The auxin biosynthesis gene, GmYUC2a, is required for root and nodule development in soybean, showing that local auxin biosynthesis contributes significantly to determine nodule development.
Keywords: Auxin, nodulation, nodule development, root development, root hair deformation, soybean
Abstract
Auxin plays central roles in rhizobial infection and nodule development in legumes. However, the sources of auxin during nodulation are unknown. In this study, we analyzed the YUCCA (YUC) gene family of soybean and identified GmYUC2a as an important regulator of auxin biosynthesis that modulates nodulation. Following rhizobial infection, GmYUC2a exhibited increased expression in various nodule tissues. Overexpression of GmYUC2a (35S::GmYUC2a) increased auxin production in soybean, resulting in severe growth defects in root hairs and root development. Upon rhizobial infection, 35S::GmYUC2a hairy roots displayed altered patterns of root hair deformation and nodule formation. Root hair deformation occurred mainly on primary roots, and nodules formed exclusively on primary roots of 35S::GmYUC2a plants. Moreover, transgenic 35S::GmYUC2a composite plants showed delayed nodule development and a reduced number of nodules. Our results suggest that GmYUC2a plays an important role in regulating both root growth and nodulation by modulating auxin balance in soybean.
Introduction
Nodulation in legumes is coordinated by two distinct developmental programs, rhizobial infection and nodule organogenesis. Flavonoids exuded by legume roots induce Nod Factor (NF) production by rhizobia, which are subsequently perceived by root epidermal cells of legume host plants to initiate rhizobial infection and nodule organogenesis (i.e. cell divisions) in the root cortex (Mathesius, 2001; Madsen et al., 2003; Wasson et al., 2006). Rhizobia enter growing root hairs and progress towards the root cortex through infection threads; simultaneously, some root cortical cells re-enter the cell cycle and contribute to the initiation of the nodule primordia (Ferguson et al., 2010, 2019). These processes eventually converge where rhizobia are released in infection droplets into the dividing cortical cells of the nodule primordia. Each process involves a highly complex gene network; however, little is known about how they are spatially regulated and integrated.
It has long been known that rhizobial infection and root cortical cell divisions are regulated by endogenous cues (e.g. plant hormones) following rhizobial recognition (Ferguson and Mathesius, 2014). For example, auxin plays a central role in nodule organogenesis, similar to its function in lateral roots (Kohlen et al., 2018). Auxin stimulates cortical cell division in response to rhizobial infection, leading to the formation of determinate or indeterminate nodules (Thimann, 1936; Van Noorden et al., 2006; Suzaki et al., 2012). The distribution of auxin and its peak concentration have been studied during nodule primordium formation and development in various legumes, including Medicago truncatula, Lotus japonicus, and soybean (Pacios-Bras et al., 2003; Bustos-Sanmamed et al., 2013; Turner et al., 2013). However, external application of auxin or inhibitors of auxin biosynthesis or action typically prevents nodule development (Thimann, 1936; Kuppusamy et al., 2009; reviewed in Ferguson and Mathesius, 2003). Thus, auxin gradients and local auxin maxima, which are crucial for nodule initiation and development, are precisely controlled. The auxin response of cortical cells is also altered during nodule initiation and nodule primordium formation (Mathesius et al., 1998; Suzaki et al., 2012). Nodule numbers are regulated by auxin receptors and auxin response factor genes (ARF genes), which are targeted by miR160/miR167 and miR393, respectively, with some differences between determinate and indeterminate legume nodule development (Turner et al., 2013; Nizampatnam et al., 2015; Wang et al., 2015; Cai et al., 2017).
Evidence indicates that the distribution and maxima of auxin are regulated mainly by local auxin transport. Early studies indicated that synthetic auxin transport inhibitors such as 2,3,5-triiodobenzoic acid or 1-N-naphthylphthalamic acid triggered the formation of nodule-like structures or pseudonodules in the absence of rhizobia in some legumes (Hirsch et al., 1989). This suggests that the inhibition of auxin transport is sufficient to establish local auxin maxima that can activate programs governing nodule initiation and organogenesis. A recent computational study supported the notion that auxin transport is required for nodule initiation and development, and that reduced auxin export plays a dominant role in auxin accumulation at sites of nodule initiation (Deinum et al., 2012). Previously, it was shown that MtLAX1 and MtPIN, which encode auxin import and export proteins, respectively, are expressed during nodulation (de Billy et al., 2001), and silencing MtPIN genes reduced nodule numbers (Huo et al., 2006). Recently, MtLAX2, which is a paralog of Arabidopsis AUX1, was reported to positively regulate nodulation in M. truncatula (Roy et al., 2017). These results confirm the importance of auxin transport in nodule development, but how these transporters (especially PIN proteins) are regulated and subcellularly localized in response to rhizobial infection and nodule formation is unknown.
Using M. truncatula, Breakspear et al. (2014) demonstrated that auxin signaling is also required for rhizobial infection of root hairs, with several auxin-responsive genes, including ARF16a, GH3.1, IAA9, and SAUR1, being induced following inoculation. The expression of auxin-responsive marker genes was also shown to be induced by rhizobial infection of root hairs, and mutation of ARF16a impaired early infection events, further indicating that auxin is required for infection. Collectively, this suggests that increased auxin levels and auxin signaling are necessary for rhizobial infection, and, importantly, that the pathway of auxin-mediated rhizobial infection is conserved in both determinate and indeterminate nodulating legumes (Breakspear et al., 2014). Recently, in Lotus japonicus, an optimized DII-based auxin accumulation sensor was used, and confirmed the appearance of auxin accumulation in root hairs in response to NF (Nadzieja et al., 2018). The precise source of auxin and the molecular mechanisms controlled by auxin signaling are yet to be fully determined.
An array of Arabidopsis mutants was used to establish that genes involved in auxin metabolic pathways are differentially regulated during various development processes (Ljung, 2013). YUCCA (YUC) flavin monooxygenases have been discovered in Arabidopsis and confirmed to be responsible for synthesizing indole-3-acetic acid (IAA) from indole-3-pyruvic acid (IPyA) (Mashiguchi et al., 2011; Stepanova et al., 2011; Won et al., 2011), and appear to be involved in the major pathways for IAA biosynthesis in plants (Zhao, 2012). Identification and analysis of YUC genes in various species including Arabidopsis (Cheng, 2006), petunia (Tobeña-Santamaria et al., 2002), and rice (Yamamoto et al., 2007; Woo et al., 2007) have illustrated their phylogenetic relationship and crucial roles in plant development (Zhao, 2008; Mashiguchi et al., 2011; Poulet and Kriechbaumer, 2017). Changes in expression levels of YUC genes have a great impact on auxin biosynthesis, leading to defects in root development (Mashiguchi et al., 2011; Stepanova et al., 2011; Won et al., 2011). For example, the root phenotype of the Atyuc1D mutant is similar to that of known auxin overproduction mutants with shorter primary roots and numerous long root hairs (Zhao et al., 2001). Overexpression of YUCCA2 and YUCCA3 also causes a similar root phenotype (Zhao et al., 2001). Single or double knockout alleles of YUCCA and YUCCA2 show no obvious phenotypic differences, suggesting that there is likely to be redundancy between their roles in plant development (Zhao et al., 2001).
Here, we report our findings from studies designed to determine whether auxin biosynthesis in roots and root hairs contributes to rhizobial infection and nodule organogenesis in soybean. By initially analyzing publicly available expression data, we identified the differential expression of a large family of YUCCA genes in various organs and tissues of soybean plants. In particular, we systematically analyzed the function of GmYUC2a, which is highly expressed in soybean nodules. We found that GmYUC2a regulates auxin biosynthesis, and its overexpression results in severe growth defects in primary root elongation, lateral root development, and root hair elongation. Our results further revealed that GmYUC2a is responsive to rhizobial infection, and its promoter exhibited strong activity in maturing nodules; additionally, GmYUC2a mediates not only early nodule development, but also nodule senescence. Collectively, our findings demonstrate that GmYUC2a-mediated local auxin biosynthesis contributes to rhizobial infection and nodule organogenesis, including nodule senescence, in soybean nodules.
Materials and methods
Phylogenetic analysis
All sequences were downloaded from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html). Using ClustalX 1.83 (Thompson et al., 1997) and MEGA6.0 (Tamura et al., 2011), a phylogenetic tree was constructed based on the amino acid sequences (http://phytozome.jgi.doe.gov/pz/portal.html) of the homologous proteins of AtYUC family members in soybean and M. truncatula with 1000 bootstraps.
Gene structure analysis
Exon–intron structure information for GmYUC genes was obtained using Phytozome v12.0. A gene structure schematic diagram was made using the Gene Structure Display Server (Guo et al., 2007).
Heatmaps
Expression data for all GmYUC genes were collected from SoyBase (http://soybase.org/soyseq/;Severin et al., 2010). Heatmap Illustrator (v1.0) was used to generate heatmaps of differentially expressed GmYUC genes (Deng et al., 2014).
Plant materials and growth conditions
Soybean (Glycine max [L.] Merrill) cv Williams 82 plants were grown in a greenhouse (16 h of light/8 h of dark; 25 °C; 50% relative humidity) in vermiculite and irrigated with a nitrogen-deficient solution as described by Wang et al. (2009). Bradyrhizobium diazoefficiens USDA110 was used for all nodulation experiments (OD600=0.08). To analyze gene expression in response to rhizobial infection, roots of 10-day-old plants were inoculated with 30 ml of a suspension of B. diazoefficiens. The plants were watered every 7 d, alternating between a nitrogen-deficient nutrient solution and water. Different soybean tissues were harvested at specified time points after inoculation. For root samples, the de-nodulated roots were used for real-time quantitative reverse transcription PCR (RT–qPCR) assays.
RNA extraction and RT–qPCR
To monitor transcript levels of GmYUC2a, total RNA was extracted from leaves, roots, and nodules using Trizol reagent (Tiangen Biotech [Beijing] Co. Ltd, Beijing, China), and 2 μg of total RNA treated with DNase I (Invitrogen, Carlsbad, CA, USA) were used to synthesize the first cDNA strand with a FastQuant RT Kit (Tiangen Biotech [Beijing] Co. Ltd). RT–qPCR was performed using SuperReal PreMix Plus (SYBR Green; Tiangen Biotech [Beijing] Co. Ltd) with gene-specific primers. The housekeeping gene GmELF1b was used as a reference (Jian et al., 2008), and relative expression was measured using the 2–ΔΔCT method (Livak and Schmittgen, 2001). All primers used in this study are listed in Supplementary Table S3 at JXB online. In order to ensure the primer specificity of GmYUC2a, GmYUC2b, and GmYUC2c used for RT–qPCR, the products were amplified via PCR and then ligated to the T-Vector pMD-19 (Takara Bio Inc. Code No. 3271) for sequencing. All primers used in qRT–PCR are included in Supplementary Table S3.
Vector construction
To generate the promoter::GUS (β-glucuronidase) reporter fusion construct of GmYUC2a, the genomic region upstream of the GmYUC2a coding sequence (1467 bp) was amplified by PCR from Williams 82 genomic DNA and cloned upstream of the GUS coding sequence in pCAMBIA3301 using BamHI and BglII. To make the overexpression construct of GmYUC2a, the coding sequence of GmYUC2a (1248 bp) was amplified by PCR using KOD DNA polymerase (Toyobo, Osaka, Japan) from Williams 82 cDNA and inserted into the binary vector pTF101-GFP directly under the control of the Cauliflower mosaic virus 35S promoter (35S) sequence using SmaI and BamHI. To make the RNAi construct, a 122 bp segment of the GmYUC2a coding sequence was amplified from Williams 82 cDNA by PCR and cloned into pTCK303 using KpnI/SpeI and BamHI/SacI in the sense and antisense orientations. Gene-specific primers are provided in Supplementary Table S3.
DNA extraction and characterization of transgenic roots
DNA was extracted from hairy root samples using a modified version of the cetyltrimethylammonium bromide (CTAB) method (Porebski et al., 1997). The Bar or HygromycinB (Hyg) gene was used for molecular validation of the transformed hairy roots according to the selective marker in the binary vectors utilized for Agrobacterium rhizogenes-mediated hairy root transformation (Kereszt et al., 2007). The Bar- and Hyg-specific primers used are listed in Supplementary Table S3.
Soybean hairy root transformation and subsequent B. diazoefficiens inoculation
Soybean hairy root transformation was performed as described previously with modifications (Kereszt et al., 2007; Jian et al., 2009; Wang et al., 2014). Agrobacterium rhizogenes strain K599 was used for transformation. Transgenic composite plants were transferred to pots (13 cm×10 cm×8.5 cm) containing vermiculite and watered with nitrogen-deficient nutrient solution, grown for 7 d (16 h of light/8 h of dark; 25 °C; 50% relative humidity), and then inoculated with 30 ml of B. diazoefficiens USDA110 (OD600=0.08) per plant.
Root hair deformation and nodulation assays
To analyze root hair deformation and nodule formation, transgenic roots were collected and stained with methylene blue. Root hair deformation was analyzed under a light microscope at 3, 6, and 10 days after inoculation (DAI) as described previously (Wang et al., 2014). Nodule numbers were determined at specified time points after rhizobial inoculation.
Histochemical localization of GUS expression
To examine tissue- and cell-specific promoter activity of GmYUC2a, the GUS reporter gene was expressed under the control of the GmYUC2a promoter (1467 bp located directly upstream of GmYUC2a), and transgenic roots and nodules were stained as described previously for the histochemical localization of GUS expression (Jefferson et al., 1987).
Auxin response assay
For gene expression analysis in response to exogenous auxin application, soybean seeds (cv Williams 82) were germinated in vermiculite irrigated once with a low-nitrate solution (Wang et al., 2009). Seven-day-old seedlings were treated with or without 1 μM 2,4-dichlorophenoxyacetic acid (2,4-D) or 1-naphthaleneacetic acid (NAA) (Turner et al., 2013; Wang et al., 2015). 6-Benzyladenine (6-BA; 5 μM) was used as a control for analyzing the expression of GmNIN. After 24 h, the roots were collected and used for analyzing gene expression. For phenotypic analysis of hairy roots in response to 2,4-D, composite plants harboring K599 were transplanted into vermiculite and watered with the low-nitrate solution containing different concentrations of 2,4-D (0, 0.2, and 0.4 μM). Ten days after transplanting, the hairy root phenotypes were noted and root length measured.
Imaging and histological analysis of nodules
Nodules from transgenic roots were analyzed using an Olympus microscope (SZ2-ILST; Olympus Corp., Tokyo, Japan). To produce transverse and longitudinal sections, roots and nodules at different stages after inoculation were collected and fixed in FAA solution, and then dehydrated in a graded alcohol series (Charon et al., 1997). Nodule sections 25 μm thick were produced using a vibratome (VT1200S; Leica, Wetzlar, Germany), mounted between a slide and coverslip, and observed with an Olympus microscope (CX31; Olympus Corp., Tokyo, Japan).
Measurement of IAA
To determine the IAA content, transgenic hairy roots harboring an empty vector (EV) or the 35S::GmYUC2a construct were collected 10 d after transplantation. IAA was extracted and the IAA content was measured using LC–tandem MS as described by Fu et al. (2012) and Sun et al. (2015).
Statistical analysis
Data were analyzed using Student’s t-tests or one-way ANOVAs with SigmaPlot 10.0 or GraphPad Prism 5. Statistically significant differences are marked in the figures with ‘***’ (P<0.001) or ‘ns’ (not significant; P>0.05). Different letters were used to indicate significant differences by the Student–Newman–Kuels test (P<0.05).
Results
Phylogenetic analysis of YUCCA family genes in Arabidopsis, soybean, and M. truncatula
YUCCA proteins regulate the limiting step of auxin biosynthesis. To identify the YUCCA gene family of soybean and M. truncatula, BLAST searches of the Glycine max (v2.0) and M. truncatula (Mt4.0 v1) genomes were conducted using protein sequences of 11 Arabidopsis YUCCA-encoding genes (www.arabidopsis.org). Fourteen putative MtYUC-and 22 putative GmYUC-encoding loci were identified based on the assigned nomenclature/classification, and each showed high sequence similarity (Supplementary Fig. S1; Supplementary Table S1).
The soybean genome has experienced duplication and diploidization (Schmutz et al., 2010). To analyze the evolutionary relationships of YUCs among Arabidopsis, soybean, and M. truncatula, a phylogenetic tree was constructed, which was generated in MEGA6.0 with 1000 bootstraps using amino acid sequences (Supplementary Dataset S1). Based on protein similarity and the phylogenetic tree (Supplementary Fig. S1A; Supplementary Table S1), the soybean YUC proteins were named according to the YUC family cluster from Arabidopsis. As shown in Supplementary Fig. S1A, the GmYUCs were divided into five subfamilies: GmYUC5/8/9 (GmYUC5-8-9a/b/c/d), GmYUC3/7 (GmYUC3-7a/b), GmYUC2/6 (GmYUC2a/b/c/d, GmYUC6a/b/c/d), GmYUC1/4 (GmYUC1-4a/b/c/d), and GmYUC10/11 (GmYUC10a/b, GmYUC11a/b). The homologous YUC family genes in M. truncatula were more closely related to those of soybean than those of Arabidopsis (Supplementary Fig. S1A), consistent with them both being legumes. To further understand their evolutionary relationship, the gene structures were analyzed with the Gene Structure Display Server using genomic and coding sequences (Supplementary Dataset S1). Strong conservation between the gene structures was found, with each composed of four exons and three introns (Supplementary Fig. S1B).
GmYUC genes show divergent expression patterns in soybean
Given that the GmYUC gene family is large and each GmYUC gene has a variable number of duplicates, we speculated that GmYUC genes play divergent roles in the control of auxin biosynthesis in a tissue-/organ-specific pattern to modulate plant growth and environmental responses. To explore the expression patterns of GmYUC genes, we analyzed the expression of 22 GmYUC genes in different tissues, mainly including young leaf, flower, pod, pod shell, root, nodule (20–25 d after rhizobial inoculation), and seeds collected at different developmental stages, using RNA sequencing (RNA-Seq) data from SoyBase (http://soybase.org/;Severin et al., 2010). Interestingly, nearly all GmYUC genes were expressed at low levels in the tissues/organs examined, yet in many cases their mRNAs were barely detected (Supplementary Fig. S2; Supplementary Table S2). Six of the genes, GmYUC2a/b/c, GmYUC5, GmYUC6d, and GmYUC11d were expressed in nodules, with GmYUC2a being the most highly expressed (Supplementary Fig. S2; Supplementary Table S2). Hence, our findings do suggest divergent expression patterns for the GmYUC genes.
GmYUC2a expression during rhizobial inoculation and nodule organogenesis
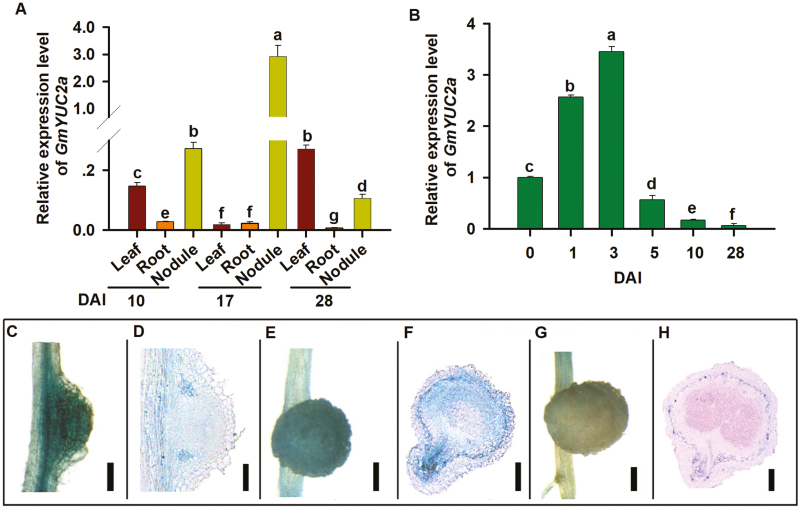
To verify the nodule-specific expression of GmYUC2a, GmYUC2a expression was determined in leaves, roots (de-nodulated), and nodules at 10, 17, and 28 DAI with B. diazoefficiens. As shown in Fig. 1A, GmYUC2a was much more highly expressed in nodules than in leaves and roots at 10 d or 17 d after rhizobial infection, and the expression of GmYUC2a was highest in the nodules at 17 DAI compared with 10 and 28 DAI (Fig. 1A). This suggests a crucial role for GmYUC2a in soybean nodulation. Using an inoculation time-course study, GmYUC2a expression was found to reach its peak at 3 DAI (Fig. 1B), then rapidly decreased down to its pre-infection level by 5 DAI, where it remained throughout subsequent stages of nodule development (Fig. 1B). Thus, GmYUC2a may act to modulate rhizobial infection and early nodule formation.
Fig. 1.
The expression pattern of GmYUC2a in different tissues and in response to rhizobial infection. (A) The expression of GmYUC2a in leaves, roots (de-nodulated), and nodules at 10, 17, and 28 DAI with B. diazoefficiens. (B) GmYUC2a expression in roots at 0, 1, 3, 5, 10, and 28 DAI. GmELF1b was used as a reference gene to determine relative expression. All experiments consisted of three independent biological replicates. Root sections 4 cm from the root tip were collected from six plant samples and were used in each biological replicate. Data are the average of three biological repeats. Error bars indicate the SE. Different letters indicate significant differences by the Student–Newman–Kuels test (P<0.05). (C–H) GUS activity in B. diazoefficiens-inoculated transgenic roots and nodules (C, 10 DAI, scale bar=600 μm; D, 10 DAI, scale bar=300 μm; E, 28 DAI, scale bar=600 μm; F, 28 DAI, scale bar=800 μm; G, 55 DAI, scale bar=1 mm; H, 55 DAI, scale bar=1 mm). (This figure is available in color at JXB online.)
To examine the tissue-specific promoter activity of GmYUC2a during nodulation, we generated transgenic hairy roots expressing GmYUC2a pro::GUS. Interestingly, in emerging nodules at 10 DAI, the promoter of GmYUC2a showed uniformly higher activity in all nodule tissues (Fig. 1C, D) and, in nodules close to maturation at 28 DAI, GmYUC2a was observed to have high promoter activity in the cortex and infection zone/nitrogen fixation zone (Fig. 1E, F). Finally, in older nodules near senescence, GmYUC2a promoter activity was substantially decreased, with GUS activity restricted to the vascular bundles of nodules (Fig. 1G, H). Taken together, these data suggest that GmYUC2a might be involved in root development, rhizobial infection, nodule organogenesis, and nodule senescence.
GmYUC2a overexpression dramatically affects the growth of roots and root hairs
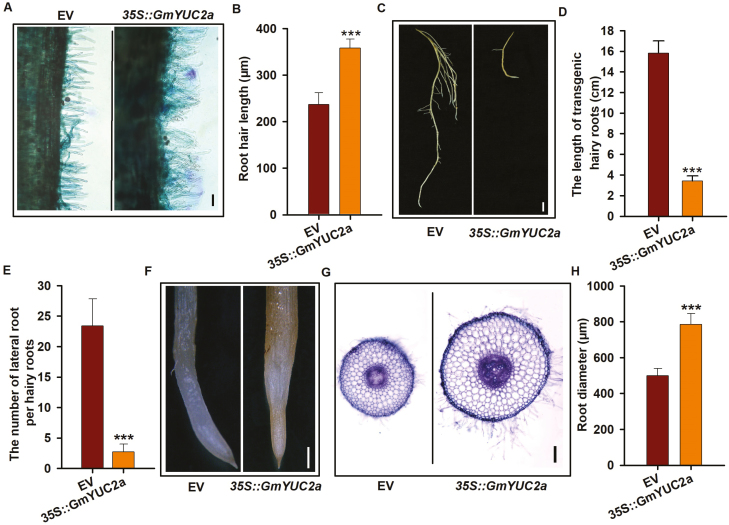
To functionally characterize GmYUC2a, we generated transgenic composite plants overexpressing GmYUC2a under the control of the 35S promoter (Supplementary Fig. S3A). Phenotypic analysis showed that root hairs of 35S::GmYUC2a transgenic roots were significantly longer than those of control roots, and the root hair density was shown to be clearly increased (Fig. 2A, B; Supplementary Fig. S3B). Notably, severe defects in root growth were also observed in 35S::GmYUC2a plants. The length of 35S::GmYUC2a roots was reduced by up to 80% (Fig. 2C, D) and the lateral root number was shown to be clearly reduced by ~75.6% (Fig. 2E). The apical meristem of 35S::GmYUC2a roots became thinner, whereas the root elongation and maturation zones were significantly thicker than vector-only control roots (Fig. 2F–H). These root phenotypes of the 35S::GmYUC2a lines mirrored those of Arabidopsis plants overexpressing YUCCA, which overproduce auxin (Zhao et al., 2001). These results indicate that GmYUC2a has a similar function to Arabidopsis YUCCA genes in auxin biosynthesis. To confirm this, we ectopically expressed GmYUC2a in Arabidopsis ecotype Col-0 plants and found that this caused a severe short root phenotype (Supplementary Figs. S4A–C), in agreement with previous Arabidopsis results (Zhao et al., 2001). In addition, the hairy roots harboring the EV showed a similar phenotype in response to exogenous auxin (2,4-D) treatment (Supplementary Figs. S5A, B). Together, these data suggests that GmYUC2a may modulate root growth by stimulating auxin biosynthesis.
Fig. 2.
GmYUC2a regulates the growth of roots and root hairs. (A, B) The root hair phenotype (A, scale bar=100 μm) and root hair length (B) in 35S::GmYUC2a transgenic roots and the EV control at 10 d after transplantation. (C, D) EV and 35S::GmYUC2a hairy roots at 10 d after transplantation (C, scale bar=5 mm). (E) The number of lateral roots in the transgenic hairy roots harboring EV and 35S::GmYUC2a. (F–H) Comparison of the root diameter of maturation zones between 35S::GmYUC2a and EV transgenic roots at 10 d after transplantation (F, scale bar=500 μm); (G) cross-section of transgenic roots harboring 35S::GmYUC2a or EV and stained with methylene blue (scale bar=100 μm); (H), diameters of 35S::GmYUC2a and EV transgenic roots. All values are the averages ±SD from three independent experiments. Three independent biological replicates were performed using 15 hairy roots per replicate. Student’s t-tests were used to determine significance: ***P<0.001. (This figure is available in color at JXB online.)
GmYUC2a overexpression increases auxin biosynthesis and enhances the auxin sensitivity of infected roots
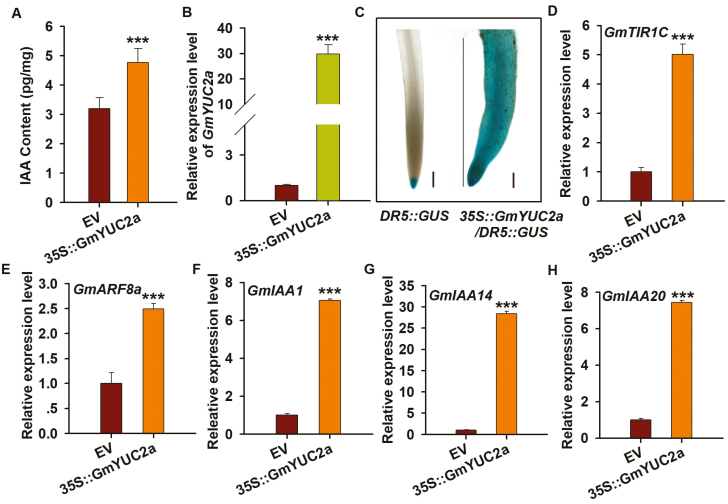
To test whether GmYUC2a controls auxin biosynthesis in soybean, we first measured the auxin content of A. rhizogenes-mediated hairy roots. As shown in Fig. 3A, the auxin content was markedly increased in 35S::GmYUC2a roots compared with EV control roots at 10 d after transplantation, suggesting that GmYUC2a positively regulates auxin synthesis in soybean. Next, we ectopically overexpressed GmYUC2a in wild-type Arabidopsis Col-0 plants expressing DR5::GUS, which can be used to detect visually the endogenous level and spatial distribution of auxin (Ulmasov et al., 1997). As shown in Fig. 3B and C, the roots of the DR5::GUS lines overexpressing GmYUC2a were significantly thicker than those of the control plants, indicating that GmYUC2a was successfully overexpressed. In DR5::GUS lines co-transformed with EV, GUS activity was mainly observed in root tips (Fig. 3C). In sharp contrast, GUS activity was strong and uniformly distributed in all cells/tissues of 35S::GmYUC2a transgenic roots (Fig. 3C). These results demonstrate that GmYUC2a is a functional soybean ortholog of AtYUC2 that controls auxin biosynthesis. To determine whether GmYUC2a affects plant responses to auxin, we analyzed the expression of key auxin signaling genes in transgenic hairy roots. Transcript levels of GmTIR1C, GmARF8a, and GmIAA1/14/20 were significantly up-regulated in 35S::GmYUC2a transgenic roots compared with EV control roots (Fig. 3D–H). Taken together, these results demonstrate the crucial role of GmYUC2a in auxin biosynthesis and plant responses to auxin in soybean.
Fig. 3.
GmYUC2a overexpression increases the auxin content of transgenic roots and regulates the expression of auxin-responsive genes. (A) Measurement of the IAA content in 35S::GmYUC2a and EV transgenic roots at 10 d after transplantation. (B) The detection of GmYUC2a expression in EV and 35S::GmYUC2a transgenic hairy roots. (C) Histochemical staining of GUS activity in DR5::GUS and DR5::GUS overexpressing GmYUC2a transgenic Arabidopsis roots at 10 d after transplantation. Scale bar=500 μm. (D–H) The transcript levels of GmTIR1C, GmARF8a, and GmIAA genes in EV and 35S::GmYUC2a transgenic hairy roots. The gene expression levels were normalized against the geometric mean of the soybean reference gene GmELF1b. Three independent biological replications were performed and five transgenic hairy roots showing overexpression of GmYUC2a were used per replicate. All values are the averages ±SD from three independent experiments. Statistically significant differences (Student’s t- test) are indicated as ***P<0.001. (This figure is available in color at JXB online.)
GmYUC2a regulates root hair deformation in response to rhizobial infection
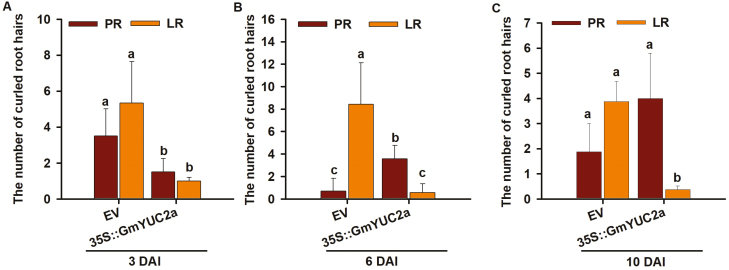
Root hairs represent the main sites of rhizobial infection (Madsen et al., 2003). In order to investigate whether GmYUC2a is involved in root hair infection during early nodulation, a thorough analysis of root hair deformation was conducted. Few deformed root hairs were found either in the primary or lateral hairy roots of the 35S::GmYUC2a composite plants in the condition without rhizobial inoculation (Supplementary Figs S6; S7A–C). In contrast, upon rhizobial infection, deformed root hairs were mainly observed on lateral roots of EV control roots, peaking at 6 DAI (Fig. 4A–C). This is consistent with our observation that most nodules formed on these lateral roots. Intriguingly, there was a sharp contrast between the numbers of deformed root hairs in 35S::GmYUC2a and the vector control during infection. At 3 DAI, the numbers of deformed root hairs in primary roots and lateral roots in the hairy roots harboring 35S::GmYUC2a were similar to those harboring the EV. However, the 35S::GmYUC2a hairy roots exhibited a significantly lower number of deformed root hairs compared with the EV control roots. (Fig. 4A; Supplementary Fig. S7A). Surprisingly, the number of deformed root hairs in the primary roots of 35S::GmYUC2a plants increased substantially at 6 DAI, although the total number of deformed root hairs of 35S::GmYUC2a roots was still lower than that of the EV control roots (Fig. 4B; Supplementary Fig. S7B). At 10 DAI, the total number of deformed root hairs in primary roots of 35S::GmYUC2a plants was comparable with that of vector control roots, and the number of deformed root hairs in the lateral roots of 35S::GmYUC2a composite plants remained low (Fig. 4C; Supplementary Fig. S7C). These observations suggest that the levels of GmYUC2a expression and auxin may positively regulate rhizobial infection of root hairs.
Fig. 4.
GmYUC2a regulates root hair deformation in response to rhizobial infection. (A–C) GmYUC2a overexpression reduced the numbers of deformed and curled root hairs on the primary root (PR) and lateral roots (LR) at 3, 6, and 10 DAI. Segments (2 cm) of hairy roots overexpressing GmYUC2a below the root–hypocotyl junction were cut and stained with 1% (w/v) methylene blue. Considerably curled root hairs were counted (n=10–12 hairy roots). All values are the averages ±SD from three independent experiments. Different letters indicate significant differences by Student–Newman–Kuels test (P<0.05). (This figure is available in color at JXB online.)
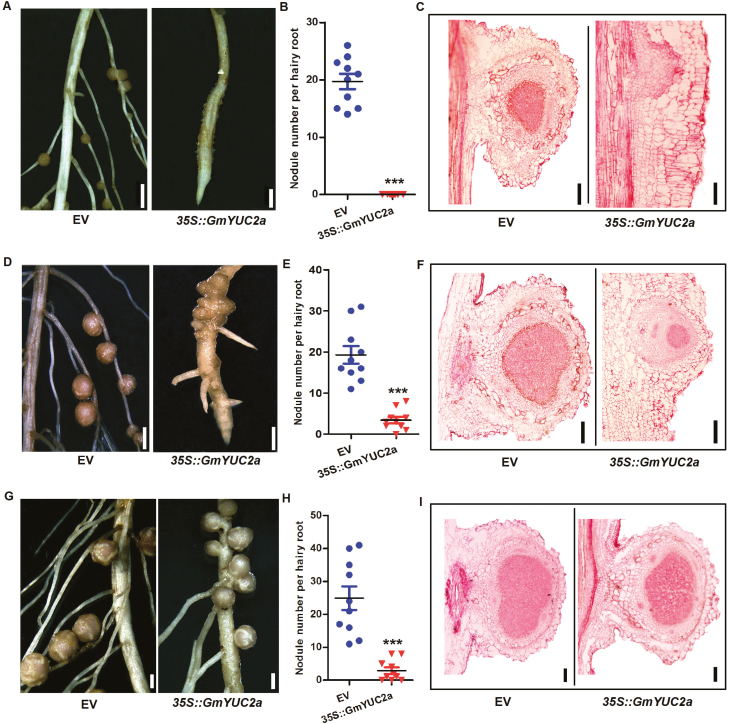
GmYUC2a overexpression delays nodule organogenesis
To further investigate whether GmYUC2a modulates nodule organogenesis, we monitored nodule development in 35S::GmYUC2a transgenic roots. At 10 DAI, there were ~20 nodules on the EV control lateral roots (Fig. 5A–C). In contrast, no lateral roots or nodules were observed on the primary roots of 35S::GmYUC2a plants (Fig. 5A, B; Supplementary Fig. S8A). However, longitudinal root sections revealed that nodule primordia had formed on the primary roots of plants expressing 35S::GmYUC2a (Fig. 5C).
Fig. 5.
GmYUC2a overexpression delays nodule organogenesis. (A–I) Nodules of individual hairy roots expressing the EV or 35S::GmYUC2a at 10, 17, and 28 DAI. (A) Scale bar=1500 μm; (D) EV, scale bar=1500 μm; 35S::GmYUC2a, scale bar=1000 μm; (G) scale bar=1500 μm. (B, E, H) Quantitative analysis of the nodule number per hairy root expressing the EV and 35S::GmYUC2a (n=10–12 hairy roots). All values are the averages ±SD from three independent experiments. Statistically significant differences (Student’s t-test) are indicated as ***P<0.001. (C, F, I) Transverse nodule sections stained with safranin O from EV control and 35S::GmYUC2a plants. (C) EV, scale bar=150 μm; 35S::GmYUC2a, scale bar=100 μm; (F) EV, scale bar=250 μm; 35S::GmYUC2a, scale bar=150 μm; (I) EV, scale bar=350 μm; 35S::GmYUC2a, scale bar=350 μm. (This figure is available in color at JXB online.)
At 17 DAI, the EV control roots continued to exhibit ~20 mature nodules each (Fig. 5D–F; Supplementary Fig. S8B). In contrast, the nodules on the 35S::GmYUC2a roots were just starting to emerge, densely packed and restricted to the primary nodulation zone (Fig. 5D–F). These nodules matured in appearance by 28 DAI (Fig. 5G–I; Supplementary Fig. S8C), indicating that overexpression of GmYUC2a and subsequent increased auxin biosynthesis may have delayed nodule development. In particular, we also estimated the ratio of the number of primary root nodules to that of total nodules at 17 DAI. As shown in Supplementary Fig. S8D, overexpression of GmYUC2a resulted in the significant increase of nodule numbers appearing in the primary roots.
Next, we generated composite transgenic plants with reduced GmYUC2a expression using RNAi, and evaluated their root and nodule development. Neither root nor nodule development was significantly affected (Supplementary Fig. S9A–C) by a reduction in GmYUC2a, indicating the redundancy of GmYUC genes in the regulation of soybean nodulation, consistent with observations made in Arabidopsis where AtYUC genes can function redundantly with one another to regulate root development (Cheng, 2006; Cheng et al., 2007).
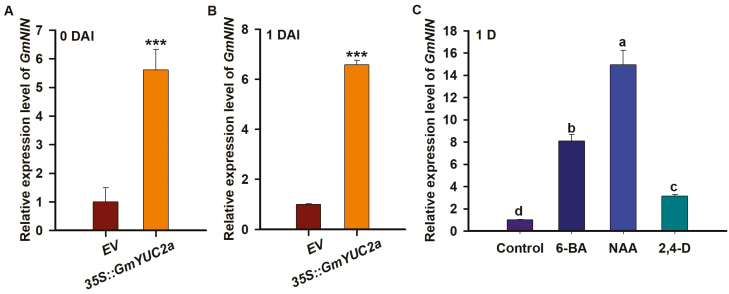
Overexpression of GmYUC2a regulates rhizobial infection and nodule development through modulating GmNIN expression
Nodule Inception (NIN) has been confirmed to be a master regulator of rhizobial infection and nodule organogenesis in legume (Schauser et al., 1999; Marsh et al., 2007; Griesmann et al., 2018). To test whether GmYUC2a overexpression affects nodulation via the NIN-mediated signaling pathway, we analyzed the expression patterns of GmNIN. Interestingly, qPCR revealed that the expression level of GmNIN was clearly increased in the hairy roots harboring 35S::GmYUC2a in the absence or presence of rhizobia (Fig. 6A, B). Based on this result, we tried to analyze whether GmNIN could be induced by exogenous auxin. As shown in Fig. 6C, 6-BA was used as a positive control and it was found to increase the expression level of GmNIN. Furthermore, GmNIN expression was stably induced in response to 2,4-D and NAA at 24 h after treatment. These data indicate that GmYUC2a has a conserved role in auxin synthesis and might regulate rhizobial infection and the development of nodules by modulating GmNIN.
Fig. 6.
GmYUC2a overexpression and exogenous auxin induce the expression of GmNIN. (A and B) GmNIN expression in 35S::GmYUC2a transgenic roots inoculated with B. diazoefficiens USDA110. (C) GmNIN expression in roots treated with 6-BA, NAA, 2,4-D, or an equal volume of distilled water (control) for 24 h. Three independent biological replications were performed; the gene expression levels were normalized against the geometric mean of the soybean reference gene GmELF1b. All experiments were conducted three times. Error bars indicate the SD. Statistically significant differences (Student’s t-test) are indicated as ***P<0.001. (This figure is available in color at JXB online.)
Discussion
Auxin is a central regulator of plant growth and is essential for root development (Benkova and Bielach, 2010; Marhavy et al., 2013). Decades of research have shown that auxin is required for rhizobial infection and nodule organogenesis (de Billy et al., 2001; Ferguson and Mathesius, 2003, 2014; Huo et al., 2006; Roy et al., 2017), but it is not well understood how auxin is synthesized and regulated in the nodule. Here, we show that GmYUC2a of soybean is induced by rhizobia and expressed in maturing nodules; it functions in auxin biosynthesis to regulate root growth and nodule organogenesis. These results demonstrate that local auxin biosynthesis is critical for legume nodulation.
YUC genes encode flavin monooxygenases, which catalyze the last step of IAA biosynthesis (IPyA to IAA) in the indole-3-pyruvate pathway (Zhao et al., 2001). Prior to our work, no YUC gene had been functionally characterized in soybean. The soybean YUC gene family is composed of 22 members that can be divided into four subgroups. Our findings demonstrate that overexpression of GmYUC2a, a putative ortholog of Arabidopsis YUC2 which catalyzes the conversion of IPyA to IAA (Mashiguchi et al., 2011; Stepanova et al., 2011), elevated the auxin content of the root and caused stunted root growth with enhanced root hair elongation. Ectopic overexpression of GmYUC2a in Arabidopsis produced growth defects that were similar to those of AtYUC2-overexpressing plants (Zhao et al., 2001), indicating that GmYUC2a is a functional ortholog of Arabidopsis YUC2.
Previous studies have highlighted a role for auxin in nodule primordium formation and rhizobial infection (e.g. Hirsch et al., 1989; Wasson et al., 2006; Rightmyer and Long, 2011; Turner et al., 2013; Breakspear et al., 2014). Computational and pharmacological data indicate that auxin transport may be a major contributor to the increase in auxin during nodule primordium formation (Deinum et al., 2012). Genetic evidence has also been reported by Roy et al. (2017). However, direct evidence for the source of that auxin is lacking, and there is no report on the involvement of auxin biosynthesis in legume nodulation. Here, we showed that GmYUC2a was expressed in soybean roots and the maturing nodules of soybean. Taking into account the functions of GmYUC2a in root development and root hair elongation, it is apparent that GmYUC2a plays a conserved role in root growth regulation. Interestingly, GmYUC2a overexpression reduced the number of deformed root hairs, which occurred mainly on the primary roots. Our results suggest that although GmYUC2a is an important regulator, it is not the only YUC gene to modulate auxin biosynthesis during rhizobial infection. Indeed, knockdown of GmYUC2a did not affect nodulation, and two additional GmYUC genes were found to be highly expressed in soybean nodules based on RNA-Seq data. Since both the gradient and maxima of auxin are important for cell growth and differentiation, it is conceivable that auxin biosynthesis is dynamically controlled.
Local increases in auxin in the pericycle and surrounding cells control lateral root initiation (Benkova and Bielach, 2010; Marhavy et al., 2013), whereas increases in cortical cells are reported to be important for nodule initiation (Suzaki et al., 2012). Our results show that local auxin biosynthesis catalyzed by GmYUC2a plays an important role in nodule organogenesis. Upon rhizobial infection, GmYUC2a promoter activity was uniformly strong in all tissues of maturing nodules, but was only expressed in vascular tissue of mature nodules. We therefore propose that the role of GmYUC2a-mediated auxin biosynthesis in nodule development is in cell elongation and nodule expansion, as has been demonstrated for auxin in other tissues (Perrot-Rechenmann, 2010). The disappearance of GmYUC2a from the nitrogen fixation zone of senescing nodules suggests that local auxin biosynthesis is also important for sustaining and maintaining nodule function. In addition, the specific pattern of promoter activity of GmYUC2a might provide a reasonable explanation for why overexpression of GmYUC2a leads to a significant delay in nodule development.
Phytohormones, such as auxin and cytokinin, have long been shown to play a role in legume nodulation (Libbenga et al., 1973; Tirichine et al., 2006, 2007; Wasson et al., 2006; Murray et al., 2007; Plet et al., 2011; Suzaki et al., 2012; Turner et al., 2013; Wang et al., 2015; Cai et al., 2017). With regards to auxin, recent studies have reported that genes involved in auxin signaling and transport are correlated with soybean nodule development (Turner et al., 2013; Wang et al., 2015; Cai et al., 2017). Changes in auxin transport have been proposed to determine auxin accumulation in nodule primordia (Suzaki et al., 2013). Concomitantly, specific patterns of auxin accumulation could be associated with auxin biosynthesis induced by rhizobial infection to regulate nodule development (Suzaki et al., 2012, 2013). Moreover, auxin accumulation in root hairs and the expression of LjTar1, which is involved in auxin biosynthesis, are up-regulated in response to the Nod factor signaling pathway (Nadzieja et al., 2018).
NIN has a well-established role in rhizobial infection and nodule organogenesis through the LHK1-dependent cytokinin signaling pathway (Schauser et al., 1999; Marsh et al., 2007; Tirichine et al., 2007; Murray et al., 2007; Suzaki et al., 2013). Up-regulation of LjTar1 appeared in the Ljnin-2 mutant in response to Nod factor inoculation (Nadzieja et al., 2018). However, in soybean, we found that GmNIN showed a stable increase in expression in response to exogenous auxin treatment and the overexpression of GmYUC2a, which is involved in auxin biosynthesis (Fig. 6A–C). This indicates that GmNIN expression could potentially be regulated by auxin production. Up-regulation of GmNIN in response to an increased auxin content may lead to the dedifferentiation and proliferation of cortical cells required for nodule organogenesis (Suzaki et al., 2012). Constitutive expression of NIN also results in the formation of spontaneous nodule structures by regulating localized auxin responses in L. japonicus (Suzaki et al., 2012; Soyano et al., 2013). Curiously, the increased abundance of GmNIN transcripts induced by GmYUC2a overexpression failed to induce spontaneous nodulation in soybean. Clearly more research is required to elucidate how auxin and other hormones, such as cytokinin and gibberellin, interact to modulate nodule development.
A role for auxin in legume nodulation has long been proposed, but the source of auxin has been elusive. Here, we provide genetic evidence for the auxin biosynthesis gene GmYUC2a as having an important contribution to local auxin production that regulates both rhizobial infection and nodule organogenesis in soybean. Rhizobia induce the expression of GmYUC genes (mainly GmYUC2a) in the root to activate auxin biosynthesis, resulting in a local increase in auxin and the onset of auxin signaling. A possible model may be that auxin signaling is integrated with other hormone signaling pathways to reactivate the cell cycle in root hairs and the cortex for infection thread formation, nodule primordium initiation, and nodule development. These findings enhance our understanding of auxin regulation of nodule development in soybean, and provide novel insights into the important legume symbiosis.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Phylogenetic relationship of YUC family proteins and the structures of their associated genes.
Fig. S2. YUCCA family gene expression in soybean.
Fig. S3. GmYUC2a transcript levels and root hair density were increased in 35S::GmYUC2a transgenic roots.
Fig. S4. GmYUC2a overexpression inhibited primary root growth in Arabidopsis.
Fig. S5. Hairy root development was affected by 2,4-D treatment.
Fig. S6. GmYUC2a overexpression did not lead to root hair deformation without rhizobial inoculation.
Fig. S7. GmYUC2a expression in EV or 35S::GmYUC2a transgenic roots of soybean.
Fig. S8. GmYUC2a transcription in transgenic roots having EV or 35S::GmYUC2a.
Fig. S9. GmYUC2a silencing did not affect nodule development.
Table S1. The YUC family genes in Glycine max.
Table S2. The expression data of GmYUC family genes in different tissues of soybean (RPKM normalized data).
Table S3. Primers used in this study.
Dataset S1. Predicted amino acid sequence of AtYUCs, GmYUCs, and MedtrYUCs.
Acknowledgements
We thank Dr Kan Wang (Iowa State University, USA) for providing the pTF101.1 vector. We are grateful to Professor Wenxin Chen (China Agricultural University) for kindly providing B. diazoefficiens USDA110. We also thank Professor Tianfu Han (Chinese Academy of Agricultural Sciences) for assistance with some experimental protocols and thank Zechuan Lin for technical help. The study was funded by the National Key Research and Development Program of China (2016YFA0500503), the National Transgenic Major Project of China (grant 2018ZX0800919B), NSFC (31730066), and Huazhong Agricultural University Scientific & Technological Self-innovation Foundation (Program No. 2015RC014).
References
- Benková E, Bielach A. 2010. Lateral root organogenesis—from cell to organ. Current Opinion in Plant Biology 13, 677–683. [DOI] [PubMed] [Google Scholar]
- Breakspear A, Liu C, Roy S, et al. . 2014. The root hair ‘infectome’ of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. The Plant Cell 26, 4680–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos-Sanmamed P, Mao G, Deng Y, et al. . 2013. Overexpression of miR160 affects root growth and nitrogen-fixing nodule number in Medicago truncatula. Functional Plant Biology 40, 1208–1220. [DOI] [PubMed] [Google Scholar]
- Cai Z, Wang Y, Zhu L, Tian Y, Chen L, Sun Z, Ullah I, Li X. 2017. GmTIR1/GmAFB3-based auxin perception regulated by miR393 modulates soybean nodulation. New Phytologist 215, 672–686. [DOI] [PubMed] [Google Scholar]
- Charon C, Johansson C, Kondorosi E, Kondorosi A, Crespi M. 1997. Enod40 induces dedifferentiation and division of root cortical cells in legumes. Procceeding of the National Academy of Sciences, USA 94, 1953–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. 2006. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes & Development 20, 1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. 2007. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. The Plant Cell 19, 2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Billy F, Grosjean C, May S, Bennett M, Cullimore JV. 2001. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Molecular Plant-Microbe Interactions 14, 267–277. [DOI] [PubMed] [Google Scholar]
- Deinum EE, Geurts R, Bisseling T, Mulder BM. 2012. Modeling a cortical auxin maximum for nodulation: different signatures of potential strategies. Frontiers in Plant Science 3, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Wang Y, Liu Z, Cheng H, Xue Y. 2014. HemI: a toolkit for illustrating heatmaps. PLoS One 9, e111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Indrasumunar A, Hayashi S, Lin MH, Lin YH, Reid DE, Gresshoff PM. 2010. Molecular analysis of legume nodule development and autoregulation. Journal of Integrative Plant Biology 52, 61–76. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Mathesius U. 2003. Signaling interactions during nodule development. Journal of Plant Growth Regulation 22, 47–72. [Google Scholar]
- Ferguson BJ, Mathesius U. 2014. Phytohormone regulation of legume–rhizobia interactions. Journal of Chemical Ecology 40, 770–790. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Mens C, Hastwell AH. 2019. Legume nodulation: the host controls the party. Plant, Cell & Environment 42, 41–51. [DOI] [PubMed] [Google Scholar]
- Fu J, Chu J, Sun X, Wang J, Yan C. 2012. Simple, rapid, and simultaneous assay of multiple carboxyl containing phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Analytical Science 28, 1081–1087. [DOI] [PubMed] [Google Scholar]
- Griesmann M, Chang Y, Liu X, et al. . 2018. Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science 361, 144. [DOI] [PubMed] [Google Scholar]
- Guo A, Zhu Q, Chen X, Luo J. 2007. GSDS: a gene structure display server. Hereditas 29, 1023–1026. [PubMed] [Google Scholar]
- Hirsch A, Bhuvaneswari T, Torrey J, Bisseling T. 1989. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Procceeding of the National Academy of Sciences, USA 86, 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X, Schnabel E, Hughes K, Frugoli J. 2006. RNAi phenotypes and the localization of a protein::GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. Journal of Plant Growth Regulation 25, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Hou W, Wu C, Liu B, Liu W, Song S, Bi Y, Han T. 2009. Agrobacterium rhizogenes-mediated transformation of Superroot-derived Lotus corniculatus plants: a valuable tool for functional genomics. BMC Plant Biology 9, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Liu B, Bi Y, Hou W, Wu C, Han T. 2008. Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Molecular Biology 9, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kereszt A, Li D, Indrasumunar A, Nguyen CD, Nontachaiyapoom S, Kinkema M, Gresshoff PM. 2007. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nature Protocols 2, 948–952. [DOI] [PubMed] [Google Scholar]
- Kohlen W, Ng JLP, Deinum EE, Mathesius U. 2018. Auxin transport, metabolism, and signalling during nodule initiation: indeterminate and determinate nodules. Journal of Experimental Botany 69, 229–244. [DOI] [PubMed] [Google Scholar]
- Kuppusamy KT, Ivashuta S, Bucciarelli B, Vance CP, Gantt JS, Vandenbosch KA. 2009. Knockdown of CELL DIVISION CYCLE16 reveals an inverse relationship between lateral root and nodule numbers and a link to auxin in Medicago truncatula. Plant Physiology 151, 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbenga KR, van Iren F, Bogers RJ, Schraag-Lamers MF. 1973. The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta 114, 29–39. [DOI] [PubMed] [Google Scholar]
- Livak K, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Method 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Ljung K. 2013. Auxin metabolism and homeostasis during plant development. Development 140, 943–950. [DOI] [PubMed] [Google Scholar]
- Madsen EB, Madsen LH, Radutoiu S, et al. . 2003. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425, 637–640. [DOI] [PubMed] [Google Scholar]
- Marhavý P, Vanstraelen M, De Rybel B, Zhaojun D, Bennett MJ, Beeckman T, Benková E. 2013. Auxin reflux between the endodermis and pericycle promotes lateral root initiation. The EMBO Journal 32, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JF, Rakocevic A, Mitra RM, Brocard L, Sun J, Eschstruth A, Long SR, Schultze M, Ratet P, Oldroyd GE. 2007. Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiology 144, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, et al. . 2011. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA 108, 18512–18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U. 2001. Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. Journal of Experimental Botany 52, 419–426. [DOI] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA. 1998. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. The Plant Journal 14, 23–34. [DOI] [PubMed] [Google Scholar]
- Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K. 2007. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315, 101–104. [DOI] [PubMed] [Google Scholar]
- Nadzieja M, Kelly S, Stougaard J, Reid D. 2018. Epidermal auxin biosynthesis facilitates rhizobial infection in Lotus japonicus. The Plant Journal 95, 101–111. [DOI] [PubMed] [Google Scholar]
- Nizampatnam NR, Schreier SJ, Damodaran S, Adhikari S, Subramanian S. 2015. microRNA160 dictates stage-specific auxin and cytokinin sensitivities and directs soybean nodule development. The Plant Journal 84, 140–153. [DOI] [PubMed] [Google Scholar]
- Pacios-Bras C, Schlaman HR, Boot K, Admiraal P, Langerak JM, Stougaard J, Spaink HP. 2003. Auxin distribution in Lotus japonicus during root nodule development. Plant Molecular Biology 52, 1169–1180. [DOI] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. 2010. Cellular responses to auxin: division versus expansion. Cold Spring Harbor Perspectives in Biology 2, a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plet J, Wasson A, Ariel F, Le Signor C, Baker D, Mathesius U, Crespi M, Frugier F. 2011. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. The Plant Journal 65, 622–633. [DOI] [PubMed] [Google Scholar]
- Porebski S, Bailey G, Baum BR. 1997. Modification of a CTAB DNA extration protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Report 15, 8–15. [Google Scholar]
- Poulet A, Kriechbaumer V. 2017. Bioinformatics analysis of phylogeny and transcription of TAA/YUC auxin biosynthetic genes. International Journal of Molecular Sciences 18, 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rightmyer AP, Long SR. 2011. Pseudonodule formation by wild-type and symbiotic mutant Medicago truncatula in response to auxin transport inhibitors. Molecular Plant-Microbe Interactions 24, 1372–1384. [DOI] [PubMed] [Google Scholar]
- Roy S, Robson F, Lilley J, et al. . 2017. MtLAX2, a functional homologue of the arabidopsis auxin influx transporter AUX1, is required for nodule organogenesis. Plant Physiology 174, 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J. 1999. A plant regulator controlling development of symbiotic root nodules. Nature 402, 191–195. [DOI] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, et al. . 2010. Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183. [DOI] [PubMed] [Google Scholar]
- Severin AJ, Woody JL, Bolon YT, et al. . 2010. RNA-Seq atlas of Glycine max: a guide to the soybean transcriptome. BMC Plant Biology 10, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Kouchi H, Hirota A, Hayashi M. 2013. Nodule inception directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLoS Genetics 9, e1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM. 2011. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. The Plant Cell 23, 3961–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Wang Y, Mou F, Tian Y, Chen L, Zhang S, Jiang Q, Li X. 2015. Genome-wide small RNA analysis of soybean reveals auxin-responsive microRNAs that are differentially expressed in response to salt stress in root apex. Frontiers in Plant Science 6, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Ito M, Kawaguchi M. 2013. Genetic basis of cytokinin and auxin functions during root nodule development. Frontiers in Plant Science 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M. 2012. Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139, 3997–4006. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV. 1936. On the physiology of the formation of nodules on legume roots. Procceeding of the National Academy of Sciences, USA 22, 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirichine L, James EK, Sandal N, Stougaard J. 2006. Spontaneous root-nodule formation in the model legume Lotus japonicus: a novel class of mutants nodulates in the absence of rhizobia. Molecular Plant-Microbe Interactions 19, 373–382. [DOI] [PubMed] [Google Scholar]
- Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Asamizu E, Tabata S, Stougaard J. 2007. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315, 104–107. [DOI] [PubMed] [Google Scholar]
- Tobeña-Santamaria R, Bliek M, Ljung K, Sandberg G, Mol JN, Souer E, Koes R. 2002. FLOOZY of petunia is a flavin mono-oxygenase-like protein required for the specification of leaf and flower architecture. Genes & Development 16, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Nizampatnam NR, Baron M, Coppin S, Damodaran S, Adhikari S, Arunachalam SP, Yu O, Subramanian S. 2013. Ectopic expression of miR160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiology 162, 2042–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U. 2006. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiology 140, 1494–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li K, Chen L, et al. . 2015. MicroRNA167-directed regulation of the auxin response factors GmARF8a and GmARF8b is required for soybean nodulation and lateral root development. Plant Physiology 168, 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li P, Cao X, Wang X, Zhang A, Li X. 2009. Identification and expression analysis of miRNAs from nitrogen-fixing soybean nodules. Biochemical and Biophysical Research Communications 378, 799–803. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang L, Zou Y, et al. . 2014. Soybean miR172c targets the repressive AP2 transcription factor NNC1 to activate ENOD40 expression and regulate nodule initiation. The Plant Cell 26, 4782–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson AP, Pellerone FI, Mathesius U. 2006. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. The Plant Cell 18, 1617–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y. 2011. Conversion of tryptophan to indole-3-acetic acid by tryptophan aminotransferases of Arabidopsis and YUCCAs in Arabidopsis. Procceeding of the National Academy of Sciences, USA 108, 18518–18523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YM, Park HJ, Su’udi M, Yang JI, Park JJ, Back K, Park YM, An G. 2007. Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio. Plant Molecular Biology 65, 125–136. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T. 2007. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiology 143, 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. 2008. The role of local biosynthesis of auxin and cytokinin in plant development. Current Opinion in Plant Biology 11, 16–22. [DOI] [PubMed] [Google Scholar]
- Zhao Y. 2012. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Molecular Plant 5, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen S, Fankhauser C, Cashman J, Cohen J, Weigel D, Chory J. 2001. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.