Abstract
Primary cilia, regulated via distinct signal transduction pathways, play crucial roles in various cellular behaviors. However, the full regulatory mechanism involved in primary cilia development during cellular differentiation is not fully understood, particularly for the sensory hair cells of the mammalian cochlea. In this study, we investigated the effects of the Rho-kinase inhibitor Y27632 and PKCα inhibitor GF109203X on primary cilia-related cell behavior in undifferentiated and differentiated temperature-sensitive mouse cochlear precursor hair cells (the conditionally immortalized US/VOT-E36 cell line). Our results indicate that treatment with Y27632 or GF109203X induced primary cilia elongation and tubulin acetylation in both differentiated and undifferentiated cells. Concomitant with cilia elongation, Y27632 treatment also increased Hook2 and cyclinD1 expression, while only Hook2 expression was increased after treatment with GF109203X. In the undifferentiated cells, we observed an increase in the number of S and G2/M stage cells and a decrease of G1 cells after treatment with Y27632, while the opposite was observed after treatment with GF109203X. Finally, while both treatments decreased oxidative stress, only treatment with Y27632, not GF109203X, induced cell cycle-dependent cell proliferation and cell migration.
Keywords: cell cycle, cell migration, Hook2, mouse cochlear cells, oxidative stress, PKCα inhibitor GF109203X, primary cilia, Rho-kinase inhibitor Y27632
Introduction
Cilia are membrane-bound hair-like structures that extend from the basal bodies located beneath the cell surface. They are usually classified into primary cilia and motile cilia according to the components of their axonemal microtubules. Primary cilia have a “9 + 0” architecture composed of nine outer microtubule doublets but no central pair of microtubules and develop via the extension of microtubules from a diplosomal centriole in various cell types, tissues, and organs.1–3 Notably, primary cilia are major cellular sensory organelles, acting as sensory antennae to compartmentalize receptors and signaling factors, and their structure, length, and function are largely tissue dependent.4,5 In the mammalian cochlea, the specialized primary cilia of the inner ear hair cells, otherwise known as the kinocilia, are largely responsible for hair bundle polarity and the hearing process, while not being directly involved in auditory perception.6 It is typically thought that the kinocilia have a “9 + 2” architecture composed of nine outer microtubule doublets and a central pair of microtubules.7 However, it is also reported that about 80% of auditory kinocilia consist of “9 + 0” microtubules, while the “9 + 2” form occurs only in less than 20%.8 In the mouse, the polarization of the kinocilium precedes the polarization of the stereociliary bundle during hair cell bundle development and the epithelial cells lose their kinocilium after the completion of stereociliary bundle structures.9,10 The natural late loss of the kinocilium in organ of Corti-hair cells does not result in aberrant development. However, mutation of kinocilia proteins affects on the normal development of organ of Corti-hair cells.10 The kinocilium of inner ear hair cells is not involved in auditory perception.9,10 The cochlear kinocilium may be critical for the emergence of hair bundle polarity and crucial for the hearing process.
As the behavior of primary cilia plays such a significant role in establishing and maintaining cellular function and polarity, deficiencies in their assembly or function have been associated with a variety of pathologies (ciliopathies), including retinal degeneration, obesity, diabetes, polycystic kidney disease, left–right asymmetry defects, and hydrocephalus.11,12 Furthermore, while proteins associated with auditory cilia have been shown to be dysfunctional in Usher syndrome patients exhibiting deafness13,14 as well as Bardet–Biedl syndrome patients with auditory deficiencies,15 the full mechanism underlying these cilia-related auditory defects is unclear.
Previous studies have shown that various signaling pathways, such as the sonic hedgehog pathway, Wnt pathway, platelet-derived growth factor (PDGF) pathway, and calcium signaling pathway, are organized by the primary cilia during development, highlighting the involvement of these organelles in the cell cycle, cell differentiation, and apoptosis.4,16–18 With regard to the regulation of the primary cilia, recent evidence indicates that autophagy induced by BIX-01294, a selective inhibitor of euchromatin histone lysine N-methyltransferase-2 (EHMT2), various proinflammatory cytokines, including tumor necrosis factor (TNF)α, interleukin (IL)1β, and interferon (IFN)γ and/or IL-10, and Rho-kinase inhibitor Y27632 all modulate primary cilia elongation.19–21 Nakakura et al.22 also reported that lithium chloride activates α-tubulin N-acetyltransferase 1 via glycogen synthase kinase (GSK)-3β inhibition and promotes α-tubulin acetylation as well as the subsequent elongation of the primary cilia. Furthermore, Gadadhar et al.23 showed that tubulin glycylation is crucial for controlling and maintaining primary cilia length, particularly during cilia dysfunction-associated human disorders. The adaptor protein Hook2 is also thought to be involved in primary cilia morphogenesis.24 In cochlear sensory hair cells, RhoA/protein kinase C (PKC)-mediated signals are known to regulate outer hair cell motility, with PKCα being the relevant isoform, and RhoA/Rho-associated protein kinase (ROCK)-mediated pathways appear to regulate the hair cells’ motile response.25,26 Unfortunately, the behavior and mechanisms of the Rho-kinase and PKCα regulating ciliogenesis is still unclear during the development and regeneration of cochlear hair cells. In the present study, we investigated the effects of the Rho-kinase inhibitor Y27632 and the PKCα inhibitor GF109203X on primary cilia-related cell behavior in undifferentiated and differentiated temperature-sensitive mouse cochlear precursor hair cells. To our knowledge, this is the first time the effects of these inhibitors have been evaluated with regard to primary cilia differentiation in the sensory hair cells of mice.
Materials and Methods
Reagents and Antibodies
The Rho-kinase inhibitor Y27632 was obtained from Nacali (Tokyo, Japan), while the PKCα inhibitor GF109203X was from Calbiochem–Novabiochem Corporation (San Diego, CA). The mouse monoclonal anti-acetylated tubulin (T7451) and rabbit polyclonal anti-γ-tubulin antibodies used in this study were from Sigma-Aldrich (St. Louis, MO). The mouse monoclonal anti-cyclinD1 antibody was from MBL (Nagoya, Japan), while the rabbit polyclonal anti-Hook2 antibody and rabbit polyclonal anti-phospho-PKCα/β (T638/641) antibody were from Novus Biologicals (Littleton, CO) and Cell Signaling Technology (Danvers, MA), respectively. Alexa 488 (green)-conjugated anti-rabbit IgG and Alexa 594 (red)-conjugated anti-mouse IgG antibodies were acquired from Molecular Probes, Inc. (Eugene, OR). The enhanced chemiluminescence western blot visualization kit was purchased from GE Healthcare UK, Ltd. (Buckinghamshire, UK).
Cell Culture and Treatments
We used the conditionally immortal cell line University of Sheffield/ventral otocyst-epithelial cell line clone 36 (US/VOT-E36) derived from ventral otic epithelial cells of the mouse at embryonic day 10.5, which was gifted from Professor Matthew Holley.27 In this culture model, cell proliferation and precursor/undifferentiated status are maintained when cultured at 33C, but the cells undergo growth arrest and differentiation at 39C. During differentiation by using E36 cells incubated at 39C, the tricellular tight junction molecules tricellulin and lipolysis-stimulated lipoprotein receptor (LSR) which are differentiated markers are induced (Supplemental Fig. 1).28 The cells were cultured in minimum essential medium (Nacalai Tesque, Inc., Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 100 µg/ml streptomycin, and 50 µg/ml amphotericin B. The cells were plated on 60-mm culture dishes (Corning Glass Works, Corning, NY) coated with rat-tail collagen (500 µg dried tendon/ml in 0.1% acetic acid) and incubated in a humidified atmosphere with 5% CO2 at 33C. After incubation at 33C for several days, the temperature was raised to 39C to induce cell differentiation. For some experiments, the cells were treated with 10 μM Y27632 or 10 μM GF109203X for the indicated number of days before analysis.21 The inhibitor stocks were prepared at 10 mM by dimethyl sulfoxide (DMSO) and stored at −30C. The inhibitors were used after dilution 1:1000 by the medium. DMSO was added to the media at a concentration of 1:1000.
Western Blot Analysis
Cells were scraped from a 35-mm dish containing 400 µl of buffer (1 mM NaHCO3 and 2 mM phenylmethylsulfonyl fluoride), collected in microcentrifuge tubes, and then sonicated for 10 sec. The protein concentrations of the samples were determined using a bicinchoninic acid protein assay (Pierce Chemical Co., Rockford, IL). The cells were lysed in NuPAGE LDS Sample Buffer (Thermo Fisher Scientific). The cell lysates (15 μg of total protein in each well) were separated by SDS-PAGE (SuperSep Ace, 5–20%, 13 wells; Wako Pure Chemicals, Osaka, Japan) and transferred onto nitrocellulose membranes (Immobilon, Millipore Co., Bedford, UK). The membranes were blocked by incubation with 4% nonfat dry milk in TBS for 1 hr at room temperature and incubated overnight at 4C with the primary antibodies (1:1000): anti-acetylated tubulin, anti-γ-tubulin, anti-cyclinD1, anti-Hook2, and anti-phospho-PKCα/β antibodies. The membranes were then washed three times and then incubated for 1 hr at room temperature with species-specific horseradish peroxidase-conjugated secondary antibodies (NA931 or NA934; GE Healthcare, Buckinghamshire, UK). Signals were detected with an enhanced chemiluminescence–plus reagent (GE Healthcare, Piscataway, NJ) in LAS3000 (Fujifilm, Tokyo, Japan). The signals were quantified using Scion Image Beta 4.02 Win (Scion Co.; Frederick, MA). Each set of results shown is representative of at least three separate experiments.
Immunocytochemistry Analysis
Cells cultured in a 35-mm glass-coated dish (Iwaki, Chiba, Japan) were fixed with cold acetone and ethanol (1:1) at −20C for 10 min, rinsed in phosphate-buffered saline (PBS), and then incubated with anti-acetylated tubulin antibody (1:400), anti-γ tubulin antibody (1:200), or anti-Hook2 antibody (1:200) at room temperature for 1 hr. Alexa Fluor 488 (green)-conjugated anti-rabbit IgG and Alexa Fluor 594 (red)-conjugated anti-mouse IgG (Invitrogen) were used as secondary antibodies. Specimens were examined with either an epifluorescence (Olympus, Tokyo, Japan) or confocal laser scanning microscope (LSM5; Carl Zeiss, Jena, Germany). A LSM5 confocal microscope was used to create maximum projections of confocal z-stacks from which cilia length was measured using image J software. At least three different mounted preparations were used to capture six fields of cells at ×63 magnification giving data for >100 cilia per subgroup.
Scanning Electron Microscopy (SEM) Analysis
For SEM, cells cultured on 15-mm cover glasses (Matsunami Glass, Ind., Ltd, Bellingham, WA) were fixed in 2.5% glutaraldehyde in 0.1 M PBS (pH 7.3) at 4C for 3 hr. After rinsing in PBS, the cells were postfixed in 1% osmium tetroxide in PBS at 4C for 3 hr, dehydrated with a graded ethanol series (70 to 100% ethanol) at 4C for 20 min in each step, and dried with butyl alcohol. The dried samples were sputter-coated with platinum and examined under a scanning electron microscope (S-4300; Hitachi, Tokyo, Japan).
Transmission Electron Microscopy (TEM) Analysis
For TEM, the cultured cells were fixed in 2.5% glutaraldehyde in PBS overnight at 4C, followed by postfixing in 2% osmium tetroxide in the same buffer. Then, the cells were dehydrated with a graded ethanol series and embedded in Epon 812. Ultrathin sections were then cut on a Sovall Ultramicrotoe MT-5000. The sections were stained with uranyl acetate followed by lead citrate and examined at 80 kV with a transmission electron microscope (H7500; Hitachi, Tokyo, Japan).
Cell Cycle Assay
Cells were treated and collected with 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA) and washed once with PBS. Then, the cells were slowly added to 1 ml of ice-cold 70% ethanol and incubated for at least 3 hr at −20C. The cells were then washed once with PBS, followed by the addition of 200 μl of Muse Cell Cycle reagent (MCH100106; Merck Millipore, MA). After incubating for 30 min at room temperature in the dark, the percentage of cells in G0/G1, S, and G2/M phases was calculated using a Muse cell analyzer (Merk Millipore, MA).
Cell Proliferation Assay
The cells were seeded onto 96-well culture plates (Corning, NY). After 5 days, proliferation was measured using a Cell Counting Kit-8 (CK04; Wako, Osaka, Japan) according to the manufacturer’s instructions. The absorption at 450 nm was measured with an iMark Microplate Reader (Bio-Rad, Hercules, CA).
Cell Migration Assay
After the cells were plated onto 35-mm dishes, they were cultured to confluence. A wound was then made in the cell layer using a plastic pipette tip (P100). At 24 hr after the scratch wound, we measured the distance of the wound using an inverted microscope imaging system (Olympus, Tokyo, Japan).
Oxidative Stress Assay
Cultured cells were treated with 0.05% Trypsin-EDTA, collected, and 500 μl of 1X Assay buffer was added. We diluted the Muse Oxidative Stress reagent (MCH100111; Merck Millipore, MA) 1:800 with 1X Assay buffer to make working solution. A 10 μl aliquot of each sample was then added to 190 μl of working solution, respectively, and incubated for 30 min at 37C. The percentage of cells that are reactive oxygen species, negative and positive in both adherent and suspension, was calculated using a Muse cell analyzer (Merk Millipore, MA).
Data Analysis
Signals were quantified using Scion Image Beta 4.02 Win (Scion Co.; Frederick, MA). Each set of results shown is representative of at least three separate experiments. Results are given as mean ± SEM. Differences between groups were tested by analysis of variance followed by a post hoc test and an unpaired two-tailed Student’s t test.
Results
Morphology of Primary Cilia in Temperature-sensitive Mouse Cochlear Precursor Hair Cells
To morphologically characterize the primary cilia in temperature-sensitive mouse cochlear precursor hair cells, we performed immunocytochemical analysis using anti-acetylated tubulin and anti-γ-tubulin antibodies as well as SEM and TEM analyses. In undifferentiated cells (incubated at 33C), acetylated tubulin-positive primary cilia were observed along with a γ-tubulin-positive basal body (Fig. 1A). Furthermore, the primary cilia structures extended from the ciliary pocket on the cell surface and had the typical “9 + 0” architecture (Fig. 1B–D). A high-density substance indicating the basal body was also observed (Fig. 1C). After differentiation (incubated at 39C), the primary cilia disappeared from the cell surface, while the γ-tubulin-positive basal bodies were still present (Fig. 1E and F).
Figure 1.
Morphological characterization of primary cilia on differentiated and undifferentiated mouse cochlear precursor hair cells. (A) Representative images showing double-immunocytochemical staining for Ac-tubulin (red) and γ-tubulin (green) in the undifferentiated US/VOT-E36 cells. Representative scanning electron microscopy (SEM) (B) and transmission electron microscopy (TEM) (C, D) of the undifferentiated cells. (E, F) Images showing double-immunocytostaining for Ac-tubulin (red) and γ-tubulin (green) in the undifferentiated cells (incubated at 33C) and differentiated cells (incubated at 39C). Scale bars = 5 μm (A), 1 μm (B, C), 0.5 μm (D), 10 μm (E, F). Abbreviation: US/VOT-E36, University of Sheffield/ventral otocyst-epithelial cell line clone 36.
Rho-kinase Inhibitor Y27632 Induces Primary Cilia Elongation in Undifferentiated Temperature-sensitive Mouse Cochlear Precursor Hair Cells
To investigate the role of Rho-kinase in temperature-sensitive mouse cochlear precursor hair cells, the cells were treated with Y27632 inhibitor for 11 days, followed by immunocytochemical, SEM, and western blotting analyses. Elongation of the acetylated tubulin-positive primary cilia structure was observed from day 1 until day 11 after treatment with Y27632 (Fig. 2A–D). In SEM analysis, elongation of primary cilia structures was confirmed from day 5 until day 11 after treatment with Y27632 (Fig. 2E). In western blotting analysis, expression of acetylated tubulin was increased from day 1 until day 11 after treatment with Y27632 (Fig. 2F and G).
Figure 2.
Rho-kinase inhibitor induces primary cilia elongation in undifferentiated mouse cochlear precursors. Images showing double-immunocytostaining for Ac-tubulin (red) and γ-tubulin (green) of undifferentiated US/VOT-E36 cells treated with Rho-kinase inhibitor Y27632 for 3 days (A) or 11 days (B). (C, D) The graph of the length of cilia labeled Ac-tubulin in A and B. (E) Scanning electron microscopy (SEM) images in undifferentiated cells treated with Y27632 for 11 days. Western blotting analysis of Ac-tubulin and γ-tubulin in undifferentiated cells treated with Y27632 for (F) 3 days or (G) 11 days. The ratio is normalized to γ-tubulin. Scale bars = 10 μm (A, B), 2 μm (E). Abbreviation: US/VOT-E36, University of Sheffield/ventral otocyst-epithelial cell line clone 36. **p<0.01 versus control.
PKCα Inhibitor GF109203X Induces Primary Cilia Elongation in Undifferentiated Temperature-sensitive Mouse Cochlear Precursor Hair Cells
To investigate role of PKCα in temperature-sensitive mouse cochlear precursor hair cells, the cells were treated with GF109203X inhibitor for 3 days, followed by immunocytochemical, SEM, and western blotting analyses. Elongation of the acetylated tubulin-positive primary cilia structure was observed from day 1 until day 3 after treatment with GF109203X (Fig. 3A and B). In SEM analysis, elongation of primary cilia structures was confirmed at day 3 after treatment with GF109203X (Fig. 3C). Furthermore, the expression of phospho-PKCα was decreased starting at day 1 after treatment with GF109203X, whereas no change of the acetylated tubulin expression was observed (Fig. 3D).
Figure 3.
PKCα inhibitor induces primary cilia elongation in undifferentiated mouse cochlear precursors. (A) Images showing double-immunocytostaining for Ac-tubulin (red) and γ-tubulin (green) in undifferentiated US/VOT-E36 cells treated with PKCα inhibitor GF109203X for 3 days. (B) Scanning electron microscopy (SEM) images in undifferentiated cells treated with GF109203X for 3 days. (C) The graph of the length of cilia labeled Ac-tubulin in A. (D) Western blotting for Ac-tubulin, phospho-PKCα and γ-tubulin in undifferentiated cells treated with GF109203X for 3 days. The ratio is normalized to γ-tubulin Scale bars = 5 μm (A), 1 μm (B). Abbreviations: PKC, protein kinase C; US/VOT-E36, University of Sheffield/ventral otocyst-epithelial cell line clone 36. **p<0.01 versus control.
Elongation of Primary Cilia by Y27632 or GF109203X in Differentiated Temperature-sensitive Mouse Cochlear Precursor Hair Cells
To investigate the effects of Rho-kinase or PKCα inhibition during temperature-sensitive mouse cochlear precursor hair cell differentiation, the cells cultured at 39C were treated with Y27632 or GF109203X for 2 days. Acetylated tubulin-positive primary cilia were present after both Y27632 and GF109203X treatment and elongation of the structure was observed at day 2 (Fig. 4A–C and 4E–G, respectively). Moreover, in the cells cultured at 39C, the acetylated tubulin was increased by Y27632 treatment (Fig. 4D) and that of phospho-PKCα were decreased by GF109203X treatment (Fig. 4H) as well as the cells cultured at 33C.
Figure 4.
Rho-kinase and PKCα inhibitors induce primary cilia elongation in differentiated mouse cochlear precursors. Differentiated US/VOT-E36 cells cultured at 39C were treated with Y27532 (A–D) or GF109203X (E–H) for 2 days. (A, E) Images showing double-immunocytostaining for Ac-tubulin (red) and γ-tubulin (green) and (B, F) scanning electron microscopy (SEM) images in differentiated cells treated with Y27532 or GF109203X for 2 days. (C, G) The graph of the length of cilia labeled Ac-tubulin in A and E. (D, H) Western blotting analysis of Ac-tubulin, γ-tubulin, and phospho-PKCα in differentiated cells treated with Y27532 or GF109203X for 2 days. The ratio is normalized to γ-tubulin. Scale bars = 10 μm (A, E), 0.5 μm (B, F). Abbreviations: PKC, protein kinase C; US/VOT-E36, University of Sheffield/ventral otocyst-epithelial cell line clone 36. **p<0.01 versus control.
Hook2 and CyclinD1 Expression Is Altered by Y27632 and GF109203X Treatment in Undifferentiated Temperature-sensitive Mouse Cochlear Precursor Hair Cells
To investigate the molecular changes induced by Rho-kinase and PKCα inhibition in undifferentiated temperature-sensitive mouse cochlear precursor hair cells, the cells were treated with Y27632 or GF109203X for 3 days and then Hook2 and cyclinD1 expression was analyzed. When the cells were treated with Y27632 or GF109203X, no changes were observed in Hook2 localization in the γ-tubulin-positive basal body (Fig. 5A). However, when Y27632-mediation elongation of the acetylated tubulin-positive primary cilia was observed (starting 1 day after treatment), the expression of both Hook2 and cyclinD1 increased (Fig. 5B and C). Following GF109203X treatment, Hook2 expression similarly increased starting at day 1 in conjunction with inhibitor-mediated elongation, but cyclin D1 expression was not altered (Fig. 5D and E).
Figure 5.
Rho-kinase and PKCα inhibitors affect Hook2 and CyclinD1 expression in undifferentiated mouse cochlear precursors. (A) Images showing double-immunocytostaining for Ac-tubulin (red) and Hook2 (green) as well as Ac-tubulin (red) and γ-tubulin (green) in undifferentiated US/VOT-E36 cells treated Y27632 or GF109203X for 3 days. (B, D) Western blotting analysis for Hook2, cyclinD1, and γ-tubulin in undifferentiated cells after treatment with Y27532 or GF109203X for 3 days. (C, E) The graph of the bands in B and D. The ratio is normalized to γ-tubulin. Scale bars = 2 μm (A). Abbreviations: PKC, protein kinase C; US/VOT-E36, University of Sheffield/ventral otocyst-epithelial cell line clone 36. *p<0.05, **p<0.01 versus control.
Y27632 and GF109203X Treatment Modulates Cell Behavior in Undifferentiated Temperature-sensitive Mouse Cochlear Precursor Hair Cells
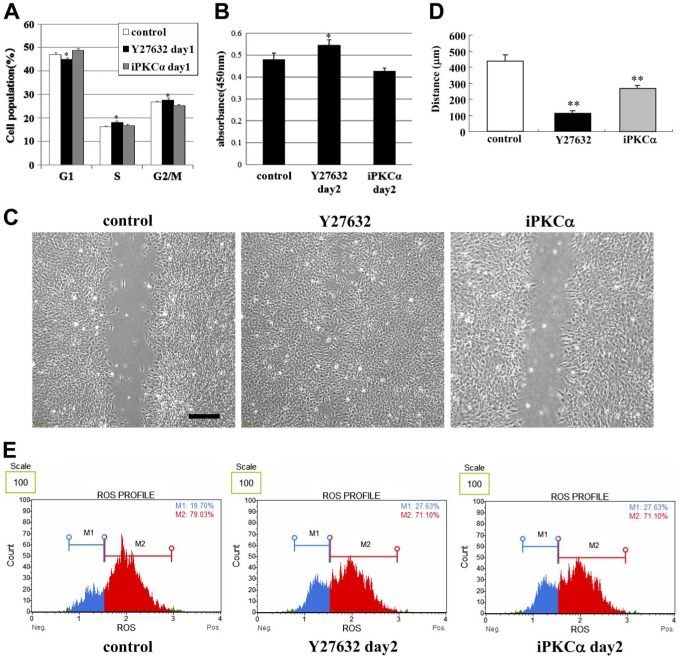
To investigate the effects of Rho-kinase and PKCα inhibition on the cell cycle, proliferation, migration, and oxidative stress in differentiated temperature-sensitive mouse cochlear precursor hair cells, the cells were treated with Y27632 or GF109203X for 2 days, followed by analysis of various cell behaviors. With regard to the cell cycle, an increase in the number of cells in the S and G2/M stages was observed along with a decrease in the number in G1 1 day after treatment with Y27632, while the opposite (i.e., an increase in the number of cells in the G1 stage and a decrease in that in G2/M) was observed at this time point after treatment with GF109203X (Fig. 6A). Furthermore, the total cell count was increased 2 days after treatment with Y27632 and decreased 2 days after treatment with GF109203X (Fig. 6B). In our cell migration assay, treatment with Y27632, but not GF109203X, promoted cell migration 2 days after treatment (Fig. 6C and D, respectively). Finally, both Y27632 and GF109203X treatments decreased the oxidative stress levels in the cultures 2 days after treatment (Fig. 6E).
Figure 6.
Rho-kinase and PKCα inhibition modulates undifferentiated mouse cochlear precursor hair cell behavior. Assays for (A) cell cycle, (B) cell proliferation, (C) cell migration, and (E) oxidative stress in undifferentiated US/VOT-E36 cells treated with Y27532 or GF109203X for 2 days. (D) The graph of the distance in C. Scale bars = 200 μm (C). Abbreviations: PKC, protein kinase C; US/VOT-E36, University of Sheffield/ventral otocyst-epithelial cell line clone 36; ROS, reactive oxygen species. *p<0.05, **p<0.01 versus control.
Discussion
Although primary cilia have been shown to play a significant role in various cellular behaviors in a multitude of cell types and tissues, the regulatory mechanisms underlying primary cilia development is still largely unknown, particularly in the context of the cochlear sensory hair cells. In the present study, we treated temperature-sensitive mouse cochlear precursor hair cells with Rho-kinase (Y27632) and PKCα (GF109203X) inhibitors to clarify their effects on primary cilia-related cell behavior during differentiation. Our results indicate that treating these cells with Y27632 and GF109203X induces primary cilia elongation of both differentiated and undifferentiated cells. Furthermore, treatment with Y27632 induces an increase in Hook2 and cyclin D1 expression, while GF109203X treatment only induces an increase in Hook2, not cyclin D1. We also observed an increase in the number of cells in the S and G2/M stages and a decrease in the number of G1 cells after treatment with Y27632 along with increased proliferation and migration. Alternatively, after GF109203X treatment, we found an increase in the number of G1 cells and a decrease in the number of cells in the G2/M stage along with decreased proliferation. Both treatments also decreased oxidative stress in the cells. To our knowledge, this is the first time the effects of these inhibitors have been evaluated with regard to primary cilia differentiation in the sensory hair cells of mice.
Primary cilia are long microtubule-based appendages that extend from the surface of most mammalian cells. In contrast to motile cilia, which are always found in multiples per cell to move fluid across the membrane surface, primary cilia are typically singular and adopt sensory roles. These sensory cilia are important for various signaling functions during the establishment and maintenance of planar cell polarity in sensory hair cells. Moreover, patients with ciliopathies affecting the inner ear are often deaf and/or suffer from balance difficulties. Mutations in genes encoding ciliary proteins, for instance, result in autosomal recessive genetic diseases, such as Bardet–Biedl syndrome or Usher syndrome.13–15 In addition, human recessive deafness DFNB66 is caused by a mutation in DCD2a, which is specific to the primary cilia of supporting cells and the kinocilia of sensory hair cells.6 Previous studies indicate that the shape of the hair bundle and polarization of the auditory hair cells are dependent on the oriented displacement of the kinocilium, which is translocated from the center to the periphery of the apical cell surface, toward its final position during differentiation and regeneration of cochlear precursor hair cells.29 The epithelial cells lose their kinocilium after the completion of stereociliary bundle structures.9,10 It is possible that the primary cilia (9 + 0 microtubules) in E36 cells, which we investigated in the present study, may be kinocilia. E36 cells were derived from ventral otic epithelial cells of the mouse at embryonic day 10.5 and the primary cilia disappeared during cell differentiation at 39C in vitro.
Cilia length is known to be regulated by multiple factors, such as various signaling cascades, inflammatory signals, and the cytoskeleton.19–22 As actin dynamics, cytoskeletal reorganization, cell motility, and migration are largely controlled by members of the Rho family of small GTPases,30 it is likely that these factors play a role in regulating cilia length. Notably, McMurray et al.21 showed that treatment with the Rho-kinase inhibitor Y27632 reduced actin stress fiber formation in mesenchymal stem cells, directly resulting in cilia elongation without changing cilia prevalence. In the present study, Y27632 treatment also induced primary cilia elongation in both differentiated and undifferentiated temperature-sensitive mouse cochlear precursor hair cells. This inhibitor, which has been used previously to promote cell cycle progression,31 also increased cell proliferation and migration. Generally, the formation of primary cilia starts during the arresting phase of cell division; hence, the elongation of primary cilia is typically promoted in the resting phase of G1 or the G2 phase of the cell cycle.32 In our Y27632-treated temperature-sensitive mouse cochlear cells, the number of cells in the S-G2 phase of the cell cycle and cyclin D1 expression were increased, indicating that the Y27632-mediated increases in cell proliferation and migration are likely dependent on cell cycle progression.
Notably, in the temperature-sensitive mouse cochlear cells treated with Y27632, we also observed increased Hook2 expression. Previous studies suggest that Hook2 exclusively associates with and functions at the centrosome, which is where the primary cilium is generated.33,34 In fact, Baron Gaillard et al.24 showed that Hook2 depletion induces disruption of ciliogenesis before the formation of the ciliary vesicle at the distal tip of the mother centriole. In this previous study, they also observed Hook2 interacting with and stabilizing pericentriolar material protein 1 (PCM1), which is essential for the recruitment of Rab8a, an important GTPase involved in membrane transport to the primary cilia.
In addition to Y27632, we also evaluated the effects of the PKCα inhibitor GF109203X. Similar to Y27632, GF109203X also induced primary cilia elongation in both differentiated and undifferentiated temperature-sensitive mouse cochlear cells. However, in contrast to our observations following Y27632 treatment, which promoted the cell cycle, treatment with GF109203X increased the number of G1 cells and decreased the number of cells in the G2/M phase. Furthermore, treatment with this inhibitor decreased cell proliferation and did not affect migration. A previous study indicates that the RhoA/ROCK/PKCα/adducing signaling cascade is a significant regulator of outer hair cells motile response.26 Our results suggest that in temperature-sensitive mouse cochlear cells, regulation of primary cilia length may be mediated by the Rho/PKCα pathway, but cell cycle-dependent migration and proliferation are not affected by PKCα signaling.
In conclusion, in the present study, we investigated the effects of the Rho-kinase inhibitor Y27632 and the PKCα inhibitor GF109203X on primary cilia-related cell behavior in undifferentiated and differentiated temperature-sensitive mouse cochlear precursor hair cells. Our results provide novel evidence that in temperature-sensitive mouse cochlear cells, Rho-kinase and PKCα-inhibition results in primary cilia elongation with increased expression of Hook2. In addition, enhanced cell cycle-dependent proliferation and migration were also found in cells treated with Y27632.
Supplemental Material
Supplemental material, DS_10.1369_0022155419841013 for Rho-kinase and PKCα Inhibition Induces Primary Cilia Elongation and Alters the Behavior of Undifferentiated and Differentiated Temperature-sensitive Mouse Cochlear Cells by Akito Kakiuchi, Takayuki Kohno, Takuya Kakuki, Yakuto Kaneko, Takumi Konno, Yukino Hosaka, Tomohiro Hata, Shin Kikuchi, Takafumi Ninomiya, Tetsuo Himi, Kenichi Takano and Takashi Kojima in Journal of Histochemistry & Cytochemistry
Acknowledgments
We thank Dr. Matthew Holley (The University of Sheffield, UK) for supplying the US/VOT-E36 cell line.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AK, TKakuki, YK, TKonno, TKohno, YH, THata, SK, and TKojima performed the experiments. AK, KT, TN, THimi, and TKojima designed the conceptual idea for this study and wrote the manuscript. All the authors approved the submission of this manuscript in its final form.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (No. 25861575).
Contributor Information
Akito Kakiuchi, Department of Otolaryngology, Sapporo Medical University School of Medicine, Sapporo, Japan; Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Takayuki Kohno, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Takuya Kakuki, Department of Otolaryngology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Yakuto Kaneko, Department of Otolaryngology, Sapporo Medical University School of Medicine, Sapporo, Japan; Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Takumi Konno, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Yukino Hosaka, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Tomohiro Hata, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Shin Kikuchi, Department of Anatomy, Sapporo Medical University School of Medicine, Sapporo, Japan.
Takafumi Ninomiya, Department of Anatomy, Sapporo Medical University School of Medicine, Sapporo, Japan.
Tetsuo Himi, Department of Otolaryngology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Kenichi Takano, Department of Otolaryngology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Takashi Kojima, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Literature Cited
- 1. Irigoin F, Badano JL. Keeping the balance between proliferation and differentiation: the primary cilium. Curr Genomics. 2011;12:285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. [DOI] [PubMed] [Google Scholar]
- 3. Hagiwara H, Ohwada N, Aoki T, Suzuki T, Takata K. The primary cilia of secretory cells in the human oviduct mucosa. Med Mol Morphol. 2008;41:193–8. [DOI] [PubMed] [Google Scholar]
- 4. Guemez-Gamboa A, Coufal NG, Gleeson JG. Primary cilia in the developing and mature brain. Neuron. 2014;82:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gulley RL, Reese TS. Intercellular junctions in the reticular lamina of the organ of Corti. J Neurocytol. 1976;5:479–507. [DOI] [PubMed] [Google Scholar]
- 6. Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falk N, Lösl M, Schröder N, Gießl A. Specialized cilia in mammalian sensory systems. Cells. 2015;4:500–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sobkowicz HM, Slapnick SM, August BK. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol. 1995;24:633–53. [DOI] [PubMed] [Google Scholar]
- 9. Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet. 2004;5:489–98. [DOI] [PubMed] [Google Scholar]
- 10. Jones C, Chen P. Primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol. 2008;85:197–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–5. [DOI] [PubMed] [Google Scholar]
- 12. Grati M, Chakchouk I, Ma Q, Bensaid M, Desmidt A, Turki N, Yan D, Baanannou A, Mittal R, Driss N, Blanton S, Farooq A, Lu Z, Liu XZ, Masmoudi S. A missense mutation in DCDC2 causes human recessive deafness DFNB66, likely by interfering with sensory hair cell and supporting cell cilia length regulation. Hum Mol Genet. 2015;24:2482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kremer H, van Wijk E, Marker T, Wolfrum U, Roepman R. Usher syndrome: molecular links of pathogenesis, proteins and pathways. Hum Mol Genet. 2006;15:R262–70. [DOI] [PubMed] [Google Scholar]
- 14. Mathur P, Yang J. Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852:406–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. [DOI] [PubMed] [Google Scholar]
- 16. Liem KF, Jr, Ashe A, He M, Satir P, Moran J, Beier D, Wicking C, Anderson KV. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol. 2012;197:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goetz SC, Anderson KV. The primary cilium: a signaling centre during vertebrate development. Nat Rev Genet. 2010;11:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin JH, Kim PS, Kim ES, Park SJ, Jo YK, Hwang JJ, Park TJ, Chang JW, Seo JH, Cho DH. BIX-01294-induced autophagy regulates elongation of primary cilia. Biochem Biophys Res Commun. 2015;460:428–33. [DOI] [PubMed] [Google Scholar]
- 20. Dummer A, Rol N, Szulcek R, Kurakula K, Pan X, Visser BI, Bogaard HJ, DeRuiter MC, Goumans MJ, Hierck BP. Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation. Pulm Circ. 2018;8(2):1-9. doi: 10.1177/2045894018764629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep. 2013;18:3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakakura T, Asano-Hoshino A, Suzuki T, Arisawa K, Tanaka H, Sekino Y, Kiuchi Y, Kawai K, Hagiwara H. The elongation of primary cilia via the acetylation of α-tubulin by the treatment with lithium chloride in human fibroblast KD cells. Med Mol Morphol. 2015;48:44–53. [DOI] [PubMed] [Google Scholar]
- 23. Gadadhar S, Dadi H, Bodakuntla S, Schnitzler A, Bièche I, Rusconi F, Janke C. Tubulin glycylation controls primary cilia length. J Cell Biol. 2017;216:2701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baron Gaillard CL, Pallesi-Pocachard E, Massey-Harroche D, Richard F, Arsanto JP, Chauvin JP, Lecine P, Krämer H, Borg JP, Le Bivic A. Hook2 is involved in the morphogenesis of the primary cilium. Mol Biol Cell. 2011;22:4549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalinec F, Zhang M, Urrutia R, Kalinec G. Rho GTPases mediate the regulation of cochlear outer hair cell motility by acetylcholine. J Biol Chem. 2008;275:28000–5. [DOI] [PubMed] [Google Scholar]
- 26. Park C, Kalinec F. PKCα-mediated signals regulate the motile responses of cochlear outer hair cells. Biophys J. 2015;108:2171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helyer R, Cacciabue-Rivolta D, Davies D, Rivolta MN, Kros CJ, Holley MC. A model for mammalian cochlear hair cell differentiation in vitro: effects of retinoic acid on cytoskeletal proteins and potassium conductances. Eur J Neurosci. 2007;25:957–73. [DOI] [PubMed] [Google Scholar]
- 28. Takano K, Kakuki T, Kaneko Y, Kohno T, Kikuchi S, Himi T, Kojima T. Histone deacetylase inhibition prevents cell death induced by loss of tricellular tight junction proteins in temperature-sensitive mouse cochlear cells. PLoS One. 2017;12:e0182291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lepelletier L, de Monvel JB, Buisson J, Desdouets C, Petit C. Auditory hair cell centrioles undergo confined Brownian motion throughout the developmental migration of the kinocilium. Biophys J. 2013;105:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. [DOI] [PubMed] [Google Scholar]
- 31. Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Hamuro J, Kinoshita S. The new therapeutic concept of using a rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011;30(Suppl 1): S54–9. [DOI] [PubMed] [Google Scholar]
- 32. Wheatley DN. Cilia in cell-cultured fibroblasts. 3. Relationship between mitotic activity and cilium frequency in mouse 3T6 fibroblasts. J Anat. 1971;110:367–82. [PMC free article] [PubMed] [Google Scholar]
- 33. Moynihan KL, Pooley R, Miller PM, Kaverina I, Bader DM. Murine CENP-F regulates centrosomal microtubule nucleation and interacts with Hook2 at the centrosome. Mol Biol Cell. 2009;20:4790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szebenyi G, Hall B, Yu R, Hashim AI, Krämer H. Hook2 localizes to the centrosome, binds directly to centriolin/CEP110 and contributes to centrosomal function. Traffic. 2007;8:32–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1369_0022155419841013 for Rho-kinase and PKCα Inhibition Induces Primary Cilia Elongation and Alters the Behavior of Undifferentiated and Differentiated Temperature-sensitive Mouse Cochlear Cells by Akito Kakiuchi, Takayuki Kohno, Takuya Kakuki, Yakuto Kaneko, Takumi Konno, Yukino Hosaka, Tomohiro Hata, Shin Kikuchi, Takafumi Ninomiya, Tetsuo Himi, Kenichi Takano and Takashi Kojima in Journal of Histochemistry & Cytochemistry