Abstract
Buckwheat (BW) constitutes a good source of bioactive components that shows anti-inflammatory effects in vitro and in vivo. The use of functional foods in the prevention and treatment of inflammatory bowel diseases (IBDs) has aroused an increasing interest. This study investigates the effect of in vitro digested BW and BW-enriched products (BW-enriched wheat breads, roasted BW groats –fermented and non-fermented-, and BW sprouts) on colon myofibroblasts, cells involved in the regulation inflammatory response in the intestine. The cells were treated with the different digested-BW products, alone or together with TNF-α (20 ng/mL), and the effects on cell migration, mitochondrial membrane potential and cell cycle, processes altered during intestinal inflammation, were investigated. A significant reduction in TNF-α-induced migration (25.5%, p<0.05) and attenuation of TNF-α-altered cell cycle (p<0.05) was observed in myofibroblasts treated with BW-enriched white wheat bread. These results contribute to extend the beneficial effects derived from BW bioactive compounds, and suggest that BW consumption can exert beneficial effects on IBDs.
Keywords: Buckwheat, Inflammation, Myofibroblasts of colon, Migration, Cell cycle
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Chron’s disease, is a multifactorial disease of unknown etiology. Here, endogenous (gene and immune) as well as exogenous (lifestyle and diet) factors have been reported to significantly impact the pathogenesis of IBD 1. Even though the relation between diet and IBD has been established, there is still an incomplete understanding of its role. Thus, some dietary habits (i.e., high animal fat consumption) can contribute to IBD development, whereas others, dietary ϖ−3 long-chain fatty acids as well as fiber rich fruit consumption can prevent or reduce the IBD pathological manifestations 2.
BW is a pseudocereal that has received increasing attention as functional food 3. The interest of the food industry in the innovation and research of functional foods has launched numerous studies into the elaboration of an important variety of both BW grains and BW-containing foodstuff, including sprouts, groats, tea, bread, noodles, and beer 4. For example, BW-enriched cookies consumption has been proven to exert hypocholesterolemic activity in humans 5, while the inclusion of BW in honey and bread formulation increases the plasma antioxidant capacity in healthy volunteers 6, 7.
Krkoskova and Mrazova8 reported that whole BW groats are composed of starch (55%), ash (2%), lipid (4%), protein (12%), carbohydrates (2%), dietary fiber (7%), and other compounds (18%) such as polyphenols. It is well known that BW is an important source of polyphenols intake. Flavone C-glucosides, including vitexin (Vi), isovitexin (IsoVi), orientin (Or) and isoorientin (IsoOr), as well as rutin (Ru) and quercetin (Q) (Figure 1) have been quantified to be in the range of 0.02 – 15.73 mg/g in BW grains and sprouts 9, 10. In the gastrointestinal tract, Ru can reach the colon in its original form, where is metabolized by the microbiota, releasing Q 11. Flavones C-glucosides have been documented to be poorly absorbed, remaining in the gastrointestinal tract up to 24 h 12. The low absorption of these compounds and the concentrations achieved in the gut suggest that Ru, quercetin, and flavone C-glucosides 12, 13 stay at the intestinal level long enough to exert their beneficial effects via regulating cell physiology, promoting the proliferation of beneficial intestinal bacteria 14, and amelioration of intestinal inflammation 15–19.
Figure 1.
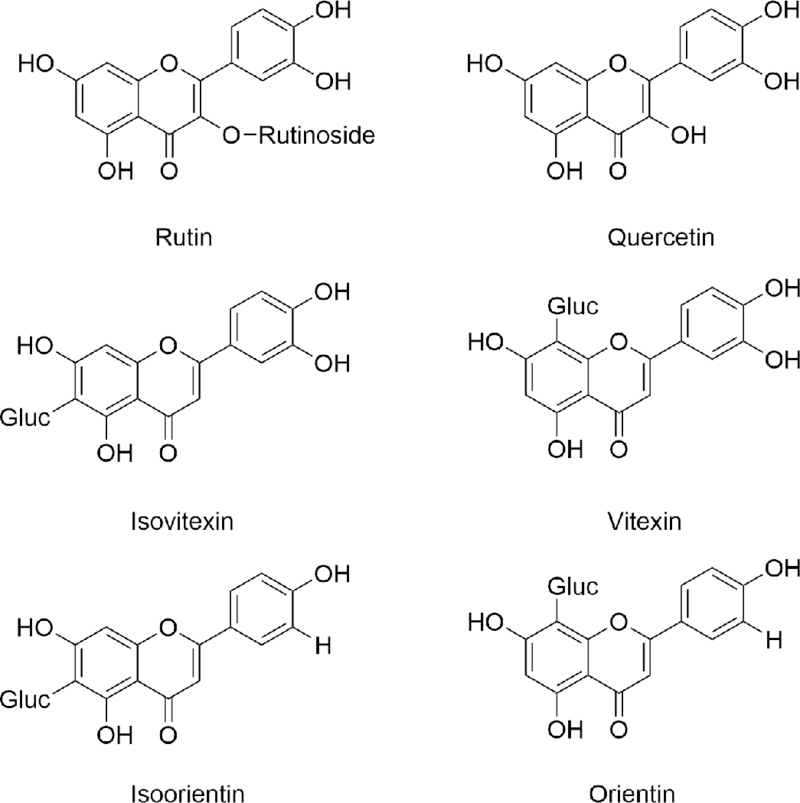
Chemical structure of polyphenols present in the different buckwheat (BW) products investigated.
A major feature of the cytokine profile in IBD is an increased production of the TNF-α, a molecule that plays a crucial role in IBD onset 20. Increased levels of TNF-α at the intestinal level leads to disruption of the physiological integrity of the intestinal barrier 21, allowing the translocation of the lumen content into the intestinal wall, where will contact with the cells of the sub-epithelial space. Here, myofibroblasts are found in the lamina propia. These cells interact with epithelial and immune cells to modulate the inflammatory response and regulate important processes such as fibrosis and tissue repair in the intestine22. The active role of myofibroblasts in the pathophysiology of IBD has resulted in an important number of in vitro studies investigating the potential anti-inflammatory effect of polyphenol-rich dietary foodstuff on intestinal fibroblasts 15, 23, 24.
Even though there is compelling in vivo evidence about the contrasting role of dietary plant foods on the onset and progression of IBD 2, 25, in vivo and in vitro studies have described that fruits and vegetables consumption can be considered a feasible therapy to ameliorate TNF-α effects on intestinal inflammation 26, 27. Hence, further studies will help clarify to what extent and how far can the pathological manifestations of IBD be controlled by dietary plant-derived bioactive compounds. Hitherto, little is known about the effect of BW and BW-enriched products on TNF-α-inflamed intestinal cells. We hypothesized that BW consumption can exert anti-inflammatory effects by modulating processes altered during intestinal inflammation. Thus, this study aims at investigating the cellular responses of myofibroblasts (CCD-18Co cells) of colon treated with TNF-α and different BW products and/or the individual flavonoids. Specifically, we explored their effects on i) cellular migration, ii) cell cycle, and iii) mitochondrial membrane potential.
Material and methods
Materials.
α-amylase (A1031–5KU, 98.3 U/mg solid), pepsin (P7000, 92 U/mg solid), pancreatin (P7545, 8xUSP), bile salts extract (B8631), MTT [3-(4,5-dimethylthyazol-2-yl)-2,5-diphenyltetrazolium bromide], human recombinant TNF-α, rutin (quercetin-3-rutinoside), dihydrorhodamine 123 (DHR), propidium iodide (PI), RNAse A, and quercetin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Vitexin (apigenin-8-C-glucoside), isovitexin (apigenin-6-C-glucoside), orientin (luteolin-8-C-glucoside), and isoorientin (luteolin-6-C-glucoside) were obtained from Extrasynthese (Genay, France). Dimethyl sulfoxide (DMSO) was purchased from MERK Millipore (Darmstadt, Germany). Ultrapure milliQ water was used for the elaboration of all solutions. All other chemicals were of analytical LC-MS grade.
BW products preparation.
The elaboration process of the different BW products is described in detail in supplementary material. Two types of BW-enriched wheat bread were prepared using dark wheat flour (BIO type 2000), white wheat flour (BIO type 500), and flour obtained by milling of roasted common buckwheat groats (BWg) (variety Kora) as described elsewhere 28,29. The flour obtained from roasted groats was used to substitute dark wheat flour or white wheat flour (50% w/w) to produce BW-enriched dark wheat bread (bBW) and BW-enriched white wheat bread (b500BW), respectively. The BW-enhanced wheat breads formulation and baking conditions are shown in Supplementary Table 1. The bread powder was kept at −20 °C until analysis. Fermented roasted BW groats were elaborated as reported by Wronkowska et al 30. The fermented product was freeze-dried and kept at −20 °C. BW sprouts (BWs) from common BW were provided by the Plant Breeding Station in Palikole (Poland) and were produced as described by Zielinska et al 31. Roasted BWg were obtained from a Polish local company (Olsztyn, Poland) and the material was freeze-dried, ground and kept at −20 °C 28.
Flavonoids extraction.
50 mg of each freeze-dried BW products was extracted with 1 mL of 60% aqueous methanol containing 0,4% trifluroacetic acid. Each stage of extraction consisted of 30 s sonication (VC 750, Sonics & Materials, USA), followed by 30 s of vortexing and centrifugation at 13,200 × g for 10 min at 4°C in a centrifuge 5415R (Eppendorf, Germany). The supernatants were collected in 5 mL flask. The extraction process was performed in duplicate and repeated 5 times. The extracted samples were filtered (0.44 μm) and directly analyzed by HPLC coupled to SPD-M20A DAD detector (Shimadzu, Japan) to determine the flavonoid content in the different BW products (Table 1).
Table 1.
HPLC-DAD analysis of the concentration of flavonoids in the different buckwheat (BW) products
| Concentration (μg/g) | Concentration (μg/mg) | ||||
|---|---|---|---|---|---|
| bBW | b500BW | fBWg | roasted BWg | BWs | |
| Orientin | - | - | - | - | 3.51 ± 0.18 |
| Isoorientin | - | - | - | - | 7.88 ± 0.53 |
| Vitexin | - | - | - | - | 3.15 ± 0.11 |
| Isovitexin | - | - | - | - | 5.19 ± 0.14 |
| Rutin | 25.49 ± 2.61 | 35.45 ± 2.09 | 73.95 ± 6.00 | 59.45 ± 0.91 | 2.94 ± 0.07 |
Abbreviations: bBW: Bread baked from “BIO” wheat flour and roasted BWF (50/50%);b500BW: Bread baked from wheat flour type 500 and roasted BWF (50/50%);fBWg: ‘Tempeh’ type product from dehulled roasted BW; BWg: Roasted BW groats; -: no detected.
In vitro digestion.
The lyophilized and milled BW products as well as a control sample (water) were subjected to an in vitro digestion as previously described 32 with some modifications. The procedure consisted of three stages: salival (pH 7.0), gastric (pH 2.0) and intestinal digestion (pH 7.5). 100 and 50 mg of BWs (BWs100 and BWs50, respectively) and 1 g of the rest of BW products were diluted in 10 mL of deionized water. 250 μL of α-amylase 98,3 U/mg solid diluted in 1 mM CaCl2 solution (pH 7.0) was added to each sample and incubated under shaking in a water bath at 37°C for 5 min. In the gastric digestion the pH was reduced to 2.0 using HCl (6 N). Then, 0.025 gr pepsin (diluted in 0,1N HCl; 92 U/mg protein) was added per 0.5 g sample, and the mixture was incubated for 120 min. After the incubation, the pH was raised to 6,0 with 1M NaHCO3 followed by the addition of 2.5 mL of a mixture containing pancreatin (activity 8x USP) and bile salts extract in 0,1M NaHCO3. The pH was then adjusted to 7,5 with 1M NaHCO3 and the samples incubated at 37°C for 120 min. The digestive enzymes were inactivated by heating (100 °C for 4 min), cooled and centrifuged at 13300 × g for 60 min at 4°C in a MPV-350R centrifuge (MPW Med. Instruments, Warsaw, Poland). The same digestion process was run in parallel of control samples containing water and a mixture of salt/enzymes. The supernatants were stored at – 20 °C until analysis and cellular assays were performed. For cell experiments, the digested products were sterilized (0.22 μm), diluted in DMEM and added to the cells.
Analysis of BW extracts by HPLC-diode array detector (DAD) analysis.
Prior to analysis, 250 μL ACN per 100 μL of digested solution was added followed by vortex and centrifugation at 15000 × rpm for 10 min (MPW 351R Centrifuge, Med. Instruments, Warsaw, Poland). The supernatant was evaporated in a vacuum concentrator plus (Eppendorf AG, Warsaw, Poland), and the samples diluted in 150 μL water:ACN (1:1 v/v). The samples were analyzed with a HPLC coupled to DAD (Shimadzu corp., Tokyo, Japan) to determine flavonoids composition. The separation was performed in a C18 column using water/ACN/formic acid (90:5:5) as mobile phase A, and water/ACN/formic acid (15:80:5) as phase B at 0.2 mL/min flow rate. The gradient started with 4% of solvent B. The concentration of B was increased up to 6% at 20 min, 11% at 35 min, 17% at 42 min and 80% at 45 min. This concentration was kept up to min 46, and then the initial conditions were re-established reaching 4% of B at 47 min. These conditions were maintained up to min 75. The flavonoids were identified according to their absorption spectra. The quantification was performed using pure external standards. This analysis was repeated 3 times (n = 3).
Cell line, culture conditions and cell treatment.
The myofibroblasts of colon CCD-18Co cell line was purchased from the American Type Culture Collection (ATTCC) (Rockville, MD, USA). Cells were routinely seeded at 6,000 cells cm−2 and grown in Eagle’s minimum essential medium (EMEM) containing 10% v/v Fetal Bovine Serum (FBS) (Sigma, Saint Louis, USA), 2mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and sodium bicarbonate (1.5 g L−1). The medium was also supplemented with penicillin (100 U mL−1) and streptomycin (100 μg mL−1). A pH between 7.2–7.4, 37 ºC of temperature and 5% CO2/ 95% air atmosphere at constant humidity were the conditions to grow the cells. The myofibrobasts were subcultured using Trypsin-EDTA (0.25%–0.03%) after reaching 80% confluence. Population doubling levels (PDL) between 33 and 35 and passages ranging from 15 – 17 were used for all the experiments.
Cell viability assay.
Cell viability was evaluated by Trypan blue dye exclusion test. CCD-18Co cells were seeded in 6-well plates at 6,000 cells/cm2 and were grown until confluence (5 – 6 days). Prior to the treatment of the cells, the digested BW products or control (salts + enzymes) were filtered (0.22 μm). The cells were treated with volumes ranging from 10 – 100 μL of the different digested BW products or control per 3 mL of medium in the presence or absence of TNF-α (20 ng/mL) for 48 h. Next, the cells were trypsinized and collected in 1 mL culture medium. 25 μL of the cell suspension was diluted in 75 μL of trypan blue, placed in a Neubauer hemocytometer (Fisher, Poznan, Poland) and cell viability determined. Viability results were expressed as percent of the control values. All experiments were repeated 3 times (n=3). Each experiment was performed in duplicate (2 wells per treatment).
Cell migration.
The migration assay was performed following a previously described method 33. Confluent myofibroblasts were incubated in culture medium containing 0.1% (v/v) FBS for 24 h. Next, a horizontal scratch was made in the center of the well by scrapping the surface using a sterile ‘tip’, the cells were washed with PBS and treated as follows: i) first group: addition of 990 μL fresh medium (0.1% FBS v/v) and 10 μL digested bBW, b500BW, BWg, and fBWg; ii) second group: addition of 950 μL fresh medium (0.1% FBS v/v) and 50 μL digested BWs (50 – 100 mg). Myofibroblasts treated with 10 or 50 μL of control digesta (salts + enzymes) were considered as controls of the first and second group, respectively.
In a second set of assays, the migration experiment was repeated treating the cells with the individual flavonoids present in the different BW products. The scratched cells were treated with three different concentrations (10, 1, and 0.1 μM) of rutin (Ru), vitexin (Vi), isovitexin (IsoVi), orientin (Or), and isoorientin (IsoOr). The cells were also treated with Q, at the same concentrations, since it is released from Ru in colon. Control cells were treated with an equivalent concentration of DMSO (0.5% v/v).
Different fields of the scratched area were photographed using a CKX41SF microscope (Olympus Corp., Tokyo, Japan) coupled to a DP25 digital color camera (Olympus, Tokyo, Japan). From 5 – 6 pictures from each well (2 wells per treatment) were taken of different sections of the scratched surface and the quantification was performed as previously described34
Results are displayed as mean ± SD of 3 – 5 independent experiments (n = 3 – 5). Each experiment was performed in duplicate (2 wells per treatment).
Mitochondrial enzyme activity (MTT test).
In order to evaluate the impact of BW-enriched products on mitochondrial functionality, the maximum volumes of BW products and control (salt + enzymes) without cytotoxic effects were also tested by MTT. After the treatment with the digested-BW products, the medium was removed, the cells were washed with PBS and 200 μL MTT (1 mg/mL) added to the cells followed by incubation for 4 h at 37 °C. The MTT was removed and 100 μL DMSO added to dilute the formazan crystals. The formazan generated was determined at 570 and 690 nm (background subtraction) using a microplate reader (SynergyH1, Biotek, Warsaw, Poland). The results are shown as percentage (%) of control cells + SD. The experiments were repeated 3 times (n = 3) and each experiment was performed in triplicate (3 wells per treatment).
Cell cycle.
The cells were seeded at 6,000 cells/cm2 in 6-wells plates, incubated for 2 days, and treated with the different digested BW products or control digesta as described before. After 48 h, cell culture medium was removed and added to a cytometer tube. The cells were rinsed with 1 mL PBS, which was mixed with the culture medium added previously to the cytometer tube. The cells were then trypsinized, added to the cytometer tube, and centrifuged at 125 × g for 7 min. The supernatant was discarded, the pellet re-suspended in 200 μL of PBS, and fixed in 2 mL ice-cold methanol (70:30, v/v) for 30 min. The pelleted cells (125 × g for 7 min) were diluted in 200 μL PBS containing 100 μg/mL of RNase and 40 μg/mL of propidium iodide (PI), and incubated 30 min at 37 °C. Cell cycle was analysed using a flow cytometer (BD LSR Fortessa Cell Analyzer; Erembodegem, Belgium) and FACS Diva Version 6.2 software (BD Bioscience, Warsaw, Poland). Cell cycle analysis of each treatment (2 plates per experiment) was repeated three times (n = 3). Results are displayed as mean ± SD.
Mitochondrial membrane potential (Δψm).
The analysis was performed using dihydrorhodamine (DHR, 20 mM in DMSO) and a PI solution (0.05 mg/ml PI; 1 mg/ml RNase A diluted in PBS) double labeling as described35. After treatment with the digested-BW products, the cells were rinsed twice with PBS, trypsinized and incubated with 1 ml DHR solution (5 μM in PBS) for 30 min at 37 °C in darkness. Next, the DHR solution was aspirated, and the cells incubated with 500 μl PI staining solution for additional 15 min under the same conditions. DHR (λex = 488 nm and λem = 525 nm) and PI (λexc = 488 nm and λem = 620 nm) fluorescent intensities were determined by flow cytometry (FACSAria II cell sorter). PI/DHR double negative cell ratio was used to determine Δψm changes. A minimum of 10,000 cells per treatment was analyzed in each replicate. The experiments were repeated 3 times (n = 3) and each experiment was performed in duplicate (2 wells per treatment).
Statistical analysis.
Results are displayed as mean ± standard deviation (SD). Prism 5 (GraphPad, La Jolla, CA, USA) was used for the analysis of significant differences. Kolmogorov Smirnov was used to determine the normal distribution of the data. ANOVA (1-way) followed by Dunnett post hoc analyses were performed to evaluate equality of variances and statistical differences between the treatments. Values p < 0.05 were indicated as statistically significant.
Results
Phenolic compounds in BW products before and after in vitro digestion.
The flavonoids composition determined in 50 mg of the different freeze-dried BW products investigated is shown in table 1. Despite flavones of C-glucosides were only detected in BWs, previous studies have described the presence of Vi (3.55 ± 0.16 μg/g) and IsoVi (1.60 ± 0.01 μg/g) in BWg 28. The HPLC analysis of the in vitro-digested BW products showed no additional peaks formation compared to the chromatograms of non-digested samples. This observation may evidence that the compounds analyzed were stable to the proteolytic activity of gastrointestinal enzymes and harsh gastrointestinal conditions. Thus, unless microbial activity can degrade these compounds, our data suggest that the bioactive compounds from BW could reach the colon in their original form.
Cell viability.
Myofibroblasts treated for 48 h with TNF-α (20 ng/mL) together with bBW, b500BW, BWg, fBWg or BWs exhibited cell viability values above 90% (Supplementary Figure S1). This value was similar to that observed for cells treated with equivalent volumes of the control digests (water + salts + enzymes) in the presence or absence of TNF-α. Based on these findings, for the rest of the experiments, cells were treated with the largest volume of bBW (1.8 nM Ru), b500BW (2 nM Ru), BWg (3 nM Ru), fBWg (4.5 nM Ru) and BWs100 (0.14 μM Or, 0.58 μM IsoOr; 0.28 μM Vi; 0.09 μM IsoVi; 0.71 μM Ru).
Effects on the migration of TNF-α-treated myofibroblasts.
We evaluated whether the treatment of the myofibroblasts with the digested-BW products affected their capacity to migrate in absence of TNF-α. As shown, cell migration was significantly (p<0.05) reduced by bBW (50.3%) and BWs100 (57.6%) (Figures 2A and B). In the presence of TNF-α (20 ng/mL) the colon fibroblasts migration was moderate, but significantly increased by 38.68% and 46.67% (p < 0.05) compared to the cells exposed to 10 and 50 μL digested control, respectively (Figures 2C and D). The TNF-α-induced migration was mainly ameliorated by b500BW (25.5%; p<0.05) (Figure 2C). Similarly, BWs100 reduced by 17.3% the migration in comparison with the TNF-α-treated cells (Figure 2D).
Figure 2.
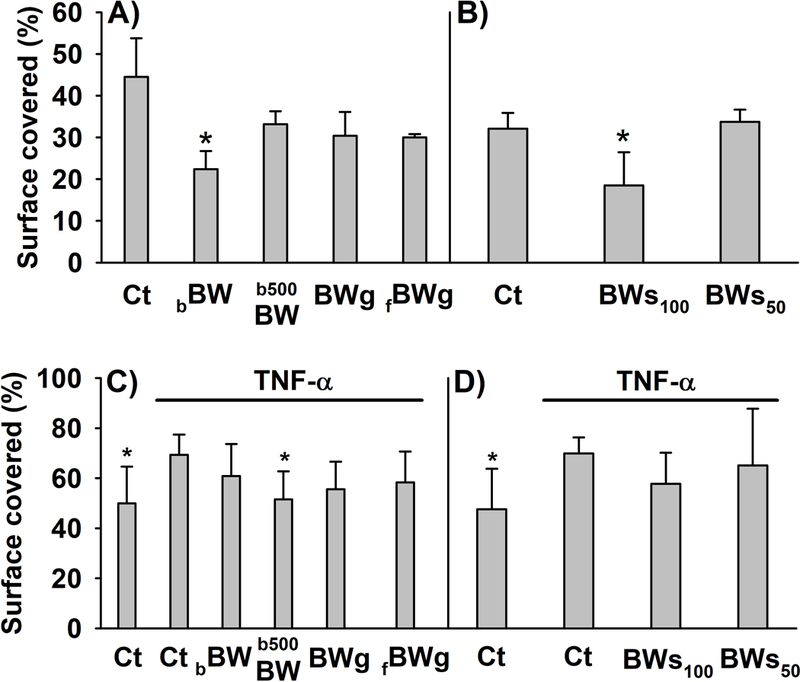
Effect of the different buckwheat (BW) products investigated on colon myofibroblasts migration in absence (A,B) or presence (C,D) of the pro-inflammatory cytokine TNF-α (20 ng/mL) for 48 h of exposure. The histograms display the ability of the myofibroblasts to migrate as percent of scratched area covered. The cells corresponding to the histograms A and B were treated with 10 or 50 μL, respectively, of the digested-BW products and control digesta (Ct). The histograms C and D were also treated with 10 or 50 μL, respectively, of the digested-BW products and control digesta in the presence or absence (Ct) of TNF-α (20 ng/mL). The average ± SD from 3 – 5 different experiments are showed. * indicates statistically significant differences (p < 0.05) from Ct (A,B) and TNF-α-treated cells (C,D). Abbreviations: Ct: control digesta; bBW: bread baked from “BIO” wheat flour and roasted BW flour (50/50%); b500BW: bread baked from wheat flour type 500 and roasted BW flour (50/50%); BWg: roasted BW groats; fBWg: ‘Tempeh’ type product from dehulled roasted BW; BWs100: solution obtained from the digestion of 100 mg of BW sprouts; BWs50: solution obtained from the digestion of 50 mg of BW sprouts.
Attempting to determine whether the flavonoids detected in the different BW products were responsible (at least in part) for the effects observed, the myofibroblasts of colon were co-treated with TNF-α (20 ng/mL) and Ru, Q, Vi, IsoVi, Or, and IsoOr at concentrations from 10 to 0.1 μM. Q showed a strong inhibitory effect on TNF-α-induced migration at 10 μM. Ru exerted a slightly non-significant reduction (16%) of cell migration at 10 μM, while the flavones C-glucosides had no significant effects on cell migration (Figure 3). These results indicate Ru and Q are not responsible for the effects observed, since the concentration needed to reduce TNF-α-induced migration is higher than that detected in the different BW products.
Figure 3.
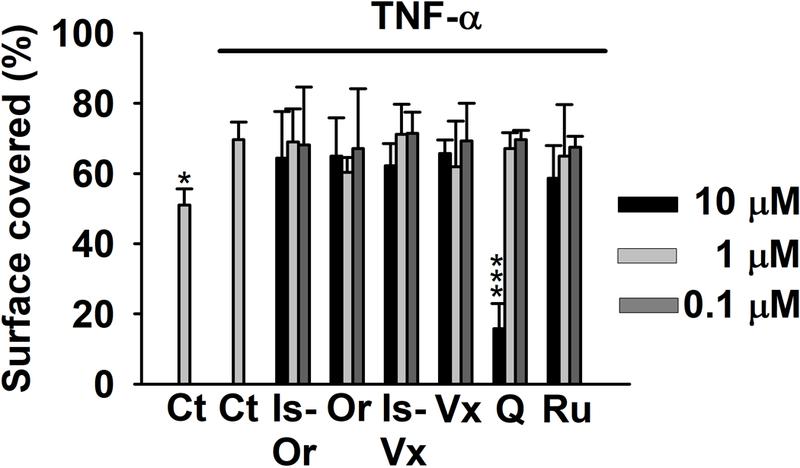
Effect of isoorientin (IsOr), orientin (Or), isovitexin (IsVx), vitexin (Vx), quercetin (Q), and rutin (Ru) on TNF-α-treated myofibroblasts of colon (20 ng/mL) for 48 h. Control cells (Ct) were treated with equivalent volume of DMSO (0.5% v/v). The histograms display the ability of the myofibroblasts to migrate as a percent of scratched area covered after 48 h compared with time 0 (initial scratched area). The average ± SD from three different experiments (n = 3) are showed. Each experiment was performed in duplicate (2 wells per treatment). * (p < 0.05) and *** (p < 0.01) indicates statistically significant differences from TNF-α-treated cells.
Effects on cell cycle distribution.
We next investigated the effects of the digested-BW products on the cell cycle of TNF-α-treated cells (Figure 4). In this assay BWs50 was discarded because it had no effect on TNF-α-induced migration. After the incubation of the myofibroblasts with TNF-α together with 10 or 50 μL of control digesta for 48 h there was quantified a decreased number of cells in phase G0/G1 (11.29% and 13.12%, respectively). This was accompanied by increased numbers of cells in phase S (76.3 and 122%, respectively) and G2/M (68 and 87.5%). The effects of TNF-α on cell cycle were slightly mitigated by b500BW reducing the number of cells in phase S (15.24%, p<0.05) and G2/M.
Figure 4.
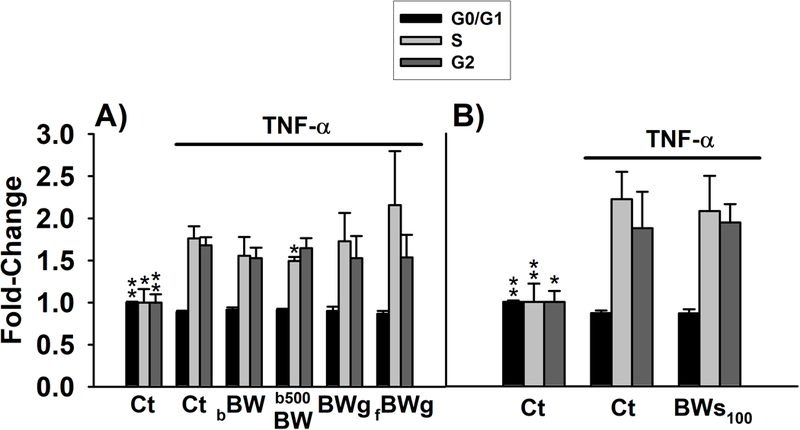
Analysis of the cell cycle in myofibroblasts CCD-18Co co-stimulated with TNF-α (20 ng/mL) and the different digested-buckwheat (BW) products for 48 h. Cells in exponential phase were treated with the different digested-BW products, at non-toxic concentrations, in the presence of TNF-α (20 ng/mL). Control cells were exposed to 10 and 50 μL of control digesta (Ct) (A and B, respectively). Values are expressed as fold-change (mean ± SD) of G0/G1, S, and G2 phases observed in control cells (set as 1.0). * (p < 0.05) and ** (p < 0.05) indicates statistically significant differences from TNF-α-treated cells. Abbreviations: Ct: control digesta; bBW: bread baked from “BIO” wheat flour and roasted BW flour (50/50%); b500BW: bread baked from wheat flour type 500 and roasted BW flour (50/50%); BWg: roasted BW groats; fBWg: ‘Tempeh’ type product from dehulled roasted BW; BWs100: solution obtained from the digestion of 100 mg of BW sprouts.
The influence of Ru and Q on myofibroblastś cell cycle was also investigated. In comparison to the TNF-α-treated cells, Ru at 10 μM reduced the number of cells in S (20.8%, p<0.05) and G2/M, while there was no effect on G0/G1 phase. Q at 1 μM had no effect on G0/G1 phase, but slightly decreased the number of cells in phase S and G2/M (Figure 5). Considering that the cells treated with the different BW products were exposed to Ru concentrations ranging from 1.80 nM to 0.71 μM, our results indicate that the effects exerted by the different BW products on cell cycle are not related to their Ru content. However, its effects might be mediated through Q (its hydrolysis product), since it modulated cell cycle to a similar extent than BW products at 1 μM (similar to the concentration of Ru detected in BW sprouts).
Figure 5.
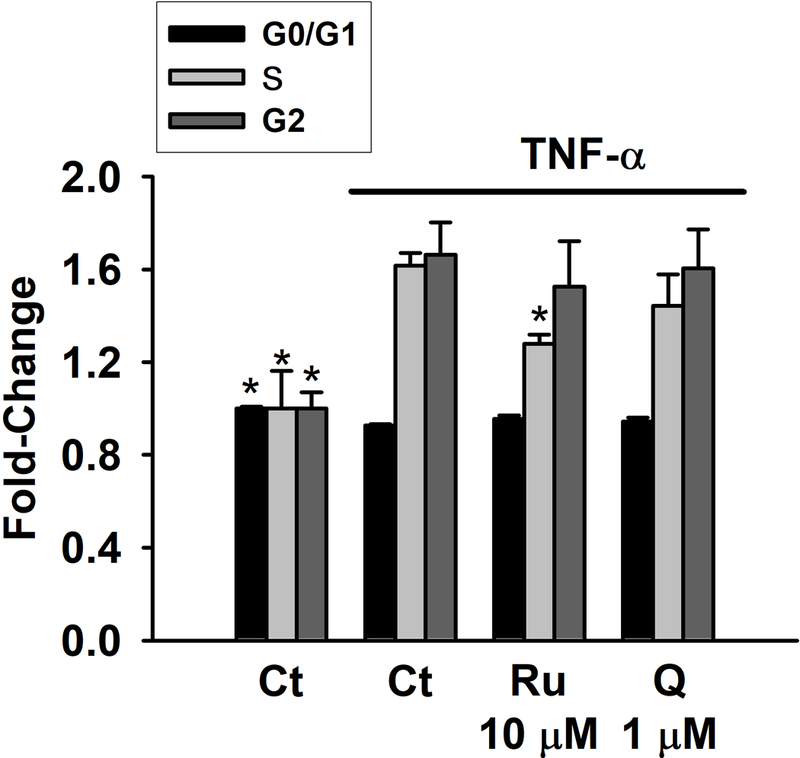
Analysis of the cell cycle in myofibroblasts CCD-18Co co-stimulated with TNF-α (20 ng/mL) and rutin (Ru) or quercetin (Q) for 48 h. Cells in exponential phase were treated with the 10 μM Ru or 1 μM Q in the presence of TNF-α (20 ng/mL). Control cells (Ct) were treated in parallel with equivalent volume of DMSO (0.5% v/v). Values are expressed as fold-change (mean ± SD) of G0/G1, S, and G2 phases observed in control cells (set as 1.0). * (p < 0.05) indicates statistically significant differences from TNF-α-treated cells.
Effects on mitochondrial enzyme activity and mitochondrial membrane potential.
The effects on MTT transformation and Δψm in cells co-treated with TNF-α (20 ng/mL) and BW products for 48 h are displayed in Figures 6 and 7, respectively. TNF-α had no effect on mitochondrial activity (Figure 6A) or Δψm (Figure 7A) in the presence of 0.33% (v/v) digested control. A substantial reduction of 31.54% in MTT conversion (Figure 6B) along with an increase IP/DHR ratio (Figure 7B) was observed when the concentration of the digested control solution was 1.67% (v/v) in relation to control (Figure 6B). The treatment with BW-enhanced breads (bBW and b500BW) or BWg (fermented and non-fermented) also reduced the MTT conversion from 17 – 25% (Figure 6A), while increased the IP/DHR ratio (fold change: 1.37 – 2.18) compared to TNF-α (Figure 7A). BWs100 caused a higher reduction in formazan formation (44.33%; p<0.05) in comparison to the other BW products (Figure 6), but no effect on Δψm compared to TNF-α-treated cells was observed (Figure 7B).
Figure 6.
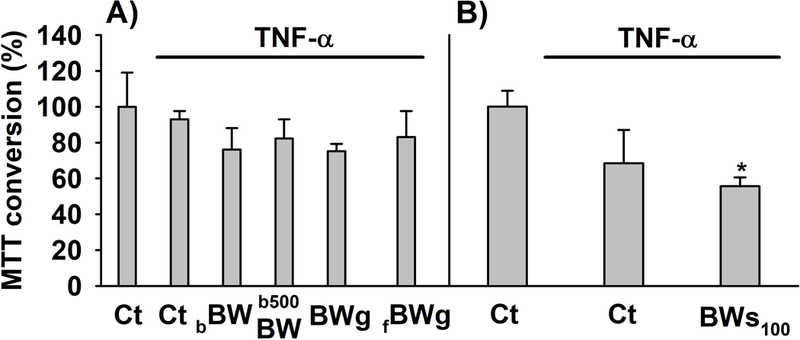
MTT conversion in myofibroblasts of colon co-treated with TNF-α (20 ng/mL) alone or in combination with the different digested-buckwheat (BW) products for 48 h. (A) myofibroblasts co-treated with TNF-α and the BW-enhanced breads (bBW and b500BW) or BW groats (BWg, fBWg) and (B) BW sprouts (BWs100). The results are expressed as percent of the control cells (set as 100%). The experiments were obtained from three independent experiments (n = 3). Each experiment was performed in triplicates. * p < 0.05 compared to cells treated with digesta control. Abbreviations: Ct: control digesta; bBW: bread baked from “BIO” wheat flour and roasted BW flour (50/50%); b500BW: bread baked from wheat flour type 500 and roasted BW flour (50/50%); BWg: roasted BW groats; fBWg: ‘Tempeh’ type product from dehulled roasted BW; BWs100: solution obtained from the digestion of 100 mg of BW sprouts.
Figure 7.
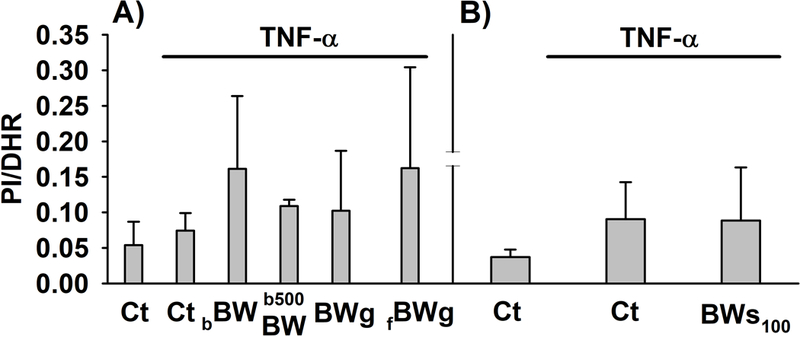
Mitochondrial membrane potential (Δψm) in myofibroblasts of colon co-treated with TNF-α (20 ng/mL) alone or in combination with the different digested-buckwheat (BW) products for 48 h. (A) myofibroblasts co-treated with TNF-α and the BW-enhanced breads (bBW and b500BW) or BW groats (BWg, fBWg) and (B) BW sprouts (BWs100). The results are expressed as the average of the IP/DHR ratio obtained from three independent experiments (n = 3) and each assay analyzed in duplicate. Abbreviations: Ct: control digesta; bBW: bread baked from “BIO” wheat flour and roasted BW flour (50/50%); b500BW: bread baked from wheat flour type 500 and roasted BW flour (50/50%); BWg: roasted BW groats; fBWg: ‘Tempeh’ type product from dehulled roasted BW; BWs100: solution obtained from the digestion of 100 mg of BW sprouts; IP: propidium iodide; DHR: dihydrorhodamine.
Discussion
Currently, the treatment of IBD comprises the use of anti-inflammatory pharmacological agents, assuming side-effects and decreased quality of life of patients. An appropriate diet could bring great benefits decreasing side-effects in patients suffering of IBD in terms of reducing inflammation and medication 36. BW has been proposed as a promising food to be included in the diet of patients experiencing intestinal inflammation37. The anti-inflammatory effects at intestinal level of BW have been reported in animal models of intestinal inflammation by improving gut permeability, attenuating colonic mucosa inflammation, and modulating expression of tight junction proteins38. Nevertheless, there is a lack of understanding in relation to i) the cellular mechanisms by which BW exerts its inflammatory activity, and ii) the molecule(s) responsible of the benefits observed.
In agreement with previous studies39, our results showed that the phenolics present in BW (such as Ru and Q) are stable during in vitro digestion. These results indicate that the flavonoids, unless microbial activity modifies them, can reach the colon in their original physicochemical form and exert their beneficial effects.
Myofibroblasts migration is a core mechanism during intestinal inflammation that involves the movement of the intestinal fibroblasts to the inflamed area, contributing to tissue repair. During intestinal chronic inflammation, the fibroblasts are permanently activated synthesizing abnormal amounts of extracellular matrix, which can then lead to intestinal fibrosis, and express receptors for molecules involved in the regulation of the migration (i.e., TNF-α and its receptor)40. The role of TNF-α on cell migration is very complex, and its effects vary depending on diverse factors such as tissue source, cell type, concentration, and exposure time 41. In this context, there have been described dissimilar effects of TNF-α regulating myofibroblasts function either reducing42 or promoting cell migration 33. Under the conditions of the study, TNF-α (20 ng/mL; 48 h) increased the migration of the colon myofibroblasts compared with untreated cells. Regulation of intestinal fibroblasts migration by natural products has been proposed as a mechanism to attenuate intestinal inflammation43. In this study, it has been shown the modulatory effect of different BW products on TNFα-stimulated myofibroblasts. The b500BW product exerted a strong inhibitory effect on TNF-α-induced migration (Figure 3). According to studies where polyphenols reduced myofibroblasts migration in the absence of pro-inflammatory stimulus 44, the BW products reduced fibroblasts migration in the absence of TNF-α. Overall, these results suggest that the modulatory effects of BW products involve additional signaling pathways other than the TNFα one. Previous research efforts have shown the potential of dietary bioactive compounds (such as polyphenols) to modulate the aryl hydrocarbon receptor (AhR) 45. The AhR is a pleiotropic nuclear factor, expressed in intestinal fibroblasts, of critical importance in the inhibition of TNF-α-induced-collagen synthesis in the gut 46. The hypothesized mechanism of polyphenols interaction with AhR could explain the positive effects of BW products through the reduction of TNF-α-induced fibroblasts migration. Additionally, it should not be ruled out that the effect of the different digested-BW products on TNF-α-induced fibroblasts migration might be exerted through innate immune receptors such as Toll-like receptor (TLR)-4 47, growth factors 48, as well as COX-2 and PGE2 33. These effects on cell migration might suppose an important immunonutritional contribution of BW-derived products against the undesirable effects (such as excessive fibrosis) resulting from the dysregulation of the intestinal homeostasis during chronic inflammation.
This study points out the importance of investigating the effects of whole products on the intestinal cells, BW and BW-enhanced products, containing mixtures of phenolic compounds with other nutrients. These complex mixtures exemplify a closer approximation to the content that can be found in the intestinal lumen after consuming BW products. Nevertheless, a study which parallels the whole products together with their individual phenolic compounds can help elucidate the molecules responsible of the biological effects exerted by the products. Like studies showing that Ru and Q reduce migration in intestinal cells 49, 50, our results displayed a slight (16%) and strong (77.34%), respectively, inhibitory effect of these compounds on migration of TNF-α-stimulated cells at 10 μM (but not at 1 μM or lower). Despite 10 μM is a concentration that can be achieved in colon after consuming foodstuff containing Ru and Q 13, the results suggest that these compounds are not responsible for the effects observed in our in vitro study, since their concentrations are lower in the BW products investigated. Therefore, other compounds different than polyphenols such as fiber 51, and/or BW protein52, might be contributing to reduce the TNF-α effect.
Chronic inflammation disrupts the cell cycle of intestinal cells, thus altering intestinal homeostasis and inducing intestine impairment53. Here, dietary interventions preventing cell biological alterations could have great benefits regulating intestinal homeostasis 54. To date, numerous studies have investigated the effect of polyphenols and polyphenols-containing foods on cancer cells 55, 56, while only a few studies have investigated the role of food products on cells cycle regulation in non-cancerous cell lines 57. In line with studies describing the ability of TNF-α to alter fibroblasts’ cell cycle 58, our results showed that the CCD-18Co myofibroblasts exposed to TNF-α (20 ng/mL) altered the physiological cellular distribution in the different cell cycle phases (Figure 4). An important mechanism to protect intestinal cells from the undesirable effect of oxidative agents is the prevention of cell cycle perturbation by natural products59. The BW products reduced the effect of TNF-α on cell cycle progression. This effect was independent of their Ru content, since its concentration in the BW products is below 10 μM, which is the concentration required to reduce the TNF-α-induced cell cycle alteration. Q, a phenolic compound that comes from the hydrolysis of Ru, has been reported to be a potent regulator of cell cycle in numerous intestinal cell lines 60. In this study, Q at 1 μM exhibited a modest capacity to decrease TNF-α effect on fibroblastś cell cycle by reducing about 10% the number of cells in phase S. Although this effect was rather moderate, it has to be considered that the continuous exposure of intestinal cells to Q due to the repeated consumption of BW products could help prevent/regulate cell cycle alteration during intestinal inflammation. A possible mechanism by which BW products could regulate the TNF-α-altered cell cycle checkpoint function is the modulation of cyclins 61, 62 as well as AhR63, 64.
Besides fibroblasts migration and cell cycle, TNF-α also promotes inflammation through the generation of reactive oxygen species (ROS) in the mitochondria, reducing the mitochondrial membrane potential and causing cell death 65, 66. In this study, TNF-α (20 ng/mL, 48 h) affected Δψm only in the presence of 1.67% (v/v) digested control. Co-treatment with the BW products increased cells sensitivity to TNF-α at the mitochondrial level. In agreement with our results, García-Nebot et al. (2011) reported a reduction in Δψm in Caco-2 cells exposed to digested caseinophosphopeptides59. By contrast, a blackberry extract suppressed acrylamide-induced alteration of Δψm67. These contradictory results could be explained by the generation of unidentified molecules generated during the in vitro digestion, exerting dissimilar effects on Δψm. We also have to consider that, despite the vast majority of studies has described BW as an antioxidant food 68, 69, the pro-oxidant activity of methanolic BW extracts has also been reported 70, what could partially explain the alteration of Δψm observed.
The alteration exerted on cell migration and cell cycle in the myofibroblasts by TNF-α is in concordance with the connection established between cell cycle and cell migration regulation reported in different cellular models71, 72. The attenuation of the effect of the pro-inflammatory cytokine on cell cycle alteration exerted by some of the BW products investigated was accompanied by a reduction of cell migration in TNF-α-stimulated cells, indicating a link between these two processes. However, this effect was less clear in other products like fBWg, where the effect of TNF-α was mitigated on cell migration but not on cell cycle. These results highlight the need of more studies in order to define the role of BW against chronic diseases such as IBDs, the molecule(s) involved in this response, and the mechanisms associated.
Conclusion
There is a growing number of in vitro studies investigating the biological activity of BW and/or BW-derived products. However, a limited number of investigations have looked at their anti-inflammatory activity. A PubMed search for “buckwheat and inflammation” will give less than 20 studies in the past 20 years. This indicates that the anti-inflammatory activity of BW is a field that needs to be explored. This study investigated for the first time the role of different digested BW products as well as their individual flavonoids in a TNF-α-treated myofibroblasts of colon cell model. In general, under the conditions of our study, the different BW products were able to modulate the response of the myofibroblasts in the presence of TNF-α. Our study also highlights the importance of testing in parallel bioactive rich nutritional products and their bioactive flavonoids to determine which compound(s) are responsible for the effects observed. However, the in vitro results obtained in this study cannot be extrapolated to in vivo models. Thus, it has to be considered that the concentration of polyphenols (such as Ru and Q) reached in the gastrointestinal tract after prolonged consumption of BW can be higher than that used in this study.
In conclusion, our results showed that the whole BW products reduced the effect of TNF-α on migration and cell cycle in the myofibroblasts, independently of their polyphenols content. Although the response observed in some cases was modest (expected for natural foodstuff), the effect exerted by BW products on the processes investigated might represent an alternative strategy against the detrimental effects caused during chronic intestinal inflammation. However, the study of mechanisms related to the inflammatory process (cytokines production, transcription factors, and/or pathways involved), as well as more human trials, and animal studies need to be performed to determine the benefits of BW against intestinal chronic diseases.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by REFRESH project (FP7-REGPOT-2010–1-264103) – Unlocking the potential of the Institute of Animal Reproduction and Food Research for strengthening integration with the European Research Area and region development; postdoctoral award 16POST30690001 from the American Heart Association (to J.A.G.-B.); J.M.L.L. is the holder of a “Ramon y Cajal” contract RyC-2015–18083 (Immunonutritional-based imprinting on non-alcoholic fatty liver disease).
Footnotes
CONFLICT OF INTEREST
Authors declare no competing financial or personal interest, nor having an association with any individuals or organizations that could have influenced inappropriately the submitted work.
References
- 1.Knight-Sepulveda K, Kais S, Santaolalla R and Abreu MT, Diet and Inflammatory Bowel Disease, Gastroenterol Hepatol (N Y), 2015, 11, 511–520. [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis JD and Abreu MT, Diet as a Trigger or Therapy for Inflammatory Bowel Diseases, Gastroenterology, 2017, 152, 398–+. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZL, Zhou ML, Tang Y, Li FL, Tang YX, Shao JR, Xue WT and Wu YM, Bioactive compounds in functional buckwheat food, Food Res Int, 2012, 49, 389–395. [Google Scholar]
- 4.Giménez-Bastida JA, Piskula M and Zielinski H, Recent advances in development of gluten-free buckwheat products, Trends Food Sci Tech, 2015, 44, 58–65. [Google Scholar]
- 5.Wieslander G, Fabjan N, Vogrincic M, Kreft I, Janson C, Spetz-Nystrom U, Vombergar B, Tagesson C, Leanderson P and Norback D, Eating Buckwheat Cookies Is Associated with the Reduction in Serum Levels of Myeloperoxidase and Cholesterol: A Double Blind Crossover Study in Day-Care Centre Staffs, Tohoku J Exp Med, 2011, 225, 123–130. [DOI] [PubMed] [Google Scholar]
- 6.Schramm DD, Karim M, Schrader HR, Holt RR, Cardetti M and Keen CL, Honey with high levels of antioxidants can provide protection to healthy human subjects, J Agric Food Chem, 2003, 51, 1732–1735. [DOI] [PubMed] [Google Scholar]
- 7.Bojnanska T, Francakova H, Chlebo P and Vollmannova A, Rutin Content in Buckwheat Enriched Bread and Influence of its Consumption on Plasma Total Antioxidant Status, Czech J Food Sci, 2009, 27, S236–S240. [Google Scholar]
- 8.Krkoskova B and Mrazova Z, Prophylactic components of buckwheat, Food Res Int, 2005, 38, 561–568. [Google Scholar]
- 9.Kreft S, Knapp M and Kreft I, Extraction of rutin from buckwheat (Fagopyrum esculentumMoench) seeds and determination by capillary electrophoresis, J Agric Food Chem, 1999, 47, 4649–4652. [DOI] [PubMed] [Google Scholar]
- 10.Wiczkowski W, Szawara-Nowak D, Debski H, Mitrus J and Horbowicz M, Comparison of flavonoids profile in sprouts of common buckwheat cultivars and wild tartary buckwheat, Int J Food Sci Tech, 2014, 49, 1977–1984. [Google Scholar]
- 11.Olthof MR, Hollman PC, Buijsman MN, van Amelsvoort JM and Katan MB, Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans, J Nutr, 2003, 133, 1806–1814. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Tie X, Bao B, Wu X and Zhang Y, Metabolism of flavone C-glucosides and p-coumaric acid from antioxidant of bamboo leaves (AOB) in rats, Br J Nutr, 2007, 97, 484–494. [DOI] [PubMed] [Google Scholar]
- 13.Manach C, Morand C, Demigne C, Texier O, Regerat F and Remesy C, Bioavailability of rutin and quercetin in rats, FEBS Lett, 1997, 409, 12–16. [DOI] [PubMed] [Google Scholar]
- 14.Power KA, Lu JT, Monk JM, Lepp D, Wu W, Zhang C, Liu R, Tsao R, Robinson LE, Wood GA and Wolyn DJ, Purified rutin and rutin-rich asparagus attenuates disease severity and tissue damage following dextran sodium sulfate-induced colitis, Mol Nutr Food Res, 2016, 60, 2396–2412. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Sarrias A, Larrosa M, Tomas-Barberan FA, Dolara P and Espin JC, NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts, Br J Nutr, 2010, 104, 503–512. [DOI] [PubMed] [Google Scholar]
- 16.Espley RV, Butts CA, Laing WA, Martell S, Smith H, McGhie TK, Zhang J, Paturi G, Hedderley D, Bovy A, Schouten HJ, Putterill J, Allan AC and Hellens RP, Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice, J Nutr, 2014, 144, 146–154. [DOI] [PubMed] [Google Scholar]
- 17.Nafees S, Rashid S, Ali N, Hasan SK and Sultana S, Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFkappaB/MAPK pathway, Chem Biol Interact, 2015, 231, 98–107. [DOI] [PubMed] [Google Scholar]
- 18.Romier-Crouzet B, Van De Walle J, During A, Joly A, Rousseau C, Henry O, Larondelle Y and Schneider YJ, Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells, Food Chem Toxicol, 2009, 47, 1221–1230. [DOI] [PubMed] [Google Scholar]
- 19.Kaulmann A, Legay S, Schneider YJ, Hoffmann L and Bohn T, Inflammation related responses of intestinal cells to plum and cabbage digesta with differential carotenoid and polyphenol profiles following simulated gastrointestinal digestion, Mol Nutr Food Res, 2016, 60, 992–1005. [DOI] [PubMed] [Google Scholar]
- 20.West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, Coccia M, Gortz D, This S, Stockenhuber K, Pott J, Friedrich M, Ryzhakov G, Baribaud F, Brodmerkel C, Cieluch C, Rahman N, Muller-Newen G, Owens RJ, Kuhl AA, Maloy KJ, Plevy SE, Oxford IBDCI, Keshav S, Travis SPL and Powrie F, Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease, Nat Med, 2017, 23, 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT and Turner JR, Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression, Am J Pathol, 2005, 166, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roulis M and Flavell RA, Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease, Differentiation, 2016, 92, 116–131. [DOI] [PubMed] [Google Scholar]
- 23.Angel-Morales G, Noratto G and Mertens-Talcott S, Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: potential role of microRNA-126, Food Funct, 2012, 3, 745–752. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Banerjee N, Barnes RC, Pfent CM, Talcott ST, Dashwood RH and Mertens-Talcott SU, Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo, Mol Carcinog, 2017, 56, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasson L, Canova C, Vettorato MG, Savarino E and Zanotti R, Influence of Diet on the Course of Inflammatory Bowel Disease, Digest Dis Sci, 2017, 62, 2087–2094. [DOI] [PubMed] [Google Scholar]
- 26.Kaulmann A and Bohn T, Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls, Oxid Med Cell Longev, 2016, 2016, 9346470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen V, Hansen AK and Heitmann BL, Potential Impact of Diet on Treatment Effect from Anti-TNF Drugs in Inflammatory Bowel Disease, Nutrients, 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zielinski H, Michalska A, Amigo-Benavent M, del Castillo MD and Piskula MK, Changes in protein quality and antioxidant properties of buckwheat seeds and groats induced by roasting, J Agric Food Chem, 2009, 57, 4771–4776. [DOI] [PubMed] [Google Scholar]
- 29.Szawara-Nowak R, Koutsidis G, Wiczkowski W and Zielinski H, Evaluation of the in vitro inhibitory effects of buckwheat enhanced wheat bread extracts on the formation of advanced glycation end-products (AGEs), Lwt-Food Sci Technol, 2014, 58, 327–334. [Google Scholar]
- 30.Wronkowska M, Honke J and Piskula MK, Effect of Solid-State Fermentation with Rhizopus Oligosporus on Bioactive Compounds and Antioxidant Capacity of Raw and Roasted Buckwheat Groats, Ital J Food Sci, 2015, 27, 424–431. [Google Scholar]
- 31.Zielinska D, Szawara-Nowak D and Zieliński H, Determination of the antioxidant activity of rutin and its contribution to the antioxidant capacity of diversified buckwheat origin material by updated analytical strategies, Polish Journal of Food and Nutrition Science 2010, 60, 315–321. [Google Scholar]
- 32.Delgado-Andrade C, Conde-Aguilera JA, Haro A, de la Cueva SP and Rufian-Henares JA, A combined procedure to evaluate the global antioxidant response of bread, J Cereal Sci, 2010, 52, 239–246. [Google Scholar]
- 33.Saini S, Liu T and Yoo J, TNF-alpha stimulates colonic myofibroblast migration via COX-2 and Hsp27, J Surg Res, 2016, 204, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gimenez-Bastida JA, Gonzalez-Sarrias A, Vallejo F, Espin JC and Tomas-Barberan FA, Hesperetin and its sulfate and glucuronide metabolites inhibit TNF-alpha induced human aortic endothelial cell migration and decrease plasminogen activator inhibitor-1 (PAI-1) levels, Food & Function, 2016, 7, 118–126. [DOI] [PubMed] [Google Scholar]
- 35.Cilla A, Laparra JM, Alegria A, Barbera R and Farre R, Antioxidant effect derived from bioaccessible fractions of fruit beverages against H2O2-induced oxidative stress in Caco-2 cells, Food Chem, 2008, 106, 1180–1187. [Google Scholar]
- 36.Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR and Cave D, An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report, Nutr J, 2014, 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Cagno R, De Angelis M, Auricchio S, Greco L, Clarke C, De Vincenzi M, Giovannini C, D’Archivio M, Landolfo F, Parrilli G, Minervini F, Arendt E and Gobbetti M, Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients, Appl Environ Microbiol, 2004, 70, 1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu LN, Cai XT, Yan J, Luo Y, Shao M, Lu Y, Sun ZG and Cao P, In Vivo and In Vitro Antinociceptive Effect of Fagopyrum cymosum (Trev.) Meisn Extracts: A Possible Action by Recovering Intestinal Barrier Dysfunction, Evid-Based Compl Alt, 2012, DOI: Artn98380110.1155/2012/983801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hur SJ, Park SJ and Jeong CH, Effect of buckwheat extract on the antioxidant activity of lipid in mouse brain and its structural change during in vitro human digestion, J Agric Food Chem, 2011, 59, 10699–10704. [DOI] [PubMed] [Google Scholar]
- 40.Rieder F and Fiocchi C, Intestinal fibrosis in inflammatory bowel disease - Current knowledge and future perspectives, J Crohns Colitis, 2008, 2, 279–290. [DOI] [PubMed] [Google Scholar]
- 41.Apostolaki M, Armaka M, Victoratos P and Kollias G, Cellular mechanisms of TNF function in models of inflammation and autoimmunity, Curr Dir Autoimmun, 2010, 11, 1–26. [DOI] [PubMed] [Google Scholar]
- 42.Drygiannakis I, Valatas V, Sfakianaki O, Bourikas L, Manousou P, Kambas K, Ritis K, Kolios G and Kouroumalis E, Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: implication in intestinal fibrosis, J Crohns Colitis, 2013, 7, 286–300. [DOI] [PubMed] [Google Scholar]
- 43.Gimenez-Bastida JA, Martinez-Florensa M, Espin JC, Tomas-Barberan FA and Garcia-Conesa MT, A Citrus Extract Containing Flavanones Represses Plasminogen Activator Inhibitor-1 (PAI-1) Expression and Regulates Multiple Inflammatory, Tissue Repair, and Fibrosis Genes in Human Colon Fibroblasts, J Agr Food Chem, 2009, 57, 9305–9315. [DOI] [PubMed] [Google Scholar]
- 44.Gimenez-Bastida JA, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan F, Espin JC and Garcia-Conesa MT, Intestinal Ellagitannin Metabolites Ameliorate Cytokine-Induced Inflammation and Associated Molecular Markers in Human Colon Fibroblasts, J Agr Food Chem, 2012, 60, 8866–8876. [DOI] [PubMed] [Google Scholar]
- 45.Xue Z, Li D, Yu W, Zhang Q, Hou X, He Y and Kou X, Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor, Food Funct, 2017, 8, 1414–1437. [DOI] [PubMed] [Google Scholar]
- 46.Monteleone I, Zorzi F, Marafini I, Di Fusco D, Dinallo V, Caruso R, Izzo R, Franze E, Colantoni A, Pallone F and Monteleone G, Aryl hydrocarbon receptor-driven signals inhibit collagen synthesis in the gut, Eur J Immunol, 2016, 46, 1047–1057. [DOI] [PubMed] [Google Scholar]
- 47.Liu HM, Liao JF and Lee TY, Farnesoid X receptor agonist GW4064 ameliorates lipopolysaccharide-induced ileocolitis through TLR4/MyD88 pathway related mitochondrial dysfunction in mice, Biochem Biophys Res Commun, 2017, 490, 841–848. [DOI] [PubMed] [Google Scholar]
- 48.Barrientos S, Brem H, Stojadinovic O and Tomic-Canic M, Clinical application of growth factors and cytokines in wound healing, Wound Repair Regen, 2014, 22, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han M, Song Y and Zhang X, Quercetin Suppresses the Migration and Invasion in Human Colon Cancer Caco-2 Cells Through Regulating Toll-like Receptor 4/Nuclear Factor-kappa B Pathway, Pharmacogn Mag, 2016, 12, S237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben Sghaier M, Pagano A, Mousslim M, Ammari Y, Kovacic H and Luis J, Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells, Biomed Pharmacother, 2016, 84, 1972–1978. [DOI] [PubMed] [Google Scholar]
- 51.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM and Chan AT, A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis, Gastroenterology, 2013, 145, 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gimenez-Bastida JA and Zielinski H, Buckwheat as a Functional Food and Its Effects on Health, J Agr Food Chem, 2015, 63, 7896–7913. [DOI] [PubMed] [Google Scholar]
- 53.Bhattacharyya S, Borthakur A, Dudeja PK and Tobacman JK, Carrageenan induces cell cycle arrest in human intestinal epithelial cells in vitro, J Nutr, 2008, 138, 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKernan DP and Egan LJ, The intestinal epithelial cell cycle: uncovering its ‘cryptic’ nature, Curr Opin Gastroenterol, 2015, 31, 124–129. [DOI] [PubMed] [Google Scholar]
- 55.Chen D, Daniel KG, Kuhn DJ, Kazi A, Bhuiyan M, Li L, Wang Z, Wan SB, Lam WH, Chan TH and Dou QP, Green tea and tea polyphenols in cancer prevention, Front Biosci, 2004, 9, 2618–2631. [DOI] [PubMed] [Google Scholar]
- 56.Gupta SC, Kim JH, Prasad S and Aggarwal BB, Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals, Cancer Metast Rev, 2010, 29, 405–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harishkumar M, Masatoshi Y, Hiroshi S, Tsuyomu I and Masugi M, Revealing the mechanism of in vitro wound healing properties of Citrus tamurana extract, Biomed Res Int, 2013, 2013, 963457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darzynkiewicz Z, Williamson B, Carswell EA and Old LJ, Cell cycle-specific effects of tumor necrosis factor, Cancer Res, 1984, 44, 83–90. [PubMed] [Google Scholar]
- 59.Garcia-Nebot MJ, Cilia A, Alegria A and Barbera R, Caseinophosphopeptides exert partial and site-specific cytoprotection against H2O2-induced oxidative stress in Caco-2 cells, Food Chem, 2011, 129, 1495–1503. [Google Scholar]
- 60.Araujo JR, Goncalves P and Martel F, Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines, Nutr Res, 2011, 31, 77–87. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez-Sarrias A, Ma H, Edmonds ME and Seeram NP, Maple polyphenols, ginnalins A-C, induce S- and G2/M-cell cycle arrest in colon and breast cancer cells mediated by decreasing cyclins A and D1 levels, Food Chem, 2013, 136, 636–642. [DOI] [PubMed] [Google Scholar]
- 62.Liang YC, Lin-Shiau SY, Chen CF and Lin JK, Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate, J Cell Biochem, 1999, 75, 1–12. [PubMed] [Google Scholar]
- 63.Puga A, Marlowe J, Barnes S, Chang CY, Maier A, Tan Z, Kerzee JK, Chang X, Strobeck M and Knudsen ES, Role of the aryl hydrocarbon receptor in cell cycle regulation, Toxicology, 2002, 181–182, 171–177. [DOI] [PubMed] [Google Scholar]
- 64.Woo H, Lee J, Park D and Jung E, Protective Effect of Mulberry (Morus alba L.) Extract against Benzo[a]pyrene Induced Skin Damage through Inhibition of Aryl Hydrocarbon Receptor Signaling, J Agric Food Chem, 2017, 65, 10925–10932. [DOI] [PubMed] [Google Scholar]
- 65.Baregamian N, Song J, Bailey CE, Papaconstantinou J, Evers BM and Chung DH, Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis, Oxid Med Cell Longev, 2009, 2, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim JJ, Lee SB, Chung JS and Yoo YD, TNF-alpha-induced ROS Production Triggering Apoptosis is Directly Linked to Romo1 and Bcl-X-L, Free Radical Bio Med, 2009, 47, S44–S45. [DOI] [PubMed] [Google Scholar]
- 67.Chen W, Su H, Xu Y and Jin C, In vitro gastrointestinal digestion promotes the protective effect of blackberry extract against acrylamide-induced oxidative stress, Sci Rep, 2017, 7, 40514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szawara-Nowak D, Baczek N and Zielinski H, Antioxidant capacity and bioaccessibility of buckwheat-enhanced wheat bread phenolics, J Food Sci Tech Mys, 2016, 53, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malgorzata W, Konrad PM and Zielinski H, Effect of roasting time of buckwheat groats on the formation of Maillard reaction products and antioxidant capacity, Food Chem, 2016, 196, 355–358. [DOI] [PubMed] [Google Scholar]
- 70.Przybylski R, Lee YC and Eskin NAM, Antioxidant and radical-scavenging activities of buckwheat seed components, J Am Oil Chem Soc, 1998, 75, 1595–1601. [Google Scholar]
- 71.Boehm M and Nabel EG, Cell cycle and cell migration - New pieces to the puzzle, Circulation, 2001, 103, 2879–2881. [DOI] [PubMed] [Google Scholar]
- 72.Bonneton C, Sibarita JB and Thiery JP, Relationship between cell migration and cell cycle during the initiation of epithelial to fibroblastoid transition, Cell Motil Cytoskeleton, 1999, 43, 288–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


