Abstract
Glioblastoma (GB) is the most frequently occurring and aggressive primary brain tumor. Glioma stem cells (GSCs) and astrocytoma cells are the predominant malignant cells occurring in GB besides a highly heterogeneous population of migrating, neovascularizing and infiltrating myeloid cells that forms a complex tumor microenvironment (TME). Cross talk between the TME cells is pivotal in the biology of this tumor and, consequently, adaptor proteins at critical junctions of signaling pathways may be crucial. Scaffold proteins (scaffolins or scaffoldins) integrate external and internal stimuli to regulate various signaling pathways, interacting simultaneously with multiple proteins involved. We investigated by double and triple immunofluorescence the localization of IQGAP1, AmotL2, and FKBP51, three closely related scaffoldins, in malignant cells and TME of human GB tumors. We found that IQGAP1 is preferentially expressed in astrocytoma cells, AmotL2 in GSCs, and FKBP51 in white blood cells in human GB tumors. As GSCs are specially the target for novel therapies, we will investigate in further studies whether AmotL2 inhibition is effective in the treatment of GB.
Keywords: AmotL2, astrocytoma cells, FKBP51, glioblastoma microenvironment, glioma stem cells, IQGAP1, pericytes, scaffold proteins, tumor-associated macrophages (TAMs)
Introduction
In physiological conditions, the immune and vascular systems are tightly regulated through a complex network of signal transduction pathways. In neoplasm, this network disintegrates and, as a result, immunity and angiogenesis are dysregulated and are ineffective or even support malignant cells to proliferate, invade, and metastasize because the tumor microenvironment (TME) has changed in both structure and function.1
Glioblastoma (GB) is the most frequently occurring and aggressive primary brain tumor, characterized by a poor outcome; after surgery and chemoradiotherapy, the median survival of patients is 15 months; the overall survival at 2 years after diagnosis is less than 10% and after 5 years is less than 2%.2–4 In GB, the cancer cell population consists of mainly astrocytoma cells and glioma stem cells (GSCs),5,6 whereas the cellular TME consists of astrocytes, microglial cells,1,7,8 pericytes,9 telocytes,10 endothelial cells, and neurons (mainly axons). Cross talk between these cell types is pivotal in the biology of GB and, as a consequence, signaling pathways have been widely studied in GB. The commitment during the continuous stemness-related cell reprogramming of GSCs is tightly regulated by the local TME within the so-called GSC niches that have been found to be both perivascular and hypoxic11–14; whereas, Hira et al. argued that the GSC niches are periarteriolar12,13 and are a mimic of hematopoietic stem cell niches in the bone marrow.14,15 However, very little is known about scaffold proteins involved in the signaling processes between astrocytoma cells, GSCs, and TME cells.
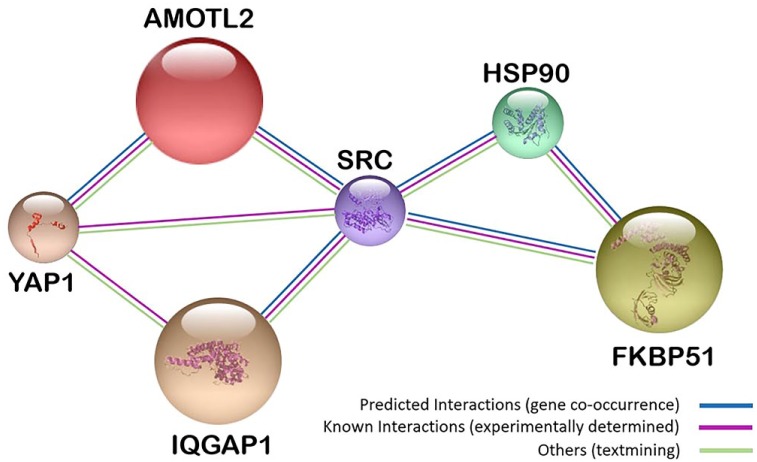
Previously, we have shown that scaffoldin IQ motif containing GTPase activating protein 1 (IQGAP1) is among those genes that show largest changes in expression level in circulating white blood cells after chemotherapy.16 String analysis showed a relationship between IQGAP1 and other scaffoldins, angiomotin-like 2 (AmotL2) and FK506-binding protein 51 (FKBP51) (Fig. 1). Furthermore, these proteins showed variations in their expression levels in colorectal cancer and their subcellular localization varied among different cancer cell types.23–25 Consequently, we decided to study these three scaffoldins in GB.
Figure 1.
String analysis17 of interactions between multimodular scaffoldins with ubiquitous expression AmotL2, IQGAP1, and FKBP51, which are involved in development, cell proliferation, and angiogenesis.14,18–22 Abbreviations: AmotL2, angiomotin-like 2; IQGAP1, IQ-motif-containing GTPase-activating protein 1; FKBP51, immunophilin, FK506-binding protein 51; YAP1, yes-associated protein 1; SRC, proto-oncogene tyrosine-protein kinase; HSP90, heat-shock protein 90.
Scaffold proteins (scaffolins or scaffoldins) promote proximity and relative orientation of multiple modular molecular partners that are committed in specific tasks, usually in a stable complex with a specific subcellular localization, regulating their activity in a complex and dynamic way, involving activation and repression of activity. Scaffold proteins function in, among others, intracellular signaling, cell motility and cell polarity, protein sorting, stabilization and localization of plasma membrane proteins, enzymatic reactions, and protein recycling. These multiple functions of scaffold proteins indicate pivotal roles of these proteins in cell fate, migration, tumorigenesis, tumor progression, and angiogenesis.26–29
Because of its complex subcellular localization, association with other types of proteins and its involvement in multiple cellular processes, IQGAP1 is the most versatile of the three scaffoldins and it is ubiquitously expressed in cells and tissues.30,31 IQGAP1 modulates several cellular functions, including cell cycle, morphology, adhesion, and motility, by linking elements of the cytoskeleton,32,33 facilitating the organization in space and time and the coordinated activation of structural and signaling molecules.34,35 Associated with molecular partners, IQGAP1 accumulates in the plasma membrane at the invasive front in several tumor types,24,36–39 thus contributing to cell fate, polarization, migration, tumorigenesis, angiogenesis, and tumor progression.36,39
AmotL2 belongs to the Amot family of proteins, together with Amot and AmotL1. Amot is expressed in two isoforms (AMOTp80 and AMOTp130) which are primarily localized to tight junctions.40,41 Human AmotL2 occurs as two isoforms with a molecular mass of 100 and 60 kDa,40 respectively. AmotL2 regulates cell-cell interactions to maintain asymmetrical apical-basal polarity, prevents endothelial detachment, and promotes vascular tube formation.42 Loss of polarity, epithelial-mesenchymal transition (EMT) and angiogenesis are crucial for GB. EMT is associated with loss of cell polarity and epithelial organization. However, whether EMT is a cause or a consequence of tumor progression is yet to be established.42
FKBP51 is a multifunctional immunophilin protein and is a member of the peptidyl-prolyl isomerase (PPI) superfamily of proteins,43 which catalyzes the cis-trans transformation of peptidyl-prolyl imide bonds in target proteins.44 Three distinct classes of proteins are included in this superfamily: the cyclosporin A-binding proteins, the parvulin-like PPIs, and the FK506-binding proteins (FKBP12, FKBP51, and FKBP52).45 FKBP51 travels between the mitochondria, cytoplasm, and nucleus,44,46,47 it modulates a variety of signaling pathways and is considered as a molecular integrant of the adaptation process.48 FKBP51 has been considered as a co-chaperone.18 Altered expression levels of FKBP51 have been reported in a number of tumor types.19,23
FKBP51 exerts relevant roles in antineoplastic therapy responses and in tumorigenesis20,21 through its effects on steroid receptor maturation22 and through its regulation of several signaling pathways, such as NF-kB,22 PKA,49 transforming growth factor β,50 and Akt.51
Figure 1 shows gene-expression co-occurrence and experimentally determined interactions between the three scaffold proteins and the proteins YAP1, SRC, and HSP90.17 These interactions, along with their ubiquitous expression at cell and tissue levels, and their involvement in development, cell proliferation, and angiogenesis,41,52–56 make of IQGAP1, AmotL2, and FKBP51 a set of attractive proteins for diagnostic staging and monitoring therapeutic effects. These scaffoldins may also be involved in the cross talk between astrocytoma cells, GSCs, and TME cells. We performed double, triple, and quadruple fluorescence immunolabeling and confocal microscopy for a “cell by cell” analysis in paraffin-embedded tissue sections of GB to obtain high quality data on the potential role of IQGAP1, AmotL2, and FKBP51 in intercellular signaling between TME cells, astrocytoma cells, and GSCs.
Materials and Methods
Tumor Tissues
The study was approved by the Ethical Committee of Nuestra Señora de Candelaria University Hospital (HUNSC); Santa Cruz de Tenerife, Canary Islands, Spain (no. 198/2008, approved on 16/09/2008) and the Ethics Committee of La Laguna University (La Laguna, Canary Islands, Spain). All patients were treated in the HUNSC in the period 2007–2017 and provided informed consent before entering the study for the diagnosis and research performed on tissue specimens. Clinical and pathological data were collected from 41 patients, 50 to 70 years of age, 33 primary GB (14 males and 19 females), and eight secondary GB (six males and two female). The main locations of the tumors were frontal lobe (38.5%), temporal lobe (43.6%), and parietal lobe (15.4%), all tumors were of Grade IV GB according to the World Health Organization classification. The mean survival was 19 months. GB samples were obtained after initial surgery before patients received radiation and/or chemotherapy. Paraffin-embedded tissue samples and corresponding clinical data were used ensuring patient anonymity.
Antibodies
Primary Antibodies
Rabbit anti-human polyclonal antibody against IQGAP1 (dilution 1:250; #ABT186 EMD; Millipore, Billerica, MA); rabbit polyclonal antibody against FKBP51 (dilution 1:50; #ab46002; Abcam, Cambridge, UK); rabbit polyclonal antibody against AMOTL2 (dilution 1:50; #LS-C178611; LifeSpan BioSciences, Seattle, WA); mouse monoclonal anti-human cluster of differentiation (CD)31 (ready-to-use; #IR610; Dako, Glostrup, Denmark); mouse monoclonal anti-human CD34 Class II Clone QBEnd10 (ready-to-use; #IR632, Dako); mouse monoclonal anti-NeuN (MAB377, dilution 1:150; Chemicon International, Temecula, CA); mouse monoclonal antibody against human nestin (dilution 1:25; #MAB1259; R&D Systems, Minneapolis, MN); goat polyclonal antibody against Iba1 (dilution 1:500; #ab107159; Abcam); mouse monoclonal anti-glial fibrillar acidic protein (GFAP) (dilution 1:100; #G3896; Sigma, MO); phalloidin-tetramethylrhodamine B isothiocyanate (phalloidin-TRITC) (dilution 1:500; #sc-301530; Santa Cruz Biotechnology Inc., Dallas, TX).
Secondary Antibodies
Fluorescein isothiocyanate (FITC)-conjugated goat polyclonal antibody against rabbit IgG (dilution 1:200; #F9887; Sigma-Aldrich); goat polyclonal antibody against mouse IgG DyLight 650 (dilution 1:100; #ab97018; Abcam); Cy3 AffiniPure Donkey Anti-goat IgG (H+L) (dilution 1:400; #705-165-147; Jackson Immunoresearch, West Grove, PA). Table 1 lists the primary antibodies that were used in our study to detect functional markers of various cell types.
Table 1.
Antibodies Applied in the Present Study.
| Antibody | Marker of |
|---|---|
| Anti-Iba1 | Microglia/Macrophages |
| Anti-GFAP | Astrocytes/Astrocytoma cells |
| Anti-Nestin | Neural stem cells/Glioblastoma stem cells |
| Anti-CD31 Anti-CD34 |
Endothelial cells/Monocyte-derived macrophages/pericytes Endothelial cells/Macrophages/telocytes |
| Anti-NeuN | Mature neurons |
| Phalloidin-TRITC | F-actin |
Double/Triple Immunofluorescence Colocalization
Immunofluorescence staining of 10% formalin-fixed paraffin-embedded tissue sections was performed as previously described.38 Briefly, 5 μm-thick tissue sections were deparaffinized in xylene and hydrated in a graded series of alcohol baths. Heat-induced epitope retrieval was achieved by heating samples in sodium citrate buffer (pH 6.0) at 120C for 10 min in an autoclave. Once nonspecific sites were blocked with 5% bovine serum albumin or normal donkey serum in Tris-buffered saline for 1 hr at room temperature, tissue sections were incubated simultaneously with a mixture of two/three distinct primary antibodies (rabbit against human target 1, mouse against human target 2, goat against human target 3) overnight at 4C. Sections were then incubated for 1 hr at room temperature in the dark with a mixture of two/three secondary antibodies raised in different species and conjugated to different fluorochromes. For actin staining, tissue sections were incubated in the dark for 1 hr at room temperature with phalloidin-TRITC. Sections were mounted with ProLongDiamond antifade mountant containing DAPI (4′,6-diamidino-2-phenylindole) to visualize cell nuclei (Molecular Probes; Thermo Fisher Scientific, Waltham, MA). Sections were analyzed using a FV1000 (Olympus Corporation, Tokyo, Japan) and a SP8 (Leica Microsystems, Wetzlar, Germany) confocal microscope.
Negative controls were performed in the absence of the primary antibodies. Immunoreactivity was absent when the primary antibodies were omitted. In some experiments, nonspecific fluorescence occurred in erythrocytes in the lumen of blood vessels.
Image Analysis and Statistical Analysis
Two independent observers evaluated the specimens blindly. Staining intensities were graded as absent (–), faint (+), moderate (++), or strong (+++). These cut-offs were established by consensus between each investigator following an initial survey of all blindly coded sections. In cases where scoring data differed by more than one unit, the observers reevaluated the sections to reach a consensus. In other cases, means of the scoring data were calculated.
Results
IQGAP1
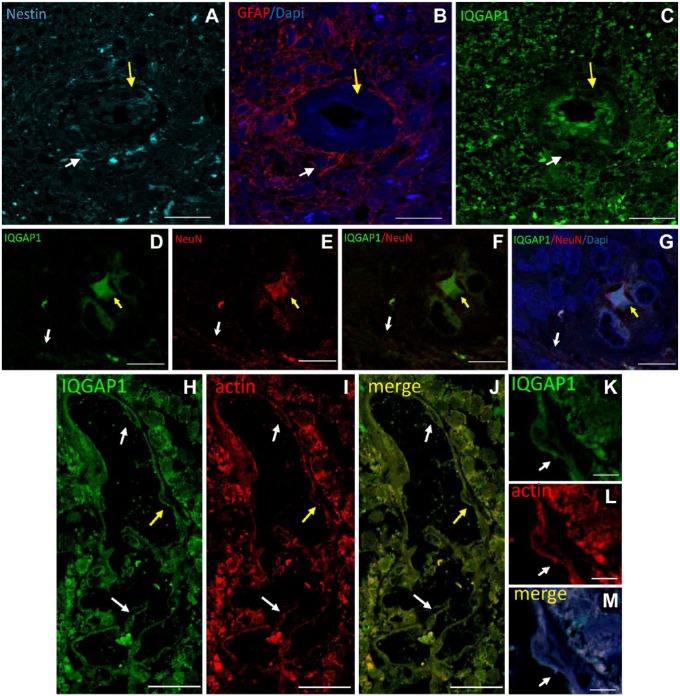
Immunostaining of IQGAP1, nestin, and GFAP in GB sections revealed the presence of nestin+ cells around arterioles (Fig. 2A, arrows). Some of these nestin+ cells co-expressed GFAP (Fig. 2A and B, white arrows) and others did not (Fig. 2A and B, yellow arrows). IQGAP1 was not expressed by most nestin+ cells, whereas endothelial cells of arterioles showed distinct expression of IQGAP1 but not of nestin and GFAP (Fig. 2A–C). Double immunostaining of IQGAP1 and the neuronal differentiation marker NeuN showed that IQGAP1 was expressed in the soma of NeuN+ neurons (Fig. 2D–G, yellow arrow) but not in axons (Fig. 2D–G, white arrow). Furthermore, double labeling of IQGAP1 and F-actin filaments using phalloidin showed that IQGAP1 and actin were expressed in endothelial cells of capillaries and/or sinusoids and in GB cells around these types of blood vessels (Fig. 2H–M, arrows).
Figure 2.
Representative images of immunolabeling of IQGAP1, nestin, and GFAP in human GB tissue sections. (A–C) Serial sections immunostained for nestin (A, cyan), GFAP (B, red), and IQGAP1 (C, green). (A) Around a tumor-associated arteriole, nestin+ and nestin– cells are present. (B) In the periarteriolar area, nestin+/GFAP+ cells (white arrow) and nestin+/GFAP– are present (yellow arrow). (C) IQGAP1-positive immunolabeling is mainly present in the cytoplasm and nuclei of endothelial cells and in tumor tissue around arterioles in a similar localization pattern as GFAP. Arrows indicate cells adjacent to the tunica adventitia of the arteriole; white arrow points at a nestin+/GFAP+/IQGAP1– cell; yellow arrow points at nestin+/GFAP–/IQGAP1+. (D–G) Double immunostaining of IQGAP1 (D, green), the mature neuron marker NeuN (E, red), IQGAP1 and NeuN merged image (F), and IQGAP1, NeuN and DAPI merged image (G). White arrow points at a NeuN+ traversing axon. Yellow arrow points at a NeuN+/IQGAP1+ neuronal soma. (H–J) Capillary or sinusoid showing double immunolabeled IQGAP1 (green) and actin (red). (J) Merged image. Arrows point at IQGAP1+/actin+ endothelial cells. (K–L) Higher magnification of the endothelial cell identified with the yellow arrow in Panels H to J. Bar = 20 µm (A–C); bar = 25 µm (D–J); bar = 5 µm (K–M). Abbreviations: IQGAP1, IQ-motif-containing GTPase-activating protein 1; GFAP, glial fibrillar acidic protein; GB, glioblastoma.
AmotL2
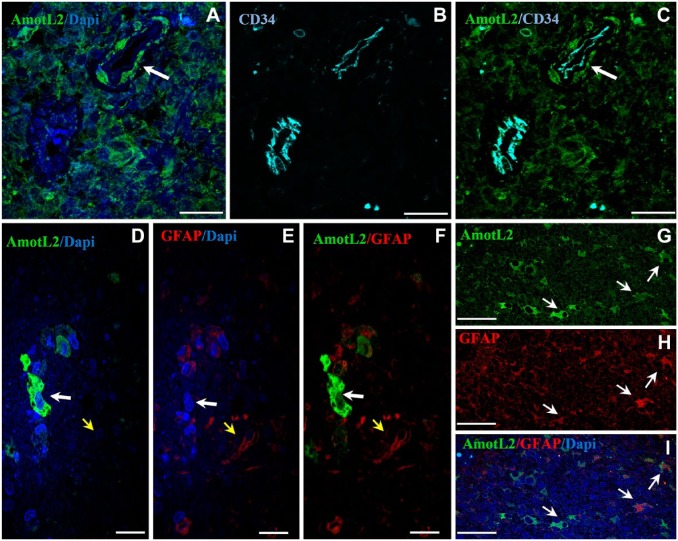
In Fig. 3A–C, a meandering arteriole with CD34+ endothelium is dissected twice. AmotL2+ cells are present adjacent to the tunica adventitia of the upper right part of the dissected arteriole but not of the lower left part of the dissected arteriole. The AmotL2+ cells that are indicated by the arrow in Fig. 3A–C are likely GSCs in a periarteriolar niche. The tunica media is much thinner here than in Fig 2A–C.
Figure 3.
Representative images of immunolabeling of AmotL2, CD34, and GFAP in human GB sections. (A–C) Double immunolocalization of AmotL2 (green) and CD34 (cyan). Arrow points at AmotL2+ cells probably GSCs in the periarteriolar niche around the tunica adventitia of an arteriole. These cells are not present in the second section of the arteriole area of the CD34+ arteriole. Tumor tissue around arteriole is also positive for AmotL2 staining. (D–I) Double immunostaining of AmotL2 (green) and GFAP (red). (D–F) White arrow points at an AmotL2+/GFAP– cell; yellow arrow points at an AmotL2–/GFAP+ cell. (G–I) Arrows point at AmotL2+/GFAP+ cells. Bar = 25 µm (A–C); bar = 20 µm (D–F); bar = 50 µm (G–I). Abbreviations: AmotL2, angiomotin-like 2; GFAP, glial fibrillar acidic protein; GB, glioblastoma; GSC, glioma stem cell.
Strong AmotL2 immunostaining was also observed in cytoplasm and protrusions of differentiated GB cells. To assess the nature of these cells, the astrocyte-lineage marker GFAP was used in double immunofluorescence experiments (Fig. 3D–I). Most cells were either AmotL2+/GFAP– or AmotL2–/GFAP+. Whereas, cells that were both AmotL2+/GFAP+ occurred less frequently (Fig. 3D–I).
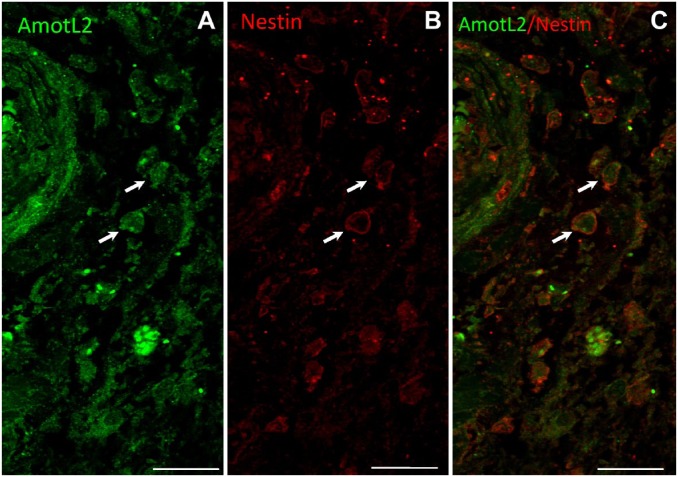
To further demonstrate the expression of AmotL2 by GSCs, double immunostaining of AmotL2 (green) and nestin (red) was performed. Figure 4 shows AmotL2+/nestin+ cells adjacent to the tunica adventitia of an arteriole that may well be GSCs.
Figure 4.
Representative images of double immunostaining of AmotL2 (green) and nestin (red) and a merged image of a human GB tissue section. Arrows point at AmotL2+/nestin+ cells adjacent to the tunica adventitia of an arteriole that are likely GSCs. Bar = 20 µm. Abbreviations: AmotL2, angiomotin-like 2; GB, glioblastoma; GSCs, glioma stem cells.
FKBP51
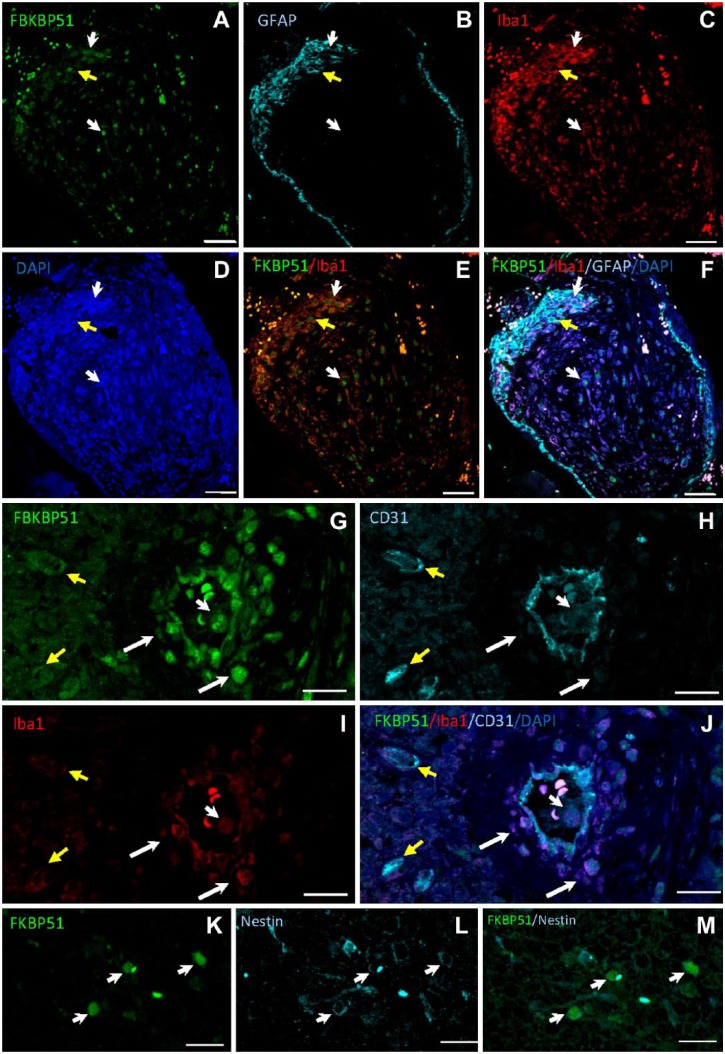
Nuclear FKBP51 staining was observed in a subpopulation of Iba1+ macrophages/microglia in human GB tissue samples (Fig. 5A–F, white arrows), whereas a less intense immunostaining was also found in the cytoplasm of other macrophages/microglia. Nuclear FKBP51 immunolabeling was also found in GFAP+ cells (Fig. 5A–F, yellow arrow). FKBP51+ cells were observed in the wall of arterioles but not around capillaries. CD31+ endothelial cells co-expressed FKBP51 and Iba1 proteins. FKBP51+/Iba1+ cells were also detected in the wall of arterioles (Fig. 5G–J, white arrows). FKBP51+/CD31+/Iba1– cells were observed at the edge of perivascular zones (Fig. 5G–J, yellow arrows). In several cells, an intense FKBP51 immunostaining was also detected in actin-rich core-like cellular structures (Fig. 5G–J, short white arrow) and in nestin+ cells (Fig. 5K–M).
Figure 5.
Human GB tissue sections immunolabeled for FKBP51, Iba1, GFAP, and CD31. (A–D) Iba1+ cells (red; macrophages/microglia) and GFAP+ astrocytoma cells (cyan; yellow arrow) show FKBP51 nuclear staining. (G–J) FKBP51 (green), CD31 (cyan), and Iba1 (red) immunostaining in endothelial cells of an arteriole and capillaries whereas FKBP51+/Iba1+/CD31– cells are mainly located in the arteriolar wall (long white arrows). Endothelial cells of the arteriole and the capillaries are FKBP51+/CD31+/Iba1– (yellow arrows). Short white arrows point at an immune cell with a FKBP51+ nucleus, probably a polymorphonuclear white blood cell in the lumen of the arteriole. (K–M) Arrows point at nestin+/FKBP51+ cells. Please note, that the nonspecific dot-like fluorescence in all images which are shown as white fluorescence in images F and I are erythrocytes. Bar = 50 µm (A–F); bar = 20 µm (G–J); bar = 25 µm (K–M). Abbreviations: GB, glioblastoma; FKBP51, immunophilin, FK506-binding protein 5; GFAP, glial fibrillar acidic protein; DAPI, 4′,6-diamidino-2-phenylindole.
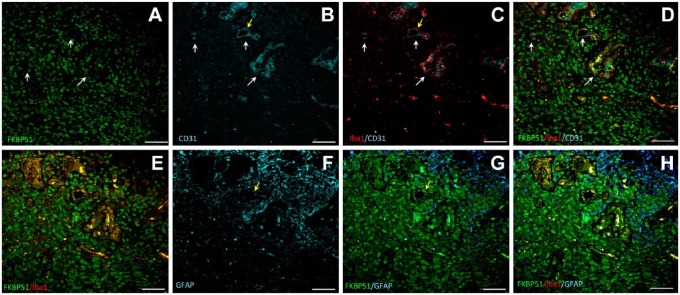
In highly vascularized tissue areas, nuclear FKBP51 immunostaining was present in most cells (Fig. 6). In between, Iba1+ macrophages/microglia and CD31+ endothelial cells were found (Fig. 6, arrows in A–D).
Figure 6.
Representative images of triple immunolabeling for FKBP51, Iba1, CD31, and for FKBP51, Iba1, GFAP proteins in a human GB tissue section. Two serial sections are shown from the same patient, labeled for FKBP51/Iba1/CD31 (A–D) and FKBP51/Iba1/GFAP (E–H). Cells in the highly vascularized tissue area exhibit strong FKBP51 immunostaining (A). Several clusters of cells expressing Iba1 and FKBP51 in the vascularized tissue area (arrows in A-D). Bar = 25 µm. Abbreviations: FKBP51, immunophilin, FK506-binding protein 51; GFAP, glial fibrillar acidic protein; GB, glioblastoma.
Table 2 summarizes protein localization and intensity in GB cells and TME of the three scaffoldins analyzed in this study.
Table 2.
Protein Expression Levels and Localization of IQGAP1, AmotL2, and FKBP51 in GB Microenvironment.
| GB | IQGAP1 | AmotL2 | FKBP51 |
|---|---|---|---|
| Astrocytoma cells | +++/– m, n |
+++/– c, m, ne |
+++/++ n |
| ECs | +++/++ m, p/i, c, n |
+++ m, c |
+++/++ c, n, p/i |
| Perivascular cells | ++/+ m, c |
+++ m, c |
+++/+ c, n, p/i |
| TAMs | +++ m, p/i, n |
+++ c |
+++ n, c, p/i |
| GSCs | ++/– M |
+++/++ c, m, ne |
++/– c |
| Neurons | +++/++ s, d |
nd | nd |
Localization: m, membranes; p/i, podosome/invadosome; c, cytoplasm; n, nucleus; s, soma; d, dendritic spines; ne, nuclear envelope. Intensity: +++, strong; +++/–, variable, strong to absent; ++/-, moderate to absent; +++/++, strong to moderate; ++/+, moderate to weak; +++/+, strong to weak: nd, not detected. Data are based on the analysis of 246 tissue sections (six tissue sections/patient) by two different observers. Abbreviations: IQGAP1, IQ-motif-containing GTPase-activating protein 1; AmotL2, angiomotin-like 2; FKBP51, immunophilin, FK506-binding protein 51; GB, glioblastoma; ECs, endothelial cells; TAMs, tumor-associated macrophages; GSCs, glioma stem cells.
Discussion
The panel of antibodies as cell markers has provided clues to identify undifferentiated cells (GSCs), differentiated malignant cells (astrocytoma cells), and differentiated cell types of the TME. Nestin is an intermediate filament protein and a (not specific) marker of neural stem/progenitor cells in the developing central nervous system (CNS) and GSCs.12,57 It has been reported that nestin in particular is not a specific marker for neuronal stem cells and GSCs and that other GSCs marker have to be detected besides nestin, such as CD133, SOX-2, and CD95. Nestin is also expressed in astrocytes and endothelial cells as was shown in the present study as well. However, in this exploring study to establish the role of scaffoldins in GB, the nestin staining in combination with their localization in relationship to arterioles was sufficient to come to conclusions with respect to expression patterns of scaffoldins in GB. Nestin+/GFAP– GSCs have been found to be intermixed with nestin+/GFAP+ cells, indicating clearly committed cells such as astrocytoma cells and astrocyte-like cells. Nestin was also expressed in endothelial cells of GB which is in agreement with recent reports,12,57 specifically, nestin is expressed in proliferative endothelial cells, but not in mature vasculature. Nestin-positive GSCs were found in periarteriolar niches, as shown in Fig. 2A–C (arrow in 2A) and Fig. 4A–C in agreement with recent reports.12–14,58 This localization pattern mimics that of hematopoietic stem cells in periarteriolar niches in the bone marrow.15
The expression patterns of IQGAP1 in human GB are rather ubiquitous. However, our study indicates that nestin+ cells are expressing low levels of IQGAP1 or no IQGAP1 at all. Therefore, our immunofluorescence study indicates that IQGAP1 is expressed in GFAP+ differentiated astrocytoma cells rather than in GSCs. Moreover, endothelial cells also express considerable amounts of IQGAP1. IQGAP1 was expressed at cell membranes of endothelial cells, which points at a role for IQGAP1 in podosomes/invadosomes.30,38
In nonmalignant cells, IQGAP1 was found to be expressed in differentiated cells such as neurons and particularly in the soma of the neurons and not in the axons. Neurons in GB are scarce and are, usually, observed as transversal axons in tissue sections.59 In our studies, two distinct localization patterns of NeuN were observed, in traversing fibers and in neuronal soma. These NeuN+ neuron-like cells are either remaining neurons or neural precursor-derived cells. Endogenous neural precursors have been reported to have an antitumor response by specifically targeting GSCs,60 suggesting that the microenvironment of the CNS controls proliferation of glial cells and cancer cells. Failure of this system may enable cancer progression as is the case with PD-L1 expression by neurons nearby GB, which is associated with better prognosis for patients.59 Recently, a regulatory role for FKBP51s (FKBP51 spliced isoform) in PD-L1 expression in glioma has been reported.18
In contrast to the expression of IQGAP1, high AmotL2 expression is associated with nestin+ GSCs rather than with nestin– astrocytoma cells. As GSCs are the cancer cell type to be targeted therapeutically, AmotL2 expression and its inhibition need to be studied in the future as a possible manner to realize more effective therapy of GB. Of particular interest for future research is to investigate whether taxane-sensitive tumors in taxane chemotherapy61 modify the expression of AmotL2 as a consequence of drug effect as a more accurately modulated treatment.62 Presently, GB treatment does not include any taxane drug.
In GB glomerular blood vessels, a different AmotL2 expression pattern was observed. CD34+ endothelial cells of arterioles were surrounded by AmotL2+ cells, whereas, endothelial cells that were cubic-shaped were not surrounded by cells that expressed AmotL2. AmotL2+ cells may well be GSCs in periarteriolar niches.
Based on AmotL2 expression, two types of astrocytoma cells can be defined, AmotL2+ and AmotL2–. To our knowledge, a possible function of angiomotin in astrocytoma cells or astrocyte-like (GFAP+ cells) is unknown. Recently, Zhang et al.63 reported that malignant pericytes expressing protein GT198 give rise to cancer cells contributing to GB growth. A complementary role of AmotL2 in this process should be investigated.
FKBP51 was expressed in virtually all types of cells of GB tumors with different cellular and subcellular localization patterns. GFAP+ astrocytoma cells expressed FKBP51 in nuclei. A subpopulation of Iba1+ macrophages showed high intranuclear levels of FKBP51, whereas in other tumor-associated macrophages (TAMs)/glioma-infiltrating macrophages (GIMs), this FKBP51 was only present in the cytoplasm at low levels. FKBP51+/CD31+/Iba1– cells were observed adjacent to the lumen of arterioles.
In highly vascularized tissue areas (glomerular areas), FKBP51 is expressed in all cell types of the GB TME. Near blood vessels, cells are organized in clusters expressing Iba1 and/or FKBP51 and/or CD31. Iba1+/CD31+ cells could represent a common cell type of pericyte/TAMs becoming endothelial cells expressing FKBP51, with different subcellular localization patterns, pointing at an involvement of FKBP51 in pericyte/TAM to endothelial cell differentiation. Around these newly formed blood vessels, GFAP+ astrocytoma cells and Iba1+ intrinsic inflammation-activated microglia are abundant. These facts are in agreement with recent data on periarteriolar niches of GSCs,12–14,58 which are important targets for novel GB treatment.64–67
Stem cells are best defined functionally, but the differentiation processes leading to heterogeneous cell populations are not well understood. In addition, EMT processes have to be considered. In GB, a similar process can be observed leading to the so-called glial-mesenchymal transition (GMT).68,69 In theory, GSCs can differentiate to any type of GB cell. GSCs can transdifferentiate into pericytes that are recruited toward endothelial cells and thus become vascular pericytes that may actively remodel perivascular niches.70 Pericytes are considered as progenitor cells, a source of undifferentiated mesenchymal cells with macrophage-like potential and may form endothelial cells or can become fibroblasts/myofibroblasts.9 In CNS, pericytes are essential components of the blood-brain barrier and in conditions in which the blood-brain barrier becomes leaky, pericytes are involved in the phagocytosis of foreign proteins that pass the endothelial layer.71–73
In the TME of GB, the differentiation commitment presents a variable array; some clusters have very few uncommitted cells, while others contain a large number of uncommitted cells or cells in intermediary steps of differentiation.74 CD133+ GSCs are a subpopulation (cluster) of cancer cells that are resistant to therapy and contribute to tumor recurrence. GSCs are able to secrete chemoattractants for mesenchymal stem cells (MSCs). MSCs response are not well characterized yet and it is not clear whether this tropism is beneficial for patients for drug delivery or immunoregulation or that MSCs contribute to tumor growth.75 Co-expression of CD133 and scaffoldins, especially IQGAP1, needs to be studied to understand any implication of these pathways modulators in chemokines or exosome production by CD133+ GSCs versus CD133– GSCs.
The finding that GSCs in periarteriolar niches are positive for AmotL2 is a promising fact for this protein to be used as a therapeutic target in the future. In our study, the limited number of cases and the heterogeneity of samples does not allow us to establish any correlation between expression levels or cellular or subcellular localization patterns of scaffoldins. However, the consistent and high expression levels of IQGAP1, AmotL2, and FKBP51 in endothelial and perivascular cells are an indication of their pivotal role in angiogenesis and cell migration. Therefore, further studies are needed to understand functions of the three scaffoldins investigated here.
In the GB tumor specimens examined in this study, the plasticity of the TME was confirmed by the fact that none of the markers investigated was specifically expressed by the various cell types, including pericytes, TAMs, and endothelial cells. The expression and localization analyses of the three scaffold protein studied, in correlation with the expression of the different markers used, suggest an involvement of these scaffoldins in cell differentiation and in GMT. The three scaffold proteins provide tantalizing pieces of the puzzle of tumor biology and, as a consequence, this study has to be expanded in areas such the intriguing role of telocytes and their patterns of localization and migration during GB evolvement and expansion, or to get deeper in the biochemical (live cells) and topological bases that drive GSCs to commit either to pericytes or glioma cells to show the roles of these scaffoldins in these processes.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors have contributed to this article as follows: conceptualization (DR, MM, PM-V), methodology (DR, MM, JA, AM, PM-V), validation (DR, MM, M-d-CM, PM-V), formal analysis (DR, PM-V), investigation (DR, M-d-CM), resources (MM, NDP-R, M-d-CM), data curation (MM, M-d-CM), writing-original draft preparation (DR, JA, AM, PM-V), writing-review and editing (DR, JA, MM, M-d-CM, AM, CJFvN, PM-V), visualization (DR, PM-V), supervision (PM-V, AM, MM, M-d-CM), project administration (PM-V), and funding acquisition (PM-V, JA).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant FIS PI11/00114 to Pablo Martín-Vasallo and grant FIS PI12/00729, Spain, to Julio Ávila. Supported partially by grant MCT-FEDER 2003/2004 (Olympus FV-1000) and IMBRAIN-FP7-REGPOT-2012-37 awarded to the Institute of Biomedical Technologies and the Center of Biomedical Research of the Canary Islands at Universidad de La Laguna.
Contributor Information
Deborah Rotoli, UD of Biochemistry and Molecular Biology; Instituto de Tecnologías Biomédicas de Canarias; Universidad de La Laguna, San Cristóbal de La Laguna, Spain; Istituto per l’Endocrinologia e l’Oncologia Sperimentale Gaetano Salvatore, Naples, Italy; Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz, Spain.
Manuel Morales, Oncología Médica; Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz, Spain; Oncología Médica, Hospiten Rambla, Santa Cruz, Spain.
María-del-C. Maeso, Servicio de Anatomía Patológica Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz, Spain.
Julio Ávila, UD of Biochemistry and Molecular Biology; Instituto de Tecnologías Biomédicas de Canarias; Universidad de La Laguna, San Cristóbal de La Laguna, Spain.
Natalia D. Pérez-Rodríguez, Oncología Médica Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz, Spain.
Ali Mobasheri, Department of Regenerative Medicine, State Research Institute Center for Innovative Medicine, Vilnius, Lithuania.
Cornelis J. F. van Noorden, Department of Medical Biology, Cancer Center Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands; Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; Department of Genetic Toxicology and Cancer Biology, National Institute of Biology, Ljubljana, Slovenia.
Pablo Martín-Vasallo, UD of Biochemistry and Molecular Biology; Instituto de Tecnologías Biomédicas de Canarias; Universidad de La Laguna, San Cristóbal de La Laguna, Spain.
Literature Cited
- 1. Brandao M, Simon T, Critchley G, Giamas G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia. Epub 2018 September 21. doi: 10.1002/glia.23520. [DOI] [PubMed] [Google Scholar]
- 2. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. [DOI] [PubMed] [Google Scholar]
- 3. Lee E, Yong RL, Paddison P, Zhu J. Comparison of glioblastoma (GBM) molecular classification methods. Semin. Cancer Biol. 2018;53:201–11. doi: 10.1016/j.semcancer.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 5. Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–17. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Godlewski J, Ferrer-Luna R, Rooj AK, Mineo M, Ricklefs F, Takeda YS, Nowicki MO, Salinska E, Nakano I, Lee H, Weissleder R, Beroukhim R, Chiocca EA, Bronisz A. MicroRNA signatures and molecular subtypes of glioblastoma: the role of extracellular transfer. Stem Cell Rep. 2017;8:1497–505. doi: 10.1016/j.stemcr.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Vleeschouwer S, Bergers G. Chapter 16: glioblastoma: to target the tumor cell or the microenvironment? In: De Vleeschouwer S1, editor. Glioblastoma. Brisbane, Australia: Codon Publications; 2017:315–40. [PubMed] [Google Scholar]
- 8. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–69. [DOI] [PubMed] [Google Scholar]
- 10. Diaz-Flores L, Gutierrez R, Gonzalez-Gomez M, Diaz-Flores L, Jr, Valladares F, Rancel N, Saez FJ, Madrid JF. Telocyte behaviour during inflammation, repair and tumour stroma formation. Adv Exp Med Biol. 2016;913:177–91. doi: 10.1007/978-981-10-1061-3_12. [DOI] [PubMed] [Google Scholar]
- 11. Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 12. Hira VV, Ploegmakers KJ, Grevers F, Verbovsek U, Silvestre-Roig C, Aronica E, Tigchelaar W, Turnsek TL, Molenaar RJ, Van Noorden CJ. CD133+ and nestin+ glioma stem-like cells reside around CD31+ arterioles in niches that express SDF-1alpha, CXCR4, osteopontin and cathepsin K. J Histochem Cytochem. 2015;63:481–93. doi: 10.1369/0022155415581689. [DOI] [PubMed] [Google Scholar]
- 13. Hira VVV, Aderetti DA, Van Noorden CJF. Glioma stem cell niches in human glioblastoma are periarteriolar. J Histochem Cytochem. 2018;66:349–58. doi: 10.1369/0022155417752676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hira VVV, Wormer JR, Kakar H, Breznik B, van der Swaan B, Hulsbos R, Tigchelaar W, Tonar Z, Khurshed M, Molenaar RJ, Van Noorden CJF. Periarteriolar glioblastoma stem cell niches express bone marrow hematopoietic stem cell niche proteins. J Histochem Cytochem. 2018;66:155–73. doi: 10.1369/0022155417749174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hira VVV, Van Noorden CJF, Carraway HE, Maciejewski JP, Molenaar RJ. Novel therapeutic strategies to target leukemic cells that hijack compartmentalized continuous hematopoietic stem cell niches. Biochim Biophys Acta. 2017;1868:183–98. doi: 10.1016/j.bbcan.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 16. Morales M, Ávila J, Gonzalez-Fernandez R, Boronat L, Soriano ML, Martin-Vasallo P. Differential transcriptome profile of peripheral white cells to identify biomarkers involved in oxaliplatin induced neuropathy. J Pers Med. 2014;4:282–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Arrigo P, Russo M, Rea A, Tufano M, Guadagno E, Del Basso De, Caro ML, Pacelli R, Hausch F, Staibano S, Ilardi G, Parisi S, Romano MF, Romano S. A regulatory role for the co-chaperone FKBP51s in PD-L1 expression in glioma. Oncotarget. 2017;8:68291–304. doi: 10.18632/oncotarget.19309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukaide H, Adachi Y, Taketani S, Iwasaki M, Koike-Kiriyama N, Shigematsu A, Shi M, Yanai S, Yoshioka K, Kamiyama Y, Ikehara S. FKBP51 expressed by both normal epithelial cells and adenocarcinoma of colon suppresses proliferation of colorectal adenocarcinoma. Cancer Invest. 2008;26:385–90. [DOI] [PubMed] [Google Scholar]
- 20. Gallo LI, Lagadari M, Piwien-Pilipuk G, Galigniana MD. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J Biol Chem. 2011;286:30152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L, Lou Z, Wang L. The role of FKBP5 in cancer aetiology and chemoresistance. Br J Cancer. 2011;104:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab. 2011;22:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rotoli D, Morales M, Del Carmen MM, Del Pino GM, Morales A, Ávila J, Martin-Vasallo P. Expression and localization of the immunophilin FKBP51 in colorectal carcinomas and primary metastases, and alterations following oxaliplatin-based chemotherapy. Oncol Lett. 2016;12:1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rotoli D, Morales M, Maeso MDC, Garcia MDP, Gutierrez R, Valladares F, Ávila J, Diaz-Flores L, Mobasheri A, Martin-Vasallo P. Alterations in IQGAP1 expression and localization in colorectal carcinoma and liver metastases following oxaliplatin-based chemotherapy. Oncol Lett. 2017;14:2621–8. doi: 10.3892/ol.2017.6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rotoli D, Morales M, Ávila J, Maeso MDC, Garcia MDP, Mobasheri A, Martin-Vasallo P. Commitment of scaffold proteins in the onco-biology of human colorectal cancer and liver metastases after oxaliplatin-based chemotherapy. Int J Mol Sci. 2017;18:891. doi: 10.3390/ijms18040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bendris N, Schmid SL. Endocytosis, metastasis and beyond: multiple facets of SNX9. Trends Cell Biol. 2017;27:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buday L, Tompa P. Functional classification of scaffold proteins and related molecules. FEBS J. 2010;277:4348–55. doi: 10.1111/j.1742-4658.2010.07864.x. [DOI] [PubMed] [Google Scholar]
- 28. Garbett D, Bretscher A. The surprising dynamics of scaffolding proteins. Mol Biol Cell. 2014;25:2315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herrero-Garcia E, O’Bryan JP. Intersectin scaffold proteins and their role in cell signaling and endocytosis. Biochim Biophys Acta. 2017;1864:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abel AM, Schuldt KM, Rajasekaran K, Hwang D, Riese MJ, Rao S, Thakar MS, Malarkannan S. IQGAP1: insights into the function of a molecular puppeteer. Mol Immunol. 2015;65:336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Liang W, Yang Y, Pan Y, Yang Q, Chen X, Singhal PC, Ding G. IQGAP1 regulates actin cytoskeleton organization in podocytes through interaction with nephrin. Cell Signal. 2015;27:867–77. doi: 10.1016/j.cellsig.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erickson JW, Cerione RA, Hart MJ. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem. 1997;272:24443–7. [DOI] [PubMed] [Google Scholar]
- 33. Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:7940–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malarkannan S, Awasthi A, Rajasekaran K, Kumar P, Schuldt KM, Bartoszek A, Manoharan N, Goldner NK, Umhoefer CM, Thakar MS. IQGAP1: a regulator of intracellular spacetime relativity. J Immunol. 2012;188:2057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal. 2012;24:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson M, Sharma M, Henderson BR. IQGAP1 regulation and roles in cancer. Cell Signal. 2009;21:1471–8. [DOI] [PubMed] [Google Scholar]
- 37. Nabeshima K, Shimao Y, Inoue T, Koono M. Immunohistochemical analysis of IQGAP1 expression in human colorectal carcinomas: its overexpression in carcinomas and association with invasion fronts. Cancer Lett. 2002;176:101–9. [DOI] [PubMed] [Google Scholar]
- 38. Rotoli D, Perez-Rodriguez ND, Morales M, Maeso MD, Ávila J, Mobasheri A, Martin-Vasallo P. IQGAP1 in podosomes/invadosomes is involved in the progression of glioblastoma multiforme depending on the tumor status. Int J Mol Sci. 2017;18:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bratt A, Wilson WJ, Troyanovsky B, Aase K, Kessler R, Van Meir EG, Holmgren L. Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene. 2002;298:69–77. [DOI] [PubMed] [Google Scholar]
- 41. Huang H, Lu FI, Jia S, Meng S, Cao Y, Wang Y, Ma W, Yin K, Wen Z, Peng J, Thisse C, Thisse B, Meng A. Amotl2 is essential for cell movements in zebrafish embryo and regulates c-Src translocation. Development. 2007;134:979–88. [DOI] [PubMed] [Google Scholar]
- 42. Mojallal M, Zheng Y, Hultin S, Audebert S, van HT, Johnsson P, Lenander C, Fritz N, Mieth C, Corcoran M, Lembo F, Hallstrom M, Hartman J, Mazure NM, Weide T, Grander D, Borg JP, Uhlen P, Holmgren L. AmotL2 disrupts apical-basal cell polarity and promotes tumour invasion. Nat Commun. 2014;5:4557. doi: 10.1038/ncomms5557. [DOI] [PubMed] [Google Scholar]
- 43. Erlejman AG, De Leo SA, Mazaira GI, Molinari AM, Camisay MF, Fontana V, Cox MB, Piwien-Pilipuk G, Galigniana MD. NF-kappaB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: a role for peptidyl-prolyl isomerase activity. J Biol Chem. 2014;289:26263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–7. [DOI] [PubMed] [Google Scholar]
- 45. Shaw PE. Peptidyl-prolyl isomerases: a new twist to transcription. EMBO Rep. 2002;3:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barik S. Immunophilins: for the love of proteins. Cell Mol Life Sci. 2006;63:2889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–9. [DOI] [PubMed] [Google Scholar]
- 48. Rein T. FK506 binding protein 51 integrates pathways of adaptation: FKBP51 shapes the reactivity to environmental change. Bioessays. 2016;38:894–902. [DOI] [PubMed] [Google Scholar]
- 49. Toneatto J, Guber S, Charo NL, Susperreguy S, Schwartz J, Galigniana MD, Piwien-Pilipuk G. Dynamic mitochondrial-nuclear redistribution of the immunophilin FKBP51 is regulated by the PKA signaling pathway to control gene expression during adipocyte differentiation. J Cell Sci. 2013;126:5357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Romano S, D’Angelillo A, D’Arrigo P, Staibano S, Greco A, Brunetti A, Scalvenzi M, Bisogni R, Scala I, Romano MF. FKBP51 increases the tumour-promoter potential of TGF-beta. Clin Transl Med. 2014;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han H, Yang B, Wang W. Angiomotin-like 2 interacts with and negatively regulates AKT. Oncogene 2017;36:4662–9. doi: 10.1038/onc.2017.101. [DOI] [PubMed] [Google Scholar]
- 53. Kumar R, Moche M, Winblad B, Pavlov PF. Combined x-ray crystallography and computational modeling approach to investigate the Hsp90 C-terminal peptide binding to FKBP51. Sci Rep. 2017;7:14288. doi: 10.1038/s41598-017-14731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS One. 2008;3:e3848. doi: 10.1371/journal.pone.0003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sayedyahossein S, Li Z, Hedman AC, Morgan CJ, Sacks DB. IQGAP1 binds to yes-associated protein (YAP) and modulates its transcriptional activity. J Biol Chem. 2016;291:19261–73. doi: 10.1074/jbc.M116.732529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Li Z, Xu P, Huang L, Tong J, Huang H, Meng A. Angiomotin-like2 gene (amotl2) is required for migration and proliferation of endothelial cells during angiogenesis. J Biol Chem. 2011;286:41095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 2010;58:721–30. doi: 10.1369/jhc.2010.955609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aderetti DA, Hira VVV, Molenaar RJ, Van Noorden CJF. The hypoxic peri-arteriolar glioma stem cell niche, an integrated concept of five types of niches in human glioblastoma. Biochim Biophys Acta. 2018;1869:346–54. doi: 10.1016/j.bbcan.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 59. Liu Y, Carlsson R, Ambjorn M, Hasan M, Badn W, Darabi A, Siesjo P, Issazadeh-Navikas S. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J Neurosci. 2013;33:14231–45. doi: 10.1523/JNEUROSCI.5812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chirasani SR, Sternjak A, Wend P, Momma S, Campos B, Herrmann IM, Graf D, Mitsiadis T, Herold-Mende C, Besser D, Synowitz M, Kettenmann H, Glass R. Bone morphogenetic protein-7 release from endogenous neural precursor cells suppresses the tumourigenicity of stem-like glioblastoma cells. Brain. 2010;133:1961–72. doi: 10.1093/brain/awq128. [DOI] [PubMed] [Google Scholar]
- 61. Perry MC, Doll DC, Freter CE. Chemotherapy source book. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. Available from: http://site.ebrary.com/id/10825358.
- 62. Garcia JA. HIFing the brakes: therapeutic opportunities for treatment of human malignancies. Sci STKE. 2006;2006:e25. doi: 10.1126/stke.3372006pe25. [DOI] [PubMed] [Google Scholar]
- 63. Zhang L, Wang Y, Rashid MH, Liu M, Angara K, Mivechi NF, Maihle NJ, Arbab AS, Ko L. Malignant pericytes expressing GT198 give rise to tumor cells through angiogenesis. Oncotarget. 2017;8:51591–607. doi: 10.18632/oncotarget.18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Angara K, Rashid MH, Shankar A, Ara R, Iskander A, Borin TF, Jain M, Achyut BR, Arbab AS. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol Histopathol. 2017;32:917–28. doi: 10.14670/HH-11-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Diaz-Flores L, Gutierrez R, Garcia-Suarez MP, Saez FJ, Gutierrez E, Valladares F, Carrasco JL, Diaz-Flores L, Jr, Madrid JF. Morphofunctional basis of the different types of angiogenesis and formation of postnatal angiogenesis-related secondary structures. Histol Histopathol. 2017;32:1239–79. doi: 10.14670/HH-11-923. [DOI] [PubMed] [Google Scholar]
- 66. Dimberg A. The glioblastoma vasculature as a target for cancer therapy. Biochem Soc Trans. 2014;42:1647–52. doi: 10.1042/BST20140278. [DOI] [PubMed] [Google Scholar]
- 67. Lv M, Shen Y, Yang J, Li S, Wang B, Chen Z, Li P, Liu P, Yang J. Angiomotin family members: oncogenes or tumor suppressors? Int J Biol Sci. 2017;13:772–81. doi: 10.7150/ijbs.19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mahabir R, Tanino M, Elmansuri A, Wang L, Kimura T, Itoh T, Ohba Y, Nishihara H, Shirato H, Tsuda M, Tanaka S. Sustained elevation of Snail promotes glial-mesenchymal transition after irradiation in malignant glioma. Neuro Oncol. 2014;16:671–85. doi: 10.1093/neuonc/not239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Z, Zhang S, Siu TL, Huang S. Glioblastoma multiforme formation and EMT: role of FoxM1 transcription factor. Curr Pharm Des. 2015;21:1268–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–52. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Baker RN, Cancilla PA, Pollock PS, Frommes SP. The movement of exogenous protein in experimental cerebral edema. An electron microscopic study after freeze-injury. J Neuropathol Exp Neurol. 1971;30:668–79. [DOI] [PubMed] [Google Scholar]
- 72. Cancilla PA, Baker RN, Pollock PS, Frommes SP. The reaction of pericytes of the central nervous system to exogenous protein. Lab Invest. 1972;26:376–83. [PubMed] [Google Scholar]
- 73. van Deurs B. Observations on the blood-brain barrier in hypertensive rats, with particular reference to phagocytic pericytes. J Ultrastruct Res. 1976;56:65–77. [DOI] [PubMed] [Google Scholar]
- 74. Levenson R, Housman D. Commitment: how do cells make the decision to differentiate? Cell. 1981;25:P5–6. [DOI] [PubMed] [Google Scholar]
- 75. Pavon LF, Sibov TT, de Souza AV, da Cruz EF, Malheiros SMF, Cabral FR, de Souza JG, Boufleur P, de Oliveira DM, de Toledo SRC, Marti LC, Malheiros JM, Paiva FF, Tannus A, de Oliveira SM, Chudzinski-Tavassi AM, de Paiva Neto MA, Cavalheiro S. Tropism of mesenchymal stem cell toward CD133(+) stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res Ther. 2018;9:310. doi: 10.1186/s13287-018-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]