Abstract
Objectives:
Cardiovascular disease (CVD) is the leading cause of mortality in the United States. The risk for developing CVD is usually calculated and communicated to patients as a percentage. The calculation of heart age—defined as the predicted age of a person’s vascular system based on the person’s CVD risk factor profile—is an alternative method for expressing CVD risk. We estimated heart age among adults aged 30-74 in New York City and examined disparities in excess heart age by race/ethnicity and sex.
Methods:
We applied data from the 2011, 2013, and 2015 New York State Behavioral Risk Factor Surveillance System to the non–laboratory-based Framingham risk score functions to calculate 10-year CVD risk and heart age by sex, race/ethnicity, and selected sociodemographic groups and risk factors.
Results:
Of 6117 men and women in the study sample, the average heart age was 5.7 years higher than the chronological age, and 2631 (43%) adults had a predicted heart age ≥5 years older than their chronological age. Mean excess heart age increased with age (from 0.7 year among adults aged 30-39 to 11.2 years among adults aged 60-74) and body mass index (from 1.1 year among adults with normal weight to 11.8 years among adults with obesity). Non-Latino white women had the lowest mean excess heart age (2.3 years), and non-Latino black men and women had the highest excess heart age (8.4 years).
Conclusions:
Racial/ethnic and sex disparities in CVD risk persist among adults in New York City. Use of heart age at the population level can support public awareness and inform targeted programs and interventions for population subgroups most at risk for CVD.
Keywords: Behavioral Risk Factor Surveillance System, cardiovascular diseases, risk factors, health status disparities, ethnic groups, gender
Cardiovascular disease (CVD) is the leading cause of death in the United States.1 Surveillance data indicate that pervasive disparities exist for CVD and its underlying risk factors.2 Identification and management of persons with high-risk CVD and population-level interventions to address cardiovascular disparities and reduce CVD risk factors are important risk-reduction strategies.2,3 Risk-assessment tools, such as the Framingham Risk Score (FRS), can detect persons at high CVD risk for primary prevention.4-6 The FRS allows health care providers to present CVD risk information to patients, facilitating informed treatment decision making and increased compliance with therapeutic decisions.7 However, the FRS communicates CVD risk in a 10-year period as a percentage, which may not resonate with patients.8,9
Heart age is an alternate way to convey CVD risk.10 It is defined as the predicted age of a person’s vascular system based on the person’s CVD risk factor profile. Heart age is calculated by identifying the chronological age of a person with the same predicted FRS-derived 10-year CVD risk but normal levels of CVD risk factors. Heart age greater than chronologic age (excess heart age) connotes increased CVD risk. Several studies suggest that heart age may be more meaningful than the FRS in conveying CVD risk and motivating patients to adopt healthier cardiovascular lifestyles.11,12 Estimating heart age at the population level may identify geographic regions and populations in greatest need of CVD prevention and motivate action for interventions at the local level.13 National estimates indicate that a considerable burden of excess heart age exists in the United States, including substantial racial/ethnic, sociodemographic, and state-level disparities in heart age among adults.13 However, limited research is available on population-level estimates of heart age in smaller geographic areas. CVD risk for smaller regions of the country, such as counties or cities, can differ substantially from the national average and may have important implications for local health policy.14 Estimating heart age and examining CVD risk variability by population subgroups at the local level may improve priority-setting efforts to address health disparities and provide opportunities to educate and empower the public to better manage CVD risk and promote population-level prevention.15,16
The objectives of our study were to (1) estimate heart age by using the FRS, (2) characterize increased CVD risk across various sociodemographic factors, and (3) measure the amount of excess heart age attributable to modifiable CVD risk factors (eg, body mass index [BMI], systolic blood pressure, and smoking) among adults in New York City.
Methods
We used data from the New York State Behavioral Risk Factor Surveillance System (BRFSS),17 a random-digit-dial telephone survey that uses a multistage sampling design to select a state-specific sample from the noninstitutionalized US civilian population aged ≥18. The BRFSS monitors risk behaviors and factors that contribute to leading causes of morbidity and mortality. A CVD-specific module is conducted in odd-numbered years. We used weighted BRFSS data collected from New York City in 2011, 2013, and 2015 combined, and we limited study participants to adults aged 30-74 with no history of CVD (coronary heart disease, myocardial infarction, stroke). Of 12 058 BRFSS participants, 7485 met the age criteria for heart age estimation and had no self-reported history of CVD. We excluded 1368 participants because of missing covariates used for calculations, leaving 6117 participants (2502 men and 3615 women) for analysis.
We used the methods of Marma and Lloyd-Jones18 to derive heart age equations and calculate heart age (chronological age of a person with the same predicted CVD risk but all risk factors at the normal level: systolic blood pressure = 125 mm Hg, BMI = 22.5 kg/m2, no hypertension treatment, no smoking, and no diabetes).10 Briefly, we first used non–laboratory-based sex-specific FRS risk function regression equations10,19 to calculate the 10-year risk for developing CVD. The non–laboratory-based FRS uses common office-based variables obtained in primary care (age, sex, BMI, systolic blood pressure, use of antihypertensive medication, smoking status, and diabetes status). Second, we entered the normal levels of risk factors (defined previously) into the FRS equations and then solved for age, leaving risk probability as an independent variable, which resulted in sex- and hypertension treatment–specific health age equations. Thus, the estimated 10-year risk in these equations was matched with the age at which the risk was equivalent but all other risk factors were normal. For example, according to the FRS, a hypothetical 50-year-old man who smokes, has a systolic blood pressure of 140 mm Hg, and is treated for hypertension has an estimated 10-year CVD risk of 27%. The heart age of this person would be 75 years, because this is the age at which a man with normal risk factor levels would also have an estimated CVD risk of 27%. We then entered the estimated CVD risk into the appropriate heart age equations and calculated heart age.
The FRS uses systolic blood pressure in risk estimation, but because measured systolic blood pressure is unavailable in the BRFSS, we used an established method15,20 to estimate systolic blood pressure. Briefly, Yang et al15 developed multivariable regression models to predict systolic blood pressure based on information from the National Health and Nutrition Examination Survey 2005-2010. Systolic blood pressure was the dependent variable in these models; age, race/ethnicity, educational attainment, centered-log BMI, physical activity level, annual household income, smoking status, alcohol consumption, health insurance, diagnosed diabetes, and use of antihypertensive medication were independent variables. We applied these model parameters to the comparable variables among BRFSS participants in New York City to predict systolic blood pressure.
We validated the systolic blood pressure predictive models by applying their regression coefficients to participants recruited for the New York City Heart Follow-Up Study (HFUS).21 The New York City Department of Health and Mental Hygiene conducted the HFUS in 2010 to assess sodium intake among the New York City population. Important to this validation, the HFUS contains objectively measured values of CVD risk factors. HFUS participants completed an interview with questions on hypertension status and antihypertensive medication use, collected urine during a 24-hour period to measure sodium intake, and consented to an in-home medical examination that included anthropometric measurements and 3 seated blood pressure readings using the Omron HEM-907XL device (Omron Healthcare Inc, Kyoto, Japan). Overall, 1656 HFUS participants provided complete 24-hour urine samples. To validate the models, we predicted each person’s systolic blood pressure by using the models and then compared it with the average of the person’s HFUS systolic blood pressure measurements. Overall, the mean predicted systolic blood pressure was 120.3 mm Hg (95% confidence interval [CI], 119.4-121.2 mm Hg) and the mean measured systolic blood pressure was 121.8 mm Hg (95% CI, 120.3-123.3 mm Hg). The mean difference corresponded to about 1.5 mm Hg (median, –0.8 mm Hg; interquartile range, –9.2 to 7.5 mm Hg). In addition, the log systolic blood pressure values that were included when calculating heart age were identical (4.8 for both measurement methods).
Measurements
We calculated mean excess heart age and prevalence of excess heart age by using the following variables: age group (30-39, 40-49, 50-59, 60-74), race/ethnicity (non-Latino white, non-Latino black, Latino), education (<high school, high school, >high school), annual household income (<$30 000, ≥$30 000), general health (good or better health: respondents who reported having excellent, very good, or good health; poor health: respondents who reported having fair or poor health), health insurance status (yes, no), BMI (normal/underweight: BMI <25 kg/m2; overweight: BMI 25 to <30 kg/m2; obese: BMI ≥30 kg/m2), current smoker (yes, no), hypertension (yes, no), suboptimal blood pressure control (yes, no), suboptimal blood pressure control among persons without hypertension (yes, no), and borough of residence (Bronx, Brooklyn, Manhattan, Queens, Staten Island). We defined the physical activity variable as whether the respondent reported doing ≥150 minutes per week of physical activity (yes, no). We created the suboptimal blood pressure control variable as a composite measure that included adults who were not taking hypertension medication and had a predicted systolic blood pressure >125 mm Hg and adults who were taking hypertension medication and had a predicted systolic blood pressure ≥140 mm Hg. We based suboptimal blood pressure control among persons without hypertension only on adults not taking hypertension medication with a predicted systolic blood pressure >125 mm Hg. All measurements, with the exception of predicted systolic blood pressure and suboptimal blood pressure control variables, were self-reported.
Statistical Analysis
We generated descriptive statistics of CVD risk factors by using means for continuous variables and percentages for categorical variables. We calculated overall and sex-specific weighted means and 95% CIs for participants’ chronological age, heart age, excess heart age, and prevalence of excess heart age ≥5 years. To determine differences in means and percentages and calculate P values, we used the t test for continuous variables and Wald χ2 test for categorical variables. We performed bivariate linear and logistic regression analyses to test the differences in excess heart age and prevalence of excess heart age ≥5 years within each sociodemographic and risk factor subgroup and between sexes. We used multivariable linear regression models to estimate racial/ethnic disparities in the differences of excess heart age among racial/ethnic groups, adjusting for selected sociodemographic variables. All statistical tests were 2-sided, with P < .05 considered significant. We calculated relative standard errors and 95% CIs for means and percentages, and we considered any estimates with a relative standard error ≥30% to be unreliable. We conducted all analyses by using SAS version 9.422 and SAS-Callable-SUDAAN version 11.0.123 to account for survey complex sampling design and to obtain standard error estimates by Taylor series linearization. The protocol was approved by the New York City Department of Health and Mental Hygiene Institutional Review Board.
Results
Compared with men, women were slightly older (48.9 years; 95% CI, 48.4-49.5 years vs. 47.8 years; 95% CI, 47.2-48.4 years), significantly more likely to have an annual household income <$35 000 (44.5%; 95% CI, 42.1%-46.8% vs. 40.6%; 95% CI, 37.9%-43.4%) and be insured (88.8%; 95% CI, 87.2%-90.2% vs. 82.9%; 95% CI, 80.8%-84.9%), and significantly less likely to report being in good or better general health (80.4%; 95% CI, 78.6%-82.2% vs. 83.5%; 95% CI, 81.3%-85.4%) or current smokers (13.4%; 95% CI, 11.9%-15.0% vs. 18.6%; 95% CI, 16.6%-20.8%; Table 1). Compared with men, women had a significantly lower prevalence of self-reported hypertension (27.0%; 95% CI, 25.2%-28.9% vs. 30.4%; 95% CI, 28.1%-32.9%), predicted mean systolic blood pressure (120.1; 95% CI, 119.7-120.6 mm Hg vs. 124.4; 95% CI, 124.1-124.8 mm Hg), and prevalence of suboptimal blood pressure control (15.9%; 95% CI, 14.5%-17.5% vs. 25.1%; 95% CI, 22.9%-27.4%). Of 1988 adults with hypertension, a higher percentage of women than men reported use of antihypertensive medication (80.8%; 95% CI, 77.7%-83.7% vs. 65.2%; 95% CI, 60.6%-69.6%).
Table 1.
Characteristics of study population aged 30-74 (n = 6117) in New York City, by sex, New York State Behavioral Risk Factor Surveillance System, 2011, 2013, and 2015a
| Characteristic | Overall | Men | Women | ||||
|---|---|---|---|---|---|---|---|
| No. | Mean % (95% CI) | No. | Mean % (95% CI) | No. | Mean % (95% CI) | P Valueb | |
| Mean age, y | 6117 | 48.4 (48.0-48.8) | 2502 | 47.8 (47.2-48.4) | 3615 | 48.9 (48.4-49.5) | .01 |
| Race/ethnicity | .41 | ||||||
| Non-Latino white | 2967 | 36.0 (34.5-37.5) | 1309 | 37.3 (35.0-39.6) | 1658 | 34.3 (32.9-36.7) | |
| Non-Latino black | 1288 | 21.9 (20.5-23.4) | 441 | 18.9 (16.9-21.0) | 847 | 24.8 (22.9-26.8) | |
| Latino | 1363 | 27.2 (25.7-28.8) | 525 | 27.8 (25.5-30.3) | 838 | 26.7 (24.8-28.7) | |
| Otherc | 499 | 14.9 (13.4-16.4) | 227 | 16.1 (13.9-18.5) | 272 | 13.7 (12.0-15.7) | |
| Education | .29 | ||||||
| <High school | 673 | 18.8 (17.3-20.3) | 242 | 18.0 (15.8-20.4) | 431 | 19.5 (17.6-21.6) | |
| High school | 1065 | 20.6 (19.1-22.0) | 425 | 20.4 (18.4-22.7) | 640 | 20.6 (18.8-22.5) | |
| >High school | 4379 | 60.7 (59.0-62.4) | 1835 | 61.6 (58.9-64.2) | 2544 | 59.9 (57.7-62.1) | |
| Annual household income, $d | .04 | ||||||
| <35 000 | 2037 | 42.6 (40.8-44.4) | 779 | 40.6 (37.9-43.4) | 1258 | 44.5 (42.1-46.8) | |
| ≥35 000 | 3488 | 57.4 (55.6-59.2) | 1536 | 59.4 (56.6-62.1) | 1952 | 55.5 (53.2-57.9) | |
| General healthd | .03 | ||||||
| Good or better | 5064 | 81.9 (80.5-83.2) | 2118 | 83.5 (81.3-85.4) | 2946 | 80.4 (78.6-82.2) | |
| Poor | 1010 | 18.1 (16.8-19.5) | 366 | 16.5 (14.6-18.7) | 644 | 19.6 (17.8-21.4) | |
| Health insurance | <.001 | ||||||
| Yes | 5479 | 86.0 (84.7-87.2) | 2180 | 82.9 (80.8-84.9) | 3299 | 88.8 (87.2-90.2) | |
| No | 638 | 14.0 (12.8-15.3) | 322 | 17.1 (15.1-19.2) | 316 | 11.2 (9.8-12.8) | |
| Physical activitye | .07 | ||||||
| Yes | 3046 | 46.3 (44.6-48.0) | 1294 | 48.1 (45.5-50.7) | 1752 | 44.6 (42.5-46.8) | |
| No | 3071 | 53.7 (52.0-55.4) | 1208 | 51.9 (49.3-54.5) | 1863 | 55.4 (53.2-57.5) | |
| BMI, kg/m2 | .02 | ||||||
| Underweight/normal weight (BMI <25) | 2401 | 37.7 (36.0-39.3) | 818 | 32.3 (29.9-34.8) | 1583 | 42.7 (40.5-44.8) | |
| Overweight (BMI 25 to <30) | 2183 | 36.5 (34.9-38.2) | 1118 | 43.8 (41.2-46.4) | 1065 | 29.8 (27.8-31.8) | |
| Obese (BMI ≥30) | 1533 | 25.8 (24.3-27.3) | 566 | 23.9 (21.7-26.2) | 967 | 27.6 (25.6-29.6) | |
| Current smokerf | <.001 | ||||||
| Yes | 831 | 15.9 (14.7-17.3) | 398 | 18.6 (16.6-20.8) | 433 | 13.4 (11.9-15.0) | |
| No | 5286 | 84.1 (82.7-85.3) | 2104 | 81.4 (79.2-83.4) | 3182 | 86.6 (85.0-88.1) | |
| Diabetes | .98 | ||||||
| Yes | 638 | 10.5 (9.5-11.6) | 259 | 10.5 (8.9-12.2) | 379 | 10.5 (9.2-11.9) | |
| No | 5479 | 89.5 (88.4-90.5) | 2243 | 89.5 (87.8-91.1) | 3236 | 89.5 (88.1-90.8) | |
| Predicted mean systolic blood pressure, mm Hg | 6117 | 122.2 (121.9-122.5) | 2502 | 124.4 (124.1-124.8) | 3615 | 120.1 (119.7-120.6) | <.001 |
| Self-reported hypertension | .03 | ||||||
| Yes | 1888 | 28.7 (27.2-30.2) | 817 | 30.4 (28.1-32.9) | 1071 | 27.0 (25.2-28.9) | |
| No | 4229 | 71.3 (69.8-72.8) | 1685 | 69.6 (67.1-71.9) | 2544 | 73.0 (71.1-74.8) | |
| Antihypertensive medication use among persons with hypertension | <.001 | ||||||
| Yes | 1435 | 72.9 (70.0-75.6) | 571 | 65.2 (60.6-69.6) | 864 | 80.8 (77.7-83.7) | |
| No | 453 | 27.1 (24.4-30.0) | 246 | 34.8 (30.4-39.4) | 207 | 19.2 (16.3-22.3) | |
| Suboptimal blood pressure control | <.001 | ||||||
| Yes | 1351 | 20.4 (19.0-21.7) | 682 | 25.1 (22.9-27.4) | 669 | 15.9 (14.5-17.5) | |
| No | 4766 | 79.6 (78.3-81.0) | 1820 | 74.9 (72.6-77.1) | 2946 | 84.1 (82.5-85.5) | |
| Suboptimal blood pressure control among persons without hypertension | <.001 | ||||||
| Yes | 799 | 15.7 (14.3-17.2) | 413 | 19.7 (17.4-22.2) | 386 | 12.2 (10.6-13.9) | |
| No | 3430 | 84.3 (82.8-85.7) | 1272 | 80.3 (77.8-82.6) | 2158 | 87.7 (77.8-82.6) | |
| Borough of residenced | .50 | ||||||
| Manhattan | 1477 | 20.5 (19.2-21.8) | 616 | 20.5 (18.5-22.6) | 861 | 20.4 (18.8-22.2) | |
| Bronx | 880 | 16.2 (14.9-17.5) | 316 | 15.2 (13.4-17.3) | 564 | 17.0 (15.4-18.7) | |
| Brooklyn | 1635 | 29.5 (27.9-31.2) | 698 | 30.1 (27.6-32.6) | 937 | 29.0 (27.0-31.2) | |
| Queens | 1361 | 27.9 (26.3-29.6) | 576 | 28.4 (25.9-31.0) | 785 | 27.5 (25.4-29.6) | |
| Staten Island | 359 | 5.9 (95.2-6.7) | 134 | 5.8 (4.7-7.1) | 225 | 6.1 (5.2-7.1) | |
Abbreviation: BMI, body mass index.
a Data source: New York State Behavioral Risk Factor Surveillance System.17 Data were weighted for the New York City adult population.
b All comparisons were based on the t test for continuous variables and Wald χ2 test for categorical variables. All statistical tests were 2-sided, with P < .05 considered significant.
c Other includes all racial/ethnic groups other than non-Latino white, non-Latino black, and Latino.
d Data were missing for 593 persons on annual household income, 43 persons on general health, and 405 persons on borough of residence.
e Physical activity was defined as ≥150 minutes per week of physical activity.
f A current smoker was defined as someone who smoked ≥100 cigarettes in his or her lifetime and currently smoked.
Compared with non-Latino white adults, non-Latino black adults had a significantly higher prevalence of self-reported hypertension (35.0%; 95% CI, 31.7%-38.4% vs. 24.9%; 95% CI, 23.0%-27.0%), diabetes (12.6%; 95% CI, 10.5%-15.1% vs. 6.2%; 95% CI, 5.3%-7.3%), and obesity (37.0%; 95% CI, 33.5%-40.6% vs. 19.2%; 95% CI, 17.4%-21.2%) and a significantly higher predicted mean systolic blood pressure (126.0; 95% CI, 125.4-126.7 mm Hg vs. 120.9; 95% CI, 120.5-121.2 mm Hg; Table 2). In addition, non-Latino white women had better risk profiles than non-Latino white men, but the same was not true for non-Latino black and Latino women. Non-Latino black women had significantly higher levels of obesity and a similar percentage of diabetes compared with non-Latino black men, and Latino men and women had comparable levels of diabetes and obesity prevalence. The overall female advantage in CVD risk profile was driven predominantly by non-Latino white women.
Table 2.
Distribution of cardiovascular disease risk factors included in the Framingham Risk Score heart agea calculation comparing racial/ethnic groups, by sex, among adults in New York City aged 30-74, New York State Behavioral Risk Factor Surveillance System, 2011, 2013, and 2015b
| Characteristic |
Non-Latino White,
Mean % (95% CI) |
Non-Latino Black,
Mean % (95% CI) |
P Valuec for Difference Between Non-Latino White and Non-Latino Black |
Latino,
Mean % (95% CI) |
P Valuec for Difference Between Non-Latino White and Latino |
|---|---|---|---|---|---|
| Overall | |||||
| Mean age, y | 49.8 (49.3-50.4) | 48.1 (47.3-48.9) | .01 | 47.4 (46.6-48.2) | <.001 |
| Predicted mean systolic blood pressure, mm Hg | 120.9 (120.5-121.2) | 126.0 (125.4-126.7) | <.001 | 122.5 (121.9-123.1) | <.001 |
| Self-reported hypertension prevalence | 24.9 (23.0-27.0) | 35.0 (31.7-38.4) | <.001 | 30.0 (27.0-33.2) | .01 |
| Diabetes prevalence | 6.2 (5.3-7.3) | 12.6 (10.5-15.1) | <.001 | 13.4 (11.2-16.0) | <.001 |
| BMI category, kg/m2 | |||||
| Normal (BMI <25) | 45.5 (43.1-47.7) | 25.3 (22.2-28.6) | <.001 | 30.1 (27.1-33.2) | <.001 |
| Overweight (BMI 25 to <30) | 35.4 (33.2-37.6) | 37.7 (34.2-41.3) | .28 | 37.4 (34.3-40.7) | .30 |
| Obese (BMI ≥30) | 19.2 (17.42-21.2) | 37.0 (33.5-40.6) | <.001 | 32.5 (29.4-35.8) | <.001 |
| Current smoking prevalence | 14.9 (13.2-16.8) | 18.0 (15.2-21.3) | .08 | 16.4 (14.0-19.1) | .35 |
| Men | |||||
| Mean age, y | 48.9 (48.1-49.7) | 47.5 (46.2-48.8) | .07 | 46.9 (45.6-48.2) | .01 |
| Predicted mean systolic blood pressure, mm Hg | 123.6 (123.3-124.0) | 128.0 (127.1-128.8) | <.001 | 124.5 (123.8-125.2) | .03 |
| Self-reported hypertension prevalence | 29.2 (26.1-32.5) | 33.6 (28.4-39.3) | .17 | 28.2 (23.7-33.1) | .74 |
| Diabetes prevalence | 7.1 (5.7-8.9) | 10.9 (8.2-14.4) | .04 | 13.3 (10.0-17.6) | .01 |
| BMI category, kg/m2 | |||||
| Underweight/normal (BMI <25) | 34.2 (31.0-37.6) | 24.5 (19.9-29.7) | .01 | 28.0 (23.6-32.9) | .03 |
| Overweight (BMI 25 to <30) | 43.7 (40.3-47.1) | 50.9 (45.0-56.9) | .04 | 40.6 (35.6-45.7) | .32 |
| Obese (BMI ≥30) | 22.1 (19.2-25.3) | 24.6 (20.0-29.9) | .40 | 31.4 (26.7-36.6) | .01 |
| Current smoking prevalence | 16.7 (14.1-19.6) | 21.5 (16.7-27.2) | .11 | 19.1 (15.4-23.5) | .33 |
| Women | |||||
| Mean age, y | 50.8 (50.0-51.5) | 48.5 (47.4-49.6) | .01 | 47.9 (46.9-48.9) | <.001 |
| Predicted mean systolic blood pressure, mm Hg | 118.1 (117.5-118.7) | 124.7 (123.9-125.5) | <.001 | 120.5 (119.5-121.4) | <.001 |
| Self-reported hypertension prevalence | 20.7 (18.4-23.1) | 35.9 (31.9-40.2) | <.001 | 31.8 (28.0-35.9) | <.001 |
| Diabetes prevalence | 5.3 (4.2-6.7) | 13.9 (10.9-17.5) | <.001 | 13.5 (10.8-16.8) | <.001 |
| BMI category, kg/m2 | |||||
| Underweight/normal (BMI <25) | 56.5 (53.4-59.5) | 25.9 (22.0-30.2) | <.001 | 32.1 (28.3-36.2) | <.001 |
| Overweight (BMI 25 to <30) | 27.1 (24.4-30.1) | 28.3 (24.6-32.4) | .63 | 34.4 (30.5-38.6) | .01 |
| Obese (BMI ≥30) | 16.4 (14.3-18.6) | 45.8 (41.2-50.4) | <.001 | 33.5 (29.5-37.8) | <.001 |
| Current smoking prevalence | 13.1 (11.1-15.5) | 15.6 (12.4-19.5) | .24 | 13.7 (10.9-17.1) | .75 |
Abbreviation: BMI, body mass index.
a Heart age is defined by identifying the chronological age of a person with the same predicted Framingham Risk Score–derived 10-year cardiovascular disease (CVD) risk but normal levels of CVD risk factors.
b Data source: New York State Behavioral Risk Factor Surveillance System.17 Data were weighted for the New York City adult population.
c All comparisons were based on the t test for continuous variables and Wald χ2 test for categorical variables. All statistical tests were 2-sided, with P < .05 considered significant.
Overall, average excess heart age was 5.7 years (95% CI, 5.4-6.0 years), and the prevalence of excess heart age ≥5 years was 43.0% (95% CI, 41.3%-44.7%). Among men and women, excess heart age increased with age and BMI and was higher among non-Latino black and Latino men and women than among non-Latino white men and women (Table 3). Excess heart age was higher among adults with <high school education vs. >high school education, <$35 000 annual household income vs. ≥$35 000 annual household income, poor general health vs. good or better general health, no physical activity vs. physically active, self-reported hypertension vs. no self-reported hypertension, suboptimal blood pressure control vs. optimal blood pressure control, and among current smokers vs. nonsmokers. Excess heart age was lowest among adults living in Manhattan.
Table 3.
Mean excess heart age,a by sex, among adults in New York City aged 30-74 (n = 6117), New York State Behavioral Risk Factor Surveillance System, 2011, 2013, and 2015b
| Characteristic | Overall | Men | Women | P Value for Difference Between Men and Womenc | ||||
|---|---|---|---|---|---|---|---|---|
| Years (95% CI) | P Valuec | Years (95% CI) | P Valuec | Years (95% CI) | P Valuec | |||
| Total | 5.7 (0.3-1.1) | 6.8 (6.4-7.2) | 4.7 (4.2-5.1) | <.001 | ||||
| Age group, y | ||||||||
| 30-39 | 0.7 (0.3-1.1) | Ref | 3.4 (2.8-3.9) | Ref | –1.8 (–2.2 to –1.4) | Ref | <.001 | |
| 40-49 | 4.0 (3.4-4.6) | <.001 | 5.3 (4.5-6.1) | <.001 | 2.4 (1.4-3.4) | <.001 | <.001 | |
| 50-59 | 8.7 (7.9-9.5) | <.001 | 9.4 (8.4-10.4) | <.001 | 8.1 (7.0-9.3) | <.001 | .11 | |
| 60-74 | 11.2 (10.7-11.7) | <.001 | 11.3 (10.5-12.1) | <.001 | 11.1 (10.4-11.8) | <.001 | .70 | |
| Race/ethnicity | ||||||||
| Non-Latino white | 4.2 (3.8-4.6) | Ref | 6.1 (5.7-6.6) | Ref | 2.3 (1.8-2.9) | Ref | <.001 | |
| Non-Latino black | 8.4 (7.7-9.1) | <.001 | 8.4 (7.4-9.3) | <.001 | 8.4 (7.4-9.4) | <.001 | .94 | |
| Latino | 6.4 (5.8-7.0) | <.001 | 7.1 (6.4-7.9) | .03 | 5.7 (4.8-6.7) | <.001 | .02 | |
| Education | ||||||||
| <High school | 9.0 (8.1-10.0) | Ref | 9.3 (8.1-10.4) | Ref | 8.8 (7.4-10.3) | Ref | .66 | |
| High school | 7.6 (6.8-8.3) | .01 | 7.8 (6.9-8.8) | .06 | 7.3 (6.2-8.3) | .09 | .44 | |
| >High school | 4.0 (3.7-4.4) | <.001 | 5.7 (5.3-6.2) | <.001 | 2.4 (1.9-2.9) | <.001 | <.001 | |
| Annual household income, $ | ||||||||
| <35 000 | 8.1 (7.5-8.7) | Ref | 8.1 (7.3-8.9) | Ref | 8.1 (7.3-9.0) | Ref | .95 | |
| ≥35 000 | 3.9 (3.6-4.3) | <.001 | 5.9 (5.4-6.3) | <.001 | 1.9 (1.4-2.5) | <.001 | <.001 | |
| General health | ||||||||
| Good or better | 4.4 (4.1-4.7) | Ref | 5.9 (5.5-6.3) | Ref | 2.9 (2.4-3.3) | Ref | <.001 | |
| Poor | 11.5 (10.6-12.4) | <.001 | 11.2 (9.9-12.4) | <.001 | 11.8 (10.5-13.1) | <.001 | .52 | |
| Health insurance | ||||||||
| Yes | 5.7 (5.3-6.0) | Ref | 6.9 (6.4-7.4) | Ref | 4.6 (4.1-5.1) | Ref | <.001 | |
| No | 5.7 (4.9-6.5) | .89 | 6.3 (5.4-7.1) | .20 | 5.0 (3.5-6.5) | .61 | .16 | |
| Physical activityd | ||||||||
| Yes | 5.0 (4.5-5.4) | Ref | 6.4 (5.8-7.0) | Ref | 3.6 (2.9-4.2) | Ref | <.001 | |
| No | 6.3 (5.8-6.8) | <.001 | 7.2 (6.6-7.8) | .06 | 5.5 (4.9-6.2) | <.001 | <.001 | |
| BMI, kg/m2 | ||||||||
| Underweight, normal weight (BMI <25) | 1.1 (0.7-1.5) | Ref | 3.2 (2.6-3.8) | Ref | –0.3 (–0.8 to 0.2) | Ref | <.001 | |
| Overweight (BMI 25 to <30) | 6.0 (5.6-6.5) | <.001 | 6.7 (6.1-7.3) | <.001 | 5.1 (4.4-5.9) | <.001 | .002 | |
| Obese (BMI ≥30) | 11.8 (11.2-12.5) | <.001 | 11.9 (11.0-12.8) | <.001 | 11.8 (10.8-12.7) | <.001 | .86 | |
| Current smokere | ||||||||
| Yes | 15.3 (14.5-16.2) | Ref | 15.5 (14.6-16.5) | Ref | 15.1 (13.6-16.5) | Ref | .59 | |
| No | 3.9 (3.6-4.2) | <.001 | 4.8 (4.4-5.2) | <.001 | 3.0 (2.6-3.5) | <.001 | <.001 | |
| Self-reported hypertension | ||||||||
| Yes | 15.3 (14.8-15.9) | Ref | 13.6 (12.8-14.3) | Ref | 17.2 (16.5-17.9) | Ref | <.001 | |
| No | 1.8 (1.6-2.1) | <.001 | 3.8 (3.5-4.2) | <.001 | 0 (–0.3 to 0.4) | <.001 | <.001 | |
| Suboptimal blood pressure control | ||||||||
| Yes | 9.3 (8.7-9.8) | Ref | 8.4 (7.7-9.1) | Ref | 10.5 (9.6-11.5) | Ref | <.001 | |
| No | 4.8 (4.4-5.1) | <.001 | 6.3 (5.8-6.8) | <.001 | 3.5 (3.0-4.1) | <.001 | <.001 | |
| Suboptimal blood pressure control among persons without hypertension | ||||||||
| Yes | 7.6 (6.9-8.3) | Ref | 7.5 (6.6-8.4) | Ref | 7.8 (6.6-8.9) | Ref | .73 | |
| No | 0.7 (0.5-1.0) | <.001 | 2.9 (2.6-3.3) | <.001 | –1.0 (–1.4 to –0.7) | <.001 | <.001 | |
| Borough of residence | ||||||||
| Manhattan | 3.8 (3.2-4.4) | Ref | 5.0 (4.3-5.7) | Ref | 2.7 (1.7-3.7) | Ref | <.001 | |
| Bronx | 7.2 (6.4-8.0) | <.001 | 8.7 (7.7-9.8) | <.001 | 6.0 (4.8-7.1) | <.001 | .001 | |
| Brooklyn | 5.9 (5.3-6.5) | <.001 | 6.8 (6.0-7.5) | <.001 | 5.0 (4.1-6.0) | <.001 | .01 | |
| Queens | 6.0 (5.3-6.7) | <.001 | 7.3 (6.3-8.3) | <.001 | 4.8 (3.9-5.7) | .01 | <.001 | |
| Staten Islandf | 6.9 (5.6-8.2) | <.001 | 8.3 (6.7-10.0) | <.001 | 5.7 (3.7-7.7) | .01 | .04 | |
Abbreviations: BMI, body mass index; Ref, reference group.
a Heart age is defined by identifying the chronological age of a person with the same predicted Framingham Risk Score–derived 10-year cardiovascular disease (CVD) risk but normal levels of CVD risk factors. Excess heart age (heart age greater than chronological age) connotes increased CVD risk.
b Data source: New York State Behavioral Risk Factor Surveillance System.17 Data were weighted for the New York City adult population.
c P value based on linear regression model t test. All statistical tests were 2-sided, with P < .05 considered significant.
d Physical activity was defined as ≥150 minutes per week of physical activity.
e Current smoker was defined as persons who reported having smoked ≥100 cigarettes in their lifetime and currently smoked.
f Estimate may be unreliable due to a large relative standard error.
Men had a higher excess heart age than women (6.8; 95% CI, 6.4-7.2 years vs. 4.7; 95% CI, 4.2-5.1 years) and a higher proportion with excess heart age ≥5 years (47.6%; 95% CI, 45.0%-50.2% vs. 38.8%; 95% CI, 36.7%-40.9%; Table 3). Excess heart age did not differ significantly by sex for older age, lower education levels, and lower income; self-reported poor general health; not having health insurance; current smokers; or obesity. Non-Latino black men and women had equally high excess heart age (8.4 years). Among adults who had self-reported hypertension, women had significantly higher excess heart age than men (17.2 years; 95% CI, 16.5-17.9 years vs. 13.6; 95% CI, 12.8-14.3 years). We found similar trends for the estimated prevalence of excess heart age ≥5 years (Table 4).
Table 4.
Prevalence of excess heart age ≥5 years,a by sex, among adults in New York City aged 30-74 (n = 6117), New York State Behavioral Risk Factor Surveillance System, 2011, 2013, and 2015b
| Characteristic | Overall | Men | Women | P Value for Difference Between Men and Womenc | |||
|---|---|---|---|---|---|---|---|
| % (95% CI) | P Valuec | % (95% CI) | P Valuec | % (95% CI) | P Valuec | ||
| Total | 43.0 (41.3-44.7) | 47.6 (45.0-50.2) | 38.8 (36.7-40.9) | <.001 | |||
| Age group, y | |||||||
| 30-39 | 20.2 (17.4-23.2) | Ref | 29.7 (25.1-34.7) | Ref | 11.1 (8.6-14.2) | Ref | <.001 |
| 40-49 | 35.0 (31.7-38.4) | <.001 | 39.6 (34.7-44.8) | .01 | 29.5 (25.4-34.0) | <.001 | .003 |
| 50-59 | 52.5 (49.1-55.9) | <.001 | 57.3 (52.2-62.3) | <.001 | 48.5 (44.0-53.0) | <.001 | .01 |
| 60-74 | 73.1 (70.3-75.7) | <.001 | 75.4 (70.9-79.4) | <.001 | 71.3 (67.7-74.7) | <.001 | .15 |
| Race/ethnicity | |||||||
| Non-Latino white | 38.6 (36.3-40.9) | Ref | 45.5 (42.1-49.0) | Ref | 31.6 (28.9-34.5) | Ref | <.001 |
| Non-Latino black | 52.2 (48.5-55.8) | <.001 | 51.5 (45.5-57.7) | .09 | 52.6 (48.0-57.2) | <.001 | .78 |
| Latino | 46.4 (43.0-49.8) | <.001 | 49.7 (44.5-54.9) | .19 | 43.1 (38.9-47.4) | <.001 | .06 |
| Education | |||||||
| <High school | 58.4 (53.6-63.0) | Ref | 62.5 (54.9-69.5) | Ref | 54.8 (48.8-60.7) | Ref | .11 |
| High school | 51.3 (47.4-55.2) | .03 | 53.4 (47.4-59.3) | .06 | 49.4 (44.4-54.5) | .18 | .33 |
| >High school | 35.5 (33.6-37.4) | <.001 | 41.3 (38.3-44.4) | <.001 | 29.9 (27.7-32.1) | <.001 | <.001 |
| Annual household income, $ | |||||||
| <35 000 | 52.9 (49.9-55.8) | Ref | 54.7 (50.0-59.2) | Ref | 51.3 (47.5-55.0) | Ref | .27 |
| ≥35 000 | 35.8 (33.7-37.9) | <.001 | 42.5 (39.2-45.8) | <.001 | 28.8 (26.3-31.4) | <.001 | <.001 |
| General health | |||||||
| Good or better | 37.3 (35.5-39.1) | Ref | 43.1 (40.4-45.9) | Ref | 31.7 (29.5-33.9) | Ref | <.001 |
| Poor | 68.2 (64.2-72.0) | <.001 | 69.8 (63.4-75.5) | <.001 | 67.0 (61.8-71.8) | <.001 | .49 |
| Health insurance | |||||||
| Yes | 43.0 (41.2-44.8) | Ref | 47.9 (45.0-50.7) | Ref | 38.8 (36.6-41.0) | Ref | <.001 |
| No | 43.1 (38.3-47.9) | .98 | 46.2 (39.7-52.8) | .65 | 38.6 (32.0-45.8) | .97 | .12 |
| Physical activityd | |||||||
| Yes | 39.5 (37.2-41.8) | Ref | 44.8 (41.2-48.5) | Ref | 34.1 (31.3-37.0) | Ref | <.001 |
| No | 46.1 (43.7-48.8) | <.001 | 50.2 (46.6-53.9) | .05 | 42.5 (39.5-45.5) | <.001 | .001 |
| BMI, kg/m2 | |||||||
| Underweight/normal weight (BMI <25) | 25.1 (22.8-27.6) | Ref | 32.3 (28.0-37.0) | Ref | 20.0 (17.6-22.6) | Ref | <.001 |
| Overweight (BMI 25-<30) | 42.1 (39.4-44.9) | <.001 | 44.3 (40.5-48.2) | <.001 | 39.0 (35.3-42.9) | <.001 | .05 |
| Obese (BMI ≥30) | 70.5 (67.3-73.5) | <.001 | 74.2 (69.0-78.7) | <.001 | 67.5 (63.3-71.4) | <.001 | .04 |
| Current smokere | |||||||
| Yes | 92.0 (89.2-94.1) | Ref | 99.3 (96.9-99.8) | Ref | 82.6 (76.9-87.1) | Ref | <.001 |
| No | 33.7 (32.1-35.5) | <.001 | 35.8 (33.1-38.5) | <.001 | 32.0 (29.9-34.1) | <.001 | .03 |
| Self-reported hypertension | |||||||
| Yes | 90.7 (88.7-92.3) | Ref | 86.9 (83.6-89.6) | Ref | 94.6 (92.7-96.0) | Ref | <.001 |
| No | 23.9 (22.2-25.7) | <.001 | 30.4 (27.5-33.4) | <.001 | 18.1 (16.2-20.2) | <.001 | <.001 |
| Suboptimal blood pressure control | |||||||
| Yes | 65.8 (62.3-69.2) | Ref | 60.6 (55.6-65.4) | Ref | 73.5 (68.5-77.9) | Ref | <.001 |
| No | 37.2 (35.4-39.0) | <.001 | 43.2 (40.2-46.2) | <.001 | 32.2 (30.0-34.4) | <.001 | <.001 |
| Suboptimal blood pressure control among persons without hypertension | |||||||
| Yes | 58.5 (53.5-63.2) | Ref | 55.9 (49.2-62.4) | Ref | 62.1 (54.9-68.9) | Ref | .21 |
| No | 17.4 (15.8-19.3) | <.001 | 24.1 (21.2-27.3) | <.001 | 12.0 (10.3-14.0) | <.001 | <.001 |
| Borough of residence | |||||||
| Manhattan | 35.1 (31.8-38.5) | Ref | 38.4 (33.1-43.9) | Ref | 32.0 (28.0-36.2) | Ref | .07 |
| Bronx | 49.4 (45.1-53.6) | <.001 | 58.1 (51.2-64.7) | <.001 | 42.1 (37.0-47.5) | .003 | <.001 |
| Brooklyn | 43.7 (40.4-47.0) | <.001 | 48.2 (43.3-53.2) | .01 | 39.3 (35.2-43.7) | .02 | .01 |
| Queens | 44.3 (40.8-47.8) | <.001 | 49.0 (43.6-54.4) | .01 | 39.8 (35.5-44.3) | .01 | .01 |
| Staten Islandf | 50.3 (43.8-56.9) | <.001 | 55.4 (44.8-65.5) | .01 | 45.9 (37.8-54.1) | .002 | .16 |
Abbreviations: BMI, body mass index; Ref, reference group.
a Heart age is defined by identifying the chronological age of a person with the same predicted Framingham Risk Score–derived 10-year cardiovascular disease (CVD) risk but normal levels of CVD risk factors. Excess heart age (heart age greater than chronological age) connotes increased CVD risk. Prevalence of excess heart age ≥5 years indicates the proportion of population with a heart age at least 5 years older than their chronological age and therefore at greater risk for CVD event.
b Data source: New York State Behavioral Risk Factor Surveillance System.17 Data were weighted for the New York City adult population.
c P value based on logistic regression model Wald χ2 test. All statistical tests were 2-sided, with P < .05 considered significant.
d Physical activity was defined as ≥150 minutes per week of physical activity.
e Current smoker was defined as persons who reported having smoked ≥100 cigarettes in their lifetime and currently smoked.
f Estimate may be unreliable due to a large relative standard error.
After multivariate adjustment, racial differences in excess heart age between non-Latino black and non-Latino white adults were significant for almost all sociodemographic characteristics (Table 5). Racial/ethnic differences in excess heart age between non-Latino black and non-Latino white men was 1.5 years (95% CI, 0.3-2.7 years). Non-Latino black men had significantly higher excess heart age than non-Latino white men in the following subgroups: age (50-59 and 60-74), poor general health, insurance, no physical activity, overweight, and suboptimal blood pressure control. Excess heart age among Latino men and non-Latino white men was comparable, but Latino men who were obese had lower excess heart age than non-Latino white men. Racial/ethnic differences in excess heart age between non-Latino black and non-Latino white women were larger than differences observed between non-Latino black and non-Latino white men (5.1; 95% CI, 4.0-6.1 years). Compared with non-Latino white women, non-Latino black women had significantly higher adjusted excess heart age across all levels of sociodemographic variables and risk factors, except suboptimal blood pressure control among women without hypertension. Latino and non-Latino white women had comparable excess heart age, but Latino women had higher excess heart age than non-Latino white women in categories that usually protect against CVD (eg, higher education, income, good or better general health, and not smoking).
Table 5.
Multivariate adjusteda difference in excess heart ageb among racial/ethnic groups, by sex and selected sociodemographic characteristics and CVD risk factors, among adults in New York City aged 30-74 (n = 6117), New York State Behavioral Risk Factor Surveillance System, 2011, 2013, and 2015c
| Model | Difference in Excess Heart Age, y (95% CI) [P Value]d | |||
|---|---|---|---|---|
| Men | Women | |||
| Non-Latino Black vs. Non-Latino White | Latino vs. Non-Latino White | Non-Latino Black vs. Non-Latino White | Latino vs. Non-Latino White | |
| Total | 1.5 (0.3-2.7) [.01] | –0.4 (–1.4 to 0.6) [.41] | 5.1 (4.0-6.1) [<.001] | 0.9 (–0.3 to 2.0) [.13] |
| Age group, y | ||||
| 30-39 | 0.7 (–0.8 to 2.1) [.37] | 0.7 (–1.4 to 1.5) [.93] | 2.6 (1.3-3.9) [<.001] | –1.3 (2.6-0) [.06] |
| 40-49 | 0.9 (–1.8 to 3.5) [.51] | –0.6 (–2.2 to 1.0) [.45] | 6.6 (4.5-8.7) [<.001] | 0.7 (–1.3 to 2.7) [.49] |
| 50-59 | 2.4 (0.2-4.7) [.04] | –2.0 (–4.1 to 0) [.05] | 6.8 (4.2-9.3) [<.001] | 3.6 (1.2-6.1) [.003] |
| 60-74 | 2.5 (0.5-4.6) [.02] | 0.8 (–1.2 to 2.9) [.43] | 4.4 (2.9-5.8) [<.001] | 0.7 (–1.5 to 3.0) [.54] |
| Education | ||||
| <High school | 1.9 (–2.0 to 5.8) [.34] | –1.2 (–4.5 to –2.1) [.47] | 4.2 (0.3-8.0) [.04] | –1.6 (–4.9 to 1.8) [.35] |
| High school | 0.7 (–1.9 to 3.3) [.58] | –2.0 (–4.1 to –0.2) [.07] | 4.5 (2.5-6.5) [<.001] | 0.9 (–1.2 to 3.0) [.40] |
| >High school | 1.4 (0.0-2.7) [.05] | 0.4 (–0.7 to 1.4) [.53] | 5.2 (4.0-6.4) [<.001] | 1.5 (0.3-2.7) [.01] |
| Annual household income, $ | ||||
| <35 000 | 1.4 (–0.4 to 3.1) [.14] | –1.2 (–2.8 to 0.5) [.16] | 3.7 (1.7-5.6) [<.001] | –0.7 (–2.6 to 1.2) [.47] |
| ≥35 000 | 1.4 (–0.1 to 2.9) [.06] | 0.5 (–0.7 to 1.6) [.46] | 5.8 (4.6-7.0) [<.001] | 2.1 (0.8-3.4) [.002] |
| General health | ||||
| Good or better | 1.1 (–0.2 to 2.3) [.09] | –0.5 (–1.5 to 0.5) [.35] | 4.7 (3.7-5.7) [<.001] | 1.2 (0.1-2.4) [.04] |
| Poor | 5.2 (1.8-8.5) [.002] | 1.5 (–1.0 to 3.9) [.24] | 6.3 (2.9-9.7) [<.001] | –0.2 (–2.9 to 2.4) [.86] |
| Health insurance | ||||
| Yes | 1.7 (0.4-3.0) [.01] | –0.2 (–1.2 to 0.9) [.75] | 5.2 (4.1-6.3) [<.001] | 0.9 (–0.2 to 2.1) [.12] |
| No | 0.1 (2.1-2.3) [.90] | –1.6 (–3.6 to 0.4) [.13] | 4.6 (1.7-7.6) [.002] | 0.7 (–2.4 to 3.9) [.66] |
| Physical activitye | ||||
| Yes | 1.1 (−0.3 to 2.4) [.13] | –0.9 (–2.1 to 0.3) [.16] | 5.2 (3.9-6.6) [<.001] | 0.8 (–0.6 to 2.2) [.26] |
| No | 1.9 (0.2-3.7) [.03] | 0 (–1.4 to 1.4) [.98] | 4.9 (3.5-6.4) [<.001] | 0.8 (–0.6 to 2.3) [.25] |
| BMI, kg/m2 | ||||
| Underweight/normal weight (BMI <25) | 0.7 (–1.1 to 2.5) [.45] | –0.4 (–2.0 to 1.2) [.64] | 3.3 (1.9-4.7) [<.001] | 0.2 (–1.1 to 1.5) [.78] |
| Overweight (BMI 25 to <30) | 1.2 (0.1-2.4) [.04] | –0.2 (–1.5 to 1.0) [.78] | 2.3 (0.8-3.8) [.003] | 0.6 (–0.9 to 2.2) [.42] |
| Obese (BMI ≥30) | 2.1 (–0.5 to 4.8) [.11] | –2.6 (–4.3 to –0.8) [.004] | 3.5 (1.6-5.5) [<.001] | –0.5 (–2.7 to 1.6) [.62] |
| Current smokerf | ||||
| Yes | 2.4 (0.3-4.6) [.03] | 0.2 (–1.2 to 1.6) [.80] | 5.9 (2.9-8.8) [<.001] | 2.3 (–0.3 to 4.8) [.08] |
| No | 1.2 (0.4-2.1) [.004] | 0.3 (–0.6 to 1.1) [.55] | 5.0 (4.1-5.9) [<.001] | 1.3 (0.3-2.3) [.01] |
| Self-reported hypertension | ||||
| Yes | 1.9 (–0.2 to 4.0) [.07] | 0.6 (–1.0 to 2.3) [.44] | 3.0 (1.4-4.6) [<.001] | 0.5 (–1.3 to 2.3) [.56] |
| No | 1.1 (0.0-2.2) [.04] | 0 (–0.9 to 0.9) [>.99] | 3.5 (2.6-4.5) [<.001] | 0 (–0.9 to 0.9) [.97] |
| Suboptimal blood pressure control | ||||
| Yes | 2.1 (0.4-3.8) [.02] | 1.0 (–1.1 to 3.1) [.35] | 3.5 (1.0-6.0) [.01] | –0.2 (–2.6 to 2.2) [.89] |
| No | 1.5 (0.0-2.9) [.05] | –0.9 (–1.9 to 3.1) [.10] | 5.4 (4.3-6.4) [<.001] | 1.0 (–0.2 to 2.2) [.09] |
| Suboptimal blood pressure control among persons without hypertension | ||||
| Yes | 0.5 (–1.8 to 2.8) [.66] | –0.9 (–3.0 to 1.3) [.44] | 2.1 (–0.9 to 5.0) [.17] | –0.5 (–3.1 to 2.1) [.72] |
| No | 0.4 (–0.8 to 1.6) [.51] | 0.2 (–0.7 to 1.2) [.63] | 3.2 (2.2-4.1) [<.001] | 0.3 (–0.7 to 1.3) [.56] |
| Borough of residence | ||||
| Bronx | 2.4 (–0.4 to 5.1) [.09] | –0.3 (–3.0 to 2.5) [.85] | 6.6 (3.7-9.6) [<.001] | 2.5 (–0.3 to 5.4) [.08] |
| Brooklyn | 1.8 (0.2-3.3) [.03] | 0.1 (–1.7 to 1.9) [.91] | 4.4 (2.7-6.1) [<.001] | 1.5 (–0.7 to 3.7) [.19] |
| Manhattan | 2.9 (–0.2 to 6.0) [.07] | –0.3 (–2.1 to 1.5) [.76] | 5.4 (3.5-7.3) [<.001] | 3.1 (0.7-5.6) [.01] |
| Queens | –0.4 (–3.7 to 2.8) [.79] | –0.6 (–2.5 to 1.3) [.53] | 4.5 (2.5-6.5) [<.001] | –1.0 (–3.0 to 1.0) [.32] |
| Staten Islandg | –3.3 (–5.8 to 0.8) [.01] | –2.5 (–5.9 to 1.0) [.16] | 10.2 (0.9-19.4) [.03] | –2.4 (–5.7 to 0.9) [.15] |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease.
a Overall differences in excess heart age were adjusted for age, education, annual household income, general health, health insurance, physical activity, and borough of residence. Subgroup differences in excess heart age were adjusted for age, education, income, general health, health insurance status, physical activity, borough of residence, and subgroup characteristic variable by race/ethnicity interaction term to estimate racial/ethnic differences by subgroup levels.
b Heart age is defined by identifying the chronological age of a person with the same predicted Framingham Risk Score-derived 10-year CVD risk but normal levels of CVD risk factors. Excess heart age (heart age greater than chronological age) connotes increased CVD risk.
c Data source: New York State Behavioral Risk Factor Surveillance System.17 Data were weighted for the New York City adult population.
d P value based on multivariate linear regression model t test. All statistical tests were 2-sided, with P < .05 considered significant.
e Physical activity was defined as ≥150 minutes per week of physical activity.
f Current smoker was defined as persons who reported having smoked ≥100 cigarettes in their lifetime and currently smoked.
g Estimate may be unreliable due to a large relative standard error.
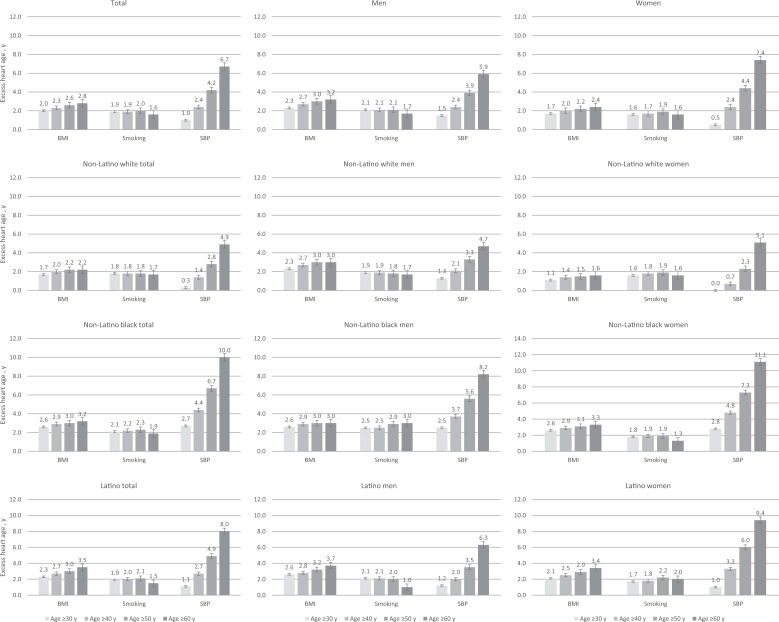
Assuming all other CVD risk factors were normal, the amount of excess heart age attributable to BMI alone for this population was 2 years (95% CI, 1.9-2.1 years), smoking alone was 1.9 years (95% CI, 1.7-2.0 years), and systolic blood pressure alone was 1.0 year (95% CI, 0.8-1.2 years). Although excess heart age effects due to BMI and smoking were relatively stable with increasing age, excess heart age effects due to systolic blood pressure had an increasing effect with increasing age (Figure). We observed similar trends among both sexes and racial/ethnic groups. However, excess heart age effects due to systolic blood pressure alone had a larger effect with older age among non-Latino black adults compared with non-Latino white adults, especially among women.
Figure.
Excess heart age attributable to modifiable cardiovascular disease (CVD) risk factors (body mass index [BMI], current smoking, systolic blood pressure [SBP]), by sex, race/ethnicity, and chronological age, among adults in New York City aged 30-74, New York State Behavioral Risk Factor Surveillance System, 2011, 2013, and 2015. Heart age is defined by identifying the chronological age of a person with the same predicted Framingham Risk Score–derived 10-year CVD risk but normal levels of CVD risk factors. Excess heart age (heart age greater than chronological age) connotes increased CVD risk. Prevalence of excess heart age ≥5 years indicates the proportion of the population with a heart age at least 5 years older than their chronological age and therefore at greater risk for CVD event. Data source: New York State Behavioral Risk Factor Surveillance System (BRFSS).17 Data were weighted for the New York City adult population. Error bars indicate 95% confidence intervals.
Discussion
Broadly, our findings are comparable to those of a national study of US adults13 that used 2011 and 2013 BRFSS data collected from all 50 states. The study reported excess heart age of 7.8 years among men and 5.4 years among women and a prevalence of excess heart age ≥5 years of 49% among men and 39% among women. The national results also showed similar differences between racial/ethnic groups: non-Latino black adults had higher excess heart age than other racial/ethnic groups, particularly non-Latino black women. Interestingly, the difference in excess heart age between non-Latino black men and non-Latino white men was significantly lower in New York City than in the national study.13
Racial/ethnic differences in cardiovascular health have been documented extensively in the literature.16,24-28 Although CVD mortality declined 28.8% in the United States from 2003-2013, racial/ethnic disparities in CVD mortality have not changed.1,16,29 Various rates of CVD prevalence are seen in the United States; non-Latino black persons have more cardiovascular events than other racial/ethnic groups.1 A higher prevalence of uncontrolled CVD risk factors also places non-Latino black persons at a greater probability of having adverse outcomes and premature mortality.16,27,30 Non-Latino black men in particular have the highest overall CVD mortality rate of any racial/ethnic group, and non-Latino black women have higher CVD mortality rates than non-Latino white women.16,31,32
We found that excess BMI accounted for 2 years of excess heart age among adults in New York City, and although excess heart age varied by age, it was a consistent finding across all age groups. This finding underscores the importance of public health interventions aimed at achieving and maintaining a healthy weight, including promoting a healthy diet, increasing physical activity, and promoting health behaviors that also can improve blood pressure control independent of weight status.33 Several community-based lifestyle interventions from the National Institutes of Health Diabetes Prevention Program have shown promise in improving diet and physical activity and reducing body weight. These interventions resulted in a 3% to 7% loss of body weight and increased moderate physical activity to at least 150 minutes per week immediately after the core curriculum, varying in length from 6-24 weeks.34 Scaling up these lifestyle interventions could mitigate excess health age by improving BMI among overweight and obese adults, reducing the incidence of diabetes, and lowering blood pressure.33,34 Current smoking also accounted for nearly 2 years of excess heart age among adults in New York City. This figure is notable because the prevalence of smoking among adults in New York City was about 14% in 201 535 and reflects the great potential that continued reductions in smoking prevalence may have on CVD risk.34,36,37 We found that systolic blood pressure accounted for 1 year of the excess heart age among adults in New York City. Although this effect was smaller than the effect of BMI and smoking, it may be explained by the low systolic blood pressure among younger adults (eg, the mean systolic blood pressure among adults aged 30-39 was 115.2 mm Hg). Having a systolic blood pressure <125 mm Hg lowered overall heart age. However, once a person develops high blood pressure (especially requiring medication), the health age effect of systolic blood pressure increases substantially, which we observed when calculating health age among adults aged >40. This increase in heart age highlights the importance of efforts to ensure higher overall systolic blood pressure control among persons with hypertension38 and policies and programs designed to reduce raised blood pressure at the population level.39-42
Limitations
This study had several limitations. First, we used model-estimated systolic blood pressure instead of measured systolic blood pressure. However, studies showed that the FRS CVD risk using mean predicted systolic blood pressure among BRFSS participants was nearly identical to that of National Health and Nutrition Examination Survey participants with measured systolic blood pressure.15 The results of our model validation component using HFUS data showed small differences between the 2 systolic blood pressure measurements. Similarly, we used the non–laboratory-based FRS to predict CVD risk, which, according to some studies, may overestimate CVD risk compared with the laboratory-based FRS.13,15 Second, we were limited in our ability to report on heart age for certain racial/ethnic subgroups, including Asian/Pacific Islanders. We used a previously published method15,20 to estimate systolic blood pressure in which the authors calculated regression coefficients for 3 racial/ethnic categories (non-Latino white, non-Latino black, Latino) and grouped all others in the “other” category. Future studies should expand heart age calculations to additional racial/ethnic subgroups. Oversampling racial/ethnic subgroups at an earlier step of data collection is needed for the sample to produce statistically reliable health estimates for these underrepresented groups. Third, we used self-reported height, weight, and diabetes status to predict CVD risk. Generally, self-report tends to overestimate height and to underestimate weight, thus reducing BMI measurements and leading to an underestimation of CVD risk. Missing adults with undiagnosed diabetes would also underestimate CVD risk. We estimated the relative contribution of modifiable CVD risk factors included in the FRS equations to the excess heart age. Thus, the relative contribution of these risk factors was calculated only among a preselected set of risk factors. Finally, the BRFSS does not collect data on heart failure or peripheral artery disease, so participants with these conditions could not be excluded. As such, we might have overestimated heart age for some participants.
Conclusions
Excess heart age is potentially a timely indicator for CVD risk surveillance at the national level rather than the local level due to the need for smaller geographies to combine survey years for robust measures. However, local prospective tracking of excess heart age is a potentially useful addition to public health surveillance efforts. Studies indicate a high level of public interest in CVD risk self-assessments when an easily understood metric such as heart age is used.43 Interactive heart age tools can be helpful as a communication instrument to initiate lifestyle change. By simplifying the concept of CVD risk, state and local jurisdictions may effectively monitor socioeconomic, racial/ethnic, and sex disparities in excess heart age to support public health initiatives and promote heart-healthy lifestyle changes.
Acknowledgments
The authors thank Rania Kanchi, MPH, New York University School of Medicine, New York, New York, and Claudia Chernov, MPH, New York City Department of Health and Mental Hygiene, Queens, New York, for their help preparing this article.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bahman P. Tabaei, MPH  https://orcid.org/0000-0001-6779-1044
https://orcid.org/0000-0001-6779-1044
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi:10.1161/CIR.0000000000000250 [DOI] [PubMed] [Google Scholar]
- 2. Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff (Millwood). 2007;26(1):38–48. doi:10.1377/hlthaff.26.1.38 [DOI] [PubMed] [Google Scholar]
- 3. Bovet P, Chiolero A, Paccaud F, Banatvala N. Screening for cardiovascular disease risk and subsequent management in low and middle income countries: challenges and opportunities. Public Health Rev. 2015;36:13 doi:10.1186/s40985-015-0013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. [DOI] [PubMed] [Google Scholar]
- 5. Siontis GC, Tzoulaki I, Siontis KC, Ioannidis JP. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ. 2012;344:e3318 doi:10.1136/bmj.e3318 [DOI] [PubMed] [Google Scholar]
- 6. Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S75]. Circulation. 2014;129(suppl 2):S49–S73. doi:10.1161/01.cir.0000437741 [DOI] [PubMed] [Google Scholar]
- 7. Webster R, Heeley E. Perceptions of risk: understanding cardiovascular disease. Risk Manag Healthc Policy. 2010;3:49–60. doi:10.2147/RMHP.S8288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soureti A, Hurling R, Murray P, van Mechelen W, Cobain M. Evaluation of cardiovascular disease risk assessment tool for the promotion of healthier lifestyles. Eur J Cardiovasc Prev Rehabil. 2010;17(5):519–523. doi:10.1097/HJR.0b013e328337ccd3 [DOI] [PubMed] [Google Scholar]
- 9. Groenewegen KA, den Ruijter HM, Pasterkamp G, Polak JF, Bots ML, Peters SA. Vascular age to determine cardiovascular disease risk: a systematic review of its concepts, definitions, and clinical applications. Eur J Prev Cardiol. 2016;23(3):264–274. doi:10.1177/2047487314566999 [DOI] [PubMed] [Google Scholar]
- 10. D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi:10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 11. Lopez-Gonzalez AA, Aguilo A, Frontera M, et al. Effectiveness of the heart age tool for improving modifiable cardiovascular risk factors in a southern European population: a randomized trial. Eur J Prev Cardiol. 2015;22(3):389–396. doi:10.1177/2047487313518479 [DOI] [PubMed] [Google Scholar]
- 12. Spiegelhalter D. How old are you, really? Communicating chronic risk through “effective age” of your body and organs. BMC Med Inform Decis Mak. 2016;16:104 doi:10.1186/s12911-016-0342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Q, Zhong Y, Ritchey M, et al. Vital signs: predicted heart age and racial disparities in heart age among U.S. adults at the state level [published correction appears in MMWR Morb Mortal Wkly Rep. 2015;64(34):960-962]. MMWR Morb Mortal Wkly Rep. 2015;64(34):950–958. [DOI] [PubMed] [Google Scholar]
- 14. Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, et al. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980-2014. JAMA. 2017;317(19):1976–1992. doi:10.1001/jama.2017.4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Q, Zhong Y, Ritchey M, et al. Predicted 10-year risk of developing cardiovascular disease at the state level in the U.S. Am J Prev Med. 2015;48(1):58–69. doi:10.1016/j.amepre.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi:10.1161/01.CIR.0000158136.76824.04 [DOI] [PubMed] [Google Scholar]
- 17. New York State Department of Health. Behavioral Risk Factor Surveillance System (BRFSS). www.health.ny.gov/statistics/brfss. Accessed September 21, 2016.
- 18. Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham Heart Study general cardiovascular risk profile. Circulation. 2009;120(5):384–390. doi:10.1161/CIRCULATIONAHA.108.835470 [DOI] [PubMed] [Google Scholar]
- 19. National Heart, Lung and Blood Institute. Framingham Heart Study. https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk. Accessed December 21, 2016.
- 20. Ezzati M, Oza S, Danaei G, Murray CJ. Trends and cardiovascular mortality effects of state-level blood pressure and uncontrolled hypertension in the United States. Circulation. 2008;117(7):905–914. doi:10.1161/CIRCULATIONAHA.107.732131 [DOI] [PubMed] [Google Scholar]
- 21. Angell SY, Yi S, Eisenhower D, et al. Sodium intake in a cross-sectional, representative sample of New York City adults. Am J Public Health. 2014;104(12):2409–2416. doi:10.2105.AJPH.2013.301542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. SAS Institute Inc. SAS [computer software]. Version 9.4 Cary, NC: SAS Institute Inc; 2016. [Google Scholar]
- 23. Research Triangle Institute. SAS-Callable-SUDAAN [computer software]. Version 11.0.1 Research Triangle Park, NC: Research Triangle Institute; 2012. [Google Scholar]
- 24. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873–898. doi:10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 25. Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. doi:10.1001/jama.2012.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation. 2012;125(15):1848–1857. doi:10.1161/CIRCULATIONAHA.111.047480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and non-cardiovascular death: the Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, and the Multi-ethnic Study of Atherosclerosis. Circulation. 2012;126(1):50–59. doi:10.1161/CIRCULATIONAHA.111.057232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewey J, Choudhry NK. The current state of ethnic and racial disparities in cardiovascular care: lessons from the past and opportunities for the future. Curr Cardiol Rep. 2014;16(10):530 doi:10.1007/s11886-014-0530-3 [DOI] [PubMed] [Google Scholar]
- 29. Romero CX, Romero TE, Shlay JC, Ogden LG, Dabelea D. Changing trends in the prevalence and disparities of obesity and other cardiovascular disease risk factors in three racial/ethnic groups of USA adults. Adv Prev Med. 2012;2012:172423 doi:10.1155/2012/172423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143–152. [PubMed] [Google Scholar]
- 31. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi:10.1161/CIR.0b013e3182009701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–245. doi:10.2174/1573403X11666141122220003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307(12):1273–1283. doi:10.1001/jama.2012.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson L. Translating the diabetes prevention program into practice: a review of community interventions. Diabetes Educ. 2009;35(2):309–320. doi:10.1177/0145721708330153 [DOI] [PubMed] [Google Scholar]
- 35. New York City Department of Health and Mental Hygiene. Epiquery: NYC interactive health data—Community Health Survey 2015. http://nyc.gov/health/epiquery. Accessed October 1, 2017.
- 36. Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38(6):600–609. doi:10.1016/j.amepre.2010.02.016 [DOI] [PubMed] [Google Scholar]
- 37. Dube SR, McClave A, James C, Caraballo R, Kaufmann R, Pechacek T. Vital signs: current cigarette smoking among adults aged ≥18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135–1140. [PubMed] [Google Scholar]
- 38. Frieden TR, Berwick DM. The “Million Hearts” initiative—preventing heart attacks and strokes. N Engl J Med. 2011;365(13):e27 doi:10.1056/NEJMp1110421 [DOI] [PubMed] [Google Scholar]
- 39. Sacks FM, Campos H. Dietary therapy in hypertension. N Engl J Med. 2010;362(22):2102–2112. doi:10.1056/NEJMct0911013 [DOI] [PubMed] [Google Scholar]
- 40. New York City Department of Health and Mental Hygiene. Sodium initiatives. https://www1.nyc.gov/site/doh/health/health-topics/national-salt-reduction-initiative.page#national-salt-reduction-initiative. Accessed September 15, 2017.
- 41. Curtis CJ, Clapp J, Niederman SA, Ng SW, Angell SY. US food industry progress during the National Salt Reduction Initiative: 2009-2014. Am J Public Health. 2016;106(10):1815–1819. doi:10.2105/AJPH.2016.303397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hardy ST, Loehr LR, Butler KR, et al. Reducing the blood pressure–related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc. 2015;4(10):e002276 doi:10.1161/JAHA.115.002276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel RS, Lagord C, Waterall J, et al. Online self-assessment of cardiovascular risk using the Joint British Societies (JBS3)-derived heart age tool: a descriptive study. BMJ Open. 2016;6(9):e011511 doi:10.1136/bmjopen-2016-011511 [DOI] [PMC free article] [PubMed] [Google Scholar]