Abstract
An early dialogue between nanomedicine developers and regulatory authorities are of utmost importance to anticipate quality and safety requirements for these innovative health products. In order to stimulate interactions between the various communities involved in a translation of nanomedicines to clinical applications, the European Commission's Joint Research Centre hosted a workshop titled “Bridging communities in the field of Nanomedicine” in Ispra/Italy on the 27th −28th September 2017. Experts from regulatory bodies, research institutions and industry came together to discuss the next generation of nanomedicines and their needs to obtain regulatory approval. The workshop participants came up with recommendations highlighting methodological gaps that should be addressed in ongoing projects addressing the regulatory science of nanomedicines. In addition, individual opinions of experts relevant to progress of the regulatory science in the field of nanomedicine were summarised in the format of a survey.
Keywords: Nanomedicine, workshop, regulatory science, critical quality attributes, immune effects, standardization
Graphical abstract
1. Background
Nanotechnology enabled health products (nanomedicines) are emerging innovative pharmaceutical products offering new diagnostic/therapeutic opportunities as well as tools for the implementation of personalised medicine. For their successful translation into clinical applications, clear regulatory pathways and suitable standardised test methods allowing their quality, safety and efficacy assessments must be available. However, the huge heterogeneity of nanomaterials, the limited availability of relevant standards and methods, the poor reproducibility of literature data and batch-to-batch variability are challenging the regulatory assessment of nanotechnology based pharmaceutical products. The increase of submissions of nanomedicinal products to competent authorities (Noorlander et al., 2015) and identified challenges when regulating such products have recently triggered a number of regulatory science activities including European research projects and international workshops (Global Summit on Regulatory Science: Nanotechnology Standards and Applications, 2016; NANoREG, 2013). In particular, the Global Summit on Regulatory Science workshops in 2015 and 2016 (GSRS15 and GSRS16), helped to identify main priority needs in the nanomedical sector such as reference materials (RMs) for drug delivery systems e.g. liposomes, and RMs relevant for surface characterisation of nanomaterials. Furthermore, methods for the identification and quantification of nanoparticles (NPs) in complex matrices, drug loading and release from drug delivery systems, NP surface characterisation and methods predicting the interaction of nanomaterials with the immune system were identified among the most needed documentary standards. Finally, regulatory scientists highlighted training needs of stakeholders, including regulators, on the state-of-the-art in nanotechnology science and related characterisation methods.
Two currently ongoing projects funded by Horizon 2020 Research and Innovation programme: the European Nanomedicine Characterisation Laboratory (EUNCL) and Regulatory Science Framework for Nano(bio)material-based Medical Products and Devices (REFINE) advance the regulatory science of nanomedicines and support the availability of appropriate test methods for their characterisation. The EUNCL (www.euncl.eu) is a research infrastructure aiming to set up a pre-clinical characterisation cascade dedicated to investigate those physical and chemical properties of nanomedicines that will have an impact on their safety and efficacy profile. The service of the EUNCL is freely accessible for public and private product developers, after an application and review process. The infrastructure offers the physical, chemical, in vitro and in vivo characterisation providing information relevant for the pharmaceutical development of nanomedicines. EUNCL keeps updating and developing new assays to provide reliable testing strategies for the assessment of the next generation nanomedicines. Whereas the EUNCL is concentrating on the characterisation of emerging nanomedicines, its sister project REFINE focusses on the development and standardization of methods needed for regulatory decision making. REFINE (www.refine-nanomed.com) is a Research and Innovation Action aiming to set up a scientific regulatory framework for nano(bio)materials used in medicinal products and/or in medical devices. The scientific framework will suggest science based integrated testing strategies using novel physicochemical and biological characterisation methods that allow the assessment of the next generation nanomedicines. Such testing strategies will be supported by a Decision Support System (DSS) taking into account the particularities of each product and responding to the needs of product developers and regulators for decision making. The DSS system will provide the user with the most efficient and reliable testing strategy combining both regulatory and scientifically-based characterisation needs. Strong and structured interactions and knowledge sharing between different communities will allow the optimisation of the development of innovative methods and tools consistent with their respective needs and requirements.
2. Objectives of the workshop
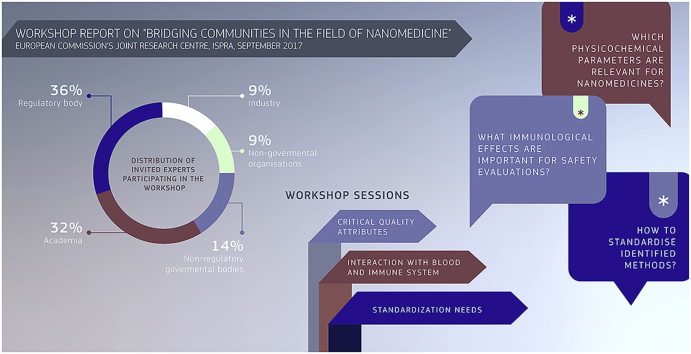
The development of a regulatory science framework for nanotechnology based medical products and devices that can address the upcoming needs for the next generation of nanomedicines requires the involvement of stakeholders from the very beginning. In particular, a continuous dialogue between the regulatory, industrial and academic community is necessary in order to discuss and identify crucial physical, chemical and biological parameters that contribute to the regulatory decision making. The European Commission's Joint Research Centre (JRC) hosted its first workshop aiming to bridge communities in the field of nanomedicine on 27–28 September 2017. It gathered 19 invited experts from regulatory institutions, industry and academia (Fig. 1) to discuss specific topics related to the regulation on nanomedicines and agree on the next steps forward in order to advance the field of nanomedicine.
Fig. 1.
Distribution of invited experts participating in the workshop.
The anticipation of regulatory needs and how they could be addressed in ongoing research projects was a main goal of the workshop, as introduced by Dr Susanne Bremer (JRC).
One major objective was related to the identification of physicochemical parameters that can have an impact on the safety and efficacy of nanomaterial based products (see text box 1). The process to determine such Critical Quality Attributes (CQAs) and their exact measurement is currently the focus of discussions in the nanomedicine community and was also the topic of the workshop's first session.
Text box1. Critical Quality Attributes.
CQAs are the physical, chemical or biological properties or any other characteristics that must be kept within a predefined range to ensure the expected quality of the product (International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, 2009)
Alt-text: Text box1
Within the second session participants elaborated on the interaction of nanomaterials with the immune system and aimed to gain a better understanding whether the existing methods and guidance are sufficient to detect immunological effects triggered by nanomedicines.
Advanced test methods are needed to characterise nanomedicines in order to ensure their quality and safety. This requires a qualification process of newly developed methods ensuring their reliability and relevance for a given purpose. During the third session of the workshop, an overview on existing standards including reference materials and on various pathways that can lead to the regulatory acceptance of test methods was presented.
Finally, the participants were asked to address the main recommendations related to each session and to answer a questionnaire in order to provide quantitative feedback.
This report provides a summary of the sessions followed by the main recommendations addressed by the participants. The results of the survey are also provided. The outcomes should contribute to the formulation of recommendations on regulatory needs that will feed into the ongoing H2020 projects REFINE and EUNCL and that could be shared internationally across the communities.
3. Critical quality attributes
The first workshop session focused on CQAs, corresponding methodologies and quality-by-design approaches. Presently, the identification of CQAs for nanomedicines remains a crucial challenge to be addressed. Dr Didier Bazile (Sanofi) presented some particular examples of CQAs in reference to nanomedicines. For the controlled delivery of a drug, the assessment of drug/nanocarrier association is particularly important to anticipate the influence of the dose on the free, protein bound and nano-associated fractions. In the translational process, the understanding and control of the drug/nanocarrier association appears as a critical point to properly extrapolate from in vitro and preclinical data to humans.
Many in vitro release techniques show some limitations to characterise and control the quality of the nanomedicines aimed at routing the drugs. Dr Bazile stressed the need to describe as CQAs other association principles (complexation, adsorption, etc.) between the various entities (small molecules, nucleic acids, peptides, proteins) and nanomedicines. A methodology to calculate the fraction of nano-encapsulated drug after manufacturing, and after the dilution in blood following intravenous administration was developed and applied to the anticancer drug cabazitaxel encapsulated in Poly(lactic)-Polyethylen glycol (PLA-PEG) NPs (Diou et al., 2015; Lakkireddy and Bazile, 2016).
In addition, other CQAs such as size, zeta potential, impurities, encapsulation efficiency and drug loading were identified as highly relevant for nanomedicines. For the intravenous route, the specific surface attributes primarily used to guarantee the colloidal stability of the nanomedicines need to be taken into account, as their interaction with blood proteins can trigger the risk of immunogenicity.
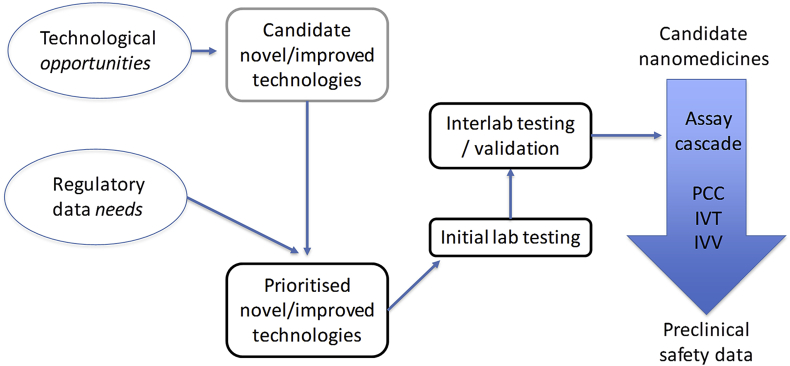
The main challenge related to the identification and assessment of CQAs is the availability and suitability of the relevant methods. Dr Sven Even Borgos (SINTEF) provided an overview of the available methods for physicochemical characterisation of nanomedicines and highlighted the gaps still persisting. Even though many methods exist, most have limitations or are not suitable for regulatory applications. He stressed the need of input from regulators and the necessity of an applicability-driven method development for the preclinical characterisation of nanomedicines (Fig. 2). Existing methods used for the regulation of other complex drugs might be also suitable for the assessment of nanomedicines.
Fig. 2.
Major criteria for prioritizing and development of methods needed for the preclinical characterisation of nanomedicines; PCC, physicochemical characterisation; IVT, in vitro characterisation; IVV, in vivo characterisation.
Dr Luigi Calzolai (JRC) presented the major challenges related to the most common methods for the assessment of size as one of the principal CQAs. Using dynamic light scattering (DLS) in batch mode as a case study, he demonstrated the limitations of the method for the case of polydisperse samples. The combination of DLS with separation and quantification methods was shown to yield a clear improvement, resulting in the particle size distribution of the nanomedicine, rather than just an average size of questionable relevance. For more sophisticated formulations, e.g., NPs functionalised with targeting moieties, the analysis of NP-bound protein (so called protein corona) structure was shown to be an upcoming challenge.
Finally, CQAs in combination with critical material attributes and critical process parameters are crucial components for the development of the Quality-by-Design (QbD) approach, which is a risk-based approach of drug development relying on the understanding of both the product formulation and the manufacturing process. This engineering approach provides a clear and efficient paradigm to manage efficacy and safety during the complete lifecycle of pharmaceutical products, from the early steps of design up to industrial production (Bastogne, 2017).
The application of the QbD approach in the development of nanomedicines was discussed by Prof Thierry Bastogne (University of Lorraine), who presented a review of 30 QbD studies in the nanomedicine field published over the last decade. The most critical material attributes, process parameters, quality variables and measurement technologies were reviewed. Nevertheless, specific deficiencies such as the absence of prior risk assessment, production scale-up, process analytical technology (PAT) and control strategy were also identified. Moreover, the statistical techniques used to apply QbD are all based on mean models and therefore do not correctly account for uncertainty, which finally leads to an underestimation of the risks. It is also important to stress that FDA and EMA now strongly recommend use of the QbD approach to develop more reliable analytical methods. Notably, the adoption of QbD is growing mainly in Asia and USA, but is still limited in Europe.
3.1. Main recommendations
Among the principal physicochemical properties identified as potential CQAs for nanomedicines, size, size distribution, physical and chemical stability, zeta potential, structure, purity and sterility, were discussed (Table 1). Challenges related to the detection and quantification of endotoxin in NP samples were underlined. CQAs could vary according to the type and characteristics of a nanomedicinal product. For drug delivery systems additional properties related to the encapsulation efficiency, drug/nanocarrier association and drug release are crucial. Relevant methods should be validated in order to demonstrate that they are reproducible and fit for purpose. Transferability of methods from other sectors is highly desirable and the concept of cross fertilisation will be further explored as a part of the REFINE project. The prioritisation of methods for further development and standardization should consider their robustness, sensitivity, speed, cost and particularly regulatory needs.
Table 1.
Summary of the major recommendations related to critical quality attributes addressed by the workshop participants.
| Main properties recognized as CQAs | Main recommendations |
|---|---|
|
|
Finally, the systematic implementation of QbD analysis must be encouraged to better control risks from the early stages of nanopharmaceutical development.
4. Interaction with blood and immune system
The immune and the blood systems are the first biological systems interacting with intravenously administered nanomedicines. The nanoparticle surface is immediately covered with a layer of blood components, forming the so-called protein corona (Neagu et al., 2017). Its adsorption kinetics and composition appear to depend on particle surface properties and will contribute to fate of material in the body. Usually, particulate material will be cleared from the circulation by immune cells via active (phagocytosis) or passive (diffusion) transport before ending up in the organs of the reticulo-endothelial system (RES), mostly in the liver and the spleen. The nanoparticle itself, its drug-load and the particle-specific protein corona will all play their part in how the product finally interacts with various immune components.
In a joint presentation, Dr Patricia Urbán (JRC) and Dr Blanka Halamoda (JRC) presented their literature reviews on the most prevalent in vivo effects of nanomaterials on the blood and on the immune system. Thrombosis was the most frequently reported effect among blood incompatibilities, whereas immunoactivation, including both activation of innate and acquired response, was the main reaction of the immune system (Halamoda-Kenzaoui and Bremer-Hoffmann, 2018). The adversities were linked to the main categories of nanomaterials employed in nanomedicine, such as inorganic, lipid based and polymer based NPs (Wicki et al., 2015). Inorganic NPs were the main category responsible for the induction of haematotoxic and immunotoxic effects, in particular thrombosis and inflammation. The most frequent adverse effects of lipid based NPs were the complement activation-related pseudoallergy (CARPA) and the activation of the adaptive immune system. The latter accompanied by the production of specific antibodies and accumulation of NPs in liver and spleen, lead to the so-called accelerated blood clearance (ABC) phenomenon, particularly noticeable after repetitive administration of PEGylated NPs. The reported adverse effects could often be linked to the physicochemical properties of NPs such as surface coating, surface chemistry and surface charge. Other properties such as size and chemical structure were also reported as having an impact on biological responses.
Mechanisms of the most relevant adverse effects of nanomedicines were discussed by Dr Neill Liptrott (University of Liverpool). He provided some deeper insights into the mechanism of CARPA, which had been previously described for several drugs already on the market. Cardiovascular and bronchopulmonary effects are caused by the pulmonary and coronary vasoconstriction, capillary leakage and systemic vasodilation (Szebeni, 2014). The role of different parameters, e.g., surface charge, size, surface coating, presence of aggregates, endotoxin contamination etc. in the activation of the complement system was investigated, but to-date the exact molecular mechanism leading to the activation is not known. Once the complement system is activated, the resulting anaphylatoxins (mainly C3a, C5a) stimulate blood cells, mast cells, basophils and tissue macrophages to release pro-inflammatory mediators responsible for the effect on the endothelial cells and smooth muscles (Szebeni, 2014). High inter-individual variability and the difficulty of finding a suitable animal model were highlighted.
The investigation of the mode of action should lead to the development of reliable testing strategies. Dr Marina Dobrovolskaia (NCL) emphasized the need for selecting appropriate methods for the safety assessment of nanomedicines. Every methodology has advantages and limitations, the understanding of which is essential for the creation of a network of assays suitable for various types of nanomaterials. Assays demonstrating a good in vitro-in vivo correlation (IVIVC) have the potential of predictivity of adverse effects in humans (Dobrovolskaia and McNeil, 2013). Case studies from the experience of NCL have demonstrated a good IVIVC of methods for acute toxicities such as haemolysis, complement activation, pyrogenicity, cytokine induction, and mononuclear phagocyte system (MPS) uptake. Fair correlation was experienced with thrombogenicity and myelosuppression, the weaker correlation being due to the multiple components and biodistribution, respectively, which cannot be accurately recapitulated in vitro using only one assay. Poor correlation was observed in immunosuppression and delayed type hypersensitivity tests because these toxicities are complex and in the absence of a reliable model, establishing of the IVIVC is needed for any given nanomaterial. Regarding the protein corona, the total protein binding is a good indication of NP stealthiness, however, it cannot accurately predict nanoparticle toxicity. Therefore, specialized immunotoxicity tests are warranted. Dr Dobrovolskaia emphasized the need to standardize methods with good IVIVC and to provide more guidance on how to detect and overcome NP interference with standardised assays.
Further aspects of the immunotoxicity assessment were provided by Dr Karin Cederbrant (Swetox). Recommended testing should include prediction of adverse effects by prioritizing the following immune functions: phagocytosis, oxidative burst, complement activation, cytokine release, and T-cell-Dependent Antibody Response (TDAR). The use of in silico modelling for the prediction of nanomedicine-induced immunogenicity was proposed as a “personalised safety” approach. With few exceptions (TDAR), animal cells or models are not recommended for prediction of human immune reactivity, especially not for immunogenicity testing.
Moreover, Dr Cederbrant discussed some aspects of the regulatory review process. Nanomedicines may be related to two or more of the following three product groups: biopharmaceuticals, low-molecular weight drugs and medical devices. This multi-facetted background makes safety prediction difficult, especially since nanomedicinal product-specific guidelines on toxicity testing are lacking. Today, safety assessment of nanomedicines requires navigation between all three sets of guidelines to find recommendations suitable for the individual drug candidate. A specific regulatory guidance, preferentially including a decision-tree model for safety studies, would be beneficial for drug developers in this area.
A further study on the suitability of the current regulatory framework for nanomedicines was presented by Dr Margriet Park from the Dutch National Institute for Public Health and the Environment (RIVM). The European regulatory framework for pharmaceuticals does not contain specific provisions for nanomedicines. In fact, a formal definition of nanomedicines does not even exist. Nevertheless, the European Medicine Agency (EMA) is well aware of the developments in this area, and has published in recent years a number of reflection papers describing general issues to be considered during the development of nanomedicines, such as the effect of coating on their stability and biodistribution (EMA/CHMP, 2013), and data requirements for intravenous iron-based nano-colloidal products (EMA/CHMP, 2015).
Considering the high level of interaction of nanomedicines with the immune system, their potential immunomodulatory effects, such as immunostimulation, immunosuppression and hypersensitivity reactions deserve adequate attention in the regulatory risk-benefit assessment. A survey of the public literature confirmed that such immunomodulatory effects have been reported for various nanomaterials, including nanomedicines (Giannakou et al., 2016). In this study, it was concluded that immunotoxic effects, such as CARPA, myelosuppression, inflammasome activation, and hypersensitivity, are not readily detected when following the current immunotoxicity testing guideline ICH-S8 for pharmaceuticals (International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, 2005). An additional shortcoming of this guideline is that it does not contain specific considerations for testing nanomedicines, which have been shown to be incompatible with a large number of commonly used toxicity assays (Guadagnini et al., 2015).
Further regulatory aspects were discussed in the presentation of Prof Rogerio Gaspar (University of Lisbon). Thanks to modified pharmacokinetic parameters and increased bioavailability, nanotechnology based agents can efficiently target tumour tissues for diagnostic and therapeutic purposes. On the other hand, the regulatory assessment of follow-on nanomedicines can pose additional challenges. Prof Gaspar stressed the need for developing new approaches, tools and standards for regulatory application and provided some insights into the perspectives of regulatory science in healthcare (Sainz et al., 2015). Major recommendations for the improvement of the translational process in the nanomedicine field included training and experience sharing on the application-driven approach to a research project and a holistic view on the from-bench-to-market evolution. A converging approach across the disciplines should be promoted.
Main recommendations.
The main recommendation of the workshop participants concerned the safety evaluation of nanomedicines, which should consider their effects on the blood and immune system. Among the crucial endpoints complement system activation, the release of cytokines, the uptake by monocytes, antigenicity, the induction of haemolysis and blood partitioning were pointed out most frequently (Table 2). Special attention should be given to potential immunogenicity of nanomedicines including the formation and composition of the compound-specific protein corona. In general, the in vitro methods with good in vitro/in vivo correlation should be prioritized for further development/standardization. Suitable models and approaches for assessing the immune functions are also needed since the current animal models are often limited in their predictivity.
Table 2.
Summary of the major recommendations related to the interaction of nanomedicines with blood and immune system.
| Most relevant endpoints | Main recommendations |
|---|---|
|
|
The use of human primary cells should be a first-hand choice. The use of realistic concentrations and physiological conditions was recommended. Furthermore, the employed methods should be suitable for testing of nanomaterials, which have been shown to interfere with many commonly used toxicity assays. The presence of endotoxin should be excluded before immunotoxicity testing as endotoxin itself may already induce an immune response (Giannakou et al., 2017).
Relevant tests should be performed in accordance with a “cause-for-concern” approach based on drug-target relationship and the drug's potential impact on specific immune-related Adverse Outcome Pathways. Finally, due to the high variability of the immune system, a more personalised approach, preferentially using emerging test systems (in vitro and in silico -modelling) was recommended.
5. Standardization needs
Documentary standards as well as reference materials are a prerequisite for the translation of nanotechnology based products to the market. A detailed analysis and evaluation of the suitability of existing standards is relevant to identify gaps hindering the regulatory approval of innovative nanotechnology based products. The session elucidated the availability of standardised methods and reference materials for nanomedicines. Furthermore, a number of standardization possibilities for analytical tests relevant for nanotechnology based products are existing and depend on the industrial sector e.g. via the OECD test guideline programme, ISO committees, the pharmacopoeia, ASTM International committees, etc. In the third session two possible pathways for the standardization were presented and currently ongoing activities in the field were summarised.
Prof Gerrit Borchard (University of Geneva) opened the session on standardization by describing the activities of the European Directorate for the Quality of Medicines & HealthCare (EDQM) with respect to the European Pharmacopoeia (Ph. Eur.). Ph. Eur. contains monographs on active substances, excipients, substances of biological origin, herbal drugs, vaccines, etc., but also general monographs on dosage forms, quality issues and standard analytical methods.
Most monographs require at least one reference standard (Ph. Eur. Chapter 5.12: chemical reference substance, herbal drugs or mixtures, biological substances), that reinforces the quality standard. Prof Borchard highlighted challenges related to the adoption of monographs for complex and heterogeneous substances, such as biotherapeutics, that could be comparable to those that will be faced for nanomedicines. While there are reference standards to formulation properties of nanomedicines such as pH, chloride content, etc., to date there are no reference standards related to CQAs specific to nanomedicines in Ph. Eur.
Text box 2. Brief description of the Ph. Eur. monograph development.
To become a monograph, a proposal should be submitted by stakeholders in cooperation with a national medical authority to the Ph. Eur. Commission. After the decision by the Commission to add the proposal to its program, a group of experts or working party is established to develop a first draft of a monograph. The draft is then published in Pharmeuropa (www.pheur.eu) for comments. The comments received are processed by National Pharmacopoeia Authorities (NPAs) and sent back to the Commission. The monograph draft, adapted by the group of experts/working party following the comments, is again submitted to the Commission for adoption. The EDQM Laboratory is assisting in this process by establishing and monitoring of reference standards, more than 2500 of which are currently available.
Alt-text: Text box2
Ph. Eur. chapter 5.12 states, “Before publication of a monograph in Pharmeuropa, the required quantities of reference standards should be supplied to the EDQM”. In the case of nanomedicines, and related follow-on products (“nanosimilars”), who is to provide these standards? This is a very important question, if one assumes that, for these complex drugs, the rule “the process is the product” also applies. EDQM is currently in the process of adapting a monograph for etanercept, a recombinant fusion protein of soluble TNF-alpha receptor and the Fc domain of a monoclonal antibody. The publication of this monograph had been postponed due to the lack of availability of the Chemical Reference Substance for the protein. EDQM has recently overcome this challenge and the monograph is now announced to be published in the Supplement 9.5 of Ph. Eur. However, the challenge of defining CQAs for nanomedicines and their reference materials remains.
Dr Valerie Zuang (JRC) elaborated further on the topic of validation of alternative test methods in view of regulatory acceptance and adoption as international standards, such as e.g. OECD Test Guidelines (TG). New test methods or approaches are either submitted to the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) via the standard test submission procedure (i.e. through the EURL ECVAM website) or in reply to a specific call. The test methods are assessed with regard to scientific and technical aspects, their regulatory relevance and impact on the 3Rs (i.e. replacement, reduction and refinement of animal use). For most human health effects or endpoints such as e.g. repeated dose systemic toxicity, the relevance of a single test method is typically evaluated with respect to its potential usefulness when combined with complementary methods, for example within an integrated testing strategy. If the submitted test method/approach is sufficiently developed and relevant for entering validation, then a study is launched. Validation may be executed by third parties or in some cases the method is transferred to the EURL ECVAM laboratories and to its Network of Validation Laboratories (EU-NETVAL) in case a ring trial is foreseen. After successful validation, a validation report is drafted and submitted to the ECVAM Scientific Advisory Committee (ESAC) for independent peer review. ESAC issues an opinion on the test method's scientific validity in context of its intended purpose. On the basis of the ESAC opinion and regulatory/stakeholder input, EURL ECVAM drafts its recommendation on the validated test method/approach. EURL ECVAM may also decide to lead on behalf of EC the regulatory acceptance process by drafting an OECD TG and an EU test method to be adopted in the EU Test Method Regulation.
Dr Matthias Roesslein (EMPA) demonstrated an alternative validation scheme, applied to the EUNCL testing cascade. The comparability of results is an essential precondition for denoting an assay as fit for purpose besides its biological relevance and scope. These are requirements for incorporating them into the collections of international standardization organisations, such as ISO, OECD or ASTM International. During its build-up phase the EUNCL could not rely on assays with the status of international standards, because currently none or very few exist for the field of nanomedicine. Therefore, EUNCL adapted the alternative approach of transferring the well-established testing cascade of the US Nanotechnology Characterisation Laboratory (NCL), which has characterised more than 350 different nanomaterial based products over the past 14 years. Key scientists of the EUNCL were directly trained on the assays by NCL specialists, after they had performed a first series of familiarization experiments using the NCL Standard Operation Procedures. Not all assays could be transferred directly one-to-one, as laboratories would employ different detection techniques. Hence it proved essential to keep the biological assay part 100% identical and only modify the detection/analytical part of the methods. The reason for this approach is the empirical nature of the biological part of the assays, which as such mainly defines the measurand. Furthermore, basic metrological principles, such as metrological traceability, measurement uncertainty and method validation, are essential to warrant proper method transfer. The overall approach was verified in an extensive inter-laboratory comparison between all involved laboratories and the NCL, investigating a recently approved nanomedicinal product. Such an assay transfer should not only employ pristine products, but it should also test the ability of a laboratory to detect any problem in a given sample.
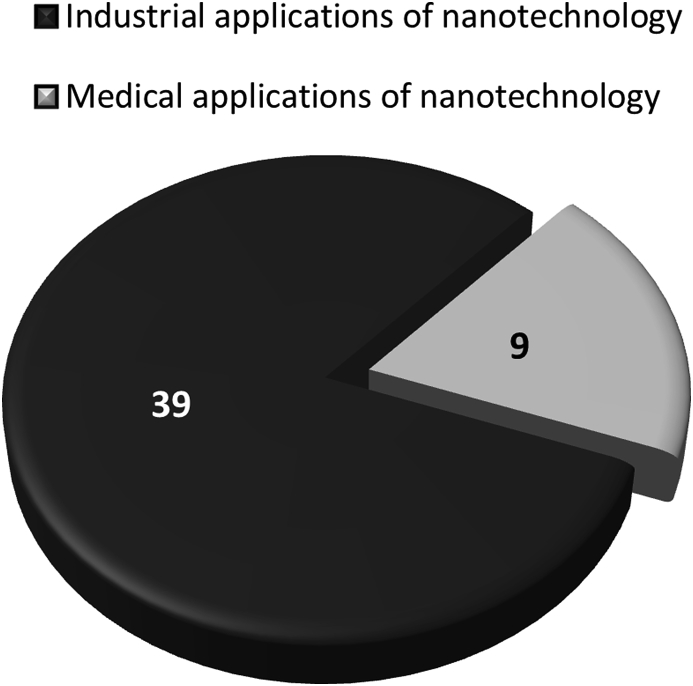
Dr Blanka Halamoda (JRC) presented an overview on existing documentary standards related to nanotechnology that could be relevant for nanomedicines (Halamoda-Kenzaoui et al., 2018). She gathered standardised test methods and guidance documents issued by international standardization bodies related to the safety assessment and the physicochemical characterisation of nanomaterials. Some of the available standardised methods for physicochemical characterisation address a specific nanomaterial category such as carbon nanotubes, but most are applicable to all nanomaterials. Size distribution is the most frequently addressed endpoint of the available test methods for nanotechnology products. For the assessment of drug delivery-specific critical parameters such as drug loading or drug release no standardised methods are available yet. Only a few of the available documentary standards were designed for nanomedicines (Fig. 3), raising the question whether the standards developed by other sectors could be applicable to the nanomedicine field and accepted by the relevant regulatory authorities. Other standardised test methods (from outside of the nanotechnology field) exist for particle characterisation or for medical device safety assessment, but their suitability for nanomaterials needs to be evaluated. Furthermore, the optimised protocols developed by NCL and EUNCL platforms for the preclinical evaluation of quality and safety of nanomedicine candidates are available but would require additional validation and standardization processes if used for regulatory purposes.
Fig. 3.
Documentary standards (including those under development) related to the characterisation of nanotechnology based products for industrial and medical applications (Halamoda-Kenzaoui et al., 2018).
Dr Vince Hackley (NIST) provided an overview on reference materials in the context of nanomedicines. Currently, the lack of well characterised, widely available RMs designed specifically for nanomedicine applications is an impediment to the commercialization and regulatory oversight of new medicines based on nanotechnology. This is a global issue, and it requires international cooperation to achieve the resources and timely response necessary to advance the field.
Dr Hackley pointed to the principal challenges for RM development in nanomedicine. One important challenge is stability (both chemical and physical), required for the RM to have sufficient shelf life; complex nanomaterial formulations can be notoriously difficult to stabilize for long term use. Furthermore, clearly defined measurands are required and appropriate validated methods must be available and widely accessible. The RM must be fit for purpose and the cost and effort required must match the need. For quality systems, traceability to the SI may be required or desired; a traceability chain can be challenging to establish. Commutability (the property of the RM that indicates it behaves sufficiently similar to a routine test sample) may be important, depending on the specific use and measurands. Finally, the sheer diversity of materials and applications within the nanomedicine landscape can present difficulties; it is unlikely that RMs can or will be developed for every possible scenario.
Text box 3. Definition and roles of reference materials.
A reference material (RM) is defined [ISO Guide 30:2015, 2.11] as a substance whose property values are sufficiently homogeneous, stable, and fit for its intended use in a measurement process. A certified RM is accompanied by a certificate or by documentation issued by an authoritative body that includes property values with associated uncertainty and traceability, obtained using metrologically valid procedures. Property values can be quantitative or qualitative. Either way, the primary role of RMs is to provide increased confidence in measurements. In this context, RMs can serve many roles, including, but not limited to, measurement calibration, assessment of methods and assays, quality control (QC) and proficiency testing, benchmarking, implementation of standard practices or methods, and critical inter-laboratory comparisons.
Alt-text: Text box3
Dr Hackley addressed major recommendations for development of RMs for nanomedicines, which would substantially benefit the nanomedicine community at large. Cooperation between regulatory agencies, industry and RM developers would greatly facilitate this process.
Main recommendations.
There was a substantial agreement among the participants on the need to develop relevant standards (both documentary and reference materials) for nanomedicines (Table 3). However, the effort to develop them should be justified by their further use.
Table 3.
Summary of the major recommendations related to the standardization session.
| Needed reference materials | Needed guidelines |
|---|---|
|
|
RMs can support a measurement framework that includes property values relevant to the regulatory process for nanomedicines and ensure quality in manufacturing and preclinical testing. Since there are currently no RMs produced specifically for nanomedicine applications (though there are RMs available that can serve to support specific aspects of nanomedicine research and development), and given the types and classes of nanoformulations submitted for regulatory approval, three specific recommendations were proposed:
-
(1)
focus on development of a generic liposome RM (liposomal formulations represent the single largest class of nanomedicine submissions to the US Food and Drug Administration at roughly 35% (D'Mello et al., 2017);
-
(2)
develop RMs with (certified) reference values for one or more critical quality attributes (size, size distribution, morphology, composition, etc.) that are stable in physiological media;
-
(3)
develop RMs with quantifiable surface-active species (e.g., ligands, coating, active pharmaceutical ingredient).
In addition, the development of guidelines for the quality and safety assessment of nanomedicines was recommended. Validated, humanised, in vitro test systems should be part of a nano-specific immunotoxicity testing strategy, applicable for various types of nanomedicines. Anticipation of hypersensitivity reactions could benefit from specific guidelines. Furthermore, a guidance document on how to detect and overcome NP interference with assays would be helpful.
Another issue concerns the selection of an appropriate regulatory framework for nanomedicines. The current legislation requires considerations of guidance documentation from at least three different areas (i.e., medical devices, low-molecular weight drugs and biopharmaceuticals). For this reason, a decision-tree model was suggested. Finally, given the limited number of standards addressing nanomedicines, the question of suitability and applicability of standards drawn from other fields should be addressed.
6. Individual opinions on requirements relevant to advance regulatory science in the field of nanomedicines (survey results)
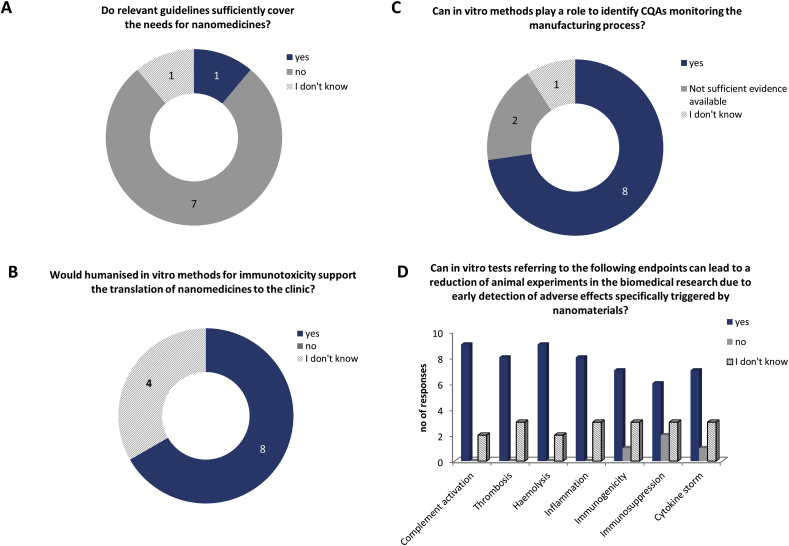
At the end of the workshop, a survey related to the topics of the workshop was performed among the participants. The respondents were encouraged to include additional information not covered by the predefined questions. The rate of participation was 63%. However, the total number of responses depends on a question, since not all the respondents had a deep knowledge related to all specific questions in the survey. A summary of the results is provided in Fig. 4, Fig. 5, Fig. 6.
Fig. 4.
Summary of the questions and responses related to critical quality attributes. The results are expressed in number of responses. The total number of respondents was 12, but the number of responses for different questions can vary since not all the questions were answered by all the respondents..
Fig. 5.
Summary of the questions and responses related to the interaction of nanomedicines with blood and the immune system. The results are expressed in number of responses. The total number of respondents was 12, but the number of responses for different questions can vary since not all the questions were answered by all the respondents.
Fig. 6.
Summary of the questions and responses related to the session on standardization. The results are expressed in number of responses. The total number of respondents was 12, but the number of responses for different questions can vary since not all the questions were answered by all the respondents.
A strong majority of respondents agreed that process analytical technologies such as data acquisition and data analysis tools, process analytical chemistry and knowledge management tools are relevant for the manufacturing process of nanomedicines and should be further developed (Fig. 4A and B). Among the physicochemical properties, size, size distribution and chemical and enzymatic stability of the product were judged as relevant and considered as CQAs for all nano-sized products. Shape, morphology, surface charge and other surface characteristics should also be assessed, but their recognition as CQAs would depend on the product category. More specific properties such as drug loading, drug release, and functionality of targeting moieties should be considered for the specific product classes or on a case-by-case basis (Fig. 4C and D).
The questions related to the session on the interaction of nanomedicines with blood and the immune system revealed that the majority of the participants felt that the current guidelines for safety assessment (e.g., ICH-S8, ISO 10993) are not sufficiently covering the needs for nanomedicines since they do not address nano-specific issues (Fig. 5A and not shown). The immune reactions (e.g., CARPA), cardiotoxicity or neurotoxicity of NPs were given as examples of safety issues not covered. In addition, guidance in the form of a decision tree model was suggested to identify appropriate regulatory pathways.
In vitro methods could help to identify CQAs and would support the translation of nanomedicines to the clinic (majority of responses) (Fig. 5B and C) if they provide reproducible results. Between 10% and 30% of the respondents did not have a strong opinion on this topic. Among the relevant endpoints, complement activation, thrombosis, haemolysis, inflammation, immunogenicity, immunosuppression and the risk of inducing a cytokine storm were all recognized as important and enabling an early detection of adverse effects of nanomedicines (Fig. 5D), confirming the recommendations discussed during the session. Immunostimulation was added as an additional endpoint to consider. For the improvement of the reliability of in vitro methods, the use of primary human cells was recommended. Furthermore, a testing strategy was suggested, taking into account the purpose of the treatment (intended patient population, treatment duration, administration route, etc.) and the type of nanomedicinal product.
In relation to the development and standardization of methods, around 8 out of 11 respondents expressed the need to develop guidance on equivalence and comparability of methods for physicochemical characterisation and 10 out of 11 judged it necessary to develop RMs for improving reliability of these methods (Fig. 6A and B). However, the balance between the effort to develop these materials and their potential future use should be taken into account. On the other hand, only 33% of respondents stated that the validation criteria for the in vitro methods are suitable for methods evaluating interaction of nanomedicines with the blood and immune system, whereas 42% considered that an additional guidance on this topic is needed (Fig. 6C). Available validation methods from other sectors could be of relevance for nanomedicines. The participants were also asked how broad the applicability of the developed methods should be. 43% of responses (6 out of 14) indicated the preference for methods applicable for specific product classes, which still need to be defined, whereas 36% (5 out of 14) would focus on methods applicable for all nano-sized products (Fig. 6D). Defining the applicability domain of a test method should be a part of the validation process. Some respondents have added that until more data become available, it is advisable to test all products and to build up a knowledge data bank. These data can then be sorted by class to identify high/low risk classes. In the end, however, new products that do not belong to a specific class will always need to be tested on a case-by-case basis.
7. Key outcomes and next steps
The workshop discussions have allowed the identification of the most relevant regulatory needs for nanomedicines. The most important parameters of the quality and safety assessment of nanomedicinal products were highlighted. Many parameters refer to the interaction of nanomedicines with the immune system, including complement system activation, cytokine release, or uptake by monocytes.
Matching of these parameters with available methodologies will allow the identification of the gaps for which additional methods need to be developed and standardised. Such an exercise will be performed within the framework of the Horizon 2020 REFINE project (www.refine-nanomed.com), aiming to develop methods for regulatory application for medicinal products and medical devices based on nanotechnology.
In addition, methods from other sectors should be evaluated for their applicability to the nanomedicine field. Such cross-fertilization, in terms of learning from the methodologies and guidelines existing in other fields, was one of the main recommendations of the workshop participants.
Standardised, fit for purpose and suitable methods should be part of a testing strategy tailored for the type of nanomedicinal product. Suitable guidance and/or early interaction with regulatory agencies could help in the development of such optimised strategy and guide the product developer through the regulatory framework.
Major recommendations.
-
•
Development/standardization of the most needed methods and reference materials for the regulatory assessment of nanomedicines
-
•
Adaptation of methods/standards from other sectors
-
•
Testing strategy
-
•
Implementation of quality-by-design approach
-
•
Early dialogue with regulatory agencies
-
•
Knowledge and experience sharing
Finally, some additional, regulatory and application-driven aspects should be part of the training in biomedical scientific disciplines to improve the process of translation of innovative medical products to the market.
Acknowledgment
The workshop was funded by the EC JRC Exploratory Research Programme. The Horizon 2020 projects: EUNCL (grant agreement no. 654190) and REFINE (grant agreement no. 761104) contributed scientifically and technically to the workshop and will benefit from the recommendations.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.yrtph.2019.04.011.
Disclaimer
The views and opinions expressed in this report are those of the authors and do not necessarily reflect the official position of their organisations.
Conflict of interest
The authors do not have any conflict of interest to declare.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Bastogne T. Quality-by-design of nanopharmaceuticals – a state of the art. Nanomed. Nanotechnol. Biol. Med. 2017;13:2151–2157. doi: 10.1016/j.nano.2017.05.014. [DOI] [PubMed] [Google Scholar]
- D'Mello S.R., Cruz C.N., Chen M.-L., Kapoor M., Lee S.L., Tyner K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017;12:523–529. doi: 10.1038/nnano.2017.67. [DOI] [PubMed] [Google Scholar]
- Diou O., Greco S., Beltran T., Lairez D., Authelin J.-R., Bazile D. A method to quantify the affinity of cabazitaxel for PLA-PEG nanoparticles and investigate the influence of the nano-assembly structure on the drug/particle association. Pharm. Res. (N. Y.) 2015;32:3188–3200. doi: 10.1007/s11095-015-1696-0. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia M. a., McNeil S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release. 2013;172:456–466. doi: 10.1016/j.jconrel.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA/CHMP . 2015. Reflection Paper on the Data Requirements for Intravenous Iron-Based Nano-Colloidal Products Developed with Reference to an Innovator Medicinal Product EMA/CHMP/SWP/620008/2012.https://www.ema.europa.eu/documents/scientific-guideline/reflection-paper-data-requirements-i [Google Scholar]
- EMA/CHMP . 2013. Reflection Paper on Surface Coatings : General Issues for Consideration Regarding Parenteral Administration of Coated Nanomedicine Products.https://www.ema.europa.eu/documents/scientific-guideline/reflection-paper-surface-coatings-general-issues-c EMA/325027/2013. [Google Scholar]
- Giannakou C., Geertsma E., de Jong R.,H., van Loveren W., R H.J.Vandebriel, VDZ Park M. Immunotoxicity testing of nanomedicinal products: possible pitfalls in endotoxin determination. Curr. Bionanotechnol. 2017;2:95–102. doi: 10.2174/2213529402666160601115600. [DOI] [Google Scholar]
- Giannakou C., Park M.V.D.Z., De Jong W.H., Van Loveren H., Vandebriel R.J., Geertsma R.E. A comparison of immunotoxic effects of nanomedicinal products with regulatory immunotoxicity testing requirements. Int. J. Nanomed. 2016;11:2935–2952. doi: 10.2147/IJN.S102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Summit on Regulatory Science . 2016. Nanotechnology Standards and Applications.https://www.astm.org/COMMIT/GSRS16 Final Report.pdf Report. pp. 1–35. [Google Scholar]
- Guadagnini R., Halamoda Kenzaoui B., Cartwright L., Pojana G., Magdolenova Z., Bilanicova D., Saunders M., Juillerat L., Marcomini A., Huk A., Dusinska M., Fjellsbø L.M., Marano F., Boland S. Toxicity screenings of nanomaterials: challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology. 2015;9:13–24. doi: 10.3109/17435390.2013.829590. [DOI] [PubMed] [Google Scholar]
- Halamoda-Kenzaoui B., Bremer-Hoffmann S. Main trends of immune effects triggered by nanomedicines in preclinical studies. Int. J. Nanomed. 2018;13:5419–5431. doi: 10.2147/IJN.S168808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halamoda-Kenzaoui B., Holzwarth U., Roebben G., Bogni A., Bremer-Hoffmann S. Mapping of the available standards against the regulatory needs for nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018:e1531. doi: 10.1002/wnan.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . 2009. ICH Harmonised Tripartite Guideline Pharmaceutical Development Q8(R2)http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1 [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human use . 2005. Immunotoxicity Studies for Human Pharmaceuticals S8.http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkireddy H.R., Bazile D. Building the design, translation and development principles of polymeric nanomedicines using the case of clinically advanced poly(lactide(glycolide))–poly(ethylene glycol) nanotechnology as a model: an industrial viewpoint. Adv. Drug Deliv. Rev. 2016;107:289–332. doi: 10.1016/j.addr.2016.08.012. [DOI] [PubMed] [Google Scholar]
- NANoREG Report on a Virtual Workshop to identify, formulate and prioritize issues/questions. 2013. https://www.rivm.nl/en/About_RIVM/Mission_and_strategy/International_Affairs/International_Projects/Completed/NANoREG/deliverables/NANoREG_D1_01_DR_Report_on_a_Virtual_Workshop_to_identify_form
- Neagu M., Piperigkou Z., Karamanou K., Engin A.B., Docea A.O., Constantin C., Negrei C., Nikitovic D., Tsatsakis A. 2017. Protein Bio-Corona: Critical Issue in Immune Nanotoxicology, Archives of Toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorlander C.W., Kooi M.W., Oomen A.G., Park M.V., Vandebriel R.J., Geertsma R.E., Agnes G. Horizon scan of nanomedicinal products. Nanomedicine. 2015;10:1–10. doi: 10.2217/nnm.15.21. [DOI] [PubMed] [Google Scholar]
- Sainz V., Conniot J., Matos A.I., Peres C., Zupancic E., Moura L., Silva L.C., Florindo H.F., Gaspar R.S., Zupančič E., Moura L., Silva L.C., Florindo H.F., Gaspar R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015;468:504–510. doi: 10.1016/j.bbrc.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol. Immunol. 2014;61:163–173. doi: 10.1016/J.MOLIMM.2014.06.038. [DOI] [PubMed] [Google Scholar]
- Wicki A., Witzigmann D., Balasubramanian V., Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J. Control. Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.