Abstract
In this work, lemon and onion biomasses commonly found in street markets are for the first time used to develop a facile, fast and low-cost one-step microwave-assisted carbonization method for synthesis of highly fluorescent carbon dots (CDs). The structure and optical properties of CDs were investigated by TEM, XRD, XRF, UV–Vis, FTIR, and fluorescence spectroscopy. CDs displayed satisfactory optical proprieties, a high quantum yield of 23.6%, and excellent water solubility, and the particle size was 4.23–8.22 nm with an average diameter of 6.15 nm. An efficient fluorescent resonance energy transfer (FRET) between the CDs and riboflavin was achieved with CDs acting as donor and riboflavin as acceptor. A linear relationship between FRET and the riboflavin concentration from 0.10 to 3.0 μg/mL was observed, allowing the development of an accurate and fast analytical method to determine this vitamin in multivitamin/mineral supplements. Despite the potential interferences in these supplements, CDs were selective for riboflavin under optimized conditions. A paired t-test at a 95% confidence level indicated no statistically significant difference between the proposed and the reference methods. Recovery test presented values ranged from 96.0% to 101.4%. The limit of detection and relative standard deviation were estimated at 1.0 ng/mL and <2.6% (n = 3), respectively. CDs were successfully synthesized in a domestic microwave oven (1450 W, 6 min), presenting satisfactory parameters when compared with results of other studies reported in the literature, suggesting that the proposed method is a potentially useful method for the synthesis of CDs and determination of riboflavin.
Keywords: Carbon dots, Microwave-assisted carbonization, Lemon-onion juice, Riboflavin, Multivitamin/mineral supplements, Fluorescence
Graphical abstract
Highlights
-
•
Lemon and onion were for the first time used to synthesize carbon dots (CDs).
-
•
CDs displayed satisfactory optical proprieties and high quantum yield of 23.6%.
-
•
A fluorescent resonance energy transfer between CDs and riboflavin was achieved.
-
•
A method was successfully developed for riboflavin quantification in supplements.
-
•
Proposed method provided improved results over previous studies.
1. Introduction
There is a promising interest in technological research involving the replacement of photoluminescent organic dyes by semiconductor or carbon nanomaterials [1]. These studies aim at the development of innovative analytical technologies, based on their optical properties with applications to several areas of knowledge, such as biology, physics, and chemistry. Fluorescent carbon dots (CDs) are promising for this purpose, when compared to their direct competitors, semiconductor quantum dots (QDs) [2,3]. CDs have already been recognized as being less cytotoxic, highly biocompatible, as well as presenting less complex aqueous synthesis routes and a high fluorescence quantum yield (QY) [1,[4], [5], [6], [7], [8], [9]].
CDs doped with heteroatoms, especially N and S, provide an efficient method to enhance their fluorescent properties including high QY, and to tune their electronic and chemical properties [7,[10], [11], [12], [13], [14], [15], [16], [17]]. For instance, Wang et al. [14] employed citric acid and cysteine to fabricate CDs with a quantum yield of 42.7% using a hydrothermal treatment. Duan et al. [7] used ethylenediamine as the carbon source and sulfamic acid as the surface passivation agent to prepare CDs with a QY of 28%. As another example, Chen et al. [17] employed hydrothermal treatment of garlic to prepare CDs with a QY of 13%, suggesting this could be an alternative for sources of carbon and surface passivation agents with heteroatoms.
Nowadays, many efforts have been made on the development of new strategies for synthesis and doping of CDs. Different physical and chemical methods, such as hydrothermal treatment, laser ablation, electrochemical oxidation and microwave treatment, have been presented in the literature [[4], [5], [6], [7]]. In general, the hydrothermal approach has been employed in many studies for CDs synthesis using various natural resources, such as leaves, fruits, grains, seeds, and even beverages such as beer, coffee, milk, and tea [1,8,9].
Other innovative research has been on the facile and rapid microwave treatment approach for the preparation of CDs from different precursors, such as hydrocarbons, carboxylic acids, esters, and biomass residues [1,7,8]. Microwave-assisted synthesis provides benefits such as simplicity, low-cost and a short reaction time thus avoiding traditional harsh conditions such as strong acids, high temperatures, and long reaction times [1].
Riboflavin, commonly known as vitamin B2, is one of the water-soluble vitamins that play a crucial role in functioning healthy humans. It is also required for a wide variety of cellular processes, such as metabolism of fats, ketone bodies, carbohydrates, proteins, nucleic acid repair process, cell apoptosis and electron transfer processes in the respiratory chain [14,18]. Although riboflavin is found in many foods, such as milk and dairy products, meat, eggs, livers, cereals and fresh leafy vegetables [18], diets lacking this vitamin cause lesions of the muco-cutaneous surfaces, intense photophobia, fatigue, slowed growth, digestive problems, angular cheilitis and anemia [14,18]. Regular daily intake of vitamin B2 is important because it is not synthesized and stored in human body in appreciable amounts [19]. During pregnancy, lactation, stress and heavy exercise, and particularly for elderly people, alcoholics and those with absorption difficulties, an increase in recommended daily intake of riboflavin (1.1 mg for men and 1.3 mg for women) is suggested [19].
If a balanced diet by foods does not provide sufficient amounts of vitamin B2, commercial multivitamin/mineral supplements containing this vitamin may be alternative. However, riboflavin is usually a part of the multinutrient formulation and an accurate and fast analytical method for determination of riboflavin in these samples is of crucial importance for assessment of compliance with the recommendations for daily riboflavin intake.
Conventional methods used for the riboflavin determination in drugs, foods, and supplements, such as electrochemistry [18], spectrophotometry [14,20], immunoassay [21], capillary electrophoresis [22] and high-performance liquid chromatography [23,24], require costly solvents, high-cost equipment, and complicated or time-consuming sample preparations [20]. In contrast, the fluorescent method is favored by many, given the method's advantages including on-site testing, faster response, greater simplicity, lower cost and higher sensitivity [14,20].
In the present study, lemon and onion juices were used for the first time to develop a simple, rapid, low-cost and one-step microwave-assisted carbonization method for synthesis of highly fluorescent CDs in a domestic microwave oven (1450 W) without further surface passivation or modification. Lemon (Citrus limon) was chosen because it is a rich source of citric (6% m/m) and L-ascorbic acids (64%, m/m) [25] and both acids have been used as carbon precursors in the preparation of carbon dots [15,26]; onion is an important source of sulfur compounds [27,28]; and ammonium hydroxide has been used as an N-dopant agent in the synthesis of CDs [15]. Transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray fluorescent (XRF), UV–Vis, Fourier-transform infrared (FTIR), and fluorescence spectroscopy were used to evaluate the structure and optical properties of the CDs. After studying their optical properties, the detection capabilities were further exploited in order to develop an analytical method for the determination of riboflavin in commercial multivitamin/mineral supplements.
2. Experimental
2.1. Chemicals and materials
All reagents were of analytical grade and freshly distilled and deionized water (18.2 MΩ cm) was used to prepare all solutions.
For the synthesis of the fluorescent CDs, the onion and lemon were purchased from a local supermarket in João Pessoa, Paraíba, Brazil and ammonium hydroxide was purchased from Sigma-Aldrich, Brazil.
The quinine sulfate purchased from Acros, USA was used as a standard for QY measurements.
A riboflavin stock solution (100.0 mg/L) was prepared daily by dissolution of solid standard (Sigma-Aldrich, Brazil) in deionized water. The standard solutions of riboflavin (0.10–3.0 μg/mL) used to build the analytical curve were prepared in deionized water from adequate dilutions of stock solution.
A pH 6.9 phosphate buffer solution composed of 0.10 mol/L potassium dihydrogen phosphate heptahydrate (Synth, Brazil) and 0.10 mol/L sodium hydroxide (Synth, Brazil) was used to build the analytical curve and analysis of samples.
For study of potentially interfering substances in the multivitamin/mineral supplements, chlorides of Zn2+ and Fe3+, sulfate of Cu2+, Fe2+, and Mg2+, nitrates of Ca2+, Na+, and K+, ascorbic acid, citric acid, sucrose, d-glucose, fructose, cyanocobalamin, biotin, niacin, thiamin, folic acid, pantothenic acid, and sodium hydroxide were used, purchased from Sigma-Aldrich, Brazil.
2.2. Apparatus
The fluorescence measurements were carried out in a Cary Eclipse fluorescence spectrophotometer (Agilent, model G9800A, USA) equipped with a 1.0 cm × 0.5 cm quartz cell, and with slit widths set at 10/10 nm. The absorption spectra were obtained on a UV–Vis spectrophotometer (Hewlett-Packard, model 8453, USA). FTIR spectra were recorded by FTIR spectrophotometer (Shimadzu, model IR Prestige-21, Japan). XRD patterns were investigated using powdered samples by X-ray diffraction (Shimadzu, model XRD 6000, Japan). Chemical composition analysis of CDs was performed using an XRF spectrometer (Shimadzu, XRF-1800, Japan). Morphologies and sizes were characterized using JEM-2100 TEM (JEOL, Japan), operating at 200 kV. For the TEM measurement, the sample was prepared by dropping diluted CDs solution on a carbon-coated copper grid.
The synthesis of the CDs was performed in a domestic microwave oven (Brastemp, model BMS45BBBNA 1450 W 2460 MHz, Brazil). Separation of the solid phase produced in the synthesis was obtained by centrifugation (HERMLE, model Z206A, Germany).
2.3. Synthesis of highly fluorescent CDs
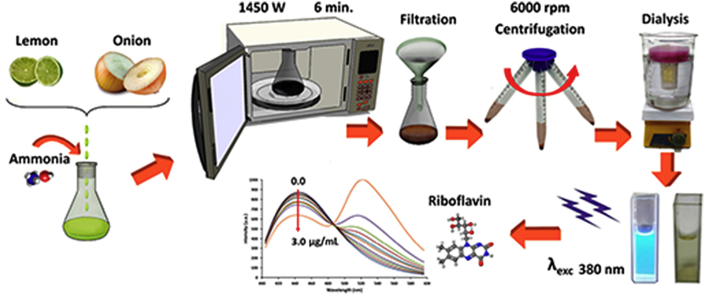
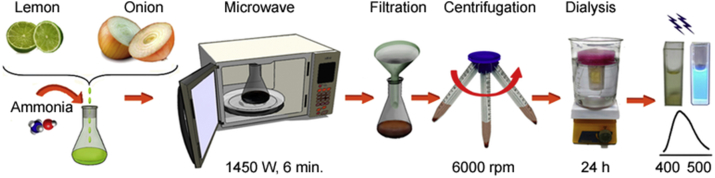
The fluorescent CDs were synthesized by a facile, rapid and one-step microwave-assisted carbonization method (Fig. 1).
Fig. 1.
Scheme of the CDs synthesis from lemon and onion juices.
In a typical synthesis, 20.0 mL of pulp-free lemon juice and 2.0 mL of pulp-free onion juice were mixed with 8.0 mL of deionized water and 10.0 mL of 25% (v/v) ammonium hydroxide solution. The mixture was transferred into a 250 mL erlenmeyer flask and heated using a domestic microwave oven (power = 1450 W) for 6 min. After cooling to room temperature, the brownish black solid was dispersed in 100 mL deionized water. Then, the mixture was centrifuged at 6000 rpm for 30 min and dialyzed against deionized water through a dialysis tubing (MWCO = 1 kDa) for 24 h to remove all un-reacted molecules. CDs powder was obtained by evaporation, re-dispersed in deionized water and stored at 4 °C for further use.
2.4. QY of CDs
The measurement of the QY of CDs was achieved by linearizing integrated fluorescence intensities (excitation at 380 nm) of CDs and of the fluorescence reference standard versus their corresponding absorbance [11]. In order to minimize the inner filter effects, absorbencies were kept below 0.05 at the excitation wavelength [29,30]. QY was then calculated by QY = QYr (Grad/Gradr) (η/ηr)2, where Grad is the slope of the curve, η is the refractive index of the solvent (1.33 for water), and subscript “r” refers to the fluorescence reference standard quinine sulfate in 0.1 mol/L H2SO4 (QYr = 0.54) [11].
2.5. Sample preparation
Nine commercially-available samples from three different brands of multivitamin/mineral supplements were purchased from local retail suppliers in João Pessoa, Paraíba, Brazil. These samples were prepared for analysis according to the following procedure described elsewhere [18,23]: five tablets of each sample were weighed and powdered in a grinding mortar, and were dissolved in 50 mL of deionized water and sonicated for 15 min. After, the sample solution was filtered to remove undissolved residue, and diluted with deionized water to the required concentration of riboflavin.
2.6. Fluorescence determination of riboflavin
For determination of riboflavin, CDs were dispersed in deionized water in a concentration of 657.3 mg/L. For the typical assay, 300 μL of CDs dispersion, and 250 μL of pH 6.9 phosphate buffer solution were added to a 2mL tube. After that, riboflavin standard solutions or sample were added, and the volume in the tube was topped up to 2.0 mL with deionized water. The concentration of CDs in the mixture was kept at 98.6 mg/L and the concentration of riboflavin varied from 0.10 to 3.0 μg/mL. After incubation for 1 min at room temperature, the fluorescence intensity was recorded at 440 nm with an excitation wavelength of 380 nm.
2.7. Reference method
The reference method for determination of riboflavin in multivitamin/mineral supplements was carried out according to AOAC method using high-performance liquid chromatography with diode-array detection [23].
3. Results and discussion
3.1. Optimization of the CDs synthesis
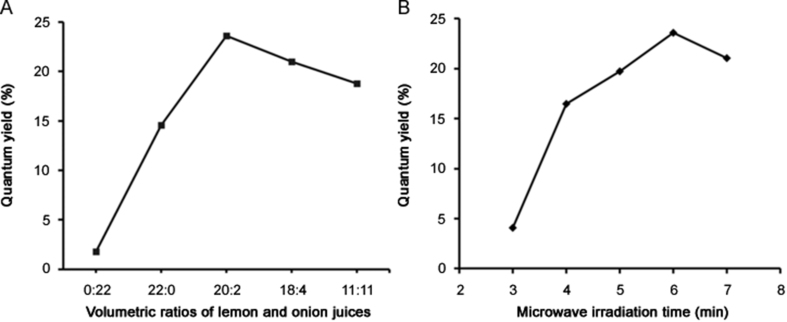
Initially, the volumetric ratio of lemon and onion juices was investigated by fixing the volumes of 25% (v/v) ammonium hydroxide solution in 10 mL and of deionized water in 8 mL. In all synthesis experiments, the total volume of the mixture, microwave irradiation time and power were also fixed at 40 mL, 6 min and 1450 W, respectively. As shown in Fig. 2A, the highest QY value of the synthesized CDs was obtained using a volumetric ratio 20:2, i.e., 20 mL of lemon juice, 2 mL of onion juice, and 10 mL of ammonium hydroxide solution. This QY (23.6%) is quite satisfactory when compared to other studies in the literature that used biomass for the synthesis of CDs [15]. In addition, this volumetric ratio was used to investigate microwave radiation time, keeping the same radiation power and volume of ammonium hydroxide solution. As can be seen in Fig. 2B, 6 min was the optimum time for CDs synthesis.
Fig. 2.
Quantum yield as a function of (A) volumetric ratios of lemon and onion juices and (B) microwave radiation time.
3.2. Characterization of CDs
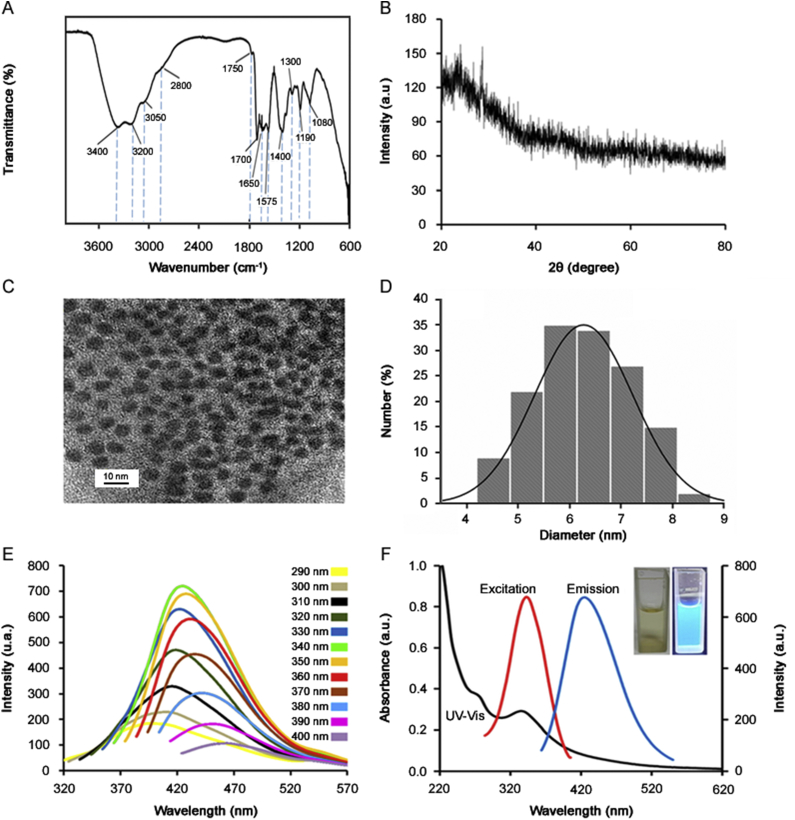
The surface functional groups of CDs obtained from different precursors were identified by FTIR spectroscopy. As shown in Fig. 3A, the peaks at 3400, 3200 and 3050 cm−1 were attributed to stretching vibrations of O–H, N–H, and C–H, respectively [31]. The peaks in the region of 1575–1700 cm−1 represented stretching vibration of C O, C N, and bending vibration of N–H [16]. Besides, the peaks in the range of 1390–1490 cm−1 can be ascribed to N–H, C–N, and COO− groups [13]. In addition, the peaks at about 1300, 1190 and 1080 cm−1 can be ascribed to the stretching vibration of C S, C–S and –SO3−, suggesting the existence of sulfonic groups [12,13,32]. The FTIR spectra demonstrate the successful incorporation of the plentiful amino group, hydroxyl, and sulfite on the surfaces of CDs, conferring high polarity and water solubility of the CDs [12].
Fig. 3.
(A) FTIR spectrum, (B) XRD pattern, (C) TEM image and (D) the particle size distribution of CDs. (E) Emission spectra of the CDs solution (32 mg/L) in pH 6.9 phosphate buffer recorded at different excitation wavelengths, from 290 to 400 nm with 10 nm increments. (F) UV–Vis absorption, excitation and emission spectra and photographs (inset) of the same CDs solution.
The XRD pattern of CDs (Fig. 3B) shows a low-intensity reflection centered at 20°, which corresponds to amorphous carbon phase in a considerably random fashion. The morphology and size of prepared CDs were observed by TEM. The TEM image (Fig. 3C) shows that CDs are nearly spherical and exhibit uniform dispersion. Fig. 3D shows that CDs presented a size range of 4.23–8.22 nm with an average diameter of 6.15 nm.
The optical properties of the synthesized CDs solution (32 mg/L) in pH 6.9 phosphate buffer are presented in Figs. 3E and F. CDs solution exhibited excitation-dependent emission behavior (Fig. 3E) similar to that reported in previous literature [15,20], which may be associated with the surface states affecting the band gap. As the excitation wavelength increased from 290 to 400 nm (with 10 nm increment), the emission gradually shifted to higher wavelengths and the most intense emission could be observed upon excitation at 340 nm.
The UV–Vis absorption spectrum (Fig. 3F) exhibits a shoulder at 280 nm which could be attributed to the π–π* transition of the aromatic sp2 domains, and absorption broad peak at 340 nm which could be ascribed to the n−π* transition of the C O bond [10,33]. The fluorescence spectra show that CDs have excitation and emission peaks at 340 nm (red line in Fig. 3F) and at 425 nm (blue line in Fig. 3F) and exhibit a yellow color in daylight and a bright-blue color under UV light at 365 nm (see inset, Fig. 3F).
The chemical composition of CDs was determined by the Kjeldahl method for N [34] and by XRF analysis for the other elements. As can be seen in Table 1, C, N, O, and S were found as major elements, while others were in minority amounts. Similar chemical composition has been usually found on CDs as discussed elsewhere [16].
Table 1.
Chemical composition of CDs determined by the Kjeldahl method for N and by XRF analysis for the other elements.
| Element | Weight (%) |
|---|---|
| C | 85.22 |
| N | 8.83 |
| O | 4.80 |
| S | 0.33 |
| Others (Cl, K, Si, P, Ca and Fe) | 0.82 |
3.3. CDs/riboflavin FRET sensing system
In order to demonstrate the feasibility of CDs/riboflavin FRET (fluorescent resonance energy transfer) sensing system for the detection of riboflavin, the fluorescence and absorption spectra of CDs, riboflavin and CDs/riboflavin were investigated.
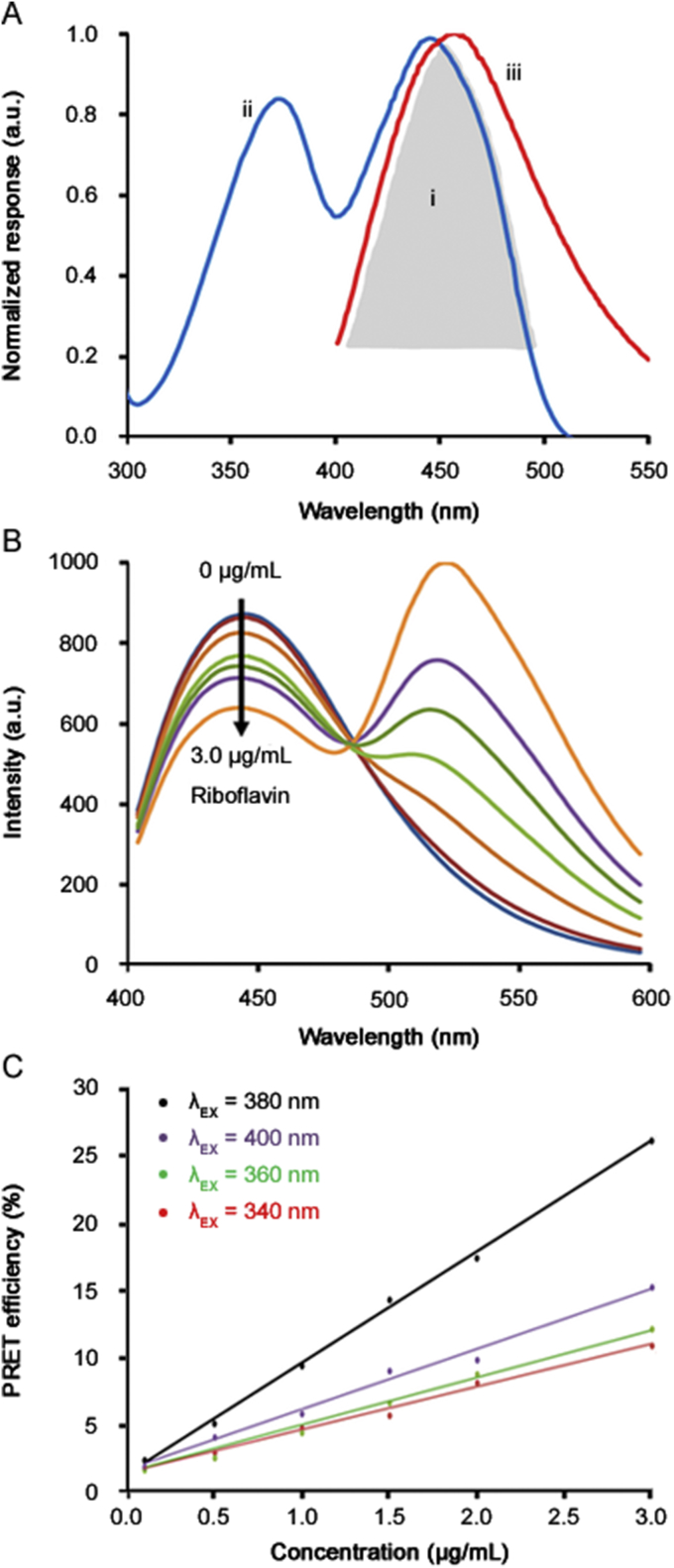
Fig. 4A shows an evident integral overlap region (Fig. 4Ai), between the absorption spectrum of riboflavin (Fig. 4Aii) and the emission spectrum of CDs excited at 380 nm (Fig. 4Aiii), indicating the possibility of a FRET process from the donor (CDs) and the acceptor (riboflavin). The presence of >C O and –NH groups in riboflavin has a strong attraction to hydroxyl and carboxyl groups on the surface of CDs to form hydrogen bonds and to decrease the spatial distance between CDs and riboflavin that successfully assists the CDs/riboflavin to act as an effective FRET pair [14,20].
Fig. 4.
(A) The integral overlap region (i), the riboflavin absorption (ii) and the CDs emission (iii) spectra. (B) Fluorescence spectra of CDs in the presence of different amounts of riboflavin, λex = 380 nm. (C) The efficiency of the FRET process as a function of the riboflavin concentration with the excitation wavelength of the CDs/riboflavin varying from 340 to 400 nm.
To evaluate better the FRET quenching, the interaction distance between CDs and riboflavin was calculated using the Förster distance described elsewhere [20,34]. The Förster distance between CDs (donor) – riboflavin (acceptor) pair was estimated at 5.89 nm, which is much below the maximum distance (∼10 nm) between a donor and an acceptor required for the FRET process indicating efficient energy transfer [35].
The emission intensity of CDs at 440 nm gradually decreases with enhancing of the riboflavin concentration, whereas the emission intensity of riboflavin at 520 nm increases, as presented in Fig. 4B. It is to be observed that the emission peak at 520 nm is due to riboflavin, which increases because of the increased concentration of the chromophore [35]. Given this, the capability of this system to detect riboflavin was then evaluated using the efficiency of the FRET process calculated by EFRET = [(F0–F)/F0] × 100 (where F and F0 represent the fluorescence intensity at 440 nm of CDs in presence and absence of the riboflavin, respectively) [13].
A gradual increase of EFRET from CDs with the addition of riboflavin can be observed in Fig. 4C, similar to other studies in the literature [14,20,[35], [36], [37]]. Although the optimal excitation for CDs is 340 nm (Fig. 3E), the excitation wavelength of the CDs/riboflavin varied from 340 to 400 nm (with increments of 20 nm) in order to find the highest sensitivity of the relationship between FRET efficiency and riboflavin concentration. As can be seen in Fig. 4C, the highest sensitivity was obtained using the excitation wavelength of 380 nm. Therefore, CDs synthetized from lemon and onion juices can successfully be used for the riboflavin determination.
3.4. Analytical application
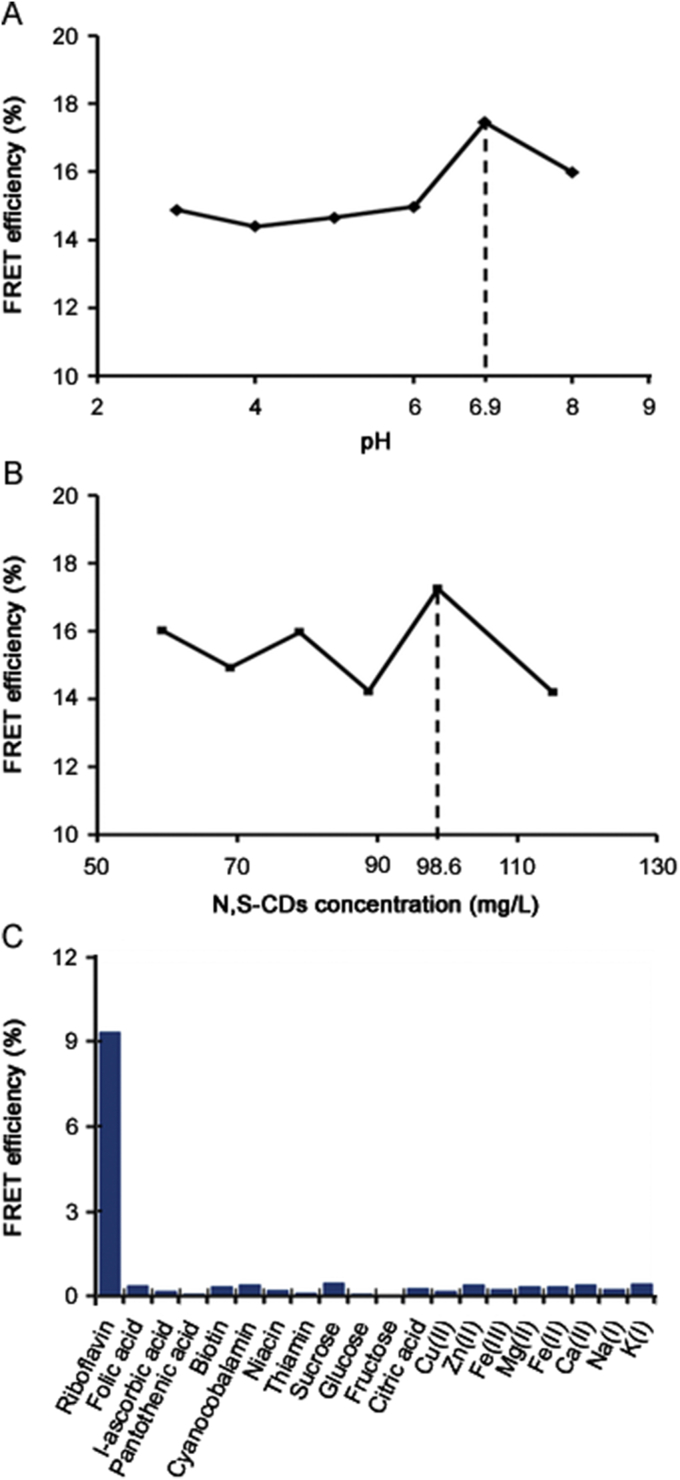
The usefulness of CDs from lemon and onion juices was investigated in the development of an analytical method to determine riboflavin in multivitamin/mineral supplements. Initially, the influence of the pH and CDs concentration on the FRET efficiency was studied by fixing the concentration of riboflavin at 2.0 μg/mL. For this purpose, pH and CDs concentration used ranged from 3.0 to 8.0 (Fig. 5A) and 59.2–115.0 mg/L (Fig. 5B). The highest FRET values were obtained using a pH 6.9 and 98.6 mg/L of CDs.
Fig. 5.
FRET efficiency as a function of (A) pH, (B) concentration of CDs, and (C) potential interfering substances (100 μg/mL).
The selectivity of the proposed method using CDs for the determination of riboflavin in multivitamin/mineral supplements was also investigated. For this purpose, potential interfering substances [18], including folic, citric, L-ascorbic and pantothenic acid, biotin, cyanocobalamin, niacin, thiamin, sucrose, d-glucose, fructose, Cu2+, Zn2+, Fe2+, Fe3+, Mg2+, Ca2+, Na+, and K+ in a ratio interferents/riboflavin 100:1.0 μg/mL were analyzed. A compound was considered “non-interfering” if the change of fluorescence intensity was ±5% as compared to that obtained in its absence [18]. As presented in Fig. 5C, the interferents studied did not show a significant change in the analytical response, indicating that the proposed method was selective under the selected experimental conditions.
An analytical curve was then built in the riboflavin concentration ranging from 0.10 to 3.0 μg/mL. An analysis of variance (ANOVA) was performed according to recommendations described elsewhere [38] to evaluate the fit for a linear model of the analytical curve. For this purpose, three authentic replicate measurements were made at each concentration level of the analytical curve. The estimated mean squares (MS) ratios of MSlack of fit/MSpure error and MSregression/MSresidual were respectively lower and higher than the F-distribution point at the 95% confidence level and the corresponding degrees of freedom. In addition, a random distribution of the residuals around zero and no heteroscedasticity were observed in the analysis of the residual plot. Therefore, no lack of fit evidence for the linear model was observed, and the linear regression was significant. Similar results were also obtained in the ANOVA of the analytical curve of reference method.
The analytical curves were then used to determine the concentration of riboflavin in nine commercial samples from three different brands of multivitamin/mineral supplements, using both the proposed and reference methods [23]. As shown in Table 2, no statistically significant differences were observed between the results at a confidence level of 95% when applying the paired t-test. The relative standard deviation (RSD %) was less than 2.6% (n = 3). The values found for correlation coefficient, limits of detection (LOD), limit of quantification (LOQ) and sensitivity of the proposed method were 0.9979, 1.0 ng/mL, 0.003 μg/mL, and 8.3279, respectively.
Table 2.
Results for determination of riboflavin concentration in multivitamin/mineral supplements.
| Sample | Proposed method (μg/mL ± SD) | Reference method (μg/mL ± SD) |
|---|---|---|
| 1 | 0.567 ± 0.003 | 0.559 ± 0.017 |
| 2 | 0.565 ± 0.015 | 0.558 ± 0.014 |
| 3 | 0.364 ± 0.009 | 0.322 ± 0.003 |
| 4 | 1.112 ± 0.019 | 1.041 ± 0.012 |
| 5 | 0.901 ± 0.017 | 0.994 ± 0.009 |
| 6 | 0.839 ± 0.010 | 0.927 ± 0.016 |
| 7 | 0.414 ± 0.007 | 0.412 ± 0.015 |
| 8 | 0.459 ± 0.004 | 0.447 ± 0.007 |
| 9 | 0.443 ± 0.003 | 0.445 ± 0.005 |
| Overall RSD (%) | 1.5 | 1.8 |
SD: standard deviation of three replicates.
RSD: relative standard deviation.
Besides the comparison with the reference method, the use of a recovery test is another means of assessing the accuracy of the proposed method. Thus, the recoveries were investigated using three different multivitamin/mineral supplements, which were spiked at three different levels. Satisfactory recovery values were obtained for three analyzed samples as can observed in Table 3.
Table 3.
Recoveries for determination of riboflavin concentration in multivitamin/mineral supplements.
| Spiked values (μg/mL) | Recovery (% ± SD) |
||
|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | |
| 0.25 | 98.2 ± 1.1 | 99.1 ± 0.2 | 101.4 ± 1.1 |
| 0.50 | 98.3 ± 0.9 | 99.3 ± 0.2 | 96.0 ± 0.4 |
| 1.0 | 100.0 ± 0.5 | 96.3 ± 1.3 | 98.8 ± 1.1 |
| Overall RSD (%) | 0.8 | 0.6 | 0.9 |
SD: standard deviation of three replicates.
RSD: relative standard deviation.
In general, the proposed method presents satisfactory parameters when compared to other reported methods which use CDs for riboflavin quantification [14,20,36,37], as shown in Table 4. The CDs were synthetized using very simple biomasses (lemon and onion juices) and a facile, fast and low-cost one-step microwave-assisted carbonization method in a domestic microwave oven.
Table 4.
Parameters of the proposed and previous carbon dots-based methods for determination of riboflavin.
| Precursor | Synthesis method | Type of modification | Quantum yield (%) | Synthesis conditions | Working range (μg/mL) | LOD (ng/mL) | Ref. |
|---|---|---|---|---|---|---|---|
| Citric acid; l-cysteine | Hydrothermal | N and S | 42.7 | Heated at 200 °C for 3 h | 0.211–2.80 | 0.7 | [14] |
| Poly (vinyl alcohol) | NaOH assisted oxidation | – | 8.5 | Refluxed in a pre-heated oil bath at 120 °C for 5 h | 0.048–0.903 | 0.44 | [20] |
| Urea and sodium citrate | Hydrothermal | N | – | Heated at 180 °C for 1 h | 0.017–1.121 | 0.33 | [36] |
| Sugar beet molasses | Homogenization | – | – | Dissolved in water and mixed until a homogeneous solution | 4.8–37.6 | – | [37] |
| Ammonium hydroxide and lemon and onion juices | Microwave | N and S | 23.6 | Microwave irradiation time for 6 min | 0.10–3.0 | 1.0 | This work |
4. Conclusion
The present study presents for the first time the use of lemon and onion biomasses easily found in public markets to develop a simple, economic and fast method for synthesis of CDs. The CDs were successfully synthesized by a one-step carbonization method using a domestic microwave oven, and characterized by TEM, XRD, UV–Vis, FTIR, and fluorescence spectroscopy. CDs exhibited excelent water solubility, high QY of 23.6% and a good linear relationship between FRET and the riboflavin concentration from 0.10 to 3.0 μg/mL. Thus, an analytical method was successfully developed to determine this vitamin in commercial multivitamin/mineral supplements. Therefore, the proposed methods for synthesis of CDs and riboflavin quantification presented satisfactory parameters when compared with other reported methods [14,20,36,37], indicating that it may be used for the determination of this vitamin.
Acknowledgments
The authors would like to thank the Brazilian agencies (CNPq and CAPES) for research fellowships and scholarships. The authors are grateful to Dr. Licarion Pinto for his help in the reference method. The English text of this paper has been revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip - TESL (Cambridge University).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Sun X., Lei Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017;89:163–180. [Google Scholar]
- 2.Lima M.B., Andrade S.I.E., Barreto I.S., et al. Automatic flow-batch approach using CdTe quantum dots for fluorescent determination of ascorbic acid in fruit juices. Food Anal. Methods. 2014;7:1598–1603. [Google Scholar]
- 3.Lima M.B., Andrade S.I.E., Barreto I.S., et al. In-line single-phase extraction for direct determination of total iron in oils using CdTe quantum dots and a flow-batch system. Anal. Methods. 2015;7:7707–7714. [Google Scholar]
- 4.Ding H., Yu S.B., Wei J.S., et al. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano. 2016;10:484–491. doi: 10.1021/acsnano.5b05406. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y.P., Zhou B., Lin Y., et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006;128:7756–7757. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- 6.Li H., He X., Kang Z., et al. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010;49:4430–4434. doi: 10.1002/anie.200906154. [DOI] [PubMed] [Google Scholar]
- 7.Duan J., Yu J., Feng S., et al. A rapid microwave synthesis of nitrogen–sulfur co-doped carbon nanodots as highly sensitive and selective fluorescence probes for ascorbic acid. Talanta. 2016;153:332–339. doi: 10.1016/j.talanta.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Hu B., Wang K., Wu L., et al. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010;22:813–828. doi: 10.1002/adma.200902812. [DOI] [PubMed] [Google Scholar]
- 9.Baptista F.R., Belhout S.A., Giordani S., et al. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015;44:4433–4453. doi: 10.1039/c4cs00379a. [DOI] [PubMed] [Google Scholar]
- 10.Lu W., Gong X., Nan M., et al. Comparative study for N and S doped carbon dots: synthesis, characterization and applications for Fe3+ probe and cellular imaging. Anal. Chim. Acta. 2015;898:116–127. doi: 10.1016/j.aca.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Kim S.H., Feng L. Highly luminescent N, S- Co-doped carbon dots and their direct use as mercury(II) sensor. Anal. Chim. Acta. 2015;890:134–142. doi: 10.1016/j.aca.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 12.Wang W., Lu Y.C., Huang H., et al. Facile synthesis of N, S-codoped fluorescent carbon nanodots for fluorescent resonance energy transfer recognition of methotrexate with high sensitivity and selectivity. Biosens. Bioelectron. 2015;64:517–522. doi: 10.1016/j.bios.2014.09.066. [DOI] [PubMed] [Google Scholar]
- 13.Sun D., Ban R., Zhang P.H., et al. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon. 2013;64:424–434. [Google Scholar]
- 14.Wang J., Su S., Wei J., et al. Ratio-metric sensor to detect riboflavin via fluorescence resonance energy transfer with ultrahigh sensitivity. Physica E Low Dimens. Syst. Nanostruct. 2015;72:17–24. [Google Scholar]
- 15.Mondal T.K., Gupta A., Shaw B.K., et al. Highly luminescent N-doped carbon quantum dots from lemon juice with porphyrin-like structures surrounded by graphitic network for sensing applications. RSC Adv. 2016;6:59927–59934. [Google Scholar]
- 16.Shen J., Shang S., Chen X., et al. Highly fluorescent N, S-co-doped carbon dots and their potential applications as antioxidants and sensitive probes for Cr (VI) detection. Sens. Actuators B Chem. 2017;248:92–100. [Google Scholar]
- 17.Chen Y., Wu Y., Weng B., et al. Facile synthesis of nitrogen and sulfur co-doped carbon dots and application for Fe(III) ions detection and cell imaging. Sens. Actuators B Chem. 2016;223:689–696. [Google Scholar]
- 18.Brezo T., Stojanovic Z., Suturovic Z., et al. Simple, rapid and selective chronopotentiometric method for the determination of riboflavin in pharmaceutical preparations using a glassy carbon electrode. Acta Chim. Slov. 2015;62:923–931. doi: 10.17344/acsi.2015.1745. [DOI] [PubMed] [Google Scholar]
- 19.Reavley N. M. Evans & Company; New York: 1999. The New Encyclopedia of Vitamins, Minerals, Supplements, and Herbs: a Completely Cross-Referenced User's Guide for Optimal Health. [Google Scholar]
- 20.Kundu A., Nandi S., Das P., et al. Facile and green approach to prepare fluorescent carbon dots: emergent nanomaterial for cell imaging and detection of vitamin B2. J. Colloid Interface Sci. 2016;468:276–283. doi: 10.1016/j.jcis.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 21.Wang P., Yin Y., Eremin S.A., et al. Indirect competitive immunoassay for detection of vitamin B2 in foods and pharmaceuticals. J. Agric. Food Chem. 2013;61:7048–7054. doi: 10.1021/jf401078t. [DOI] [PubMed] [Google Scholar]
- 22.Hu L., Yang X., Wang C., et al. Determination of riboflavin in urine and beverages by capillary electrophoresis with in-column optical fiber laser-induced fluorescence detection. J. Chromatogr. B. 2007;856:245–251. doi: 10.1016/j.jchromb.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Sim H.J., Kim B., Lee J. A systematic approach for the determination of b-group vitamins in multivitamin dietary supplements by high-performance liquid chromatography with diode-array detection and mass spectrometry. J. AOAC Int. 2016;99:1223–1232. doi: 10.5740/jaoacint.16-0093. [DOI] [PubMed] [Google Scholar]
- 24.Jin P., Xia L., Li Z., et al. Rapid determination of thiamine, riboflavin, niacinamide, pantothenic acid, pyridoxine, folic acid and ascorbic acid in vitamins with minerals tablets by high-performance liquid chromatography with diode array detector. J. Pharmaceut. Biomed. Anal. 2012;70:151–157. doi: 10.1016/j.jpba.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 25.National Nutrient Database for Standard Reference, Compilation prepared by U.S. Department of Agriculture, Agricultural (USDA), https://ndb.nal.usda.gov/ndb/search/list?qlookup=09150.
- 26.Sajid P.A., Chetty S.S., Praneetha S., et al. One-pot microwave-assisted in situ reduction of Ag+ and Au3+ ions by citrus limon extract and their carbon-dots based nanohybrids: a potential nano-bioprobe for cancer cellular imaging. RSC Adv. 2016;6:103482–103490. [Google Scholar]
- 27.Liguori L., Califano R., Albanese D., et al. Chemical composition and antioxidant properties of five white onion (allium cepa l.) landraces. J. Food Qual. 2017;2017 [Google Scholar]
- 28.Bandi R., Gangapuram B.R., Dadigala R., et al. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016;6:28633–28639. [Google Scholar]
- 29.Tian T., He Y., Ge Y., et al. One-pot synthesis of boron and nitrogen co-doped carbon dots as the fluorescence probe for dopamine based on the redox reaction between Cr(VI) and dopamine. Sens. Actuators B Chem. 2017;240:1265–1271. [Google Scholar]
- 30.Brouwer A.M. Standards for photoluminescence quantum yield measurements in solution. Pure Appl. Chem. 2011;83:2213–2228. [Google Scholar]
- 31.Madrakian T., Maleki S., Gilak S., et al. Turn-off fluorescence of amino-functionalized carbon quantum dots as effective fluorescent probes for determination of isotretinoin. Sens. Actuators B Chem. 2017;247:428–435. [Google Scholar]
- 32.Peng H., Travas-Sejdic J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009;21:5563–5565. [Google Scholar]
- 33.Lu W., Li Y., Li R., et al. Facile synthesis of N-doped carbon dots as a new matrix for detection of hydroxy-polycyclic aromatic hydrocarbons by negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. ACS Appl. Mater. Interfaces. 2016;8:12976–12984. doi: 10.1021/acsami.6b01510. [DOI] [PubMed] [Google Scholar]
- 34.Michałowski T., Asuero A.G., Wybraniec S. The titration in the kjeldahl method of nitrogen determination: base or acid as titrant? J. Chem. Educ. 2013;90:191–197. [Google Scholar]
- 35.Kundu A., Nandi S., Layek R.K., et al. Fluorescence resonance energy transfer from sulfonated graphene to riboflavin: a simple way to detect vitamin B2. ACS Appl. Mater. Interfaces. 2013;5:7392–7399. doi: 10.1021/am4017208. [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Ma Q., Wang Y., et al. Resonance energy transfer based electrochemiluminescence and fluorescence sensing of riboflavin using graphitic carbon nitride quantum dots. Anal. Chem. Acta. 2017;973:34–42. doi: 10.1016/j.aca.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 37.Dinc S. A simple and green extraction of carbon dots from sugar beet molasses: biosensor applications. Sugar Ind. 2016;141:560–564. [Google Scholar]
- 38.Draper N.R., Smith H. John Wiley and Sons; New York: 1998. Applied Regression Analysis. [Google Scholar]