Abstract
Objective
Atrial fibrillation (AF) is common in acute myocardial infarction (AMI), and galectin-3 is possibly involved in its occurrence. Galectin-3 has been shown to play a central role in fibrosis and tissue remodeling and has a role in inflammatory and proliferative responses. The aim of our study was to measure galectin-3 levels in patients with myocardial infarction and to compare its levels in patients with or without AF, in order to investigate the potential predictive role of galectin-3 in this setting.
Subjects and Methods
The study included 51 consecutive AMI patients with AF; 27 AMI patients (52.9%) had permanent/persistent AF, and 24 patients (47.1%) had paroxysmal AF. Thirty-eight consecutive AMI patients without AF were used as a control group. Blood samples were obtained from venous blood on the third day after reperfusion.
Results
Patients with AF had higher levels of C-reactive protein (p < 0.01) and galectin-3 (p < 0.05) than those without AF. Patients with high galectin-3 had 4.4 times greater odds of having AF. Galectin-3 levels were lower in patients without AF (p < 0.01) than in those with permanent/persistent AF.
Conclusion
AMI patients with AF had higher levels of galectin-3 than those without this arrhythmia. This biomarker of inflammation and fibrosis could be a potential target for treating AMI patients at high risk.
Keywords: Biomarker, C-reactive protein, Fibrosis, Myocardial infarction, Atrial fibrillation
Significance of the Study
Increased galectin-3 levels are found in patients with atrial fibrillation on the third day after myocardial infarction. Galectin-3 could be a useful prognostic biomarker in acute myocardial infarction.
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias today. The prevalence of AF is approximately 3% in the adult population, and it is independently associated with a 2-fold increased risk of all-cause mortality. Its prevalence increases with age, and AF is frequently associated with coronary artery disease [1, 2].
Acute myocardial infarction (AMI) is the leading cause of death worldwide [3, 4, 5]; the most frequent supraventricular arrhythmia in AMI is AF. Patients with any type of AF (preexisting, first time detected, new-onset AF) in AMI have more comorbidities and are at higher risk of reinfarction, strokes, heart failure, and sudden cardiac death [5].
Galectin-3 may be involved in the occurrence of AF [6, 7]. Galectin-3 is a member of the galectin family which comprises β-galactoside lectins; it is normally expressed in several cytotypes, e.g. in endothelial cells, epithelial cells, activated microglia, inflammatory cells (mainly macrophages) and various tissues including the heart [7, 8]. Multiple studies have demonstrated that serum galectin-3 levels rise immediately after AMI and decrease significantly within 5 days after the acute event [8]. Galectin-3 has been shown to be involved in ventricle remodeling and prognosis early after AMI [9].
Fibrosis is a fundamental component of adverse structural remodeling in the myocardium which is triggered by various risk factors (like those present in AMI). Galectin-3 stimulates myofibroblast proliferation and procollagen-1 deposition, which eventually contributes to cardiac fibrosis, structural remodeling, and to further cardiomyocyte dysfunction, and thus forms an ideal substrate for the onset and persistence of AF [10, 11].
The aim of our study was to compare galectin-3 levels in AMI patients with and without AF in order to investigate the potential predictive role of galectin-3 in this setting. The actual role of galectin-3 in ACS has not been fully elucidated. Fibrosis, inflammation, and myocardial dysfunction are closely related and involved in the occurrence of AF in AMI [12]. Therefore, the secondary goal of this study was to investigate the relationship between galectin-3, high-sensitivity-C-reactive protein (hs-CRP), B-type natriuretic peptide (BNP) and high-sensitivity troponin I (hs-TnI) in AMI patients with and without AF, as well as their potential role in prognosis of AF.
Subjects and Methods
Data were collected from patient medical records, the results of standard laboratory analyses, and invasive and noninvasive diagnostic procedures.
The study included 51 consecutive patients with AMI (ST-elevation myocardial infarction [STEMI] and non-ST-elevation myocardial infarction [NSTEMI]) and AF, treated at the Clinic for Cardiovascular Diseases, Nis, Serbia. AMI and AF were diagnosed according to current ESC guidelines [4, 5, 6]. Over a period of 14 months (mid-2016 to mid-2017), patients with their first AMI, without prior revascularization, were recruited for the study. The exclusion criterion was previously diagnosed decreased global contractile function of the left ventricle (LVEF < 50%). Twenty-seven of the patients (52.9%) had permanent/persistent AF that was diagnosed and treated prior to hospitalization, and 24 of the patients (47.1%) had paroxysmal AF diagnosed during hospitalization. Thirty-eight consecutive AMI patients without AF were used as a control group. There were no statistically significant differences in STEMI/NSTEMI distribution between the study and control groups (37.3 vs. 32.4%, χ2 = 0.058, p = 0.809).
All patients underwent percutaneous coronary intervention (PCI). Venous blood was obtained on the third day after AMI, when PCI was carried out in both STEMI and NSTEMI patients. Plasma was separated from whole blood by centrifugation at a temperature of 25°C for 10 min at 3,000 g and stored at −80°C for subsequent analysis. Plasma levels of galectin-3, as well as BNP, hs-CRP, and hs-TnI levels were measured using commercially available enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's instructions (Galectin-3 [ELISA, R&D Biosystems, USA], BNP [ARCHITECT Assay, Abbott, USA], hs-CRP [Beckman Coulter, USA], hs-TnI [ARCHITECT STAT High sensitive Troponin-I assay, Abbott Diagnostics, USA]). For the galectin-3 ELISA assay, the CV was 30.72 ng/mL and mean minimum detectable dose was 0.016 ng/mL. Echocardiography was performed with an ultrasonic device system (Vivid 4, GE, Chicago, IL, USA) within 24 h after hospital admission. We measured the left atrial and ventricular diameters, and they were indexed by body surface area (BSA) according to the guidelines of the American Society of Echocardiography; the LVEF was calculated from apical 2- and 4-chamber views using the Simpson's biplane method [13].
Statistical Analysis
The data obtained were analyzed using the Statistical Package for Social Sciences (SPSS 21.0; Chicago, IL, USA). The baseline characteristics are presented as frequencies or means with SDs or medians with the interquartile range for the variables deviating greatly from normal distribution. A contingent of distributional characteristics (i.e., skewness, presence of extreme values, Shapiro-Wilk test) was used to determine the variables’ normality of distribution, and therefore the use of parametric or nonparametric tests. The quantitative variables were analyzed using parametric methods (Student's t test, Pearson's r test of correlation) or nonparametric methods (Mann-Whitney U test, Jonckheere-Terpstra test, Spearman's test of correlation). The association between qualitative variables was evaluated by Fisher's exact test or χ2 test. Standard linear or binary logistic regression modeling was performed to identify significant variance predictors for the dependent variable. All independent variables were tested for significance using univariate regression modeling, and later, all the statistically significant variables were included in the multivariate analysis to determine the actual predictive value of the model and of each significant independent variable. Receiver operating characteristic (ROC) curve analyses were performed in order to determine the optimal cut-off values of galectin-3 and BNP, with the highest sensitivity and specificity. A p value of less than 0.05 was considered to be a measure of statistical significance.
Results
The clinical and biochemical characteristics of the study and control groups are shown in Table 1. There were 32 male (36.4%) and 56 female subjects (63.6%). The youngest patient was 38 years old, while the oldest was 91 (mean age 66.33 ± 11.34 years). Patients with AF had higher levels of hs-CRP (p < 0.01) and galectin-3 (p < 0.05) than those without AF. Pharmacotherapy was prescribed according to the current guidelines of the European Society of Cardiology [4, 5, 6]. Besides the difference in the frequency of amiodarone prescription (54.9% of patients with AF vs. 8.1% of patients without AF, p < 0.001), other medications were prescribed in a similar manner. There were no differences between the prevalence of diabetes mellitus (DM) in patients with AF (19.6%) and those without AF (37.8%). Also, arterial hypertension had a similar frequency in patients with AF (92.2%) and those without AF (89.2%). All other parameters, including gender and smoking habit were equally distributed between these two groups. Patients were classified as smokers (43.2%), nonsmokers (45.5%), and former smokers (11.4%); the average time as a smoker was 16.80 ± 16.92 years, and 21 smokers (35.0%) consumed more than 20 cigarettes per day.
Table 1.
Baseline characteristics of the patients
| No AF | Permanent/persistent AF | Paroxysmal AF | AF vs. no AF, p | AF vs. permanent/ persistent AF vs. paroxysmal AF, p | |
|---|---|---|---|---|---|
| Age, years | 64.30±11.85 | 69.30±9.96 | 66.13±11.73 | 0.153a | 0.220c |
| BMI | 26.62±3.89 | 26.96±5.25 | 25.70±4.67 | 0.787a | 0.594c |
| Body surface area, m2 | 1.96±0.18 | 1.92±0.23 | 1.92±0.20 | 0.404a | 0.706c |
| LVEF, % | 51.46±9.51 | 49.52±13.55 | 54.33±13.47 | 0.947a | 0.389c |
| EDD, mm | 51.73±7.08 | 56.70±8.49 | 50.95±5.74 | 0.264a | 0.028c |
| EDDI, mm/m2 | 26.39±3.85 | 29.01±3.93 | 26.76±4.04 | 0.143a | 0.073c |
| ESD, mm | 35.96±7.15 | 39.98±8.69 | 33.95±6.93 | 0.622a | 0.050c |
| ESDI, mm/m2 | 18.32±3.61 | 20.42±4.03 | 17.90±4.37 | 0.426a | 0.114c |
| LA, mm | 40.81±4.14 | 49.55±7.16 | 40.32±7.28 | 0.087a | 0.001c |
| LAI, mm/m2 | 20.90±2.51 | 25.97±5.12 | 21.79±4.72 | 0.035a | 0.007c |
| CHADSVASC score | 4.24±1.46 | 4.30±1.23 | 3.71±0.95 | 0.402a | 0.191c |
| RBC, 1012/L | 4.48±0.67 | 4.42±0.51 | 4.38±0.65 | 0.533a | 0.801c |
| Hb, g/L | 146.0 (127.0–157.0) | 137.0 (131.0–149.0) | 129.5 (121.2–146.0) | 0.122b | 0.130d |
| HCT, L/L | 40.32±5.64 | 40.44±4.29 | 39.64±5.21 | 0.817a | 0.205c |
| WBC, 109/L | 9.3 (7.2–11.0) | 8.7 (7.0–12.1) | 10.0 (7.3–12.9) | 0.666b | 0.661d |
| PLT, 109/L | 213.0 (166.5–261.5) | 232.0 (183.0–273.0) | 235.0 (186.5–327.8) | 0.219b | 0.333d |
| Glucose, mmol/L | 7.2 (5.6–9.4) | 6.8 (5.8–9.2) | 6.9 (5.5–8.5) | 0.829b | 0.936d |
| Creatinine,μmol/L | 96.2 (83.0–140.0) | 104.1 (90.5–132.4) | 92.4 (82.7–121.8) | 0.993b | 0.644d |
| Urea, mmol/L | 6.0 (4.7–10.4) | 7.4 (5.6–10.4) | 6.8 (5.0–10.8) | 0.128b | 0.285d |
| Cholesterol, mmol/L | 5.1 (4.1–6.2) | 5.3 (4.3–6.1) | 5.5 (4.8–6.1) | 0.956b | 0.801d |
| LDL, mmol/L | 3.38±1.18 | 3.27±1.12 | 3.49±1.11 | 0.998a | 0.789c |
| HDL, mmol/L | 1.0 (0.9–1.2) | 1.0 (0.8–1.2) | 1.0 (0.9–1.3) | 0.719b | 0.799d |
| Triglycerides, mmol/L | 1.5 (1.2–2.5) | 1.3(1.2–2.0) | 1.5 (1.2–2.0) | 0.429b | 0.666d |
| AST, U/L | 28.0 (19.0–55.5) | 23.0 (19.0–37.0) | 23.5 (20.2–43.2) | 0.402b | 0.685d |
| ALT, U/L | 23.0 (16.5–34.0) | 20.0 (16.0–37.0) | 20.0 (12.0–40.2) | 0.507b | 0.675d |
| hs-troponin I, ng/L | 0.6 (0.1–5.5) | 0.1 (0.1–0.4) | 0.6 (0.3–4.0) | 0.296b | 0.018d |
| hs-CRP, mg/L | 4.0 (2.1–10.0) | 10.0(4.7–23.2) | 7.6 (2.2–40.4) | 0.007b | 0.022d |
| Fibrinogen, g/L | 4.02±1.02 | 3.30±1.32 | 5.40±1.91 | 0.711a | 0.197c |
| BNP, pg/mL | 93.3 (38.7–334.0) | 159.9 (92.1–524.1) | 144.7 (68.8–838.9) | 0.098b | 0.223d |
| Galectin-3, ng/mL | 8.41±2.76 | 10.53±2.79 | 9.33±2.68 | 0.011a | 0.012c |
Data are presented as mean ± SD or median (interquartile range). BMI, body mass index; LVEF, left ventricle ejection fraction; EDD, left ventricle end-diastolic diameter; EDDI, EDD index; ESD, left ventricle end-systolic diameter; ESDI, ESD index; LA, left atrium diameter; LAI, LA index; RBC, red blood cell count; WBC, white blood cell count; PLT, platelet count; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Student's t test.
Mann-Whitney U test
ANOVA
Kruskal-Wallis test.
The average plasma level of galectin-3 was 9.31 ± 2.86 ng/mL. None of the patients had a galectin-3 concentration > 17.8 ng/mL.
The galectin-3 concentration had a negative correlation with the total body height, total body weight, BSA, hemoglobin, low-density lipoprotein cholesterol, and hs-TnI levels. It was found to be directly and significantly proportional to the following variables: age, left ventricular end-diastolic diameter index (EDDI), platelet count (PLT), urea, hs-CRP, and BNP.
Higher galectin-3 levels were observed in male patients (p < 0.001), patients with DM (p < 0.05) and arterial hypertension (p < 0.05), and patients treated with ticagrelor, compared to those treated with clopidogrel (p < 0.01).
Using standard linear regression, we developed a statistically significant model which included gender, the presence of DM, the use of ticagrelor, and EDDI. Independent covariates were gender (p < 0.01) and EDDI (p < 0.05). The odds of having elevated galectin-3 levels were higher in males by 69.3% and for every increase in EDDI (by 1 mm/m2) by 74.8%.
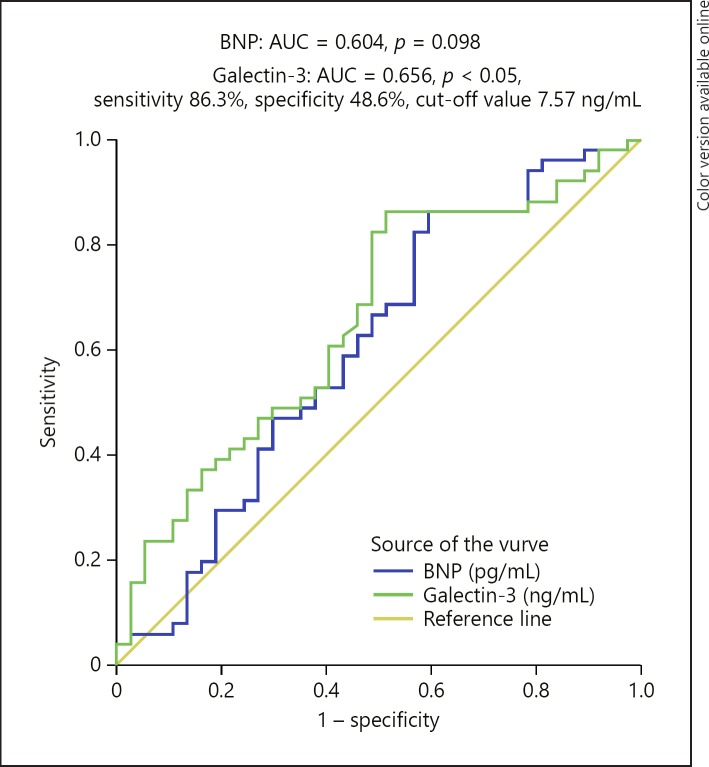
An ROC curve analysis was performed to determine the optimal cut-off values of galectin-3 and BNP concentration cut-off value for predicting AF in patients with AMI (Fig. 1). For galectin-3, the optimal cut-off value was 7.57 ng/mL (AUC = 0.656, 95% CI: 0.540–0.772, sensitivity 86.3%, specificity 48.6%, p < 0.05). In contrast to galectin-3, the AUC of the ROC curve for BNP was not statistically significant (p = 0.098).
Fig. 1.
The receiver operating characteristic curve for the ability of galectin-3 and BNP to predict AF in patients with acute myocardial infarction.
Patients with AF were classified into two groups according to the previously determined cut-off value for galectin-3. Seven patients (13.7%) had AF and low galectin-3 concentrations. Patients with low levels of galectin-3 and AF had higher hemoglobin (p < 0.05), hematocrit – HCT (p < 0.05), cholesterol (p < 0.05) and low-density lipoprotein levels (p < 0.05), which may have contributed to the occurrence of AF in spite of low galectin-3 levels. In addition, a galectin-3 concentration below 7.57 ng/mL was associated with lower urea (p < 0.05), hs-TnI (p < 0.05), hs-CRP (p < 0.05), and BNP (p < 0.01). No association was found between the galectin-3 level (below and above the cut-off value) and the frequency of comorbidities (diabetes mellitus, arterial hypertension), medications prescribed or smoking habit.
Using binary logistic regression, a model including four variables was developed. Independent variables were: age, CHADSVASC score, hs-CRP levels, and an increased galectin-3 concentration above the previously determined cut-off of 7.57 ng/mL. Patients with high galectin-3 have 4.4 times higher odds of having AF.
After dividing patients into three groups (without AF, with permanent/persistent AF and with paroxysmal AF), the quantitative characteristics of each group of patients are shown in Table 1. Levels of galectin-3 were lower in patients without AF (p < 0.01) than in patients with permanent/persistent AF, while the difference between patients with paroxysmal AF and each of the other two groups was not significant. Moreover, these two groups differed in the diameters of the left atrium and left ventricle. The left ventricle and atrial echocardiographic parameters were lower in patients without AF (left ventricle end-diastolic diameter [EDD], p = 0.028; left ventricle end-systolic diameter, p = 0.05; left atrial diameter (LA), p = 0.001; LA index, p = 0.007). Additionally, hs-TnI concentrations were lower (p = 0.0018), while the hs-CRP concentration was higher (p = 0.022) in patients with permanent/persistent AF compared with those without AF. Paroxysmal AF was associated with lower EDD, ESD, and LA, while lower levels of hs-TnI were seen in permanent/persistent AF.
The distribution of gender, comorbidities, and smoking habit was similar in each pair of groups according to the AF status. Left ventricular dilatation was more frequent among patients with permanent/persistent AF than in patients without AF (p < 0.05).
Discussion
The primary aim of this study was to compare the levels of galectin-3 in AMI patients with or without AF. We found that patients with AF had significantly higher levels of galectin-3 and hs-CRP than those without this arrhythmia. Patients with galectin-3 > 7.57 ng/mL had 4.4 times higher odds of having AF. The levels of galectin-3 positively correlated with hs-CRP and BNP. Also, patients with different types of AF (paroxysmal/persistent/permanent) did not have significantly different galectin-3 levels. Higher levels of galectin-3 were more likely to be found in males and in patients with a dilated left ventricle.
AF is very frequent in AMI patients, and these patients face an increased risk of severe complications, including heart failure, stroke, and premature death. Namely, pathophysiological mechanisms that lead to remodeling of the atrial structure and development of AF also underlie cardiac remodeling and heart failure [14, 15]. A number of studies have shown an association between high circulating levels of galectin-3 and increased risk of developing AF [14].
We examined the association between different risk factors for AF, as well as coronary artery disease, and galectin-3 levels in AMI patients with AF. In a study conducted on the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) in nondiabetic residents of Groningen, galectin-3 values were higher in females than in males and were elevated with increasing age [7]. In our study, galectin-3 was higher in male patients. This could be explained by diabetes being significantly more frequent in our male patients (59.4 vs. 40.6%, p < 0.05), and by the fact that our male participants were significantly older (70.53 ± 10.2 vs. 63.93 ± 11.33 years, p < 0.01). De Boer et al. [7] showed that galectin-3 was higher in patients with arterial hypertension and diabetes mellitus, which was also a finding in our study. However, recent studies showed that higher circulating galectin-3 concentrations were found to be associated with an increased risk of development of AF over the subsequent 10 years in age- and gender-adjusted analyses [16].
In other studies, galectin-3 has also been associated with cardiovascular risk factors except current smoking status, which was observed in our study [17]. Weigert et al. [18] found that galectin-3 levels were lower in obese patients with diabetes mellitus. Galectin-3 levels were lower in our patients with lower BSA height and weight. This could be a consequence of lower concentrations of galectin-3 being secreted by adipocytes in obese individuals, irrespective of AMI. Galectin-3 and hs-TnI were negatively correlated in our study. Moreover, any correlation between galectin-3 and TnI reported at admission was lost before hospital discharge in other studies. Galectin-3 decreases immediately after necrosis, while TnI decreases over 15 days after the acute event [8]. We measured galectin-3 on the third day after admission when it was decreasing, while TnI was in the “wash-out phase” after PCI and possibly had a secondary peak [19]. Patients with higher galectin-3 levels had ticagrelor more often than clopidogrel in therapy, which could be explained by the larger atherosclerotic burden in those patients, observed in earlier studies [20]. The average levels of galectin-3 in our patients were lower than those reported in younger healthy individuals [21]. This could be the result of dynamic changes in galectin-3 concentrations in AMI and the timing of measurement [8].
Very few studies have shown a correlation between CRP, an established marker of inflammation, and galectin-3 in AMI. One study showed that galectin-3 and hs-CRP significantly increased in patients with NSTEMI with preexisting AF, compared with patients without AF. Also, hs-CRP, but not galectin-3, was a predictor of a worse outcome during the 15-month follow-up [22]. Szadkowska et al. [23] found a weak relationship between 3 measured biomarkers: galectin-3, BNP and hs-CRP, which supports the observations that these proteins reflect different pathways in the pathophysiology of cardiac injury. Our results are in line with those findings.
Gurses et al. [24] showed that galectin-3 levels were higher in patients with persistent AF than in those with paroxysmal AF. The significant difference in galectin-3 levels between patients with persistent AF and those with paroxysmal AF may be due to the prominent structural remodeling of the left atrium in patients with persistent AF. Similarly, in our cohort, paroxysmal AF was associated with significantly lower diameters of the left ventricle and atria in comparison with those with permanent/persistent AF. Also, increased EDDI was associated with low plasma levels of galectin-3.
Considered together, age, CHADSVASC score, hs-CRP levels and galectin-3 > 7.57 ng/ml were independent predictors of the existence of AF in AMI, unlike BNP. Therefore, galectin-3 may be considered as a predictor of a worse outcome (possible development of heart failure) in AMI patients and an independent marker of the presence of AF. This could indicate patients who will need more aggressive treatment in this clinical setting.
A negative correlation between galectin-3 and LVEF in AMI patients was previously noted by other authors [25]. The independent predictors of high galectin-3 levels were male gender and EDDI in our patients. Andrejic et al. [26] found in NSTEMI patients that a left ventricular ejection fraction below 45% was a good predictor of the galectin-3 concentration in patients with acute coronary syndrome and with already decreased LVEF. Presumably, those patients with a low ejection fraction had an enlarged left ventricle or EDDI.
Interestingly, galectin-3 has a different and controversial role in the early and late phases of AMI. It contributes to the reparative processes in the infarcted area, in the early phase, which is essential for the maintenance of LVEF. In the later phase, galectin-3 may support the transition from acute to chronic inflammation and trigger cardiac fibrosis, leading to adverse ventricular remodeling and, finally, heart failure and a decrease in LVEF [27]. With the aim of highlighting the important contribution of galectin-3 to cardiac fibrosis and remodeling, a few recent studies assessing the pharmacologic inhibition of galectin-3 have suggested that this may be a method for the prevention of heart failure [28].
One of the limitations of this observational study is that we included a small number of patients. Also, we do not have the serial measurements of galectin-3 levels for comparison. Patients were not followed up immediately upon initial hospitalization. Therefore, we need more studies regarding the role of galectin-3 in AMI to be able to make more solid conclusions regarding its role in the prognosis and therapy in this setting.
Conclusion
Patients with AMI and AF have higher levels of galectin-3 than those without this arrhythmia. High concentrations of this biomarker are associated with an enlarged left atrium and ventricle, and with a higher risk of AF (paroxysmal or persistent/permanent). This biomarker of inflammation and fibrosis could be a potential target in the treatment of AMI patients at high risk. However, more prospective studies are needed to substantiate these findings.
Statement of Ethics
The Ethical Committee of the Medical Faculty, University of Nis, approved the study, which was conducted according to the guidelines of the Helsinki Declaration. All patients gave written informed consent for participation in this study before enrolment.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Funding Sources
This study was funded by Grants No. 44004 and No. III 41018, given by the Serbian Ministry of Education, Science and Technological Development.
References
- 1.Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, et al. RE-LY Atrial Fibrillation Registry Investigators Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation. 2014 Apr;129((15)):1568–76. doi: 10.1161/CIRCULATIONAHA.113.005451. [DOI] [PubMed] [Google Scholar]
- 2.Uz O, Atalay M, Doğan M, Isilak Z, Yalcin M, Uzun M, et al. The CHA2DS2-VASc score as a predictor of left atrial thrombus in patients with non-valvular atrial fibrillation. Med Princ Pract. 2014;23((3)):234–8. doi: 10.1159/000361028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998 Oct;82((7 8A)):2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 4.Roffi M, Patrono C, Collet JP, et al. ESC Scientific Document Group 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018 Jan;39((2)):119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. ESC Scientific Document Group 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016 Oct;37((38)):2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 7.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012 Jul;272((1)):55–64. doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 8.Bivona G, Bellia C, Lo Sasso B, Agnello L, Scazzone C, Novo G, et al. Short-term Changes in Gal 3 Circulating Levels After Acute Myocardial Infarction. Arch Med Res. 2016 Oct;47((7)):521–5. doi: 10.1016/j.arcmed.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Di Tano G, Caretta G, De Maria R, et al. Galectin-3 predicts left ventricular remodelling after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. Heart. 2016;0:1–7. doi: 10.1136/heartjnl-2016-309673. [DOI] [PubMed] [Google Scholar]
- 10.Zamora E, Lupón J, de Antonio M, Galán A, Domingo M, Urrutia A, et al. Renal function largely influences Galectin-3 prognostic value in heart failure. Int J Cardiol. 2014 Nov;177((1)):171–7. doi: 10.1016/j.ijcard.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Lippi G, Cervellin G, Sanchis-Gomar F. Galectin-3 in atrial fibrillation: simple bystander, player or both? Clin Biochem. 2015 Aug;48((12)):818–22. doi: 10.1016/j.clinbiochem.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Alturfan AA, Basar I, Emekli-Alturfan E, Ayan F, Koldas L, Emekli N. Galectin-3 and plasma cytokines in patients with acute myocardial infarction. Lab Med. 2014;45((4)):336–41. doi: 10.1309/LM3JZKBDA7D4QFOC. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015 Jan;28((1)):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Selcoki Y, Aydin Hİ, Celik TH, Isleyen A, Erayman A, Demircelik MB, et al. Galectin-3: A biochemical marker to detect paroxysmal atrial fibrillation? Clin Invest Med. 2016 Dec;39((6)):27528. [PubMed] [Google Scholar]
- 15.Oylumlu M, Dogan A, Ozer O, Yuce M, Ercan S, Davutoglu V. Effects of lying position on P-wave dispersion in patients with heart failure. Med Princ Pract. 2014;23((6)):556–60. doi: 10.1159/000365510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho JE, Yin X, Levy D, Vasan RS, Magnani JW, Ellinor PT, et al. Galectin 3 and incident atrial fibrillation in the community. Am Heart J. 2014 May;167((5)):729–34.e1. doi: 10.1016/j.ahj.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fashanu OE, Norby FL, Aguilar D, Ballantyne CM, Hoogeveen RC, Chen LY, et al. Galectin-3 and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2017 Oct;192:19–25. doi: 10.1016/j.ahj.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab. 2010 Mar;95((3)):1404–11. doi: 10.1210/jc.2009-1619. [DOI] [PubMed] [Google Scholar]
- 19.Ferraro S, Biganzoli E, Marano G, Santagostino M, Boracchi P, Panteghini M, et al. New insights in the pathophysiology of acute myocardial infarction detectable by a contemporary troponin assay. Clin Biochem. 2013 Aug;46((12)):999–1006. doi: 10.1016/j.clinbiochem.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Gucuk Ipek E, Akin Suljevic S, Kafes H, Basyigit F, Karalok N, Guray Y, et al. Evaluation of galectin-3 levels in acute coronary syndrome. Ann Cardiol Angeiol (Paris) 2016 Feb;65((1)):26–30. doi: 10.1016/j.ancard.2015.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Agnello L, Bellia C, Lo Sasso B, Pivetti A, Muratore M, Scazzone C, et al. Establishing the upper reference limit of Galectin-3 in healthy blood donors. Biochem Med (Zagreb) 2017 Oct;27((3)):030709. doi: 10.11613/BM.2017.030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlović M, Apostolović S, Stokanović D, Momčilović S, Jevtović-Stoimenov T, Zdravković SĆ, et al. The Association between Galectin-3 and hs-CRP and the Clinical Outcome after Non-ST-Elevation Myocardial Infarction with Preexisting Atrial Fibrillation. Sci Rep. 2017 Nov;7((1)):15106. doi: 10.1038/s41598-017-15265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szadkowska I, Wlazeł RN, Migała M, Szadkowski K, Zielińska M, Paradowski M, et al. The association between galectin-3 and clinical parameters in patients with first acute myocardial infarction treated with primary percutaneous coronary angioplasty. Cardiol J. 2013;20((6)):577–82. doi: 10.5603/CJ.2013.0157. [DOI] [PubMed] [Google Scholar]
- 24.Gurses KM, Yalcin MU, Kocyigit D, Canpinar H, Evranos B, Yorgun H, et al. Effects of persistent atrial fibrillation on serum galectin-3 levels. Am J Cardiol. 2015 Mar;115((5)):647–51. doi: 10.1016/j.amjcard.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 25.George M, Shanmugam E, Srivatsan V, Vasanth K, Ramraj B, Rajaram M, et al. Value of pentraxin-3 and galectin-3 in acute coronary syndrome: a short-term prospective cohort study. Ther Adv Cardiovasc Dis. 2015 Oct;9((5)):275–84. doi: 10.1177/1753944715578405. [DOI] [PubMed] [Google Scholar]
- 26.Andrejić O, Vučić R, Apostolović S, et al. The factors influencing galectin-3 levels in acute coronary syndrome with decreased left ventricular function. Acta facultatis medicae Naissensis. 2017;34:301–310. [Google Scholar]
- 27.Agnello L, Bivona G, Lo Sasso B, Scazzone C, Bazan V, Bellia C, et al. Galectin-3 in acute coronary syndrome. Clin Biochem. 2017 Sep;50((13-14)):797–803. doi: 10.1016/j.clinbiochem.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013 Jan;6((1)):107–17. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]