Abstract

Chemiluminescence is gradually being recognized as a powerful tool for sensing and imaging. Most known light-emitting compounds undergo chemiexcitation through spontaneous decomposition of cyclic peroxide moieties. A ground-breaking milestone in the chemistry of such compounds was achieved 30 years ago with the discovery of triggerable dioxetanes by Schaap’s group. Our group has recently developed a distinct methodology to significantly improve the light emission efficiency of such phenoxy-dioxetane luminophores under physiological conditions. Introduction of an electron-withdrawing substituent at the ortho position of the phenoxy-dioxetane resulted in an approximately 3000-fold increase of the chemiluminescence quantum yield in aqueous media. Furthermore, we discovered that the emission wavelength and the kinetics of the chemiexcitation could be determined by the electronic nature of the substituent incorporated on the dioxetane luminophore. This recent development has provided scientists with new powerful chemiluminophores that can act as single-component probes for in vivo and in vitro detection and imaging of various analytes and enzymes. This outlook describes the recent progress toward applications of synthetic chemiluminescence luminophores suitable for sensing and imaging in aqueous environments.
Short abstract
Discovery of dioxetanes which are highly emissive in water enables effective chemiluminescence bioimaging. Emission color and kinetics are also currently adjustable. For phenoxy-dioxetanes, the future is brighter than ever.
Introduction
The emission of light as a result of a chemical reaction is a spectacular phenomenon. In nature, this marvel, usually referred to as bioluminescence, occurs in the firefly and some marine organisms.1 When light is emitted through chemiexcitation due to a chemical reaction, the phenomenon is termed chemiluminescence. This unique phenomenon is increasingly recognized as a powerful tool for sensing and imaging,2 and chemiluminescence detection assays make up a progressively larger share of the multibillion-dollar market of immunoassays each year.3 The main advantage of chemiluminescence over fluorescence lies in the fact that irradiation by an external light source is not required; thus, the background signal is extremely low and the sensitivity is high.4
Small molecules known as luciferins are the active substrates used by organisms to produce light emission. Such compounds are oxidized by an enzyme (luciferase) and molecular oxygen to generate an unstable dioxetane or similar peroxide intermediate that decomposes to form an excited carbonyl molecule. The latter decays to its ground state through emission of light.5 The understanding of the luciferin mode of action enabled chemists to develop various synthetic small molecules that can undergo oxidation to form analogous dioxetanes.6,7 These unstable dioxetanes rapidly decompose to release excited molecules that decay through emission of light. Thus, insights learned from the study of nature have led to development of synthetic molecules that can emit light through chemiluminescence.
In a bioluminescence event, the excited luciferin molecule (in the presence of a luciferase) emits light with high efficiency under physiological conditions.8 In contrast, the excited states of compounds that emit light through chemiluminescence are typically poorly emissive in aqueous solution, and energy decay takes place through nonradiative pathways.9 To overcome this drawback, additional components (like surfactants) are added in order to exclude water molecules from the excited species.10,11 Until recently, small molecules that emit direct light efficiently through a chemiluminescence pathway, as single components in physiological media, were unknown. A distinct discovery by our group appears to provide a promising solution to this imperative requirement.12 This outlook is focused on the recent progress and challenges of synthetic chemiluminescence luminophores suitable for use in an aqueous environment.
Historical Perspective and Overview of Recent Developments
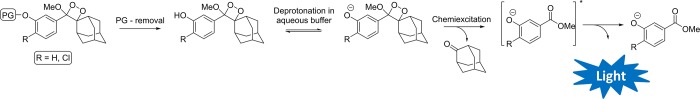
1,2-Dioxetanes have been widely explored in the past as light-emitting functionalities.13 A ground-breaking milestone in the chemistry of such compounds was achieved about 30 years ago with the discovery of triggerable dioxetanes by the Schaap group.14−16 In such compounds (Figure 1), chemiexcitation is initiated as a consequence of phenolate formation, following deprotection of phenols. Thus, by selecting the appropriate phenol-protecting group, light emission can be triggered by a specific analyte or enzyme. The first generation dioxetanes generate the chemiluminescent signal through a two-step activation pathway. In the first step, the probe is incubated with the enzyme of interest at pH 7.4 to produce a phenol-dioxetane intermediate. In the second step, the pH of the solution is raised to 10 in order to trigger the chemiexcitation of the phenol-dioxetane.17
Figure 1.
General structure and activation pathway of Schaap’s dioxetanes (PG, protecting group).
Second-generation Schaap’s dioxetanes are equipped with a chlorine substituent at the ortho position of the phenol.18 This chlorine substituent reduces the pKa of the phenol-dioxetane to allow a one-step mode of action in physiological buffer. Several dioxetane-based chemiluminescent probes have been commercialized; the most recognizable is the alkaline phosphatase substrate Lumi-Phos 530. Schaap’s dioxetanes suffer from an inherent limitation: The chemiluminescence emission of these luminophores is extremely weak in aqueous conditions. This problem can be partially solved by the addition of a surfactant (“enhancer”) to the solution.19 The surfactant reduces water-induced quenching by providing a hydrophobic environment for the chemiluminescent reaction, and consequently the light emission efficiency is considerably enhanced. Since the use of surfactants is not compatible with living systems (in terms of micelle stability and toxicity),20 the requirement for their presence limits the application of dioxetanes to test tube-based diagnostic assays.21
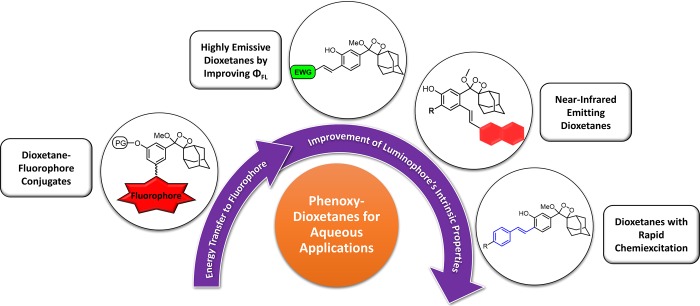
In order to make the dioxetanes generally suitable for bioimaging in live cells in culture and in animals, two fundamental obstacles must be solved. First, the dioxetane must act as a single-component, small-molecule probe. Second, the intrinsic chemiluminescence quantum yield in water must be significantly improved. Three years ago, our group began testing two different methodologies in order to address these problems. A flow diagram describing our approach, progress, and rationale in developing chemical solutions to these challenges is depicted in Figure 2.
Figure 2.
Flow diagram describing our approach and progress in developing phenoxy-dioxetane probes suitable for bioimaging.
Initially, we developed a modular synthetic route for preparation of fluorophore conjugates with phenoxy-dioxetane turn-ON chemiluminescent probes.22 In such conjugates, the chemiluminescence emission is significantly amplified through an energy-transfer mechanism that occurs under physiological conditions. Next, we realized that efficient chemiluminescence through a direct emission mode could be achieved by appropriate design of the phenoxy-dioxetanes π-electron system. Remarkably, we discovered that by introducing an electron-withdrawing acrylic substituent at the ortho position of the phenoxy-dioxetane, an incredible increase of light emission is obtained under physiological conditions.12 The substituent effect resulted in an approximately 3000-fold increase of the chemiluminescence quantum yield in aqueous media. This discovery led to development of small-molecule chemiluminescent dyes that are extremely bright in water in the absence of additives.
Incorporation of a substituent with an extended π-electron system on the excited species obtained during the chemiexcitation of the phenoxy-dioxetane probe resulted in the first chemiluminescent luminophores with a direct mode of near-infrared (NIR) light emission suitable for use under physiological conditions.23 On the basis of this notion, new dyes that luminesce via direct emission mode at various wavelengths were synthesized. The emission wavelength depends on the electronic nature of the substituent installed on the phenoxy-dioxetane probe. Finally, by installing a styryl substituent that promotes rapid chemiexcitation, we were able to design and synthesize new phenoxy-dioxetanes with up to 100-fold faster chemiexcitation kinetics than previously described compounds.24 Such phenoxy-dioxetanes are up to 16-fold more sensitive than probes with slower chemiexcitation.
With the availability of the new improved phenoxy-dioxetane luminophores, the construction of chemiluminescence probes for detection and imaging of enzymes and analytes is straightforward. Several examples were already described in the past two years,25 and numerous others will certainly follow.
Recent Progress of Triggerable Phenoxy-Dioxetanes
1. Chemiluminophores with Green Emission Light
Schaap’s dioxetanes suffer from low chemiluminescence quantum yields (ΦCL) under physiological conditions because the electronically excited benzoate species formed by chemiexcitation is a poor emitter in water; that is, it has low fluorescence quantum yields (ΦFL). Therefore, the emissive nature of the electronically excited species had to be improved in order to generate efficient chemiluminescence in aqueous media.
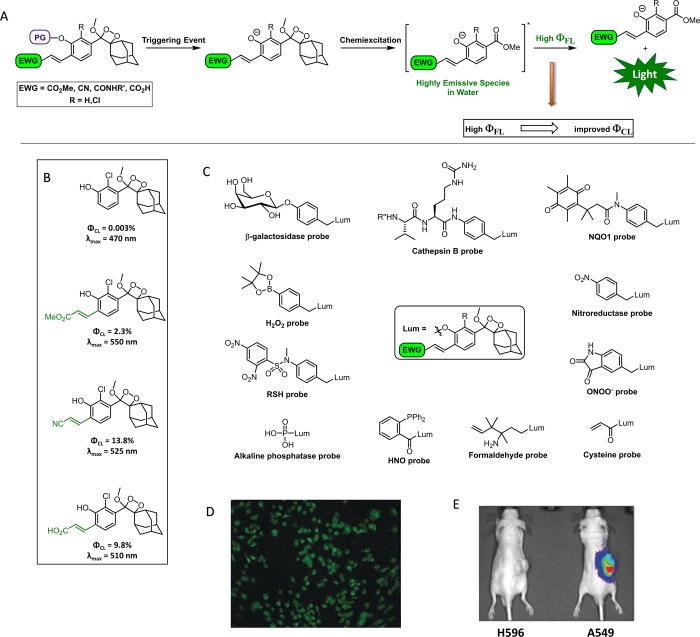
A π-conjugated donor–acceptor motif is highly prevalent in many bright fluorescent dyes.26,27 We therefore speculated that the ΦFL of phenol-based compounds could be improved by introducing an electron acceptor, an acryl group, at the ortho position of the phenolate donor. This hypothesis was examined by the preparation of several acryl-substituted phenoxy-benzoate derivatives.12 Indeed, such compounds were found to be highly emissive in water, with ΦFL of up to 40%. This observation suggested that incorporation of an acryl group into the phenoxy-dioxetane luminophore would result in an increase in its ΦCL under physiological conditions (Figure 3A). To evaluate this hypothesis, acryl-substituted phenoxy-dioxetane luminophores were synthesized, and their light emission properties under physiological conditions were measured. Remarkably, the acryl-substituted luminophores exhibit extremely bright chemiluminescence emission upon their deprotonation in pH 7.4 solution, and ΦCL’s are up to 3000-fold higher than that of the unsubstituted Schaap’s dioxetane luminophore (Figure 3B). These new luminophores emit greenish light and have maximum emission at wavelengths that range from 510 to 550 nm.
Figure 3.
(A) High ΦFL of the excited phenoxy-benzoate is achieved by incorporation of an acryl group, which improves chemiluminescence efficiency. EWG, electron-withdrawing group. (B) Structure and properties of acryl-substituted phenoxy-dioxetane luminophores, which have high ΦCL under physiological conditions. (C) Structures of various chemiluminescent probes suitable for bioimaging (Lum = luminophore). (D) RAW 264.7 macrophages imaged using a chemiluminescent probe for cathepsin B. (E) In vivo images of tumor xenografts, formed by the injection of A549 cancer cells that overexpress NQO1 vs control cell line (H596), obtained with an NQO1-specific chemiluminescent probe.
The discovery of the bright acryl-substituted phenoxy-dioxetanes is expected to revolutionize the field of chemiluminescence bioimaging. Although these luminophores were reported only two years ago, they have already been exploited for the construction of numerous efficient chemiluminescent probes (Figure 3C). Our group initially prepared a chemiluminescent probe for β-galactosidase. This probe was used to image endogenous β-galactosidase activity in LacZ-transfected cells, providing the first chemiluminescence microscopy images of living cells using 1,2-dioxetane probes.12,28 Subsequently, we prepared a chemiluminescent probe for the cysteine-protease cathepsin B, a native enzyme that is overexpressed in malignant tumors. This probe exhibited high sensitivity and further enabled chemiluminescence microscopy imaging of cathepsin B activity in cancerous living cells (Figure 3D).29 The group of Lippert synthesized a dioxetane-based probe for peroxynitrite (ONOO–) and performed a thorough mechanistic investigation of ONOO– generation from Angeli’s salt.30 In addition, they demonstrated that cellular ONOO– generated in lipopolysaccharide-stimulated macrophages could be detected with the probe. More recently, the Lippert group described a chemiluminescent probe for HNO, a reactive nitrogen species with pronounced biological activity.31 The high sensitivity of the probe enabled the real-time quantitative measurements of HNO concentration at the picomolar level. Furthermore, the dioxetane-based probe allowed monitoring of HNO production in living cells and animals. Very recently, Kim and co-workers reported an NQO1-specific chemiluminescent probe.32 This probe allows NQO1 activity to be detected in human lung cancer models both in vitro and in vivo (Figure 3E). Additional probes for chemiluminescence imaging of nitroreductase33 and cysteine34 in living animals were recently reported by the group of Zhang.
The acryl-based phenoxy-dioxetane probes are highly suitable not only for cell imaging, but also for imaging in small animals. This is despite the fact that they emit green light, which has limited tissue permeability compared to the NIR probes commonly used for fluorescence imaging in vivo.35 The imaging studies performed with these probes demonstrate the advantage of chemiluminescence over fluorescence for in vivo applications, since impediments such as autofluorescence and scattering of irradiated light are avoided. Various acryl-substituted dioxetane probes are now commercially available from Biosynth under the brand name AquaSpark.36
2. Chemiluminophores with NIR Light Emission
Although the acryl-substituted luminophores, with their green emission, can be used for some in vivo imaging applications, NIR chemiluminescence is highly preferred for animal imaging, as NIR photons penetrate mammalian tissues better than visible light.37 In order to obtain NIR-emitting luminophores, we incorporated dicyanomethylene-4H-chromene (DCMC) as an electron acceptor instead of the acrylate. The DCMC-based donor–acceptor design is known to produce NIR-emitting fluorescent dyes with good fluorescence quantum yield.38,39 We assumed that phenoxy-dioxetane luminophores bearing the DCMC acceptor at the ortho or para positions of the phenol donor would efficiently emit NIR light after chemiexcitation (Figure 4A).
Figure 4.
(A) Schematic of incorporation of the DCMC electron acceptor into the phenoxy-dioxetane scaffold to yield NIR-emitting chemiluminescent probes. (B) Structure of NIR probe for H2O2 (left), and imaging of endogenous H2O2 production in the peritoneal cavity of mice during an LPS-induced inflammatory response (right). (C) Structure of NIR probe for formaldehyde (left), and imaging of endogenous FA produced by the folate cycle through tetrahydrofolate metabolism (right).
On the basis of this design, the first phenoxy-dioxetane luminophores that emit NIR light directly (rather than due to energy transfer to a tethered NIR dye) were successfully prepared.23 As expected, these luminophores exhibit bright NIR chemiluminescence under physiological conditions. One luminophore with outstanding properties contains the DCMC acceptor at the para position of the OH group and an acrylic acid substituent at the ortho position. It was incorporated into a chemiluminescent probe for hydrogen peroxide (H2O2). The phenol functional group of the luminophore was masked with a triggering pinacol-boronate ester group (Figure 4B). This aryl-boronate ester selectively reacts with H2O2 under physiological conditions to generate the active phenolate-dioxetane, which subsequently undergoes chemiexcitation to emit NIR light. This probe enabled visualization of elevated levels of H2O2 during lipopolysaccharide-induced inflammation in mice.23 The Dvir group recently used this probe to evaluate the immune responses of mice to autologous, xenogeneic, and allogeneic implants.40
About a year ago, an NIR-emitting chemiluminescent probe for formaldehyde (FA) was prepared, using phenoxy-dioxetane substituted at the ortho position with DCMC.41 In this example, the phenol group was masked with a known FA-reactive triggering substrate,42 designed to release free phenol following a cascade reaction initiated by FA (Figure 4C). The probe enabled visualization of FA release from endogenous folate metabolism in mice and thereby provided the first in vivo evidence that folinate and tetrahydrofolate have distinct abilities to generate FA through folate cycle metabolism.
3. Chemiluminophores with Fast Chemiexcitation Kinetics
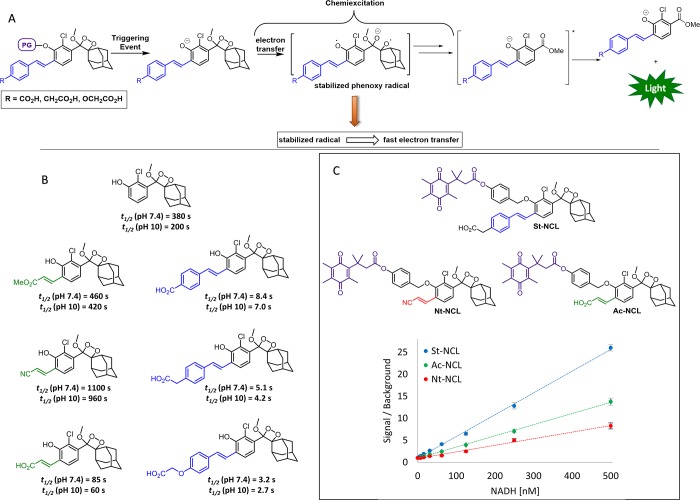
For some chemiluminescence bioassays, the absolute ΦCL of the phenoxy-dioxetane luminophore is not the primary attribute that governs the quality of a chemiluminescent probe. In these bioassays, the rate of chemiexcitation of the free phenolate-dioxetane is of critical importance. Luminophores with fast chemiexcitation kinetics are highly desired since they are expected to improve the sensitivity of chemiluminescent analytical bioassays. When photons are released within a shorter period of time, the obtained signal to background ratio (S/B) is higher and the sensitivity is better.43 Following a rational, computationally supported design, next-generation phenoxy-dioxetanes that exhibit ultrarapid chemiexcitation were recently developed.24
Previous investigations of the chemiexcitation mechanism suggested that the first step in excitation of phenoxy-dioxetane probes involves an electron transfer from the phenolate donor to the dioxetane moiety, generating a phenoxy radical species.44−47 It was therefore presumed that equipping the luminophore with a styryl substituent instead of the acryl substituent would promote more rapid chemiexcitation by increasing the radical stabilizing nature of the phenoxy moiety (Figure 5A). Styryl-substituted luminophores were synthesized, and their chemiexcitation properties were evaluated under physiological conditions and compared to the properties of the acrylate phenoxy-dioxetanes. Remarkably, the chemiexcitation of the styryl derivatives occurred extremely rapidly, about 2 orders of magnitude faster than chemiexcitation of the acrylic ester- or acrylonitrile-substituted luminophores or the unsubstituted Schaap’s dioxetane (Figure 5B). The acrylic acid-substituted derivative also exhibited quite rapid chemiexcitation; however, it was about 10 times slower than that of the styryl-substituted luminophores. Raising the pH from 7.4 to 10 did not result in substantial change in the general trend among the half-lives of luminophores (Figure 5B), thus confirming that the differences in the chemiexcitation rates are intrinsic and do not stem from different pKa values.
Figure 5.
(A) Luminophores with rapid chemiexcitation synthesized by the incorporation of a styryl substituent that stabilizes the phenoxy radical and thus promotes electron transfer. (B) Structure and chemiexcitation kinetics of the phenoxy-dioxetane luminophores. (C) Structures of NADH probes (top) and signal to background ratio plotted versus NADH concentration for each probe (bottom).
In order to demonstrate the significance of rapid chemiexcitation in bioassays, three related chemiluminescent probes for NADH were prepared by masking the phenol with a “trimethyl lock” quinone trigger.48 In such probes, DT-diaphorase-catalyzed reduction of the quinone moiety to hydroquinone by NADH initiates a cascade reaction that releases the free phenol-dioxetane. The three NADH probes differed from each other in the rates of their chemiexcitation (Figure 5C, top). As anticipated, there was a clear correlation between the chemiexcitation rate and the probe sensitivity (Figure 5C, bottom), demonstrating the importance of rapid chemiexcitation for superior probe performance.
4. Chemiluminescence Probe for Singlet Oxygen under Physiological Conditions
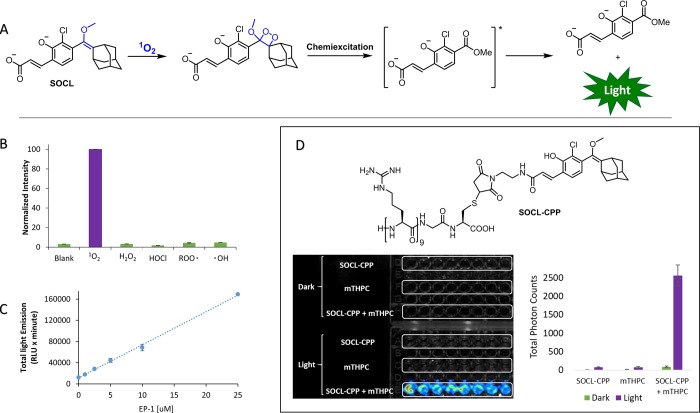
The phenol group of the triggerable phenoxy-dioxetane probes is usually protected with an enzyme- or analyte-responsive group. The chemiexcitation is initiated through the unmasking of the phenol and the release of a phenolate-dioxetane species (Figure 1). However, it was realized that phenolate-dioxetanes that efficiently emit light can also be obtained under physiological conditions by a reaction between an enol-ether precursor of the phenoxy-dioxetane and singlet oxygen (1O2). This reactivity paved the way for design of a chemiluminescent probe for 1O2.49 In light of its primary cytotoxic role in photodynamic therapy,50,51 real-time monitoring of 1O2 under biologically relevant conditions is obviously of great interest.52 The chemical strategy for monitoring of 1O2 by chemiluminescence is presented in Figure 6A. Reaction of the probe SOCL with 1O2 yields a highly emissive phenolate-dioxetane species that undergoes spontaneous chemiexcitation to produce light emission. This type of probe was found to be highly selective (Figure 6B) and sensitive (Figure 6C) for 1O2 detection. A cell-permeable analogue, SOCL-CPP, was prepared by attaching a cell-penetrating peptide (nona-arginine) to the phenoxy-enol-ether moiety (Figure 6D, top). Using SOCL-CPP, detection of intracellular 1O2 produced by the photosensitizer mTHPC upon irradiation of HeLa cells was enabled (Figure 6D, bottom).
Figure 6.
(A) Scheme for reaction of SOCL with 1O2 under physiological conditions to yield highly emissive phenolate-dioxetane, which subsequently produces chemiluminescence. (B) Selectivity of SOCL for 1O2 among other reactive oxygen species in phosphate buffered saline at pH 7.4. (C) Chemiluminescence signal upon incubation of SOCL with different concentrations of the 1O2 generator EP-1. (D) Structure of the cell-permeable probe SOCL-CPP (top) and chemiluminescence images obtained of HeLa cells incubated with SOCL-CPP and mTHPC and then irradiated (bottom). On the right is the quantification of light intensities emitted from the cells.
5. Monitoring Drug Release by Chemiluminescence
Numerous clinically approved anticancer drugs cause systemic toxicity and are consequently characterized by a narrow therapeutic window. The use of prodrugs that can be activated selectively in the tumor environment can mitigate toxicity.53 Theranostic prodrugs, in which drug release is coupled with a fluorescent signal, offer an additional advantage as they allow monitoring of drug release noninvasively in real time.54,55
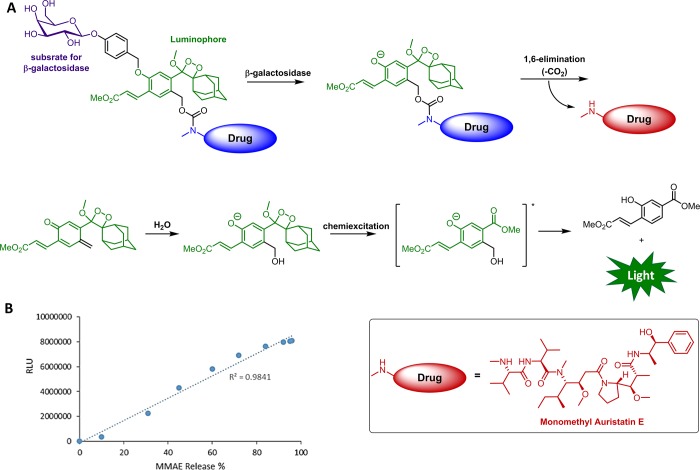
As chemiluminescence offers advantages over fluorescence for in vivo imaging, the first theranostic prodrug with chemiluminescent emission output was recently developed using our phenoxy-dioxetane as an assembly scaffold. The structure and activation mechanism of this theranostic prodrug are presented in Figure 7A.56 The prodrug is comprised of a phenoxy-dioxetane luminophore and the antineoplastic drug monomethyl auristatin E (MMAE) and is designed to be activated by β-galactosidase. Following the removal of the phenol masking group by β-galactosidase, 1,6-elimination and decarboxylation take place rapidly to release the active drug and a quinone-methide species.57 The phenoxy-dioxetane structure is then restored by the addition of a water molecule to the electrophilic quinone-methide. Subsequent chemiexcitation produces the light emission signal.
Figure 7.
(A) Molecular structure and activation mechanism of the chemiluminescent prodrug. The inactive MMAE is indicated by the blue ellipse, and the free active MMAE is shown in red. (B) Correlation between MMAE release (determined by RP-HPLC) and the produced chemiluminescence signal.
For this theranostic prodrug, there is a linear correlation between the chemiluminescence signal and drug release in physiological solution (Figure 7B). In addition, when incubated with Lac-Z transfected HEK293 cells, the prodrug produced bright chemiluminescence signal and high cytotoxicity. Weak signal and minor effect on viability were observed in the control HEK293 cells that do not express the activating enzyme. In addition, the prodrug was used to produce chemiluminescence images of tumor-bearing mice. The unique para-hydroxymethyl appendage to the phenoxy-dioxetane scaffold, which enabled the stimulus-responsive carbamate linkage of the drug, has also been utilized for the construction of various systems in which the chemiluminescent signal is amplified either by self-immolative polymers58 or by chain reactions.59,60
Summary and Future Prospects
It has now been 30 years since Paul Schaap’s influential discovery of triggerable phenoxy-dioxetanes.14−16 This family of compounds is commercially useful in chemiluminescence bioassays when the appropriate additives are added to the aqueous media. Interestingly, the field had remained dormant until very recently.61,62 Our contribution to the field, first reported about two years ago, resulted from recent insights and progress in design of fluorescent molecules. Moreover, major instrumental advances have created demands for development of new advanced dyes for detection and imaging. The understanding that the radiative decay of the electronically excited species formed by chemiexcitation in water had to be improved, led us to develop the next generation of triggerable phenoxy-dioxetanes. These new luminophores emit light efficiently under physiological conditions, via a direct mode, following their chemiexcitation by a triggering event.63
In order further to improve the light emission efficiency in water and to implement these probes in additional applications, a large number of new phenoxy-dioxetane derivatives must be screened. Therefore, an operationally simple synthetic pathway to access phenoxy-dioxetane luminophores is required. The route developed 30 years ago by Schaap and co-workers relies on a hetero-McMurray-type coupling of a ketone with an ester to afford an enol ether;64,65 the intermediate product is then oxidized to furnish the target adamantyl-phenoxy-dioxetanes. Generally, this reaction involves the use of a large excess (about 10 equiv) of the coupling reagents. Furthermore, the harsh reaction conditions require the presence of the pyrophoric titanium trichloride, hampering widespread use in academic and industrial settings. In 2000, the group of Sammes reported a six-step synthesis that utilized a key Wittig–Horner cross-coupling reaction to obtain the enol-ether precursor.66 Implementation of this synthetic route by our group and others enabled the preparation of numerous adamantyl-phenoxy-dioxetane derivatives,67,68 although the route is relatively long and limited in scope. Indeed, the nucleophilic nature of the Wittig–Horner reaction precludes use of substrates with electrophilic functional groups or unprotected functional groups. Because of these limitations, a more robust and efficient synthesis should be developed to enable preparation of new dioxetane luminophores.
With an efficient synthesis in hand, numerous new dioxetane luminophores could be rapidly screened and considered. One important direction will involve the development of chemiluminescence luminophores with direct emission at various wavelengths to allow multiplexing, which is not currently feasible with chemiluminescence.69
The limitations of fluorescence for in vivo imaging result in large part from tissue autofluorescence caused by external illumination. To avoid autofluorescence, persistent luminescent nanoparticles are widely used; these nanoparticles are excited before injection and then glow inside the animal’s body for hours.70−72 With our recent demonstration that the kinetics of chemiexcitation can be controlled,48 we presume that luminophores with very slow chemiexcitation are within reach. Such “glowing” phenoxy-dioxetanes are expected to be less toxic and easier to functionalize compared to inorganic nanoparticles and thus should serve as attractive small-molecule alternatives to persistent luminescent nanoparticles.
In summary, we predict that the development of new small-molecule luminophores with direct emission mode will continue to play a major role in providing solutions to evolving problems and requirements in chemistry and biology. Further research will be required to optimize other important parameters of the dioxetanes for in vivo imaging, particularly their biodistribution stability under physiological conditions. Such optimization will probably involve the evaluation of phenoxy-dioxetanes with different scaffolds. For example, Matsumoto and co-workers have already shown that replacing the adamantyl group with other bulky moiety might increase the dioxetane thermal stability, without compromising chemiluminescence efficiency.73−75 Undoubtedly, the progress made to date in design and synthesis of phenoxy-dioxetanes luminophores has resulted in a bright outlook for application of these molecular probes for sensing and imaging.
Acknowledgments
The authors wish to thank Prof. Paul Schaap for providing personal communication and input on the historical perspective of the field.
The authors declare no competing financial interest.
References
- Shimomura O.Bioluminescence: Chemical Principles and Methods; World Scientific, 2006. [Google Scholar]
- Roda A.; Pasini P.; Mirasoli M.; Michelini E.; Guardigli M. Biotechnological Applications of Bioluminescence and Chemiluminescence. Trends Biotechnol. 2004, 22 (6), 295–303. 10.1016/S0167-7799(04)00085-X. [DOI] [PubMed] [Google Scholar]
- Transparency Market Research . Immunoassay Instruments Market; https://www.transparencymarketresearch.com/immunoassay-instruments-market.html (accessed April 11, 2019).
- Hananya N.; Shabat D. A Glowing Trajectory between Bio- and Chemiluminescence: From Luciferin-Based Probes to Triggerable Dioxetanes. Angew. Chem., Int. Ed. 2017, 56 (52), 16454–16463. 10.1002/anie.201706969. [DOI] [PubMed] [Google Scholar]
- Matsumoto M. Advanced Chemistry of Dioxetane-Based Chemiluminescent Substrates Originating from Bioluminescence. J. Photochem. Photobiol., C 2004, 5 (1), 27–53. 10.1016/j.jphotochemrev.2004.02.001. [DOI] [Google Scholar]
- Kopecky K. R.; Mumford C. Luminescence in the Thermal Decomposition of 3,3,4-Trimethyl-1,2-Dioxetane. Can. J. Chem. 1969, 47 (4), 709–711. 10.1139/v69-114. [DOI] [Google Scholar]
- Wynberg H.; Meijer E. W.; Hummelen J. C.. 1,2-Dioxetanes as Chemiluminescent Probes and Labels. In Bioluminescence and Chemiluminescence; Deluca M. A., McElroy W. D., Eds.; Academic Press, 1981; pp 687–689. [Google Scholar]
- Ando Y.; Niwa K.; Yamada N.; Enomoto T.; Irie T.; Kubota H.; Ohmiya Y.; Akiyama H. Firefly Bioluminescence Quantum Yield and Colour Change by PH-Sensitive Green Emission. Nat. Photonics 2008, 2, 44. 10.1038/nphoton.2007.251. [DOI] [Google Scholar]
- Adam W.; Bronstein I.; Edwards B.; Engel T.; Reinhardt D.; Schneider F. W.; Trofimov A. V.; Vasil’ev R. F. Electron Exchange Luminescence of Spiroadamantane-Substituted Dioxetanes Triggered by Alkaline Phosphatase. Kinetics and Elucidation of PH Effects. J. Am. Chem. Soc. 1996, 118 (43), 10400–10407. 10.1021/ja961904g. [DOI] [Google Scholar]
- Schaap A. P.; Akhavan H.; Romano L. J. Chemiluminescent Substrates for Alkaline Phosphatase: Application to Ultrasensitive Enzyme-Linked Immunoassays and DNA Probes. Clin. Chem. 1989, 35 (9), 1863–1864. [PubMed] [Google Scholar]
- Gnaim S.; Scomparin A.; Eldar-Boock A.; Bauer C. R.; Satchi-Fainaro R.; Shabat D. Light Emission Enhancement by Supramolecular Complexation of Chemiluminescence Probes Designed for Bioimaging. Chem. Sci. 2019, 10 (10), 2945–2955. 10.1039/C8SC05174G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green O.; Eilon T.; Hananya N.; Gutkin S.; Bauer C. R.; Shabat D. Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes. ACS Cent. Sci. 2017, 3 (4), 349–358. 10.1021/acscentsci.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelen J. C.; Luider T. M.; Wynberg H.. [39] Stable 1,2-Dioxetanes as Labels for Thermochemiluminescent Immunoassay. In Bioluminescence and Chemiluminescence Part B; Academic Press, 1986; Vol. 133, pp 531–557. [DOI] [PubMed] [Google Scholar]
- Schaap A. P.; Handley R. S.; Giri B. P. Chemical and Enzymatic Triggering of 1, 2-Dioxetanes. 1: Aryl Esterase-Catalyzed Chemiluminescence from a Naphthyl Acetate-Substituted Dioxetane. Tetrahedron Lett. 1987, 28 (9), 935–938. 10.1016/S0040-4039(00)95878-7. [DOI] [Google Scholar]
- Schaap A. P.; Chen T. S.; Handley R. S.; DeSilva R.; Giri B. P. Chemical and Enzymatic Triggering of 1,2-Dioxetanes. 2: Fluoride-Induced Chemiluminescence from Tert-Butyldimethylsilyloxy-Substituted Dioxetanes. Tetrahedron Lett. 1987, 28 (11), 1155–1158. 10.1016/S0040-4039(00)95313-9. [DOI] [Google Scholar]
- Schaap A. P.; Sandison M. D.; Handley R. S. Chemical and Enzymatic Triggering of 1,2-Dioxetanes. 3: Alkaline Phosphatase-Catalyzed Chemiluminescence from an Aryl Phosphate-Substituted Dioxetane. Tetrahedron Lett. 1987, 28 (11), 1159–1162. 10.1016/S0040-4039(00)95314-0. [DOI] [Google Scholar]
- Bronstein I.; Edwards B.; Voyta J. C. 1,2 Dioxetanes: Novel Chemiluminescent Enzyme Substrates. Applications to Immunoassays. J. Biolumin. Chemilumin. 1989, 4 (1), 99–111. 10.1002/bio.1170040116. [DOI] [PubMed] [Google Scholar]
- Bronstein I.; Edwards B.; Sparks A.. Improved Chemiluminescent 1,2-Dioxetanes. WO Patent 9426726, 1994.
- Bronstein I.; Edwards B.; Voyta J. C.. Improved Enhancement of Chemiluminescent Assays. WO Patent 9421821, 1994.
- Partearroyo M.; Ostolaza H.; Goñi F. M.; Barberá-Guillem E. Surfactant-Induced Cell Toxicity and Cell Lysis: A Study Using B16 Melanoma Cells. Biochem. Pharmacol. 1990, 40 (6), 1323–1328. 10.1016/0006-2952(90)90399-6. [DOI] [PubMed] [Google Scholar]
- Richard J. A.; Jean L.; Romieu A.; Massonneau M.; Noack-Fraissignes P.; Renard P. Y. Chemiluminescent Probe for the in Vitro Detection of Protease Activity. Org. Lett. 2007, 9 (23), 4853–4855. 10.1021/ol702190y. [DOI] [PubMed] [Google Scholar]
- Hananya N.; Eldar Boock A.; Bauer C. R.; Satchi-Fainaro R.; Shabat D. Remarkable Enhancement of Chemiluminescent Signal by Dioxetane–Fluorophore Conjugates: Turn-ON Chemiluminescence Probes with Color Modulation for Sensing and Imaging. J. Am. Chem. Soc. 2016, 138 (40), 13438–13446. 10.1021/jacs.6b09173. [DOI] [PubMed] [Google Scholar]
- Green O.; Gnaim S.; Blau R.; Eldar-Boock A.; Satchi-Fainaro R.; Shabat D. Near-Infrared Dioxetane Luminophores with Direct Chemiluminescence Emission Mode. J. Am. Chem. Soc. 2017, 139 (37), 13243–13248. 10.1021/jacs.7b08446. [DOI] [PubMed] [Google Scholar]
- Hananya N.; Reid J. P.; Green O.; Sigman M. S.; Shabat D. Rapid Chemiexcitation of Phenoxy-Dioxetane Luminophores Yields Ultrasensitive Chemiluminescence Assays. Chem. Sci. 2019, 10, 1380–1385. 10.1039/C8SC04280B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L. S.; Lippert A. R. Ultrasensitive Chemiluminescent Detection of Cathepsin B: Insights into the New Frontier of Chemiluminescent Imaging. Angew. Chem., Int. Ed. 2018, 57 (3), 622–624. 10.1002/anie.201711228. [DOI] [PubMed] [Google Scholar]
- Niko Y.; Didier P.; Mely Y.; Konishi G.; Klymchenko A. S. Bright and Photostable Push-Pull Pyrene Dye Visualizes Lipid Order Variation between Plasma and Intracellular Membranes. Sci. Rep. 2016, 6, 18870. 10.1038/srep18870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karton-Lifshin N.; Albertazzi L.; Bendikov M.; Baran P. S.; Shabat D. Donor–Two-Acceptor” Dye Design: A Distinct Gateway to NIR Fluorescence. J. Am. Chem. Soc. 2012, 134 (50), 20412–20420. 10.1021/ja308124q. [DOI] [PubMed] [Google Scholar]
- Eilon-Shaffer T.; Roth-Konforti M.; Eldar-Boock A.; Satchi-Fainaro R.; Shabat D. Ortho-Chlorination of Phenoxy 1,2-Dioxetane Yields Superior Chemiluminescent Probes for in Vitro and in Vivo Imaging. Org. Biomol. Chem. 2018, 16 (10), 1708–1712. 10.1039/C8OB00087E. [DOI] [PubMed] [Google Scholar]
- Roth-Konforti M. E.; Bauer C. R.; Shabat D. Unprecedented Sensitivity in a Probe for Monitoring Cathepsin B: Chemiluminescence Microscopy Cell-Imaging of a Natively Expressed Enzyme. Angew. Chem., Int. Ed. 2017, 56 (49), 15633–15638. 10.1002/anie.201709347. [DOI] [PubMed] [Google Scholar]
- Cao J.; An W.; Reeves A. G.; Lippert A. R. A Chemiluminescent Probe for Cellular Peroxynitrite Using a Self-Immolative Oxidative Decarbonylation Reaction. Chem. Sci. 2018, 9 (9), 2552–2558. 10.1039/C7SC05087A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An W.; Ryan L. S.; Reeves A. G.; Bruemmer K. J.; Mouhaffel L.; Gerberich J. L.; Winters A.; Mason R. P.; Lippert A. R. A Chemiluminescent Probe for HNO Quantification and Real-Time Monitoring in Living Cells. Angew. Chem., Int. Ed. 2019, 58 (5), 1361–1365. 10.1002/anie.201811257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S.; Won M.; Green O.; Hananya N.; Sharma A.; Jeon Y.; Kwak J. H.; Sessler J. L.; Shabat D.; Kim J. S. Chemiluminescent Probe for the In Vitro and In Vivo Imaging of Cancers Over-Expressing NQO1. Angew. Chem., Int. Ed. 2019, 58, 1739–1743. 10.1002/anie.201813032. [DOI] [PubMed] [Google Scholar]
- Sun J.; Hu Z.; Wang R.; Zhang S.; Zhang X. A Highly Sensitive Chemiluminescent Probe for Detecting Nitroreductase and Imaging in Living Animals. Anal. Chem. 2019, 91 (2), 1384–1390. 10.1021/acs.analchem.8b03955. [DOI] [PubMed] [Google Scholar]
- Sun J.; Hu Z.; Zhang S.; Zhang X. A Novel Chemiluminescent Probe Based on 1,2-Dioxetane Scaffold for Imaging Cysteine in Living Mice. ACS Sensors 2019, 4 (1), 87–92. 10.1021/acssensors.8b00936. [DOI] [PubMed] [Google Scholar]
- Hong G.; Antaris A. L.; Dai H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 0010. 10.1038/s41551-016-0010. [DOI] [Google Scholar]
- Biosynth . Aqua Spark; https://www.biosynth.com/en/technology/aquaspark-chemiluminescence/aquaspark-tm.html (accessed April 11, 2019).
- Weissleder R. A Clearer Vision for in Vivo Imaging. Nat. Biotechnol. 2001, 19, 316. 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- Wu X.; Sun X.; Guo Z.; Tang J.; Shen Y.; James T. D.; Tian H.; Zhu W. In Vivo and in Situ Tracking Cancer Chemotherapy by Highly Photostable NIR Fluorescent Theranostic Prodrug. J. Am. Chem. Soc. 2014, 136 (9), 3579–3588. 10.1021/ja412380j. [DOI] [PubMed] [Google Scholar]
- Gu K.; Xu Y.; Li H.; Guo Z.; Zhu S.; Zhu S.; Shi P.; James T. D.; Tian H.; Zhu W.-H. Real-Time Tracking and In Vivo Visualization of β-Galactosidase Activity in Colorectal Tumor with a Ratiometric Near-Infrared Fluorescent Probe. J. Am. Chem. Soc. 2016, 138 (16), 5334–5340. 10.1021/jacs.6b01705. [DOI] [PubMed] [Google Scholar]
- Edri R.; Gal I.; Noor N.; Harel T.; Fleischer S.; Adadi N.; Green O.; Shabat D.; Heller L.; Shapira A.; et al. Personalized Hydrogels for Engineering Diverse Fully Autologous Tissue Implants. Adv. Mater. 2019, 31 (1), 1803895. 10.1002/adma.201803895. [DOI] [PubMed] [Google Scholar]
- Bruemmer K. J.; Green O.; Su T. A.; Shabat D.; Chang C. J. Chemiluminescent Probes for Activity-Based Sensing of Formaldehyde Released from Folate Degradation in Living Mice. Angew. Chem., Int. Ed. 2018, 57 (25), 7508–7512. 10.1002/anie.201802143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruemmer K. J.; Walvoord R. R.; Brewer T. F.; Burgos-Barragan G.; Wit N.; Pontel L. B.; Patel K. J.; Chang C. J. Development of a General Aza-Cope Reaction Trigger Applied to Fluorescence Imaging of Formaldehyde in Living Cells. J. Am. Chem. Soc. 2017, 139 (15), 5338–5350. 10.1021/jacs.6b12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampinen J.; Raitio M.; Perälä A.; Kytöniemi V.; Harinen R.-R. Comparison of Flash and Glow ATP Assays with Thermo Scientific Varioskan Flash Luminometry. Application Note: AP-MIB-VARIO12-0108 - Thermo Scientific, 2008.
- Adam W.; Bronstein I.; Trofimov A. V.; Vasil’ev R. F. Solvent-Cage Effect (Viscosity Dependence) as a Diagnostic Probe for the Mechanism of the Intramolecular Chemically Initiated Electron-Exchange Luminescence (CIEEL) Triggered from a Spiroadamantyl-Substituted Dioxetane. J. Am. Chem. Soc. 1999, 121 (5), 958–961. 10.1021/ja982999k. [DOI] [Google Scholar]
- Adam W.; Trofimov A. V. The Effect of Meta versus Para Substitution on the Efficiency of Chemiexcitation in the Chemically Triggered Electron-Transfer-Initiated Decomposition of Spiroadamantyl Dioxetanes. J. Org. Chem. 2000, 65 (20), 6474–6478. 10.1021/jo000495a. [DOI] [PubMed] [Google Scholar]
- Yue L.; Liu Y.-J. Mechanism of AMPPD Chemiluminescence in a Different Voice. J. Chem. Theory Comput. 2013, 9 (5), 2300–2312. 10.1021/ct400206k. [DOI] [PubMed] [Google Scholar]
- Vacher M.; Fdez Galván I.; Ding B. W.; Schramm S.; Berraud-Pache R.; Naumov P.; Ferré N.; Liu Y. J.; Navizet I.; Roca-Sanjuán D.; Baader W. J.; Lindh R. Chemi- and Bioluminescence of Cyclic Peroxides. Chem. Rev. 2018, 118, 6927–6974. 10.1021/acs.chemrev.7b00649. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Leippe D.; Duellman S.; Sobol M.; Vidugiriene J.; O’Brien M.; Shultz J. W.; Kimball J. J.; DiBernardo C.; Moothart L.; et al. Self-Immolative Bioluminogenic Quinone Luciferins for NAD(P)H Assays and Reducing Capacity-Based Cell Viability Assays. ChemBioChem 2014, 15 (5), 670–675. 10.1002/cbic.201300744. [DOI] [PubMed] [Google Scholar]
- Hananya N.; Green O.; Blau R.; Satchi-Fainaro R.; Shabat D. A Highly Efficient Chemiluminescence Probe for the Detection of Singlet Oxygen in Living Cells. Angew. Chem., Int. Ed. 2017, 56 (39), 11793–11796. 10.1002/anie.201705803. [DOI] [PubMed] [Google Scholar]
- Bacellar O. I.; Tsubone M. T.; Pavani C.; Baptista S. M. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16 (9), 20523–20559. 10.3390/ijms160920523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straten D.; Mashayekhi V.; de Bruijn H.; Oliveira S.; Robinson D. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9 (2), 19. 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilby P. R. Singlet Oxygen: There Is Indeed Something New under the Sun. Chem. Soc. Rev. 2010, 39 (8), 3181–3209. 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- Giang I.; Boland E. L.; Poon G. M. K. Prodrug Applications for Targeted Cancer Therapy. AAPS J. 2014, 16 (5), 899–913. 10.1208/s12248-014-9638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstain R.; Segal E.; Satchi-Fainaro R.; Shabat D. Real-Time Monitoring of Drug Release. Chem. Commun. 2010, 46 (4), 553–555. 10.1039/B919329D. [DOI] [PubMed] [Google Scholar]
- Lee M. H.; Kim E.-J.; Lee H.; Kim H. M.; Chang M. J.; Park S. Y.; Hong K. S.; Kim J. S.; Sessler J. L. Liposomal Texaphyrin Theranostics for Metastatic Liver Cancer. J. Am. Chem. Soc. 2016, 138 (50), 16380–16387. 10.1021/jacs.6b09713. [DOI] [PubMed] [Google Scholar]
- Gnaim S.; Scomparin A.; Das S.; Blau R.; Satchi-Fainaro R.; Shabat D. Direct Real-Time Monitoring of Prodrug Activation by Chemiluminescence. Angew. Chem., Int. Ed. 2018, 57 (29), 9033–9037. 10.1002/anie.201804816. [DOI] [PubMed] [Google Scholar]
- Gnaim S.; Shabat D. Quinone-Methide Species, A Gateway to Functional Molecular Systems: From Self-Immolative Dendrimers to Long-Wavelength Fluorescent Dyes. Acc. Chem. Res. 2014, 47 (10), 2970–2984. 10.1021/ar500179y. [DOI] [PubMed] [Google Scholar]
- Gnaim S.; Shabat D. Self-Immolative Chemiluminescence Polymers: Innate Assimilation of Chemiexcitation in a Domino-like Depolymerization. J. Am. Chem. Soc. 2017, 139 (29), 10002–10008. 10.1021/jacs.7b04804. [DOI] [PubMed] [Google Scholar]
- Gnaim S.; Shabat D. Chemiluminescence Molecular Probe with Intrinsic Auto-Inductive Amplification: Incorporation of Chemiexcitation in a Quinone-Methide Elimination. Chem. Commun. 2018, 54 (21), 2655–2658. 10.1039/C8CC00521D. [DOI] [PubMed] [Google Scholar]
- Gnaim S.; Shabat D. Chemiluminescence Molecular Probe with a Linear Chain Reaction Amplification Mechanism. Org. Biomol. Chem. 2019, 17 (6), 1389–1394. 10.1039/C8OB03042A. [DOI] [PubMed] [Google Scholar]
- Cao J.; Lopez R.; Thacker J. M.; Moon J. Y.; Jiang C.; Morris S. N. S.; Bauer J. H.; Tao P.; Mason R. P.; Lippert A. R. Chemiluminescent Probes for Imaging H2S in Living Animals. Chem. Sci. 2015, 6 (3), 1979–1985. 10.1039/C4SC03516J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; Campbell J.; Liu L.; Mason R. P.; Lippert A. R. In Vivo Chemiluminescent Imaging Agents for Nitroreductase and Tissue Oxygenation. Anal. Chem. 2016, 88 (9), 4995–5002. 10.1021/acs.analchem.6b01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaim S.; Green O.; Shabat D. The Emergence of Aqueous Chemiluminescence: New Promising Class of Phenoxy 1,2-Dioxetane Luminophores. Chem. Commun. 2018, 54 (17), 2073–2085. 10.1039/C8CC00428E. [DOI] [PubMed] [Google Scholar]
- Schaap A. P.; Akhavan-Tafti H.. Chemiluminescenct Dialkyl-Substituted 1,2-Dioxetane Compounds, Methods of Synthesis and Use. WO Patent 9616137, 1996.
- Sabelle S.; Hydrio J.; Leclerc E.; Mioskowski C.; Renard P.-Y. McMurry Intermolecular Cross-Coupling between an Ester and a Ketone: Scope and Limitations. Tetrahedron Lett. 2002, 43 (20), 3645–3648. 10.1016/S0040-4039(02)00617-2. [DOI] [Google Scholar]
- Roeschlaub C. A.; Sammes P. G. Use of the Wadsworth–Emmons Reaction for Preparing Hindered Vinyl Ethers and Related 1,2-Dioxetanes. J. Chem. Soc., Perkin Trans. 2000, 1 (14), 2243–2248. 10.1039/b002101f. [DOI] [Google Scholar]
- Turan I. S.; Akkaya E. U. Chemiluminescence Sensing of Fluoride Ions Using a Self-Immolative Amplifier. Org. Lett. 2014, 16 (6), 1680–1683. 10.1021/ol5003412. [DOI] [PubMed] [Google Scholar]
- Turan I. S.; Seven O.; Ayan S.; Akkaya E. U. Amplified Chemiluminescence Signal for Sensing Fluoride Ions. ACS Omega 2017, 2 (7), 3291–3295. 10.1021/acsomega.7b00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Frei M. S.; Salim A.; Johnsson K. Small-Molecule Fluorescent Probes for Live-Cell Super-Resolution Microscopy. J. Am. Chem. Soc. 2019, 141 (7), 2770–2781. 10.1021/jacs.8b11134. [DOI] [PubMed] [Google Scholar]
- le Masne de Chermont Q.; Chanéac C.; Seguin J.; Pellé F.; Maîtrejean S.; Jolivet J.-P.; Gourier D.; Bessodes M.; Scherman D. Nanoprobes with Near-Infrared Persistent Luminescence for in Vivo Imaging. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (22), 9266–9271. 10.1073/pnas.0702427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q.; Xie C.; Zhen X.; Lyu Y.; Duan H.; Liu X.; Jokerst J. V.; Pu K. Molecular Afterglow Imaging with Bright, Biodegradable Polymer Nanoparticles. Nat. Biotechnol. 2017, 35, 1102. 10.1038/nbt.3987. [DOI] [PubMed] [Google Scholar]
- Ni X.; Zhang X.; Duan X.; Zheng H.-L.; Xue X.-S.; Ding D. Near-Infrared Afterglow Luminescent Aggregation-Induced Emission Dots with Ultrahigh Tumor-to-Liver Signal Ratio for Promoted Image-Guided Cancer Surgery. Nano Lett. 2019, 19 (1), 318–330. 10.1021/acs.nanolett.8b03936. [DOI] [PubMed] [Google Scholar]
- Matsumoto M.; Watanabe N.; Kasuga N. C.; Hamada F.; Tadokoro K. Synthesis of 5-Alkyl-1-Aryl-4,4-Dimethyl-2,6,7-Trioxabicyclo[3.2.0]Heptanes as a Chemiluminescent Substrate with Remarkable Thermal Stability. Tetrahedron Lett. 1997, 38 (16), 2863–2866. 10.1016/S0040-4039(97)00483-8. [DOI] [Google Scholar]
- Matsumoto M.; Sakuma T.; Watanabe N. Synthesis of Bicyclic Dioxetanes Bearing a 3-Hydroxy-4-Isoxazolylphenyl Moiety: New CIEEL-Active Dioxetanes Emitting Light with Remarkable High-Efficiency in Aqueous Medium. Tetrahedron Lett. 2002, 43 (49), 8955–8958. 10.1016/S0040-4039(02)02162-7. [DOI] [Google Scholar]
- Tanimura M.; Watanabe N.; Ijuin H. K.; Matsumoto M. Thermodynamic Aspects of Thermal Decomposition and Charge-Transfer-Induced Chemiluminescent Decomposition for Bicyclic Dioxetanes Bearing a 4-(Benzothiazol-2-Yl)-3-Hydroxyphenyl Moiety. J. Org. Chem. 2010, 75 (11), 3678–3684. 10.1021/jo100449m. [DOI] [PubMed] [Google Scholar]