Abstract
Sickle cell anaemia is a hereditary disease branded by an upsurge in generation of ROS, irregular iron release and little or no antioxidant activity which can lead to cellular injuries due to oxidative stress resulting in severe symptoms including anaemia and pain. The disease is caused by a mutated version of the gene that helps make haemoglobin, the protein that carries oxygen in red blood cells. We used in silico and in vitro experiments to examine the antisickling effects of rutin for the first time by means of before and after induction approaches in sickle erythrocytes. Rutin was docked against deoxy-haemoglobin and 2,3-bisphosphoglycerate mutase, revealing binding energies (-27.329 and -25.614 kcal/mol) and Ki of 0.989μM and 0.990 μM at their catalytic sites through strong hydrophobic and hydrogen bond interactions. Sickling was thereafter, induced at 3 h with 2% metabisulphite. Rutin prevented sickling maximally at 12.3μM and reversed same at 16.4μM, by 78.5% and 69.9%, one-to-one. Treatment with rutin significantly (P < 0.05) reinvented the integrity of erythrocytes membrane as evident from the practical % haemolysis compared to induced erythrocytes. Rutin also significantly (P < 0.05) prevented and reversed lipid peroxidation relative to untreated. Likewise, GSH, CAT levels were observed to significantly (P < 0.05) increase with concomitant significant (P < 0.05) decrease in SOD activity based on administration of rutin after sickling induction approach. Furthermore, FTIR results showed that treatment with rutin favourably altered the functional chemistry, umpiring from shifts and functional groups observed. It can thus be deduced that, antisickling effects of rutin may be associated with modulation of deoxy-haemoglobin, 2,3-bisphosphoglycerate mutase, alteration of redox homeostasis and functional chemistry of sickle erythrocytes.
Keywords: Cell biology, Rutin, Antisickling, Redox, Erythrocytes, Haemoglobin
1. Introduction
Sickle cell disease (SCD) is the most prevalent hereditary haematological disease in the world (Wastnedge et al., 2018). It is a disease caused by a mutated version of the gene that helps make haemoglobin, a protein that carries oxygen in red blood cells. Equally, the most common genetic cause of morbidity and mortality in Africa with a higher proportion of cases in Sub-Sahara Africa (Bello-Manga et al., 2016). In Nigeria, which has a population of about 170 million, it is estimated that about 20–30% of the population are carriers of the sickle cell trait while about 2–3% of the population have SCD (Adewoyin, 2015). The challenges associated with SCD made the World Health Organization (WHO) to declare Sickle Cell Anaemia a public health priority (Makani et al., 2013). It has been reported that poor nutrition, inadequate health care facilities and high orthodox treatment costs, are factors that further exacerbate anaemia in children with SCD (Tluway et al., 2018).
Sickle cell disease (SCD) is a condition associated with a number of disorders due to a mutation in a single nucleotide in the beta globin gene resulting in sickle shaped haemoglobin (Makani et al., 2013). Sickle shaped haemoglobin has relatively lower capacity for oxygen supply to tissues, causing damage to vascular endothelial cells resulting in altered vascular tone and vascular adhesion (Sedrak and Kondamudi, 2018). The capacity of red cells to transport oxygen from lungs to tissues is particularly dependent on the reversible binding of oxygen to Fe (II) haemoglobin (Forget and Bunn, 2013).
Any condition that affects the autoxidation of haemoglobin, results in increased generation of superoxide radical, which is more pronounced at lower oxygen pressures (Rifkind et al., 2013). Increased generation of the superoxide radical can enhance the generation and levels of other reactive oxygen species like peroxyl radical which is the predominant free radical generated in the human body, eventually causing oxidative stress (Oztas and Yalcinkaya, 2017). The increased autoxidation rate of sickle haemoglobin thus has a two-face impact on SCD pathogenesis: oxidative damage resulting in ischemia-reperfusion injury and depleted antioxidant capacity of circulating erythrocytes (Oztas and Yalcinkaya, 2017).
Another factor that contributes to increased polymerization of sickle haemoglobin, is the elevated levels of the molecule 2,3-bisphosphoglycerate (2,3-BPG), which under normal conditions, binds to deoxy-haemoglobin, thereby stabilizing it and reducing oxygen affinity (Oslund et al., 2017). However, in SCD, the binding of 2,3-BPG to deoxy-Hb S decreases the solubility of deoxy-Hb S (Poillon et al., 1995). Therefore, an agent that reduces the level and activity of 2,3-BPG mutase in SCD patients, should have anti-sickling effects by ameliorating the vaso-occlusive severity seen in SCD (Poillon et al., 1995).
Rutin (quercetin-3-rhamnosyl glucoside) is a natural flavone derivative discovered as early as the 19th century in buckwheat, but has been found to be present in other fruits and vegetables (Yang et al., 2008). It is a low molecular weight polyphenolic compound that has been studied for its antioxidant properties in relation to human health and disease prevention (Cristina Marcarini et al., 2011; Yang et al., 2008). Recently, from our laboratory we reported the antioxidant, anticlastogenic and DNA-protective effects of rutin at in vitro and in vivo levels (Muhammad et al., 2018, 2015). It has also been shown to be protective against ROS-induced oxidative stress and inflammation (Nafees et al., 2015) which are conditions implicated in SCD. It has also been reported to exert antiplatelet effects as well as strengthening the capillaries of blood vessels (Guo et al., 2007) which are properties that could be beneficial in protecting against endothelial damage due to oxidative stress in SCD. Even though recent studies (Henneberg et al., 2013) have shown that rutin as an antioxidant, protects against damage caused by excess reactive oxygen species characteristic of sickle cell anaemia treated with hydroxyurea, which suggests an additional protective effect associated with the use of flavonoids, however, the work was basically on protection and/or amelioration not on reversal against metabisulphite-induced sickling which forms the central dynamics of this study. The possible role of deoxygenated haemoglobin and that of 2,3-bisphosphoglycerate mutase were also not captured or highlighted in their studies (Henneberg et al., 2013). Furthermore, more studies (Cesquini et al., 2003) has revealed in a comparative passion that quercetin protect against sickling much better than rutin, probably due to their diverse physic-chemical properties, however, a study on before and after induction effects of rutin as well as mechanism of action related to antisickling is still inadequately addressed. Therefore, this study examined the antisickling effects of rutin for the first time by means of before and after induction approaches in sickle erythrocytes. The answers from this study will go a long way in adding to the prevailing knowledge of discovering natural products in combating and managing SCD and its metabolic complications.
2. Materials and methods
2.1. Chemicals and reagents
Rutin (610.517 g/mol, ≥94% purity) was synthetic and purchased from Sigma Chemical Co (St. Louis, MO, USA). All other chemicals/reagents used were of analytical grade until otherwise stated.
2.2. Molecular docking
The structure of a ligand; rutin (5280805) was retrieved from major ligand data base, PubChem. A PDB format of the target proteins; deoxygenated haemoglobin (PDB ID: 3WCU) and 2,3-bisphophoglycerate mutase (PDB ID: 3NFY) were gotten from protein data bank. The proteins preparation and energy minimization were done using Discovery studio 2.5v. The CHARMm-based DOCKER program was used to score the interaction between the proteins and the ligand (rutin) into the crystal structure of the receptors binding pockets. The PDB files of the best docked conformation were generated using Accerlrys discovery studio and, were imported into Chimera 1.1v to view the hydrophobic and hydrophilic interactions. The binding energies and kinetic inhibition constants were equally calculated.
3. Experimental
3.1. Ethics
All experiments were carried out under the ethical approval of ABUTH Scientific and Health Research Ethics Committee (ABUTH/HREC/UG/6), in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
3.2. Blood collection
About 5 mL of blood was collected humanely by venepuncture from seven (7) confirmed people living with sickle cell anaemia who were not in crises at Haematology Department, of Ahmadu Bello University Teaching Hospital (ABUTH), Zaria, Kaduna State, Nigeria. They were aged between 19 and 32 years of both sexes, and in reasonable stable condition. They were informed and provided their consent prior to the experiment. Blood was collected in sodium ethylenediaminetetraacetic acid bottles, and the content carefully mixed by gently rolling the bottle. Notwithstanding, all experiments were carried-out with freshly collected blood samples within 24 h of collection at 4 °C.
3.3. Extraction of erythrocytes
The obtained homozygous HbS blood samples were spun at 2000 ×g for 10 min. The resultant plasma was cast-off to obtain the erythrocytes. The erythrocytes were then suspended in phosphate buffered saline (PBS), spun and the supernatant discarded. This was repeated thrice, after which the washed erythrocytes were resuspended in PBS and immediately used for assays.
3.4. Induction of sickling, antisickling and osmotic fragility assays
Exactly 0.1mL of the washed erythrocytes were mixed with 0.1mL of freshly prepared 2% sodium metabisulphite in a clean test tube and thereafter, incubated in water-bath at 37 °C for 3 h. A drop of the mixture was then smeared on a microscope slide, fixed with 95% methanol, dried and stained with 5% Giemsa (pH 7.2) for 30 min (Egunyomi et al., 2009). It was then examined under a microscope (×100 magnification). Aliquots were taken at 30 min interval for up to 3 h. The counting of cells was carried out by viewing from different fields (5 Fields) across the slide. The numbers of both sickle and unsickle cells were counted and the percentages of cells unsickle determined using the formula:
| (%) Unsickle = Number of unsickle cells × 100/Total number of cells |
For antisickling assay using rutin at different concentrations of 4.1, 8.2, 12.3, 16.4 and 20.5μM, the above protocol was also adopted for before and after induction approaches. The before induction approach involved treating the cells with the said concentrations before induction using 2% sodium metabisulphite and vice-versa for after induction approach. Equally, osmotic fragility (haemolysis) test was conducted against after selecting the best concentration as previously described (Kraus et al., 1997; Muhammad et al., 2016). Briefly, dilutions with equal volumes of erythrocyte and phosphate buffer saline (pH 7.4) were made. This was followed by successive addition of 100 μL of rutin to the experimental tubes excluding control in which phosphate buffer saline was added. Contents of experimental tubes were mildly shaken and thereafter, allowed for 4 h incubation at 37 °C. Then, samples were spun at 2000 ×g for 5 min and absorbance determined at 540 nm. Subsequently, the same concentration with best antisickling effect was also used for redox and FTIR analyses respectively. A single control which consisted of induced sickle cells with 2% sodium metabisulphite was employed in this bioassay.
3.5. Evaluation of rutin for redox sensitive biomarkers in sickle erythrocytes
The effective antisickling concentrations (12.3μM for before and 16.4μM for after induction studies) were incubated with 100 μL sickle erythrocytes for 3 h at 37 °C as previously described in the above section (Muhammad et al., 2016). Thereafter, the cells were spun and the supernatant assayed for reduced glutathione (GSH) (Ellman, 1959) lipid peroxidation (Chowdhury and Soulsby, 2002), SOD (Kakkar et al., 1984) and catalase levels (Chance and Maehly, 1955).
3.6. Fourier transform-infrared (FTIR) spectroscopy analysis of treated sickle erythrocytes
The effective antisickling concentrations (12.3μM for before and 16.4μM for after induction studies) were incubated with 100 μL sickle erythrocytes for 3 h at 37 °C as previously described in the above section, while adopting the methods of Erukainure et al. (2017). After treatment, the samples were dehydrated under pressure and sandwiched between potassium bromide. They were then perused on FTIR spectrophotometer at room temperature (250C–28 °C) at 1000–3500 cm−1 spectral range. The peak heights were utilised in identifying the functional groups present by relating to the IR spectroscopy correlation table.
3.7. Statistical analysis
The experiments were repeated at least thrice. Where appropriate, the results were presented as mean ± standard deviation (SD). The data were analysed using student t-test with the aid of Statistical Package for Social Sciences (SPSS) software, SPSS Inc., Chicago, Standard version 20.00. P < 0.05 was considered statistically significant for differences in means using Dunnett post hoc test.
4. Results
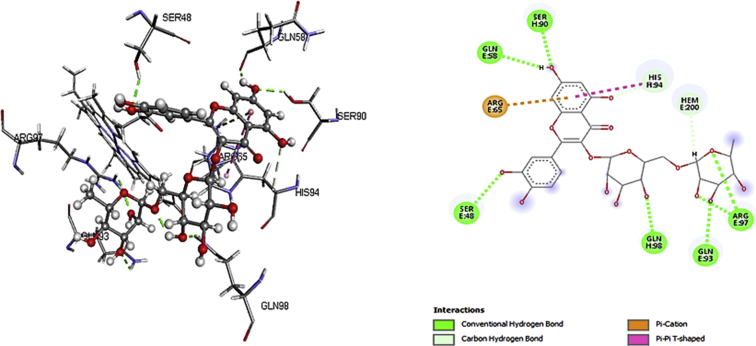
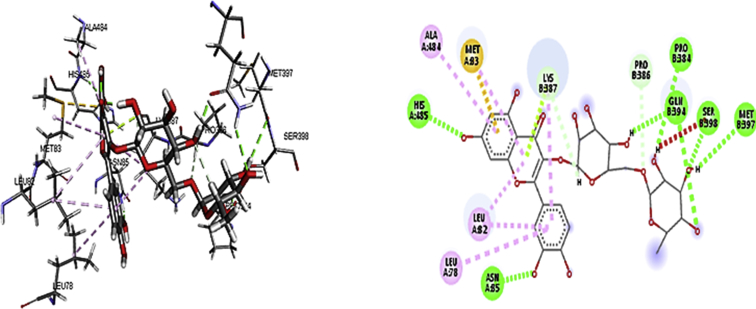
Findings from molecular docking demonstrate that rutin was able to securely dock with robust binding affinity into the active spot of the receptors; deoxygenated haemoglobin and 2,3-bisphosphoglycerate mutase (Tables 1 and 2), suggestive of a probable impounded and inhibitory properties via hydrogen and hydrophobic interactions (Figs. 1 and 2). This may as well signify the facts that rutin could have a sequestrating effects against these biomolecules.
Table 1.
The docking score (kcal/mol) and inhibition constant (μM) of rutin at the binding site of deoxygenated haemoglobin.
| Name | Score | Ki | Hydrogen bond | Hydrophobic interaction |
|---|---|---|---|---|
| Rutin | -27.329 | 0.989 | -14.283 | -3.894 |
Table 2.
The docking score (kcal/mol) and inhibition constant (μM) of rutin at the binding site of 2,3-bisphosphoglycerate mutase.
| Name | Score | Ki | Hydrogen bond | Hydrophobic interaction |
|---|---|---|---|---|
| Rutin | -25.614 | 0.990 | -8.59 | -4.50 |
Fig. 1.
3D and 2D binding interactions of rutin with deoxygenated haemoglobin.
Fig. 2.
3D and 2D binding interaction of rutin with 2, 3-bisphophoglycerate mutase.
Sequel to the predictions based on docking that rutin may actually control deoxygenated haemoglobin and 2,3-bisphosphoglycerate mutase via strong hydrophobic and hydrophilic interactions, we advanced with antisickling studies by means of before and after induction approaches. To achieve that, 2% metabisulphite was ab initio used to induce sickling because the said blood donors were not in crises as at the time of collection. From Table 3, we were able to outstandingly induce sickling at 3h by 74.2%. Essentially, 3h was adopted for our supplementary assays. Remarkably, rutin administration was able to prevent sickling maximally at 12.3μM (Table 4) and reversed same at 16.4μM (Table 5), by 78.5% and 69.9%, respectively (Fig. 3). Thus, 12.3μM and 16.4μM were selected for further analyses since the observed sickling-preventive effects were not dose-dependent. By inference, IC50 calculation is not essential instead we used the best concentrations.
Table 3.
Percentage sickling due to 2% sodium metabisulphite.
| Time interval | Sickle cells | Unsickle cells | Total cells | % Sickle | %Unsickle |
|---|---|---|---|---|---|
| 30 min. | 47.0 | 95.0 | 142.0 | 33.1 | 66.9 |
| 1 h | 56.0 | 33.0 | 89.0 | 62.9 | 37.1 |
| 1h 30 min. | 21.0 | 27.0 | 48.0 | 43.8 | 56.2 |
| 2 h | 227.0 | 160.0 | 387.0 | 58.7 | 41.3 |
| 2h 30 min. | 58.0 | 82.0 | 140.0 | 41.4 | 58.6 |
| 3 h | 132.0 | 46.0 | 178.0 | 74.2 | 25.8 |
Table 4.
Antisickling effect of rutin using before induction approach.
| Concentrations (μM) | Sickle cells | Unsickle cells | Total cells | % Sickle | %Unsickle |
|---|---|---|---|---|---|
| 4.1 | 120.0 | 201.0 | 321.0 | 37.4 | 62.6 |
| 8.2 | 157.0 | 309.0 | 466.0 | 33.7 | 66.3 |
| 12.3 | 97.0 | 354.0 | 451.0 | 21.5 | 78.5 |
| 16.4 | 147.0 | 504.0 | 651.0 | 22.6 | 77.4 |
| 20.5 | 247.0 | 691.0 | 938.0 | 26.3 | 73.7 |
Table 5.
Antisickling effect of rutin using after induction approach.
| Concentrations (μM) | Sickle cells | Unsickle cells | Total cells | % sickle | %unsickle |
|---|---|---|---|---|---|
| 4.1 | 222.0 | 280.0 | 502.0 | 44.2 | 55.8 |
| 8.2 | 333.0 | 311.0 | 644.0 | 51.7 | 48.3 |
| 12.3 | 286.0 | 410.0 | 696.0 | 41.1 | 58.9 |
| 16.4 | 106.0 | 246.0 | 352.0 | 30.1 | 69.9 |
| 20.5 | 75.0 | 129.0 | 204.0 | 36.8 | 63.2 |
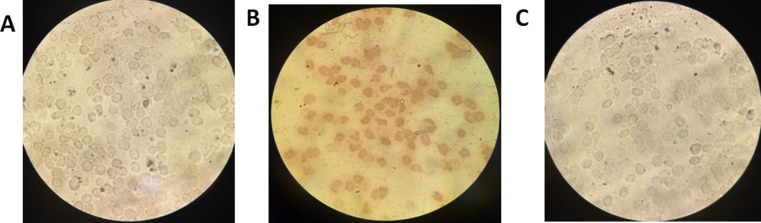
Fig. 3.
Comparative photomicrograph of induced only (A), before induction approach with 12.3μM rutin (B) and after induction approach with 16.4μM rutin (C). Stain: Giemsa, magnification ×100.
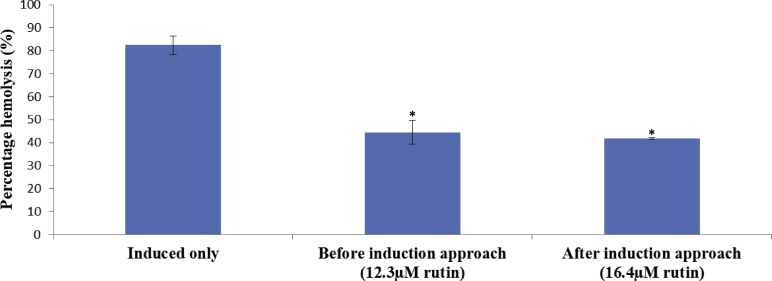
Membrane stability of erythrocytes is crucial to antisickling effects. As seen in Fig. 4, treatment with rutin significantly (P < 0.05) restored the integrity of erythrocytes membrane as evident from the observed % haemolysis relative to control (induced erythrocytes and without treatment) adjudicating from before and after induction approaches (Fig. 4).
Fig. 4.
Membrane stabilising effect of rutin on 2% metabisulphite-induced sickling in human erythrocytes. *statistically significant (P < 0.05) when compared with the induced only.
From Table 6, administration of rutin significantly (P < 0.05) prevented and reversed lipid peroxidation as evident from the level of MDA produced relative to control. The same trends were observed for GSH, CAT and SOD except that CAT and SOD activities were also significantly (P < 0.05) lowered relative to untreated, based on after induction approach.
Table 6.
Effect of rutin on redox sensitive biomarkers on sickle erythrocytes induced with 2% metabisulphite.
| Treatments | MDA (μM) | GSH (μg/mL) | CAT (U/mL) | SOD (U/mL) |
|---|---|---|---|---|
| Induced with 2% metabisulphite | 3.81 ± 0.02 | 199.15 ± 1.32 | 0.27 ± 0.03 | 2.99 ± 0.02 |
| Before induction Approach (12.3μM) | 0.87 ± 0.002∗ | 27.94 ± 1.35∗ | 0.69 ± 0.02∗ | 3.85 ± 0.09∗ |
| After induction Approach (16.4μM) | 2.19 ± 0.02∗ | 243.6 ± 1.39∗ | 0.13 ± 0.03∗ | 1.27 ± 0.08∗ |
statistically significant (P < 0.05) when compared with the induced.
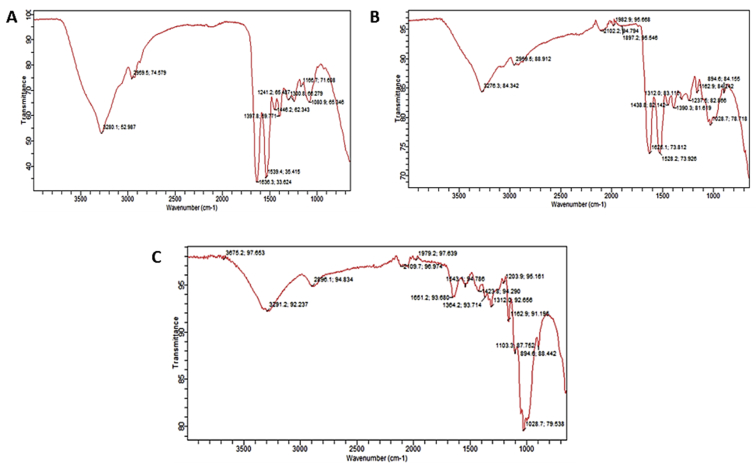
Based on the observed increase in lipid peroxidation and undesirable effects on the part of GSH, CAT and SOD vis-à-vis higher % haemolysis owing to sickling induction, it will be almost impossible that the three-dimensional structure of lipid bilayer and proteins could not be affected so also their functional chemistry. Judging from FTIR results (Tables 7, 8, and 9), it could be seen that there was a favourable alteration on the part of functional chemistry in terms of shifts (bend and stretches) (Fig. 5) and functional groups observed as compared to induced erythrocytes (Fig. 5).
Table 7.
FTIR results of induced with 2% metabisulphite/untreated sickle erythrocytes.
| Characteristic absorptions, cm−1 | Intensity, %T | Bond | Functional group |
|---|---|---|---|
| 3280.1 | 52.9 | N–H stretch | __CONH2; __CONH__ |
| 2959.5 | 74.5 | C–H stretch | C__H |
| 1397.8 | 59.7 | N=O bend | __R_NO2__ |
| 1636.3–1539.4 | 33.6–35.4 | N–H bend | __ CONH2 |
Table 8.
FTIR results of treated and induced using before induction approach (12.3μM rutin).
| Characteristic absorptions, cm−1 | Intensity, %T | Bond | Functional group |
|---|---|---|---|
| 3276.3 | 84.3 | N–H stretch | __CONH2; __CONH__ |
| 2959.5 | 92.1 | C __ H stretch | C__H |
| 1626.1–1528.2 | 73.8; 73.9 | N __ H bend | __NH2__ |
| 1028.7 | 78.7 | C __ O stretch | __ C__O__C__ |
Table 9.
FTIR results of induced and treated using after induction approach (16.4μM rutin).
| Characteristic absorptions, cm−1 | Intensity, %T | Bond | Functional group |
|---|---|---|---|
| 3291.2 | 92.2 | N–H stretch | __CONH2; __CONH__ |
| 2896.1 | 94.8 | C __ H stretch | C__H |
| 1651.2; 1543.1 | 93.0; 94 | N–H bend | __NH2 |
| 1028.7 | 79.5 | C __ O stretch | __ C__O__C__ |
Fig. 5.
Comparative FTIR peaks of induced only (A), before induction approach with 12.3μM rutin (B) and after induction approach with 16.4μM rutin (C).
5. Discussion
Sickle cell anaemia is a hereditary disease branded by an upsurge in generation of ROS, abnormal iron release and little or no antioxidant activity which can lead to cellular injuries due to oxidative stress (Cesquini et al., 2003). It is a monogenic, haematological and multi-organ ailment linked with progressive organ injury leading to chronic and acute illness (Pule et al., 2016). It is basically caused by a point mutation (A to T) in the sixth codon of the β-globin gene on chromosome 11, leading to substitution of the amino acid glutamic acid to valine (Pule et al., 2016). The resulting HbS leads to polymerization and precipitation of haemoglobin during deoxygenation or dehydration paving way for sickling exacerbated by higher level of 2,3-bisphosphoglycerate (Poillon et al., 1995), irregular adhesion of leukocytes and platelets, inflammation, hypercoagulation, haemolysis and hypoxia. This is in addition to microvascular impediment and in due course tissue damage (Mpiana et al., 2007; Imaga, 2013).
Utilization of natural products in efforts at inhibiting sickling may perhaps be as old as when the SCD was revealed (Egunyomi et al., 2009). This may as well serve as a foundation of a paradigm shift from synthetic drugs to medicinal plants which are naturally endowed with bioactive compounds harbouring strong biochemical effects including antisickling (Imaga, 2013). An example of such compounds is rutin present in buckwheat, fruits and vegetables (Yang et al., 2008). These sources are of course readily available within and around our microenvironment for consumption. In this communication here in, we examined the antisickling effects of rutin for the first time by means of before and after induction approaches in sickle erythrocytes, while using in silico and in vitro models.
Generation of deoxygenated haemoglobin and 2,3-bisphosphoglycerate mutase activity are some of the dynamics of erythrocytes metabolism likewise in SCD. Based on molecular docking conducted with rutin on deoxy-haemoglobin and 2,3-bisphophoglycerate mutase, a strong chemical interaction that successfully bind with the receptors occupying their catalytic sites was observed. Biochemical increase in deoxy-haemoglobin and 2,3-bisphophoglycerate mutase levels have been implicated in SCD (Imaga, 2013; Poillon et al., 1995; Rowley et al., 2014). Thus, targeting these proteins as clearly discovered from our in-silico studies might provide a viable strategy in combating and managing SCD in addition to offering a sign on the possible antisickling effects of rutin. This can be rationally supported based on the results from our previous study that flavonoids undoubtedly had a strong attraction to proteins via hydrophobic and hydrophilic interactions (Babangida et al., 2018). By implication, this might have exposed rutin's predisposition to allosterically bind to haemoglobin leading to possible adjustments on oxygen's affinity to haemoglobin thereby increasing cooperativity, favouring oxygenation buttressed by the observed sequestrating effects on 2,3-bisphophoglycerate mutase.
Interestingly, pre and post-treatment with rutin decreased the number of sickle erythrocytes signifying a shielding and restorative role of rutin against sickling. Arguably, the sickling process in SCD patients is characterized by unceasing polymerization of erythrocytes due to oxidative stress in addition to insufficient oxygen supply distinctive of sickle erythrocytes (Erukainure et al., 2017; Imaga, 2013; Poillon et al., 1995). The antioxidant properties of rutin can help defend against oxidative stress that further enhances the stability of erythrocyte membranes (Muhammad et al., 2018, 2015; Nafees et al., 2015). This come to an agreement with previous studies which showed that treatment with rutin frustrated oxidative stress levels in treated experiments relative to non-treated controls (Muhammad et al., 2018). Changes in the functional chemistry may as well be connected with membrane stabilizing effects of rutin currently observed bearing in mind that, erythrocytes akin to other cells composed of lipids, proteins and carbohydrates at membranous and non-membranous levels. Rutin has also been reported to have high bioavailability that enhances its antioxidant properties as currently observed based on the decreased in lipid peroxidation and increased in GSH, CAT and SOD (Muhammad et al., 2018). Equally, treatment with rutin has been reportedly associated with anti-inflammatory and protein stability effects which may be beneficial in battling SCD associated oxidative stress, inflammation and decreased haemoglobin S stability (Nafees et al., 2015). This can ease and conceivably converses excruciating vaso-occlusive actions characterized by endothelial dysfunction and initiation of inflammatory and coagulation pathway lately designated in SCD (Erukainure et al., 2017; Macharia et al., 2018; Sani et al., 2015).
6. Conclusion
The antisickling effects of rutin may be associated with modulation of deoxy-haemoglobin, 2,3-bisphosphoglycerate mutase, and changes in functional chemistry of sickle erythrocytes. Future studies are needed to understand whether rutin or its derivatives/metabolites (such as glucuronide/sulfate metabolites of rutin and quercetin) are the really effectors. Equally, studies are needed to measure the levels of deoxy-haemoglobin, 2,3-bisphosphoglycerate mutase in vitro vis-à-vis an in vivo contribution of rutin in SCD models. Similarly, studies on rutin possible toxicological effects and rutin-rich dietary intervention in experimental animal and people living with sickle cell anaemia would be necessary.
Declarations
Author contribution statement
Aliyu Muhammad: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Aliyu Dahiru Waziri: Contributed reagents, materials, analysis tools or data.
Gilead Ebiegberi Forcados, Babangida Sanusi: Conceived and designed the experiments; Wrote the paper.
Hadiza Sani, Ibrahim Malami, Ibrahim Babangida Abubakar, Hafsat Abdullahi Mohammed: Analyzed and interpreted the data.
Habeebah Yahya Oluwatoyin: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Otaru Abdulrasheed Adinoyi: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the advice and assistance of Mrs. Zeenat Bello Kudan and Miss Funmi Audu E. from the Department of Biochemistry, Ahmadu Bello University, Zaria, towards the successful completion of this work. We equally appreciate Mrs. Salamatu Sani of the Department of English and literary studies, Ahmadu Bello University, Zaria for improving manuscript's quality.
References
- Adewoyin A.S. Management of sickle cell disease: a review for physician education in Nigeria (sub-saharan Africa) Anemia. 2015:791498. doi: 10.1155/2015/791498. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babangida S., Ibrahim S., Muhammad A., Arthur D.E., Uzairu A., Garba A. The role of molecular modelling strategies in validating the effects of chrysin on sodium arsenite-induced chromosomal and DNA damage. Hum. Exp. Toxicol. 2018:1–11. doi: 10.1177/0960327117751233. [DOI] [PubMed] [Google Scholar]
- Bello-Manga H., DeBaun M.R., Kassim A.A. Epidemiology and treatment of relative anemia in children with sickle cell disease in sub-Saharan Africa. Expert Rev. Hematol. 2016;9:1031–1042. doi: 10.1080/17474086.2016.1240612. [DOI] [PubMed] [Google Scholar]
- Cesquini M., Torsoni M.A., Stoppa G.R., Ogo S.H. t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed. Pharmacother. 2003;57:124–129. doi: 10.1016/s0753-3322(03)00018-0. [DOI] [PubMed] [Google Scholar]
- Chance B., Maehly A.C. Assays of catalases and peroxidases. Methods Enzymol. 1955 doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Chowdhury P., Soulsby M. Lipid peroxidation in rat brain is increased by simulated weightlessness and decreased by a soy-protein diet. Ann. Clin. Lab. Sci. 2002 [PubMed] [Google Scholar]
- Cristina Marcarini J., Ferreira Tsuboy M.S., Cabral Luiz R., Regina Ribeiro L., Beatriz Hoffmann-Campo C., Ségio Mantovani M. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011;63:459–465. doi: 10.1016/j.etp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Egunyomi A., Moody J.O., Eletu O.M. Antisickling activities of two ethnomedicinal plant recipes used for the management of sickle cell anaemia in Ibadan , Nigeria. Afr. J. Biotechnol. 2009;8:20–25. [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959 doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erukainure O., Ajiboye J., Abbah U., Asieba G., Mamuru S., Zaruwa M.Z., Manhas N., Singh P., Islam M.S. Monodora myristica ( African nutmeg ) modulates redox homeostasis and alters functional chemistry in sickled erythrocytes. Hum. Exp. Toxicol. 2017;37:458–467. doi: 10.1177/0960327117712385. [DOI] [PubMed] [Google Scholar]
- Forget B.G., Bunn H.F. Classification of the disorders of hemoglobin. Cold Spring Harb. Perspect. Med. 2013;3:a011684. doi: 10.1101/cshperspect.a011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Wei P., Liu W. Combined antioxidant effects of rutin and vitamin C in Triton X-100 micelles. J. Pharm. Biomed. Anal. 2007;43:1580–1586. doi: 10.1016/j.jpba.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Henneberg R., Otuki M.F., Furman A.E.F., Hermann P., Nascimento A.J. do, Leonart M.S.S. Protective effect of flavonoids against reactive oxygen species production in sickle cell anemia patients treated with hydroxyurea. Rev. Bras. Hematol. Hemoter. 2013;35:52–55. doi: 10.5581/1516-8484.20130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaga N.A. Phytomedicines and nutraceuticals: alternative therapeutics for sickle cell anemia. Sci. World J. 2013;12 doi: 10.1155/2013/269659. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984 [PubMed] [Google Scholar]
- Kraus A., Roth H.P., Kirchgessner M. Influence of vitamin C, vitamin E and beta-carotene on the osmotic fragility and the primary antioxidant system of erythrocytes in zinc-deficient rats. Arch. Tierernahr. 1997;50:257–269. doi: 10.1080/17450399709386137. [DOI] [PubMed] [Google Scholar]
- Macharia A.W., Mochamah G., Uyoga S., Ndila C.M., Nyutu G., Makale J., Tendwa M., Nyatichi E., Ojal J., Shebe M., Awuondo K.O., Mturi N., Peshu N., Tsofa B., Scott J.A.G., Maitland K., Williams T.N. The clinical epidemiology of sickle cell anemia in Africa. Am. J. Hematol. 2018;93:363–370. doi: 10.1002/ajh.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani J., Ofori-Acquah S.F., Nnodu O., Wonkam A., Ohene-Frempong K. Sickle cell disease: new opportunities and challenges in Africa. ScientificWorldJournal. 2013;2013:193252. doi: 10.1155/2013/193252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpiana P.T., Tshibangu D.S.T., Shetonde O.M., Ngbolua K.N. In vitro antidrepanocytary actvity (anti-sickle cell anemia) of some congolese plants. Phytomedicine. 2007;14:192–195. doi: 10.1016/j.phymed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Muhammad A., Erukainure O.L., Malami I., Murtala U., Muhammed Z.M.K., Alahirah E.E. 2 , 5-hexanedione-induced oxidative damage and DNA Fragmentation : ameliorative role of rutin ex vivo. Toxicol. Int. 2015;22:137–146. [Google Scholar]
- Muhammad A., Ibrahim M.A., Erukainure O.L., Habila N., Idowu A.A., Ndidi U.S., Malami I., Zailani H., Kudan Z.B., Muhammad B.A. Induction of haemolysis and DNA fragmentation in a normal and malarial-infected blood by commonly - used antimalarial drugs in the North-Western region of Nigeria. Drug Metab. Lett. 2016;10:49–55. doi: 10.2174/187231281001160212150630. [DOI] [PubMed] [Google Scholar]
- Muhammad A., Arthur D.E., Babangida S., Erukainure O.L., Malami I., Sani H., Abdulhamid A.W., Ajiboye I.O., Saka A.A., Hamza N.M., Asema S., Ado Z.M., Musa T.I. Modulatory role of rutin on 2,5-hexanedione-induced chromosomal and DNA damage in rats: validation of computational predictions. Drug Chem. Toxicol. 2018 doi: 10.1080/01480545.2018.1465948. [DOI] [PubMed] [Google Scholar]
- Nafees S., Rashid S., Ali N., Hasan S.K., Sultana S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFκB/MAPK pathway. Chem. Biol. Interact. 2015;231:98–107. doi: 10.1016/j.cbi.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Oslund R.C., Su X., Haugbro M., Kee J.-M., Esposito M., David Y., Wang B., Ge E., Perlman D.H., Kang Y., Muir T.W., Rabinowitz J.D. Bisphosphoglycerate mutase controls serine pathway flux via 3-phosphoglycerate. Nat. Chem. Biol. 2017;13:1081–1087. doi: 10.1038/nchembio.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztas Y., Yalcinkaya A. Oxidative alterations in sickle cell disease: possible involvement in disease pathogenesis. World J. Hematol. 2017;6:55. [Google Scholar]
- Poillon W.N., Kim B.C., Labotka R.J., Hicks C.U., Kark J. a. Antisickling effects of 2,3-diphosphoglycerate depletion. Blood. 1995;85:3289–3296. [PubMed] [Google Scholar]
- Pule G.D., Mowla S., Novitzky N., Wiysonge C.S., Town C., Town C., Town C., Town C. A systematic review of known mechanisms of hydroxyurea- induced foetal haemoglobin for treatment of sickle cell disease. Expert Rev. Hematol. 2016;8:669–679. doi: 10.1586/17474086.2015.1078235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind J.M., Nagababu E., Ramasamy S., Luke, Ravi B. 2013. Redox Report Communications in Free Radical Research Hemoglobin Redox Reactions and Oxidative Stress. [DOI] [PubMed] [Google Scholar]
- Rowley C.A., Ikeda A.K., Seidel M., Anaebere T.C., Antalek M.D., Seamon C., Conrey A.K., Mendelsohn L., Nichols J., Gorbach A.M., Kato G.J., Ackerman H. Microvascular oxygen consumption during sickle cell pain crisis. Blood. 2014;123:3101–3104. doi: 10.1182/blood-2013-11-533406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani H.L., Malami I., Hassan S.W., Alhassan A.M., Halilu M.E., Muhammad A. Effects of standardized stem bark extract of mangifera indica L. In wistar rats with 2,4-dinitrophenylhydrazine-induced haemolytic anaemia. Pharm. J. 2015;7 [Google Scholar]
- Sedrak A., Kondamudi N.P. StatPearls Publishing; 2018. Sickle Cell Disease, StatPearls. [PubMed] [Google Scholar]
- Tluway F., Urio F., Mmbando B., Sangeda R.Z., Makubi A., Makani J. Possible risk factors for severe anemia in hospitalized sickle cell patients at muhimbili National hospital, Tanzania: protocol for a cross-sectional study. JMIR Res. Protoc. 2018;7:e46. doi: 10.2196/resprot.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastnedge E., Waters D., Patel S., Morrison K., Goh M.Y., Adeloye D., Rudan I. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J. Glob. Health. 2018;8 doi: 10.7189/jogh.08.021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Guo J., Yuan J. In vitro antioxidant properties of rutin. LWT – Food Sci. Technol. (Lebensmittel-Wissenschaft – Technol.) 2008;41:1060–1066. [Google Scholar]