epoch than is seen for many NRG1-regulated genes (55, 56). We identified a group of EGFR ligands (57, 58), including EGF, sHB-EGF, amphiregulin, epiregulin, and TGFα, that stimulated muscle Galgt2 gene expression. NRG1, which does not bind the EGFR but instead binds EGFR family members ErbB3 and ErbB4 (57, 58), in contrast, did not stimulate muscle Galgt2 expression. Similar to NRG1 receptors ErbB3 and ErbB4 (59), EGFR is localized to the NMJ in adult skeletal muscle, a finding first described by Sanes and colleagues (60) and confirmed in this study. Thus, EGFR ligands and EGFR may be involved in the localization of genes (and proteins) to the NMJ in the postnatal period, akin to NRG1’s role in embryonic muscle.
Our studies suggest that sHB-EGF controls important postnatal muscle gene expression programs and has important roles in muscle biology. Overexpression of sHB-EGF in skeletal muscle not only induced expression of mouse muscle Galgt2 expression but also upregulated the expression of utrophin, laminin α2, laminin α4, and integrin β1. Overexpression of full-length HB-EGF, in contrast, did not produce these changes, confirming the importance of HB-EGF cleavage to its soluble sHB-EGF form to mediate EGFR signaling (35, 36, 61). Interestingly, Engvall and colleagues have shown that transgenic overexpression of ADAM12, which can cleave transmembrane HB-EGF protein to create active sHB-EGF, is therapeutic in mdx mice and can increase integrin α7 and utrophin expression (62, 63). Those results may be related to the mechanism we have described here. sHB-EGF was concentrated at the NMJ in adult muscle, and deletion of mouse Hbegf decreased synaptic expression of muscle Galgt2 protein and the CT glycan, which is made by Galgt2 (12, 46, 64). Similarly, deletion of Hbegf led to increased fragmentation of postsynaptic acetylcholine receptors to a degree seen with deletion of Galgt2 (46), albeit on a slightly different time scale. Moreover, in mdx mice, Hbegf deletion modulated muscle disease phenotypes in a manner similar to deletion of Galgt2. Such phenotypes included increases in muscle hypertrophy and muscle weakness with reduced ambulation. Not all phenotypes, however, were matched between Hbegf and Galgt2. For example, perinatal systemic overexpression of sHB-EGF was lethal in mdx mice, a finding not found with GALGT2 (14). While this may be due to the use of different promoters in these experiments (cytomegalovirus [CMV] for sHB-EGF and MCK for GALGT2), HB-EGF has additional well-described functions not reported with GALGT2. For example, Hbegf-deficient mice have extensive phenotypes related to cardiac development (61, 65, 66), cardiac hypertrophy (66, 67), tumor cell growth and angiogenesis (57, 68), embryo implantation and placental development (69–71), cognitive function and synaptic plasticity (72, 73), tissue fibrosis (74–77), and intestinal health and regeneration after injury (78–82). Some of these phenotypes have been not reported in Galgt2−/− mice, although Galgt2 deletion can give rise to blood, cardiac, pregnancy, and tumor-related phenotypes (14, 31, 83–85). Similarly, GALGT2 overexpression in skeletal muscle induced the expression of some surrogate genes not induced by sHB-EGF, including Lama5 and Itga7, while other surrogate genes were induced by both.
Most of the functions described for sHB-EGF correlate with its ability to activate EGFR and downstream pathways, including PI3 kinase, Akt, mTOR, ERK, and MEK, as well as cross talk with IGF receptor 1 (IGFR1) signaling (58, 86). While we have not delineated the entire signaling pathway in muscle, we have shown that sHB-EGF activates the muscle EGFR signaling and increases Akt phosphorylation. sHB-EGF also increases muscle EGFR gene and protein expression, thereby causing the overexpression of its own receptor. This no doubt facilitates the ability of sHB-EGF to induce overexpression of other normally synaptic therapeutic genes in the extrasynaptic muscle membrane. Importantly, while sHB-EGF induces increased Mek1/2 and Erk1/2 phosphorylation in muscle cells, it did not do so in muscle tissue. This suggests that additional regulatory factors or mechanisms are involved in sHB-EGF signaling in vivo that are not involved in sHB-EGF signaling in cultured muscle cells.
We have also shown that addition of sHB-EGF to muscle cells stimulates increased occupancy of the GALGT2 promoter by TFAP4. TFAP4 overexpression is sufficient to activate the GALGT2 promoter and requires a specific binding site to do so. LMO2 overexpression could also stimulate the same site on the GALGT2 promoter, but it was less potent than TFAP4. Intriguingly, addition of sHB-EGF to muscle cells decreased LMO2 occupancy at the same time that it increased TFAP4 occupancy in ChIP experiments. As such, sHB-EGF may increase GALGT2 expression by activating a switch from a less active transcription factor (LMO2) to a more active one (TFAP4). sHB-EGF also increased endogenous Tfap4 gene expression, which may further facilitate increased TFAP4 occupancy on the GALGT2 promoter. Like sHB-EGF, TFAP4 overexpression induced mouse Galgt2 gene expression in muscle cells. Surprisingly, FOXD3 overexpression also did this. FOXD3, however, may act via TFAP4, as FOXD3 overexpression also increased endogenous mouse Tfap4 gene expression (while decreasing Lmo2). Also, unlike that of Tfap4, FoxD3 expression was not detectable in adult mouse skeletal muscle. The fact that there is also an E-box within the TFAP4/LMO2 binding site suggests that myogenin (MYOG) also activates GALGT2 expression, but we found no inductive effects from myogenin overexpression. Goldman, Yao, Schaeffer, and others have previously shown that myogenin protein is downregulated in skeletal muscle by muscle activity in a calcium- and histone deacetylase (HDAC) 4- and/or 9-dependent manner (87–92). As such, one would not expect appreciable myogenin to be present in extrasynaptic regions of a functioning muscle cell, where sHB-EGF was overexpressed in our experiments. The activation of TFAP4, however, may provide a mechanism for late-developing synaptic genes to maintain gene expression even in the presence of muscle activity by activating transcription from sites that might normally be occupied by myogenin. TFAP4 may also offset suppression of GALGT2 expression by promoter methylation, as is known to occur in gastrointestinal cancers and cancer cell lines (93, 94).
These experiments provide the first evidence that endogenous muscle proteins can activate Galgt2 gene expression in a manner that mimics its own ability to induce downstream therapeutic genes that can inhibit forms of muscular dystrophy. This work should provide avenues to understanding how to exploit endogenous muscle signaling pathways to induce expression of therapeutic muscle genes for these disorders.
MATERIALS AND METHODS
Mice.
All mice were kept and used in accordance with protocols approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children’s Hospital (Columbus, OH). C57BL/6J and mdx mice were purchased from Jackson Laboratories (Bar Harbor, ME). Hbegf−/− mice were kindly provided by Gail Besner (The Research Institute at Nationwide Children’s Hospital, Columbus OH). Hbegf−/− mice were bred with mdx mice to create stable double knockout mice (mdx::Hbegf−/− mice). Galgt2−/− mice were provided by the Consortium for Functional Glycomics and originally generated by John Lowe (Genentech, South San Francisco, CA).
Cell lines.
The mouse C2C12 cell line was grown in Dulbecco’s modified Eagle’s medium (DMEM; Mediatech, Manassas, VA) containing 20% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA) and differentiated in DMEM containing 2% horse serum (Thermo Fisher Scientific). The human endothelial kidney (HEK293) cell line was maintained in DMEM containing 10% FBS. The CHO cell line was maintained in DMEM–F-12 medium (Gibco, Gaithersburg, MD) containing 10% FBS. All cell lines were cultivated in the presence of 100 U/ml penicillin and 100 μg/ml streptomycin (Pen/Strep; Mediatech).
AAV vectors.
The cDNA region encoding the human sHB-EGF protein and the cDNA encoding the full-length transmembrane human HB-EGF were subcloned into a self-complementary (sc) AAV vector designed by Douglas McCarty (Pfizer, Raleigh, NC) (95). Both AAV vectors contained a cytomegalovirus (CMV) promoter and a simian virus 40 (SV40) polyadenylation sequence. r(sc)AAV9.CMV.sHB-EGF and r(sc)AAV9.CMV.HB-EGF vectors were made by the Viral Vector Core at Nationwide Children’s Hospital using the triple transfection method in HEK293 cells (96) and were purified using iodixanol gradients and anion-exchange chromatography (97). For sHB-EGF, the human HBEGF coding sequence contains only the first 148 amino acids (soluble form). For HB-EGF, the human HBEGF sequence contains all 208 amino acids (full-length transmembrane form) (98).
GALGT2 promoter luciferase reporter plasmids.
All constructs are depicted in Fig. 2A. The bp −5870 human GALGT2 promoter was isolated by PCR from genomic DNA and ligated into the pGL4.14[luc2/Hygro] vector (Promega, Madison, WI) containing the firefly luciferase gene at the XhoI and BglII restriction sites in the polylinker region. bp −950, −500, and −200 deletion constructs were amplified and purified from the bp −5870 plasmid by PCR. Generation of serial deletions in the bp −950 construct were also made by PCR using the ultrahigh-fidelity PfuUltra II fusion HS DNA polymerase (Agilent Technologies, Santa Clara, CA) for DNA amplification via methods previously described (99). All plasmids were verified by DNA sequencing. Plasmids were transfected into C2C12 myoblasts using Effectene (Qiagen, Germantown, MD) and selected with 200 μg/ml hygromycin B (Mediatech). Control cells were transfected with a promoterless firefly luciferase plasmid as a negative control. Positive results were also confirmed using the human GALGT2 promoter cloned into a pGL4.76[hRluc/Hygro] vector (Promega) and selected with hygromycin B, with promoterless controls also generated as described above. Successfully transfected C2C12 cells were differentiated into myotubes following 6 days in differentiation media. All lines formed healthy myotubes under these conditions.
GALGT2 promoter luciferase reporter trophic factor screen.
Reporter assays were performed in C2C12 myotube cultures stably expressing the bp −5870 GALGT2 promoter firefly luciferase reporter. These cells were plated in 96-well plates and became confluent 24 to 48 h after plating. When cells had reached confluence, fusion medium (DMEM containing 2% horse serum and Pen/Strep) was added to cells for 4 days, followed by treatment with trophic factors in serum-free DMEM for 24 h. Trophic factors screened included recombinant human epidermal growth factor (EGF; 100 ng/ml) (R&D Systems, Minneapolis, MN), recombinant soluble human heparin-binding EGF-like growth factor (sHB-EGF; 100 ng/ml) (R&D Systems), recombinant human amphiregulin (50 ng/ml) (Sigma-Aldrich, St. Louis, MO), recombinant mouse epiregulin (100 ng/ml) (Sigma-Aldrich), recombinant human transforming growth factor-α (TGFα; 100 ng/ml) (Sigma-Aldrich), recombinant human insulin-like growth factor 1 (IGF1; 100 ng/ml) (Sigma-Aldrich), insulin (solution from bovine pancreas; 3 μg/ml) (Sigma-Aldrich), recombinant human brain-derived neurotrophic factor (BDNF; 100 ng/ml) (EMD Millipore, Burlington, MA), recombinant human ciliary neurotrophic factor (CNTF; 100 ng/ml) (EMD Millipore), mouse nerve growth factor 2.5S (NGF 2.5S; 100 ng/ml) (EMD Millipore), recombinant mouse leukemia inhibitory factor (LIF; 50 ng/ml) (EMD Millipore), synthetic glucagon-like peptide 2 (10 μM) (Abbiotec, San Diego, CA), human growth hormone (HGH; 100 ng/ml; from pituitary gland) (EMD Millipore), recombinant human neurotrophin-3 (NT-3; 100 ng/ml) (R&D Systems), synthetic human peptide YY (100 nM) (Sigma-Aldrich), recombinant human glial cell line-derived neurotrophic factor (GDNF; 50 ng/ml) (Sigma-Aldrich), recombinant human hepatocyte growth factor (HGF; 100 ng/ml) (Sigma-Aldrich), human calcitonin gene-related peptide (CGRP; 1 μg/ml) (Sigma-Aldrich), synthetic human somatostatin (1 μM) (AbD Serotec, Raleigh, NC), and human neuregulin 1 (NRG1; 10 nM) (R&D Systems). Firefly luciferase activity was determined by following the manufacturer’s instructions using the Bright-Glo luciferase assay system (Promega) in a GloMax 96 microplate luminometer (Promega). Relative light units (RLU) in trophic factor-treated cells were normalized to levels for untreated cells.
Transcription factor binding motif analysis.
Potential transcription factor binding sites in the human GALGT2 promoter were analyzed using TFBIND gene tool software (http://tfbind.hgc.jp/) with a cutoff value of 0.85 (40).
Ad-mediated gene overexpression.
Ads containing human FOXD3 (NM_012183), LMO2 (BC034041), TFAP4 (BC010576), MYOG (BC053899), or GFP were purchased from Applied Biological Materials (Richmond, BC, Canada). Ad was amplified in HEK293 cells per the manufacturer’s instructions. Expression of Ad-GFP in C2C12 myotubes was used to determine the necessary multiplicity of infection (MOI) to attain >80% infection efficiency without cell death and to serve as a negative control in reporter assays. Following 4 days of differentiation, Ad was added to GALGT2 promoter luciferase reporter C2C12 cultures stably expressing either the bp −950 or the −950Δ2 GALGT2 promoter construct in serum-free medium at MOIs of 2, 4, 8, 16, 32, and 64 for 48 h in the presence or absence of sHB-EGF. Firefly luciferase activity was determined by following the manufacturer’s instructions using Steady-Glo luciferase assay systems (Promega, Madison, WI) in a GloMax 96 microplate luminometer. An MOI of 32 was required by all Ad constructs, except Ad-FOXD3 (MOI of 16), to generate maximal firefly luciferase activity without toxicity.
ChIP analysis.
C2C12 myoblasts expressing the bp −5870 GALGT2 promoter firefly luciferase reporter were plated in 10-cm dishes and differentiated in fusion medium for 6 days as described above. Once differentiated into myotubes, cells were treated with human recombinant sHB-EGF protein for 24 h or 48 h. Cross-linking and harvesting of cells was performed using the Magna ChIP A/G kit from EMD Millipore per the manufacturer’s instructions. Chromatin was sheared using 8 cycles of 3 s on/15 s off at power setting 0.5, repeated 7 times on a Misonix sonicator 3000 (Misonix, Farmingdale, NY), to generate DNA fragments with a mean size between 200 and 1,000 bp. Sonicated DNA was precleared and then incubated with LMO2 antibody (sc-10497; Santa Cruz Biotechnology), TFAP4 antibody (HPA001912; Sigma-Aldrich), or IgG control (12-371; EMD Millipore) overnight at 4°C by following Magna ChIP A/G protocol instructions (EMD Millipore). Quantitative PCR (qPCR) in a 7500 real-time PCR system (Applied Biosciences, Foster City, CA) with SsoAdvanced universal SYBR green supermix (Bio-Rad, Hercules, CA) was used to quantify DNA fragments pulled down with the antibodies compared to evaluation of input DNA, which was not incubated with antibodies. Primers were created to stably include a region along the GALGT2 promoter that was 150 to 250 bp in length: bp −951 to −726 (forward, 5’TTAAAGATTCTTGCCGGTCGTG; reverse, 5’CGCTCAAATGATTCTCCCACCT).
siRNA knockdown of transcription factors.
C2C12 myoblasts expressing the bp −5870 GALGT2 promoter firefly luciferase reporter were plated in either 96-well or 6-well dishes and differentiated in fusion medium for 6 days as described above. Once differentiated, cells were treated with recombinant human sHB-EGF and/or siRNA targeting transcription factors in serum-free DMEM for 48 h. siRNA was added 24 h prior to the addition of sHB-EGF at doses of 5 nM, 10 nM, or 20 nM and included siRNA against mouse LMO2 (SR403448), TFAP4 (SR411368), or a universal scrambled negative control (OriGene, Rockville, MD). Treated cells were monitored closely to obtain maximal knockdown of targeted transcription factor without resulting in cell death. GALGT2 promoter activation was measured by reporter firefly luciferase activity by following the manufacturer’s instructions using Steady-Glo luciferase assay systems (Promega, Madison, WI) in a GloMax 96 microplate luminometer. Cell lysates were also collected to measure the resulting mRNA expression of targeted transcription factors to ensure siRNA selectivity and activity by semiquantitative reverse transcription-PCR (qRT-PCR).
sHB-EGF inhibitor screen.
C2C12 myoblasts expressing the bp −5870 GALGT2 promoter firefly luciferase reporter were plated in 96-well plates, becoming confluent after 24 to 48 h. At this time, cells were switched to fusion medium for 2 days, followed by 2 days in fusion medium that had been precleared with heparin-agarose beads (Sigma-Aldrich) to remove endogenous heparin-binding proteins. Cells were then treated with recombinant human sHB-EGF and/or inhibitors of signaling proteins in serum-free DMEM for 24 to 48 h. Inhibitors were added 1 h prior to the addition of sHB-EGF at a range of pharmacologically relevant doses and included EGF receptor (EGFR) inhibitor (tyrphostin AG-1478; 200 nM) (Cell Signaling, Danvers, MA), phosphoinositide 3-kinase (PI3K) inhibitor (LY294002; 5 μM) (EMD Millipore), protein kinase B (Akt) inhibitor (MK-2206; 5 μM) (Selleck Chemicals, Houston, TX), mammalian target of rapamycin (mTOR) inhibitor (rapamycin; 100 nM) (EMD Millipore), Raf kinase inhibitor (vemurafenib/PLX4032/RG7204; 3 μM) (Selleck Chemicals), extracellular signal-regulated kinase (ERK) inhibitor (SCH772984; 10 μM) (Selleck Chemicals), Src inhibitor (KX2-391; 5 nM) (Selleck Chemicals), Bcr/Abl inhibitor (nilotinib/AMN-107; 1 μM) (Selleck Chemicals), STAT5 inhibitor (25 μM) (EMD Millipore), JNK inhibitor (SP600125; 25 μM) (EMD Millipore), or c-myc inhibitor (10058-F4/CAS 403811-55-2; 10 μM) (EMD Millipore). Drug concentrations shown provided maximum levels of inhibition without causing cell death. Firefly luciferase activity was determined by following the manufacturer’s instructions using Steady-Glo luciferase assay systems (Promega, Madison, WI) in a GloMax 96 microplate luminometer.
Western blot analysis. (i) Muscle cells.
C2C12 myoblasts were plated in 12-well plates and differentiated as described above. Cells were then either left untreated or treated with 10 nM recombinant human sHB-EGF in serum-free DMEM for 15, 30, or 60 min. Medium was then removed and cells were rinsed briefly with cold PBS. Cells were incubated for 30 min at 4°C in lysis buffer containing 1% NP-40, 50 mM Tris, 140 mM NaCl, 1 mM EDTA, cOmplete EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN), and PhosSTOP phosphatase inhibitors (Roche). Cell lysates were then collected using cell scrapers, rocked gently at 4°C for 45 min, and centrifuged at 21,000 × g at 4°C for 10 min. Supernatant was collected, and the resulting protein concentration was measured by bicinchoninic acid assay (Thermo Fisher Scientific). Protein (25 μg) was boiled at 100°C for 10 min in NuPAGE LDS sample buffer (Thermo Fisher Scientific) containing 0.1 M β-mercaptoethanol (BME; Fisher Scientific), separated on a Bolt 4 to 12% Bis-Tris plus gel (Thermo Fisher Scientific), and transferred to a nitrocellulose membrane. The membrane was blocked in 5% nonfat dry milk in Tris-buffered saline (TBS; 25 mM Tris, 150 mM NaCl) containing 0.05% Tween 20 and then probed overnight with antibodies to EGFR (2232; Cell Signaling), phosphorylated EGFR (Tyr1173) (53A5) (4407; Cell Signaling), Akt (9272; Cell Signaling), phosphorylated Akt (Ser473) (9271; Cell Signaling), mTOR (2972; Cell Signaling), phosphorylated mTOR (Ser2448) (2971; Cell Signaling), MEK1/2 (9122; Cell Signaling), phosphorylated MEK1/2 (Ser217/221) (41G9) (9154; Cell Signaling), Erk1/2 (p44/42 mitogen-activated protein kinase [MAPK]) (137F5) (4695; Cell Signaling), phosphorylated Erk1/2 (p44/42 MAPK) (Thr202/Tyr204) (9101; Cell Signaling), C-RAF (9422; Cell Signaling), phosphorylated C-RAF (Ser289/296/301) (9431; Cell Signaling), Src (36D10) (2109; Cell Signaling), phosphorylated Src (Tyr416) (D49G4) (6943; Cell Signaling), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; G9545; Sigma-Aldrich, St. Louis, MO). After washing, blots were probed with horseradish peroxidase-coupled secondary antibody (Jackson ImmunoResearch Laboratories), washed again, and developed using an enhanced chemiluminescence (ECL; Lumigen, Southfield, MI) method according to the manufacturer’s instructions.
(ii) Muscle tissue.
Frozen skeletal muscle blocks were cut on a cryostat (20 μm/section), and muscle tissue was digested in lysis buffer (mentioned above), also using metal lysis beads with shaking four times at 30 Hz for 30 s each time (TissueLyser II; Qiagen). Samples were then rocked gently at 4°C for 3 h and centrifuged at 21,000 × g at 4°C for 10 min. The supernatant was collected, and protein (80 μg) was prepared and immunoblotted using antibodies mentioned above, except for EGFR (A-10) (sc-373746; Santa Cruz Biotechnology).
(iii) Band quantification.
ImageJ 1.46r software (National Institutes of Health, Bethesda, MA) was used to analyze relative band density in both cell culture and skeletal muscle samples using methods we have previously described (100). Phosphorylated protein expression was normalized to total protein expression and then compared to levels for untreated control lysates. Total protein expression was normalized to the loading control (GAPDH) and again compared to untreated control lysates.
AAV-induced overexpression of HB-EGF and sHB-EGF.
The gastrocnemius muscle on the left side of 5-week-old male C57BL/6J mice was injected with 5 × 1010 vector genomes of r(sc)AAV9.CMV.HB-EGF or r(sc)AAV9.CMV.sHB-EGF in a volume of 50 μl sterile PBS using a 0.3-ml insulin syringe near the midpoint of the muscle. Muscles on the contralateral (right) side of the mouse were mock injected with an identical volume of sterile PBS. At 4 or 12 weeks postinjection, mice were sacrificed and dissected. Gastrocnemius muscles were embedded in optimal cutting termperature (O.C.T.) compound (Fisher Scientific, Pittsburgh, PA) and snap-frozen in liquid nitrogen-cooled isopentane.
qPCR of AAV vector genomes.
TaqMan qPCR was used to quantify AAV vector genome copies in treated gastrocnemius muscles and saline-treated control muscles. Frozen skeletal muscle blocks were cut on a cryostat (20 μm/section), and DNA was extracted from these shavings using the Qiagen DNeasy blood and tissue kit (Germantown, MD). DNA purity and quantity were measured using an ND-1000 spectrophotometer (NanoDrop; Thermo Fisher Scientific). qPCR was performed in a 7500 real-time PCR system with TaqMan gene expression master mix (Thermo Fisher Scientific). A vector-specific primer/probe set (forward, 5’ATCCGGTGGTGGTGCAAAT; probe, 5′/56-FAM/TCCTCAGTG/ZEN/GATGTTGCCTT/3IABkFQ/; reverse, 5’CAGCAGCTTCATACGGCC) was used to amplify a portion of the vector DNA encompassing the 3′ end of the SV40 intron and the 5′ end of the human HBEGF cDNA. No amplification of the endogenous mouse Hbegf was observed in control samples using this primer/probe set. The plasmid used to make r(sc)AAV9.CMV.sHBEGF was linearized with ClaI, repurified, and utilized to generate a standard curve from 50 to 5 million copies in log increments. The correlation coefficient of the standard curve always equaled or exceeded 0.99. Copy number was reported as vector genomes per microgram of genomic DNA assayed. Each sample was measured in duplicate, and values for each cohort were averaged.
qRT-PCR.
Relative mRNA transcript levels were assessed by qRT-PCR using the 2−ΔΔCT method for both C2C12 cells and skeletal muscle (101). C2C12 myotube cultures were rinsed in ice-cold PBS and then collected in TRIzol (Thermo Fisher Scientific) using cell scrapers. Frozen skeletal muscle blocks were cut on a cryostat (20 μm/section). Total RNA from cells or tissue was extracted, purified, and reverse transcribed as previously described (100). Samples underwent qRT-PCR in a 7500 real-time PCR system with TaqMan gene expression master mix, using eukaryotic 18S rRNA (Thermo Fisher Scientific) as an internal control. Primer/probe sets were designed (PrimerQuest DNA software) and synthesized (Integrated DNA Technologies, Coralville, IA) to recognize Homo sapiens HBEGF (forward, 5’GTGACTTGCAAGAGGCAGAT; probe, 5′/56-FAM/TATCCTCCA/ZEN/AGCCACAAGCACTGG/3IABkFQ/; reverse, 5’GTGCTCCTCCTTGTTTGGT), Mus musculus Hbegf (forward, 5’AGGACTTGGAAGGGACAGA; probe, 5′/56-FAM/AGTTGCTTT/ZEN/CTCCTCCAAGCCACA/3IABkFQ/; reverse, 5’CCCATTCCTTTCTTTGCTTGG), and Mus musculus Galgt2 (B4Galnt2) (forward, 5’GCCCACGGAGAGTTCTTTATT; probe, 5′/56-FAM/ATAATTACA/ZEN/CCCGGGCAAGAGCCC/3IABkFQ/; reverse, 5’TCCTTTGGTGGTGTTCTTACTT). Premade primer/probe sets for Mus musculus Egfr (Mm.PT.58.28853978), Erbb2 (Mm.PT.58.29549739), Erbb3 (Mm.PT.30061803), Erbb4 (Mm.PT.58.16591892), and Akt1 (Mm.PT.58.8333433) were purchased from Integrated DNA Technologies. Mus musculus primers for Lama2, Lama4, Lama5, Itga7, Itgb1, Dmd, Dag1, Utrn, and Agrn were purchased and used as previously described (10). Probes for human transcription factor genes TFAP4 (Hs01558245_m1), LMO2 (Hs00153472-m1), FOXD3 (Hs00255287_s1), and MYOG (Hs01072232_m1) were purchased from Applied Biosystems. Mouse Myog probes (Mm00446194_m1) were purchased from Applied Biosystems. Mouse Tfap4 (Mm.PT.58.5559446) and Lmo2 (Mm.Pt.58.21550807) probes were purchased from IDT. Premade primer pairs for Mus musculus Lmo2 (MP207520), Tfap4 (MP217129), and Foxd3 (MP204962) were purchased from OriGene (Rockville, MD). Relative mRNA levels were averaged for each cohort and compared to levels for PBS-injected muscle.
Immunofluorescence staining of CHO cells and skeletal muscle. (i) CHO cells.
CHO cells transfected with expression vectors for sHB-EGF or HB-EGF were grown on gelatin-coated glass coverslips using previously described methods (102). Cells were washed in PBS, fixed in 4% paraformaldehyde (PFA) for 10 min, and then fixed further with 4% PFA and 0.1% Triton X-100 for 5 min. After washing, cells were incubated with antibodies that recognize sHB-EGF (MAb 2591; R&D Systems), sHB-EGF and HB-EGF (PAb 259 NA; R&D Systems), or the intracellular C-terminal domain of HB-EGF protein (C18; Santa Cruz Biotechnology, Dallas, TX). After washing, cells were incubated with appropriate fluorophore-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), washed again, and then mounted in ProLong Gold antifade mountant with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific).
(ii) Skeletal muscle.
Unfixed frozen muscle blocks were cut on a cryostat into 8- to 10-μm sections. To probe for HB-EGF, staining was performed as described above with MAb 2591 and costained with rhodamine-conjugated α-bungarotoxin (B35451; Invitrogen, Carlsbad, CA). To probe for Galgt2 and CT glycan, slides were blocked in 3% bovine serum albumin (BSA) and then stained with a monoclonal antibody to CT glycan (CT2) or a polyclonal antibody to Galgt2 (PAB20841; Abnova, Walnut, CA). After washing in PBS, sections were incubated with appropriate fluorophore-conjugated secondary antibodies and rhodamine-conjugated α-bungarotoxin. After staining, all sections were mounted as described above. For EGFR staining, sections were fixed as described above, blocked with Mouse-On-Mouse (M.O.M.) blocking reagent (Vector Labs, Burlingame, CA) followed by a second block with 3% BSA, incubated with a monoclonal antibody to EGFR (A-10) (sc-373746; Santa Cruz Biotechnology), washed, and incubated with fluorescein isothiocyanate-conjugated secondary antibody along with rhodamine-conjugated α-bungarotoxin.
All immunofluorescence staining was visualized with a Zeiss Axioskop2 plus epifluorescence microscope using fluorophore-specific filters, and representative images were captured with a Zeiss AxioCam MRC5 camera (Carl Zeiss Microscopy, Jena, Germany). All images comparing individual stains were time matched using identical exposure settings across different experimental conditions.
Quantification of AChR microaggregates in skeletal muscle.
Quantification of AChR microaggregates based on α-bungarotoxin staining of NMJs was done as previously described (46), using 8-μm-thick cryostat cut sections of the tibialis anterior muscle. A total of 45, 37, or 75 synapses were analyzed for C57BL/6J, Galgt2−/−, and Hbegf−/− muscles, respectively.
Grip strength and ambulation treadmill.
Forelimb and hindlimb grip strength were assessed in mice using a grip strength meter (Columbus Instruments, Columbus, OH). Measurements were averaged from 5 forelimb repetitions and 10 hindlimb repetitions each day; mice were tested once per day for a week, and the daily measurements were averaged to produce a final forelimb and hindlimb measurement. To measure ambulation, mice were run on a treadmill with a 10° decline (Treadmill Simplex II; Columbus Instruments). The mice ran for 1 min at 5 m/min, which increased by 1 m/min each minute until a maximum of 15 m/min and then remained at that speed for an additional 20 min. The time that the mice remained on the treadmill, up to a total time of 30 min, was recorded. After 2 days of training, mice were tested once per day for 5 days, and the daily measurements were averaged to produce a final measurement of treadmill running time before exhaustion.
Statistics.
The significance in variance between cohorts was determined by one- or two-way analysis of variance (ANOVA), where appropriate, followed by Bonferroni’s multiple-comparison test. Kaplan-Meier survival plots were analyzed via the log-rank test.
ACKNOWLEDGMENTS
We thank Deborah Zygmunt, Davin Packer, Neha Singhal, and Yelda Serinagaoglu for technical assistance in the performance of certain experiments.
This work was funded by grants from the National Institutes of Health (R01 AR049722 and P50 AR070604) to P.T.M. M.L.C. was funded in part by a T32 training award from NIH (NS077984) and an NIH Wellstone Muscular Dystrophy Center Training Award (HD066409).
The funders have no role in study design, data collection and interpretation, or the decision to submit the work for publication. P.T.M. has a financial conflict of interest with regard to studies of rAAVrh74.MCK.GALGT2 gene therapy, as he is the inventor and is paid licensing fees by a commercial biotechnology company. None of the other authors have any conflicts to declare.
REFERENCES
- 1.Deconinck N, Tinsley J, De Backer F, Fisher R, Kahn D, Phelps S, Davies K, Gillis JM. 1997. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat Med 3:1216–1221. doi: 10.1038/nm1197-1216. [DOI] [PubMed] [Google Scholar]
- 2.Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. 1998. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med 4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- 3.Rafael JA, Tinsley JM, Potter AC, Deconinck AE, Davies KE. 1998. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat Genet 19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- 4.Burkin DJ, Wallace GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. 2001. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol 152:1207–1218. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. 2005. Transgenic expression of α7β1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol 166:253–263. doi: 10.1016/S0002-9440(10)62249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gawlik K, Miyagoe-Suzuki Y, Ekblom P, Takeda S, Durbeej M. 2004. Laminin alpha1 chain reduces muscular dystrophy in laminin alpha2 chain deficient mice. Hum Mol Genet 13:1775–1784. doi: 10.1093/hmg/ddh190. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard JR, Lin S, McKee KK, Meinen S, Crosson SC, Sury M, Hobbs S, Maier G, Yurchenco PD, Ruegg MA. 2017. Linker proteins restore basement membrane and correct LAMA2-related muscular dystrophy in mice. Sci Transl Med 9:eaal4649. doi: 10.1126/scitranslmed.aal4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moll J, Barzaghi P, Lin S, Bezakova G, Lochmuller H, Engvall E, Muller U, Ruegg MA. 2001. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature 413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- 9.Chicoine LG, Rodino-Klapac LR, Shao G, Xu R, Bremer WG, Camboni M, Golden B, Montgomery CL, Shontz K, Heller KN, Griffin DA, Lewis S, Coley BD, Walker CM, Clark KR, Sahenk Z, Mendell JR, Martin PT. 2014. Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin alpha2 surrogates. Mol Ther 22:713–724. doi: 10.1038/mt.2013.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon JH, Chandrasekharan K, Xu R, Glass M, Singhal N, Martin PT. 2009. The synaptic CT carbohydrate modulates binding and expression of extracellular matrix proteins in skeletal muscle: partial dependence on utrophin. Mol Cell Neurosci 41:448–463. doi: 10.1016/j.mcn.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia B, Martin PT. 2002. Modulation of agrin binding and activity by the CT and related carbohydrate antigens. Mol Cell Neurosci 19:539–551. doi: 10.1006/mcne.2001.1095. [DOI] [PubMed] [Google Scholar]
- 12.Smith PL, Lowe JB. 1994. Molecular cloning of a murine N-acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte-specific CT oligosaccharide differentiation antigen. J Biol Chem 269:15162–15171. [PubMed] [Google Scholar]
- 13.Montiel MD, Krzewinski-Recchi MA, Delannoy P, Harduin-Lepers A. 2003. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Acalpha2-3Galbeta-R beta1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: evidence for an unusual extended cytoplasmic domain. Biochem J 373:369–379. doi: 10.1042/bj20021892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu R, Jia Y, Zygmunt DA, Martin PT. 2019. rAAVrh74.MCK.GALGT2 protects against loss of hemodynamic function in the aging mdx mouse heart. Mol Ther 27:636–649. doi: 10.1016/j.ymthe.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ervasti JM, Campbell KP. 1991. Membrane organization of the dystrophin-glycoprotein complex. Cell 66:1121–1131. doi: 10.1016/0092-8674(91)90035-W. [DOI] [PubMed] [Google Scholar]
- 16.Ervasti JM, Campbell KP. 1993. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia B, Hoyte K, Kammesheidt A, Deerinck T, Ellisman M, Martin PT. 2002. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev Biol 242:58–73. doi: 10.1006/dbio.2001.0530. [DOI] [PubMed] [Google Scholar]
- 18.Xu R, Chandrasekharan K, Yoon JH, Camboni M, Martin PT. 2007. Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am J Pathol 171:181–199. doi: 10.2353/ajpath.2007.060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu R, DeVries S, Camboni M, Martin PT. 2009. Overexpression of Galgt2 reduces dystrophic pathology in the skeletal muscles of alpha sarcoglycan-deficient mice. Am J Pathol 175:235–247. doi: 10.2353/ajpath.2009.080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon JH, Johnson E, Xu R, Martin LT, Martin PT, Montanaro F. 2012. Comparative proteomic profiling of dystroglycan-associated proteins in wild type, mdx, and Galgt2 transgenic mouse skeletal muscle. J Proteome Res 11:4413–4424. doi: 10.1021/pr300328r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen HH, Jayasinha V, Xia B, Hoyte K, Martin PT. 2002. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc Natl Acad Sci U S A 99:5616–5621. doi: 10.1073/pnas.082613599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas PJ, Xu R, Martin PT. 2016. B4GALNT2 (GALGT2) gene therapy reduces skeletal muscle pathology in the FKRP P448L mouse model of limb girdle muscular dystrophy 2I. Am J Pathol 186:2429–2448. doi: 10.1016/j.ajpath.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin PT, Scott LJ, Porter BE, Sanes JR. 1999. Distinct structures and functions of related pre- and postsynaptic carbohydrates at the mammalian neuromuscular junction. Mol Cell Neurosci 13:105–118. doi: 10.1006/mcne.1999.0737. [DOI] [PubMed] [Google Scholar]
- 24.Hoyte K, Kang C, Martin PT. 2002. Definition of pre- and postsynaptic forms of the CT carbohydrate antigen at the neuromuscular junction: ubiquitous expression of the CT antigens and the CT GalNAc transferase in mouse tissues. Brain Res Mol Brain Res 109:146–160. doi: 10.1016/S0169-328X(02)00551-X. [DOI] [PubMed] [Google Scholar]
- 25.Hoch W, Ferns M, Campanelli JT, Hall ZW, Scheller RH. 1993. Developmental regulation of highly active alternatively spliced forms of agrin. Neuron 11:479–490. doi: 10.1016/0896-6273(93)90152-H. [DOI] [PubMed] [Google Scholar]
- 26.Patton BL, Miner JH, Chiu AY, Sanes JR. 1997. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol 139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohlendieck K, Ervasti JM, Matsumura K, Kahl SD, Leveille CJ, Campbell KP. 1991. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron 7:499–508. doi: 10.1016/0896-6273(91)90301-F. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. 1992. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360:588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- 29.Kamakura K, Tadano Y, Kawai M, Ishiura S, Nakamura R, Miyamoto K, Nagata N, Sugita H. 1994. Dystrophin-related protein is found in the central nervous system of mice at various developmental stages, especially at the postsynaptic membrane. J Neurosci Res 37:728–734. doi: 10.1002/jnr.490370607. [DOI] [PubMed] [Google Scholar]
- 30.Tome FM, Matsumura K, Chevallay M, Campbell KP, Fardeau M. 1994. Expression of dystrophin-associated glycoproteins during human fetal muscle development: a preliminary immunocytochemical study. Neuromuscul Disord 4:343–348. doi: 10.1016/0960-8966(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 31.Xu R, Singhal N, Serinagaoglu Y, Chandrasekharan K, Joshi M, Bauer JA, Janssen PM, Martin PT. 2015. Deletion of Galgt2 (B4Galnt2) reduces muscle growth in response to acute injury and increases muscle inflammation and pathology in dystrophin-deficient mice. Am J Pathol 185:2668–2684. doi: 10.1016/j.ajpath.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezniczek GA, Konieczny P, Nikolic B, Reipert S, Schneller D, Abrahamsberg C, Davies KE, Winder SJ, Wiche G. 2007. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J Cell Biol 176:965–977. doi: 10.1083/jcb.200604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin PT, Kaufman SJ, Kramer RH, Sanes JR. 1996. Synaptic integrins in developing, adult, and mutant muscle: selective association of alpha1, alpha7A, and alpha7B integrins with the neuromuscular junction. Dev Biol 174:125–139. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- 34.Stocksley MA, Chakkalakal JV, Bradford A, Miura P, De Repentigny Y, Kothary R, Jasmin BJ. 2005. A 1.3 kb promoter fragment confers spatial and temporal expression of utrophin A mRNA in mouse skeletal muscle fibers. Neuromuscul Disord 15:437–449. doi: 10.1016/j.nmd.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. 1992. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem 267:6205–6212. [PubMed] [Google Scholar]
- 36.Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. 2008. Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands. Cancer Sci 99:214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higashiyama S, Nanba D. 2005. ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim Biophys Acta 1751:110–117. doi: 10.1016/j.bbapap.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. 1991. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 39.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. 2015. Proteomics. Tissue-based map of the human proteome. Science 347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 40.Tsunoda T, Takagi T. 1999. Estimating transcription factor bindability on DNA. Bioinformatics 15:622–630. doi: 10.1093/bioinformatics/15.7.622. [DOI] [PubMed] [Google Scholar]
- 41.Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, Wilson JM, Sweeney HL. 2008. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther 19:1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Zheng H, Wang B, Qiao C, Howard JF Jr, Xiao X. 2010. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther 18:1501–1508. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao C, Yuan Z, Li J, Tang R, Li J, Xiao X. 2012. Single tyrosine mutation in AAV8 and AAV9 capsids is insufficient to enhance gene delivery to skeletal muscle and heart. Hum Gene Ther Methods 23:29–37. doi: 10.1089/hgtb.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Qiao C, Bogan J, Bogan D, Tang R, Li JB, Chen C, Zheng H, Dow J, Kornegay J, Xiao X. 2011. Efficient long-term bodywide expression of an AAV9-minidystrophin in the muscle and heart of young adult GRMD dogs after intravascular injection without immune suppression. Mol Ther 19:S21. [Google Scholar]
- 45.Singhal N, Martin PT. 2011. Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol 71:982–1005. doi: 10.1002/dneu.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singhal N, Xu R, Martin PT. 2012. Distinct contributions of Galgt1 and Galgt2 to carbohydrate expression and function at the mouse neuromuscular junction. Mol Cell Neurosci 51:112–126. doi: 10.1016/j.mcn.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin PT, Xu R, Rodino-Klapac LR, Oglesbay E, Camboni M, Montgomery CL, Shontz K, Chicoine LG, Clark KR, Sahenk Z, Mendell JR, Janssen PM. 2009. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am J Physiol Cell Physiol 296:C476–C488. doi: 10.1152/ajpcell.00456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu R, Camboni M, Martin PT. 2007. Postnatal overexpression of the CT GalNAc transferase inhibits muscular dystrophy in mdx mice without altering muscle growth or neuromuscular development: evidence for a utrophin-independent mechanism. Neuromuscul Disord 17:209–220. doi: 10.1016/j.nmd.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu R, Jia Y, Zygmunt DA, Cramer ML, Crowe KE, Shao G, Maki AE, Guggenheim HN, Hood BC, Griffin DA, Peterson E, Bolon B, Cheatham JP, Cheatham SL, Flanigan KM, Rodino-Klapac LR, Chicoine LG, Martin PT. 2018. An isolated limb infusion method allows for broad distribution of rAAVrh74.MCK.GALGT2 to leg skeletal muscles in the Rhesus macaque. Mol Ther Methods Clin Dev 10:89–104. doi: 10.1016/j.omtm.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raith M, Valencia RG, Fischer I, Orthofer M, Penninger JM, Spuler S, Rezniczek GA, Wiche G. 2013. Linking cytoarchitecture to metabolism: sarcolemma-associated plectin affects glucose uptake by destabilizing microtubule networks in mdx myofibers. Skelet Muscle 3:14. doi: 10.1186/2044-5040-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfendner E, Rouan F, Uitto J. 2005. Progress in epidermolysis bullosa: the phenotypic spectrum of plectin mutations. Exp Dermatol 14:241–249. doi: 10.1111/j.0906-6705.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 52.Patton BL, Connoll AM, Martin PT, Cunningham JM, Mehta S, Pestronk A, Miner JH, Sanes JR. 1999. Distribution of ten laminin chains in dystrophic and regenerating muscles. Neuromuscul Disord 9:423–433. doi: 10.1016/S0960-8966(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 53.Heller KN, Montgomery CL, Janssen PM, Clark KR, Mendell JR, Rodino-Klapac LR. 2013. AAV-mediated overexpression of human alpha7 integrin leads to histological and functional improvement in dystrophic mice. Mol Ther 21:520–525. doi: 10.1038/mt.2012.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo SA, Zhu X, Marchionni MA, Burden SJ. 1995. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature 373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- 55.Schaeffer L, de Kerchove d'Exaerde A, Changeux JP. 2001. Targeting transcription to the neuromuscular synapse. Neuron 31:15–22. doi: 10.1016/S0896-6273(01)00353-1. [DOI] [PubMed] [Google Scholar]
- 56.Burden SJ. 1993. Synapse-specific gene expression. Trends Genet 9:12–16. doi: 10.1016/0168-9525(93)90066-Q. [DOI] [PubMed] [Google Scholar]
- 57.Roskoski R., Jr. 2014. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res 79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Singh B, Carpenter G, Coffey RJ. 2016. EGF receptor ligands: recent advances. F1000Res 5:2270. doi: 10.12688/f1000research.9025.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu X, Lai C, Thomas S, Burden SJ. 1995. Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J 14:5842–5848. doi: 10.1002/j.1460-2075.1995.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moscoso LM, Chu GC, Gautam M, Noakes PG, Merlie JP, Sanes JR. 1995. Synapse-associated expression of an acetylcholine receptor-inducing protein, ARIA/heregulin, and its putative receptors, ErbB2 and ErbB3, in developing mammalian muscle. Dev Biol 172:158–169. doi: 10.1006/dbio.1995.0012. [DOI] [PubMed] [Google Scholar]
- 61.Yamazaki S, Iwamoto R, Saeki K, Asakura M, Takashima S, Yamazaki A, Kimura R, Mizushima H, Moribe H, Higashiyama S, Endoh M, Kaneda Y, Takagi S, Itami S, Takeda N, Yamada G, Mekada E. 2003. Mice with defects in HB-EGF ectodomain shedding show severe developmental abnormalities. J Cell Biol 163:469–475. doi: 10.1083/jcb.200307035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kronqvist P, Kawaguchi N, Albrechtsen R, Xu X, Schroder HD, Moghadaszadeh B, Nielsen FC, Frohlich C, Engvall E, Wewer UM. 2002. ADAM12 alleviates the skeletal muscle pathology in mdx dystrophic mice. Am J Pathol 161:1535–1540. doi: 10.1016/S0002-9440(10)64431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moghadaszadeh B, Albrechtsen R, Guo LT, Zaik M, Kawaguchi N, Borup RH, Kronqvist P, Schroder HD, Davies KE, Voit T, Nielsen FC, Engvall E, Wewer UM. 2003. Compensation for dystrophin-deficiency: ADAM12 overexpression in skeletal muscle results in increased alpha 7 integrin, utrophin and associated glycoproteins. Hum Mol Genet 12:2467–2479. doi: 10.1093/hmg/ddg264. [DOI] [PubMed] [Google Scholar]
- 64.Conzelmann A, Kornfeld S. 1984. A murine cytotoxic T lymphocyte cell line resistant to Vicia villosa lectin is deficient in UDP-GalNAc:beta-galactose beta 1,4-N-acetylgalactosaminyltransferase. J Biol Chem 259:12536–12542. [PubMed] [Google Scholar]
- 65.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. 2003. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J 22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, Nanba D, Higashiyama S, Hori M, Klagsbrun M, Mekada E. 2003. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci U S A 100:3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, Asanuma H, Sanada S, Matsumura Y, Takeda H, Beppu S, Tada M, Hori M, Higashiyama S. 2002. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med 8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 68.Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW. 2004. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res 64:5283–5290. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- 69.Lim HJ, Dey SK. 2009. HB-EGF: a unique mediator of embryo-uterine interactions during implantation. Exp Cell Res 315:619–626. doi: 10.1016/j.yexcr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. 1994. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120:1071–1083. [DOI] [PubMed] [Google Scholar]
- 71.Jessmon P, Leach RE, Armant DR. 2009. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev 76:1116–1127. doi: 10.1002/mrd.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oyagi A, Hara H. 2012. Essential roles of heparin-binding epidermal growth factor-like growth factor in the brain. CNS Neurosci Ther 18:803–810. doi: 10.1111/j.1755-5949.2012.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oyagi A, Moriguchi S, Nitta A, Murata K, Oida Y, Tsuruma K, Shimazawa M, Fukunaga K, Hara H. 2011. Heparin-binding EGF-like growth factor is required for synaptic plasticity and memory formation. Brain Res 1419:97–104. doi: 10.1016/j.brainres.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Guo Y, Ding Q, Chen L, Ji C, Hao H, Wang J, Qi W, Xie X, Ma J, Li A, Jiang X, Li X, Jiang H. 2017. Overexpression of heparin-binding epidermal growth factor-like growth factor mediates liver fibrosis in transgenic mice. Am J Med Sci 354:199–210. doi: 10.1016/j.amjms.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Huang G, Besner GE, Brigstock DR. 2012. Heparin-binding epidermal growth factor-like growth factor suppresses experimental liver fibrosis in mice. Lab Investig 92:703–712. doi: 10.1038/labinvest.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Means AL, Ray KC, Singh AB, Washington MK, Whitehead RH, Harris RC Jr, Wright CV, Coffey RJ Jr, Leach SD. 2003. Overexpression of heparin-binding EGF-like growth factor in mouse pancreas results in fibrosis and epithelial metaplasia. Gastroenterology 124:1020–1036. doi: 10.1053/gast.2003.50150. [DOI] [PubMed] [Google Scholar]
- 77.Lian H, Ma Y, Feng J, Dong W, Yang Q, Lu D, Zhang L. 2012. Heparin-binding EGF-like growth factor induces heart interstitial fibrosis via an Akt/mTor/p70s6k pathway. PLoS One 7:e44946. doi: 10.1371/journal.pone.0044946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu X, Radulescu A, Zorko N, Besner GE. 2009. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology 137:221–230. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radulescu A, Zorko NA, Yu X, Besner GE. 2009. Preclinical neonatal rat studies of heparin-binding EGF-like growth factor in protection of the intestines from necrotizing enterocolitis. Pediatr Res 65:437–442. doi: 10.1203/PDR.0b013e3181994fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen CL, Yu X, James IO, Zhang HY, Yang J, Radulescu A, Zhou Y, Besner GE. 2012. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Investig 92:331–344. doi: 10.1038/labinvest.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng J, El-Assal ON, Besner GE. 2005. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg 14:167–174. doi: 10.1053/j.sempedsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 82.El-Assal ON, Radulescu A, Besner GE. 2007. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery 142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Dall'Olio F, Malagolini N, Chiricolo M, Trinchera M, Harduin-Lepers A. 2014. The expanding roles of the Sd(a)/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim Biophys Acta 1840:443–453. doi: 10.1016/j.bbagen.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 84.Mohlke KL, Purkayastha AA, Westrick RJ, Smith PL, Petryniak B, Lowe JB, Ginsburg D. 1999. Mvwf, a dominant modifier of murine von Willebrand factor, results from altered lineage-specific expression of a glycosyltransferase. Cell 96:111–120. doi: 10.1016/S0092-8674(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 85.Kawamura YI, Kawashima R, Fukunaga R, Hirai K, Toyama-Sorimachi N, Tokuhara M, Shimizu T, Dohi T. 2005. Introduction of Sd(a) carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res 65:6220–6227. doi: 10.1158/0008-5472.CAN-05-0639. [DOI] [PubMed] [Google Scholar]
- 86.Taylor SR, Markesbery MG, Harding PA. 2014. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) and proteolytic processing by a disintegrin and metalloproteinases (ADAM): a regulator of several pathways. Semin Cell Dev Biol 28:22–30. doi: 10.1016/j.semcdb.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 87.Tang H, Veldman MB, Goldman D. 2006. Characterization of a muscle-specific enhancer in human MuSK promoter reveals the essential role of myogenin in controlling activity-dependent gene regulation. J Biol Chem 281:3943–3953. doi: 10.1074/jbc.M511317200. [DOI] [PubMed] [Google Scholar]
- 88.Tang H, Goldman D. 2006. Activity-dependent gene regulation in skeletal muscle is mediated by a histone deacetylase (HDAC)-Dach2-myogenin signal transduction cascade. Proc Natl Acad Sci U S A 103:16977–16982. doi: 10.1073/pnas.0601565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang H, Macpherson P, Marvin M, Meadows E, Klein WH, Yang XJ, Goldman D. 2009. A histone deacetylase 4/myogenin positive feedback loop coordinates denervation-dependent gene induction and suppression. Mol Biol Cell 20:1120–1131. doi: 10.1091/mbc.e08-07-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang H, Macpherson P, Argetsinger LS, Cieslak D, Suhr ST, Carter-Su C, Goldman D. 2004. CaM kinase II-dependent phosphorylation of myogenin contributes to activity-dependent suppression of nAChR gene expression in developing rat myotubes. Cell Signal 16:551–563. doi: 10.1016/j.cellsig.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 91.Mejat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L. 2005. Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat Neurosci 8:313–321. doi: 10.1038/nn1408. [DOI] [PubMed] [Google Scholar]
- 92.Cohen TJ, Waddell DS, Barrientos T, Lu Z, Feng G, Cox GA, Bodine SC, Yao TP. 2007. The histone deacetylase HDAC4 connects neural activity to muscle transcriptional reprogramming. J Biol Chem 282:33752–33759. doi: 10.1074/jbc.M706268200. [DOI] [PubMed] [Google Scholar]
- 93.Dohi T, Kawamura YI. 2008. Incomplete synthesis of the Sda/Cad blood group carbohydrate in gastrointestinal cancer. Biochim Biophys Acta 1780:467–471. doi: 10.1016/j.bbagen.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 94.Kawamura YI, Toyota M, Kawashima R, Hagiwara T, Suzuki H, Imai K, Shinomura Y, Tokino T, Kannagi R, Dohi T. 2008. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology 135:142–151. doi: 10.1053/j.gastro.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 95.McCarty DM. 2008. Self-complementary AAV vectors; advances and applications. Mol Ther 16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 96.Xiao X, Li J, Samulski RJ. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 72:2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clark KR, Liu X, McGrath JP, Johnson PR. 1999. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther 10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- 98.Abraham JA, Damm D, Bajardi A, Miller J, Klagsbrun M, Ezekowitz RA. 1993. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem Biophys Res Commun 190:125–133. doi: 10.1006/bbrc.1993.1020. [DOI] [PubMed] [Google Scholar]
- 99.Hemsley A, Arnheim N, Toney MD, Cortopassi G, Galas DJ. 1989. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res 17:6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cramer ML, Shao G, Rodino-Klapac LR, Chicoine LG, Martin PT. 2017. Induction of T-cell infiltration and programmed death ligand 2 expression by adeno-associated virus in rhesus macaque skeletal muscle and modulation by prednisone. Hum Gene Ther 28:493–509. doi: 10.1089/hum.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 102.Parkhomovskiy N, Kammesheidt A, Martin PT. 2000. N-acetyllactosamine and the CT carbohydrate antigen mediate agrin-dependent activation of MuSK and acetylcholine receptor clustering in skeletal muscle. Mol Cell Neurosci 15:380–397. doi: 10.1006/mcne.2000.0835. [DOI] [PubMed] [Google Scholar]