Abstract
Pantoprazole sodium, a substituted benzimidazole derivative, is an irreversible proton pump inhibitor which is primarily used for the treatment of duodenal ulcers, gastric ulcers, and gastroesophageal reflux disease (GERD). The monographs of European Pharmacopoeia (Ph. Eur.) and United States Pharmacopoeia (USP) specify six impurities, viz.; impurities A, B, C, D, E and F, respectively for its active pharmaceutical ingredient (API). The identification and synthesis of all impurities except impurity E are well described in the literature; however, there is no report related to impurity E. The prospects to the formation and controlling of impurity E up to ≤0.03% in the synthesis of pantoprazole sodium sesquihydrate (PAN) were discussed in detail for the first time. The present work described the journey towards the successful development of an optimal preparation procedure of dimer impurity E. The most plausible mechanism involved in the formation of impurity E has been proposed.
Keywords: Pantoprazole sodium sesquihydrate, Impurity E, Oxidation, Enrichment, Column chromatography, Characterization, HPLC
Graphical abstract
Highlights
-
•
An optimal preparation procedure for Impurity E (dimer) of pantoprazole is reported for the first time.
-
•
Prospects to the formation and controlling of Impurity E in the synthesis of pantoprazole API were discussed in detail.
-
•
The plausible mechanism for the formation of Impurity E is discussed.
-
•
Oxidation of sulfide to sulfoxide has been carried out in water.
1. Introduction
Pantoprazole [1] is the international non-proprietary name of a substituted benzimidazole which is the active ingredient of a pharmaceutical product that is marketed as sodium salt in the United States and sold under the brand name Protonix by Pfizer Inc. Pantoprazole is chemically known as 5-(difluoromethoxy)-2-[{(3,4-dimethoxypyridin-2-yl)methyl}sulfinyl]-1H benzimid-azole [2,3]. It is a proton pump inhibitor used to treat ulcers, gastroesophageal reflux disorder (GERD), erosive esophagitis and Zollinger-Ellison syndrome [1,4]. The conventional process [1,[5], [6], [7], [8]] for the synthesis of pantoprazole comprises the condensation of 2-chloromethyl-3,4-dimethoxypyridinium hydrochloride (2) with 5-(difluoromethoxy)-1H-benzimidazole-2-thiol (3) in the presence of an inorganic base, to yield 5-(difluoromethoxy)-2-[[(3,4-dimethoxypyridin-2 yl)met-hyl]sulfanyl]-1H-benzimidazole or pantoprazole sulfide (4), which upon further oxidation with a suitable oxidizing agent eventually leads to pantoprazole sulfoxide (5). Several other methods [[9], [10], [11], [12], [13], [14]] are well reported in different patent literatures for the preparation of pantoprazole.
The structures of pantoprazole sodium sesquihydrate (PAN) (6) and its corresponding pharmacopoeial dimer impurity E (mixture of the stereoisomers of 6,6′-bis(difluoromethoxy)-2,2′-bis[[(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl]-1H,1′H-5,5′-bibenzimidazolyl, 1) are shown in Fig. 1. The other impurities of PAN described in the monographs of European Pharmacopoeia (Ph. Eur.) [15] and United States Pharmacopoeia (USP) [16] are 5-(difluoromethoxy)-2-[[(3,4-dimethoxypyridin-2-yl)met-hyl]sulfonyl]-1H-benzimidazole (Impurity A, 7), 5-(difluoromethoxy)-2-[[(3,4-dimethoxypyridin-2-yl)met-hyl]sulfanyl]-1H-benzimidazole (Impurity B, 4), 5-(difluoromethoxy)-1H-benzimidazole-2-thiol (Impurity C, 3), 5-(difluoromethoxy)-2-[(RS)-[(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl]-1-methyl-1H-benzimidazole (Impurity D, 8), and 6-(difluoromethoxy)-2-[(RS)-[(3,4-dimethoxypyridin-2-y-l)methyl]sulfinyl]-1-methyl-1H-benzimidazole (Impurity F, 9) (Scheme 1). Among these impurities, the impurity C (3) is one of the key starting materials while impurity B (4) is the sulfide intermediate formed due to the coupling of 2-chloromethyl-3,4-dimethoxypyridinium hydrochloride (2) and 5-(difluoromethoxy)-1H-benzimidazole-2-thiol (3). Impurity A (7) is the over-oxidation product of pantoprazole sulfoxide (5) while impurities D (8) and F (9) resulted due to the N-methylation of benzimidazole ring of pantoprazole sulfoxide (5). The identification, synthesis, characterization and analytical procedures of above mentioned impurities are well reported in the literature [[17], [18], [19]]. Though, impurity E (1) is a known pharmacopoeial impurity of PAN, the study towards its formation mechanism and control in active pharmaceutical ingredient (API) has not been reported in the literature to date, to the best of our knowledge. It has not been assigned a Chemical Abstracts Service (CAS) number and there is no report on its mechanism of formation or its synthesis. This attracted our attention towards the development of an efficient method for the formation of this important and expensive [20] pharmacopoeial impurity.
Fig. 1.
Structure of pantoprazole sodium sesquihydrate (6) and its dimer impurity E (1).
Scheme 1.
Synthetic scheme of pantoprazole sodium sesquihydrate (6) and its pharmacopoeial impurities.
In recent years, the impurity profile of a drug substance becomes more important for marketing approval and this work is done as part of a drug development process. Regulatory agencies worldwide are demanding the characterization of unknown impurities to ensure their non-genotoxicity, identification and control to establish the quality, safety and efficacy of drug substance. Different regulatory agencies such as International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), United States Food and Drug Administration (USFDA), and Canadian Drug and Health Organizations are accentuating the purity requirement of API and identification of impurities, and have published some guidelines on impurities [21], products [22], and residual solvents [23]. The ICH has set a high standard for the purity of drug substances [24]. If the dose is less than 2 g/day, then impurities over 0.10% are expected to be identified, qualified and controlled. If the dose exceeds 2 g/day, then the qualification threshold is lowered to 0.05%. It is therefore essential to monitor and control the impurities in both the drug substance and the drug products.
Continuing our interest in the process research and development of API [[25], [26], [27], [28]] and their impurity profiling [[29], [30], [31]] as a means of pharmaceutical analysis to manufacture high quality drug, herein, we report our investigation towards the formation and control of potential dimer impurity E (1) in the synthesis of PAN, and a most optimal preparation procedure of dimer impurity. The data obtained will facilitate the optimization of manufacturing processes and the quality control of PAN.
2. Experimental
2.1. Materials and methods
All the starting materials, reagents and solvents used in the process were purchased from commercial suppliers with optimum purity and used without further purification. NMR spectra were recorded on Bruker 400 MHz spectrometers with TMS as the internal standard. Chemical shifts are expressed in parts per million (δ, ppm). MS were recorded on VelosPro from Thermo Scientific LC-Mass spectrometer. The IR spectra were recorded on a Shimadzu IR Affinity-I FT-IR spectrophotometer (Shimadzu Corporation, North America, USA) over the range of 4000–400 cm−1 by pressed pellet method using KBr. The HPLC method given in the monograph of Ph. Eur. of PAN [15] was referred for the separation of all possible related substances of PAN. HPLC analyses were conducted using Waters 2695 with UV detector, hypersil ODS column (125 mm × 4.0 mm, 5 μm), solvent system of acetonitrile (ACN) and NaOH solution (40 mg/L) in the ratio 1:1 (%, v/v), wavelength (290 and 305 nm), flow rate of 1.0 mL/min and run time of 55 min. Purified water by Milli-Q system (Millipore, USA) was used for the preparation of samples, reference solutions and mobile phases. Isolated yields refer to yields corrected for purity on the basis of HPLC assay using standards.
2.2. General experimental procedure for the synthesis of pantoprazole sodium sesquihydrate (6)
A 1 L four-necked round bottom flask (RBF) equipped with a mechanical stirrer, a reflux condenser, a thermometer and a pressure-equalizing funnel was charged with 5-difluoromethoxy-2-mercaptobenzimidazole (3) (0.1387 mol, 30 g) in a mixture of water (300 mL) and NaOH (0.2775 mol, 11.1 g) under stirring. The reaction mass was stirred at 25–30 °C and an aqueous solution of 2-chloromethyl-3,4-dimethoxypyridinium hydrochloride (2) (0.1387 mol, 31.09 g in 60 mL of water) was added dropwise over a period of 2–3 h. The stirring was continued for an additional 1 h at 25–30 °C. When the reaction was considered complete as determined by HPLC analysis, the precipitated solids were filtered under reduced pressure and washed with water, thus yielding the wet cake (145 g) of pantoprazole sulfide (4). We charged the wet cake of pantoprazole sulfide (4) into an aqueous solution of NaOH (0.2081 mol, 8.325 g dissolved in 150 mL of water) at 25–30 °C. The reaction mass was cooled to 0–5 °C and an aqueous solution of sodium hypochlorite (NaOCl) (120.5 g; assay 9.0% w/w) was added dropwise over a period of 2–3 h and then stirred for 1 h. Progress of reaction was monitored by HPLC. After completion of reaction, the reaction mixture was quenched with 5.0% Na2S2O5 solution (105 mL). We charged dichloromethane (DCM) (150 mL) and water (120 mL) and adjusted the pH of reaction mass between 7.5 and 8.0 using 2M HCl solution. Layers were separated and the aqueous layer was extracted with DCM (60 mL). The combined DCM layers were distilled off under vacuum at 30–35 °C to obtain a red-brown colored residue of pantoprazole sulfoxide (5). The residue was dissolved in ACN (150 mL) and cooled to 20–25 °C. Aqueous solution of NaOH (0.1387 mol, 5.55 g dissolved in 6.0 mL of water) was added dropwise followed by addition of a seed crystal of PAN. The contents were stirred for 2 h at 20–25 °C and then cooled to 0–5 °C for 3 h. The reaction mass was filtered, and washed with chilled ACN (15 mL) and the obtained solid was dried under vacuum at 35–40 °C; 50.4 g (84% yield and 99.92% HPLC purity) of almost white powder of PAN (6) having water content 6.72% (lit. [15] between 5.9 and 6.9%) was obtained; 1H NMR (400 MHz, D2O): δ 7.95 (d, J = 5.6 Hz, 1H, Ar-CH), 7.50 (d, J = 8.8 Hz, 1H, Ar-CH), 7.31 (d, J = 1.6 Hz, 1H, Ar-CH), 6.89 (dd, J = 2.0 Hz, 1H, Ar-CH), 6.83 (d, J = 6.0 Hz, 1H, Ar-CH), 6.65 (t, J = 74.8 Hz, 1H, -OCHF2), 4.67 (d, J = 12.8 Hz, 1H, -CH2), 4.48 (d, J = 12.8 Hz, 1H, -CH2), 3.73 (s, 3H, -OCH3), 3.55 (s, 3H, -OCH3); 13C NMR (100 MHz, DMSO‑d6): 164.1, 158.4, 146.9, 146.7, 145.9, 144.6, 144.5, 144.3, 117.6, 117.6 (t, J = 256.0 Hz, -OCHF2), 111.2, 108.0, 107.6, 57.14, 56.0; 19F NMR (376.55 MHz, DMSO‑d6): δ −79.39 (d, J = 75.3 Hz); IR (KBr): ν3555, 3483, 3368, 3198, 2997, 2941, 2845, 1653, 1589, 1568, 1491, 1464, 1449, 1427, 1375, 1362, 1306, 1277, 1229, 1211, 1171, 1121, 1088, 1074, 1042, 986, 961, 937, 837, 816, 806, 797, 775, 752, 710, 679, 644, 631, 583, 554, 525 cm−1; MS m/z calculated for C16H15F2N3O4S 383.37, found 384.18 (M + H)+, 382.20 (M-H)-.
2.3. Acid-base treatment of mother liquor (ML) residue
The filtrate obtained in the above procedure after the filtration of PAN API (6) was concentrated under vacuum at 40–45 °C to get brown colored residue (54 g) having 1.67% (HPLC area) of Imp E (1). It was charged into a cleaned and dried 1 L four-necked RBF in water (250 mL). DCM (250 mL) was charged after stirring for 10–15 min at 20–30 °C. The pH of reaction mass was adjusted between 7.5 and 8.0 using 2M HCl. Layers were separated and the aqueous layer was extracted with DCM. We combined all DCM layers and concentrated under vacuum at 40–45 °C to get brown solid of pantoprazole sulfoxide (5) which was charged into a cleaned and dried 500 mL four-necked RBF in ACN (140 mL). The reaction mass was stirred for 10–15 min and then cooled to 5–10 °C. Charged NaOH solution (5.0 g dissolved in 5.5 mL of water) and stirred for 10–15 min at 5–10 °C. Allowed the reaction mass to come to 20–30 °C and stirred for 2–3 h. Cooled to 0–5 °C and stirred for 3–4 h at this temperature. Filtered the solid and washed the wet cake with ACN (40 mL) to get off-white solid of PAN (6). The filtrate obtained was concentrated under vacuum at 40–45 °C to get ML residue (34 g), which was enriched with Imp E up to 3.61% (HPLC area).
2.4. Purification of ML residue for the isolation of impurity E (1)
The ML residue (10 g) obtained in the above procedure was subjected to column chromatography. The slurry of ML residue was prepared with silica gel (100–200 mesh) in a mixture of DCM (18 mL) and methanol (2 mL). The slurry was charged into the column packed with silica gel and eluted with alkaline DCM (1% triethylamine in DCM). Anhydrous sodium sulfate was added into column for absorbing the moisture of solvents. Column was eluted with DCM and the collected fractions were monitored by TLC which indicated the presence of pantoprazole sulfide (4) as a major spot. The polarity of mobile phase was increased up to 0.5% of methanol in DCM for the complete elution of sulfide (4). Subsequently, polarity was increased up to 2.0% of methanol in DCM. The elution of other two spots were started between sulfide (4) and pantoprazole sulfoxide (5). Until complete separation of these spots, column was eluted with the same mobile phase. After collection of these two spots, polarity was increased up to 5.0% of methanol in DCM. Elution of sulfoxide (5) was started as confirmed through TLC and HPLC. Column was eluted with the same polarity until complete separation of sulfoxide (5). Later, polarity was increased up to 10% of methanol in DCM, concentrated the collected fractions, and analyzed the content of Imp E (1) through HPLC. Results indicated that Imp E started to elute. The same polarity was used to collect the fractions which upon concentration revealed the content of Imp E (1) ranging between 0.10% and 8.44% (HPLC area). Finally, the column was washed with methanol. The corresponding fractions collected were combined and concentrated under vacuum at 40–45 °C. The residue obtained was extracted with DCM which upon concentration afforded pure Imp E (1) (389 mg, 92.73% HPLC purity) as a brown solid; 1H NMR (400 MHz, CDCl3): 12.82 (bs, 2H, -NH); 8.03 (d, J = 5.2 Hz, 2H, Ar-CH), 7.20–7.66 (m, 4H, Ar-CH), 6.67 (d, J = 5.2 Hz, 2H, Ar-CH), 6.35 (t, J = 74.4 Hz, 2H, -OCHF2), 4.72–4.84 (dd, J = 12.8, 12.8 Hz, 4H, -CH2), 3.77 (s, 6H, -OCH3), 3.72 (s, 6H, -OCH3); 13C NMR (100 MHz, CDCl3): 158.9, 155.3, 146.1, 145.6, 143.7, 141.2, 132.3, 126.4, 122.9, 116.8 (t, J = 257.0 Hz, -OCHF2), 115.3, 109.9, 108.2, 102.9, 61.4, 57.8, 55.8; 19F NMR (376.55 MHz, DMSO‑d6): δ −80.64 (d, J = 478.22 Hz); IR (KBr): ν 3424, 3107, 3005, 2945, 2843, 2791, 2600, 1722, 1707, 1690, 1659, 1628, 1584, 1549, 1491, 1464, 1447, 1427, 1381, 1337, 1302, 1277, 1234, 1172, 1157, 1125, 1069, 1040, 993, 935, 878, 847, 822, 797, 758, 731, 712, 660, 627, 567, 519 cm−1; MS m/z calculated for C32H28F4N6O8S2 764.72, found 765.17 (M + H)+, 763.22 (M-H)-.
3. Results and discussion
Our initial efforts in this direction started with the synthesis of Imp E (1) by assuming that difluoromethoxy (CHF2O-) group imparts a unique property to the benzimidazole moiety of pantoprazole, which promotes the biaryl formation. We carried out some experiments with pantoprazole sulfide (5) using NaOCl/NaOH/ACN reagent system in the presence of azobisisobutyronitrile as a free-radical initiator for the synthesis of Imp E by assuming that the reaction proceeded via free-radical mechanism. However, desired results were not obtained and resulted in the formation of side products other than Imp E. Neelamegam et al. [32] reported the dimerization of phenols and naphthols using an aqueous solution of NaOCl. When we tried the same reaction conditions having sulfoxide (5) as a substrate, N-chloro derivative of sulfoxide was obtained instead of Imp E.
Furthermore, during the process development of an environmentally-benign, facile, and cost-effective technology [33] for the synthesis of bulk drug, PAN, we found some interesting results related to Imp E as discussed below.
3.1. Effect of solvent on impurity E (1) content in the oxidation of pantoprazole sulfide (4) to pantoprazole sulfoxide (5)
As a part of our optimization study, we tried various solvents such as ethyl acetate (EtOAc), ACN, acetone and DCM to study the reaction rate of oxidation of sulfide (4) to sulfoxide (5) using NaOCl as an oxidant. Table 1 indicates that HPLC conversion of sulfide to sulfoxide is optimal using EtOAc, ACN and DCM, respectively. However, using EtOAc and DCM, biphasic reaction mass was obtained, which led to difficulty in the monitoring of reaction as sulfide (4) is soluble in organic solvent while sulfoxide (5) being sodium salt in the reaction mixture will remain soluble in aqueous phase. Acetone led to poor conversion by exploiting the same reaction conditions. While, in the case of ACN, homogeneous reaction mass was obtained throughout the reaction and resulted in excellent conversion (99.18%; by HPLC). Later, to our surprise, almost analogous conversion was obtained along with an increased content of Imp E (1) (0.94%; by HPLC) when the reaction was carried out in neat water (Table 1, entry 5). The only difference between using water and organic solvents arose in the impurity profile of sulfoxide (5) wherein the content of Imp E (1) was found comparatively high as that obtained using organic solvents. Thus, Table 1 clearly reveals that the more the polar solvent used, the more will be the content of Imp E (1), suggesting a free radical mechanism for its synthesis during the oxidation of sulfide (4) to sulfoxide (5).
Table 1.
Effect of solvent on Impurity E (1) content in the oxidation of pantoprazole sulfide (4) to pantoprazole sulfoxide (5).a
| Entry | Solvent | HPLC conversion (%) |
|||||
|---|---|---|---|---|---|---|---|
| Sulfoxide (5) | Imp A (7) | Sulfide/Imp B (4) | Imp C (3) | Imp D + F (8,9) | Imp E (1) | ||
| 1 | Acetone | 27.48 | 0.05 | 70.76 | nd | nd | nd |
| 2 | EtOAc | 93.26 | 0.10 | 0.09 | nd | nd | nd |
| 3 | DCM | 93.49 | 0.01 | 0.04 | nd | 0.04 | 0.06 |
| 4 | ACN | 99.18 | 0.02 | 0.11 | nd | nd | 0.14 |
| 5 | Water | 97.27 | 0.24 | 0.13 | nd | 0.05 | 0.94 |
nd: not detected.
Reaction condition: 5-(difluoromethoxy)-1H-benzimidazole-2-thiol (3), 10 mmol; NaOH, 25 mmol; NaOCl, 10.5 mmol, assay 9.0%; solvent, 10 mL at 0–5 °C for 3–4 h.
3.2. Effect of concentration of NaOH on impurity E (1) content in the oxidation of pantoprazole sulfide (4) to pantoprazole sulfoxide (5)
After selecting water as a suitable candidate in the oxidation of sulfide (4) to sulfoxide (5) with respect to the content of Imp E (1), we studied the effect of concentration of NaOH on the content of Imp E. It is quite obvious from Table 2 that the quality of product is strongly dependent on the concentration of NaOH. It has been observed that the optimum conversion and increased content of Imp E (1) (97.27%; sulfoxide (5) and 0.94%; Imp E (1), by HPLC, Table 2, entry 5) were obtained when 25 mmol of NaOH was used with respect to 10 mmol of starting material. The decrease in the concentration of NaOH resulted in less conversion of sulfide (4) to sulfoxide (5) with a reduced content of Imp E (1) (Table 2, entries 1–4). Thus, concentration of NaOH does play an important role, suggesting that highly basic conditions support the oxidation of sulfide (4) to sulfoxide (5) as well as dimerization [32] of sulfoxide (5) using water as a reaction medium.
Table 2.
Effect of concentration of NaOH on Impurity E (1) content in the oxidation of pantoprazole sulfide (4) to pantoprazole sulfoxide (5).a
| Entry | NaOH (mmol) | HPLC conversion (%) |
|||||
|---|---|---|---|---|---|---|---|
| Sulfoxide (5) | Imp A (7) | Sulfide/ Imp B (4) | Imp C (3) | Imp D + F (8,9) | Imp E (1) | ||
| 1 | 5 | 93.88 | 0.15 | 0.08 | nd | nd | 0.31 |
| 2 | 10 | 95.45 | nd | 0.13 | nd | nd | 0.57 |
| 3 | 15 | 96.12 | 0.05 | 0.20 | nd | nd | 0.64 |
| 4 | 20 | 96.50 | 0.03 | 0.03 | nd | 0.06 | 0.84 |
| 5 | 25 | 97.27 | 0.24 | 0.13 | nd | 0.05 | 0.94 |
| 6 | 30 | 95.01 | 0.05 | 0.18 | nd | nd | 0.89 |
nd : not detected.
Reaction conditions: 5-(difluoromethoxy)-1H-benzimidazole-2-thiol (3), 10 mmol; NaOCl, 10.5 mmol, assay 9.0%; water, 10 mL at 0–5 °C for 3 h.
3.3. Effect of solvent on impurity E (1) content in the formation of PAN (6) from pantoprazole sulfoxide (5)
Next, we explore the ideal solvent for the formation of sodium salt of pantoprazole sulfoxide (5), i.e., pantoprazole sodium sesquihydrate (6) along with the desired yield and quality as per the monographs of Ph. Eur. [15] and USP [16]. The solvents ACN, DCM, EtOAc and acetone were screened. Table 3 concludes that optimal yield (84%) and purity (99.92%; by HPLC) and other parameters of PAN (6) as per international standards were achieved using ACN as a solvent. DCM led to off-white product with an increased content of Imp E (1). An almost white powder with good yield (82%) was obtained using acetone as a solvent; however, the product fails with respect to assay by potentiometry (90.43% w/w) which is not adequate as per the International standards. While using EtOAc, yield was less and the product fails with respect to description (off-white). Hence, Table 3 establishes that when pantoprazole sulfoxide (5) (prepared using water as a solvent (Table 1, entry 4)) was subjected to sodium salt formation using ∼46% (w/w) aqueous solution of NaOH, a pure API (>99.9% HPLC purity) substantially free from Imp E (1) (<0.03%) was obtained using ACN as a solvent.
Table 3.
Effect of sont on Impurity E (1) content in the formation of PAN (6) from pantoprazole sulfoxide (5).a
| Entry | Parameters | Solvent |
As per PAN Ph. Eur. monograph [15] | |||
|---|---|---|---|---|---|---|
| ACN | DCM | EtOAc | Acetone | |||
| 1 | Appearance | Almost white | Off-white | Off-white | Almost white | White or almost white powder |
| 2 | Water content (%, w/w by KF) | 6.72 | 6.9 | 6.71 | 7.38 | Between 5.9 and 6.9 |
| 3 | Assay by potentiometry (%, w/w) | 100.45 | 100.55 | 99.99 | 90.43 | NLT 99 and NMT 101 |
| 4 | Purity by HPLC | |||||
| a) Pantoprazole 6 | 99.92 | 99.63 | 99.85 | 99.79 | NLT 99.5 | |
| b) Imp A (7) | 0.03 | 0.02 | 0.02 | 0.08 | NMT 0.2 | |
| c) Imp B (4) | 0.02 | 0.01 | nd | nd | NMT 0.1 | |
| d) Imp C (3) | nd | nd | 0.01 | nd | NMT 0.1 | |
| e) Imp D+F (8,9) | nd | 0.02 | nd | nd | NMT 0.2 | |
| f) Imp E (1) | 0.03 | 0.14 | 0.03 | 0.10 | NMT 0.1 | |
| 5 | Yield (%) | 84 | 83 | 77 | 82 | NMT 99.0 |
nd : not detected.
NLT : Not less than.
NMT : Not more than.
Reaction conditions: 5-(difluoromethoxy)-1H-benzimidazole-2-th (3), 10 mmol; NaOH, 10 mmol; water, 0.43 mL; solvent, 10 mL at temp 0–5 °C and 20–25 °C for 4–5 h.
3.4. Enrichment of impurity E (1)
The HPLC analysis of ML residue obtained after the filtration of PAN API (6) revealed that it contained 1.67% (HPLC area) of Imp E (1). The obtained ML residue was charged into a mixture of DCM and water at room temperature. The pH was adjusted between 7.5 and 8.0 using 2M HCl, thereby converting the sodium salts of PAN (6) and Imp E (1), respectively, again into free base. The layers were separated, followed by the extraction of aqueous layer with DCM. A light-brown solid was obtained upon concentration of the collected DCM layers comprising the free base of PAN (6) and Imp E (1). Further, it was treated with ∼46% (w/w) aqueous solution of NaOH to form sodium salt of pantoprazole free base and Imp E using ACN as a solvent. The filtration of the reaction mass resulted in PAN (6) as an off-white solid free from Imp E (1) while the filtrate obtained indicated 3.61% (by HPLC) of Imp E (1) after concentrating the ML residue (Fig. 2 A). In this way, the content of Imp E has been enriched to 3.61% from 1.67% through acid-base treatment.
Fig. 2.
HPLC chromatograms of (A) mother liquor residue enriched with Imp E (1) after acid-base treatment and (B) isolated Imp E (1).
3.5. Isolation of Impurity E (1)
The obtained ML residue enriched with Imp E was subjected to column chromatography. Neutral alumina was used as an adsorbent for the separation of Imp E (1) using DCM and methanol as eluents. Initially, the column was eluted with DCM as an eluent and subsequently eluted with a mixture of DCM and methanol by slowly increasing the methanol percentage in the mobile phase. In this way, pantoprazole sulfide (4), sulfoxide (5) and other impurities were separated from Imp E (1) while running the column for three days. On the fourth day, the fraction obtained by using only methanol as an eluent afforded merely 0.71% (HPLC purity) of Imp E. It was found that the product degraded due to holding up on alumina up to four days. When the purification was tried to perform in a single day by means of flash column chromatography, only 3.61% content (by HPLC) of Imp E was obtained. Accordingly, desired results were not obtained which were actually expected in a day.
After having hands-on experience with alumina, silica gel was tried as an adsorbent by using the similar mobile phase and tried to separate all spots in one day. Herein, the elution and separation were similar as that obtained using alumina by exploiting the mobile phase comprising of DCM and methanol. Consecutively, the fractions obtained after methanol washing were found to contain increasing content of Imp E (13.48%, 45.84%, 48.59%, and 60.17%, respectively). The subsequent methanol fraction collected afforded an excellent HPLC purity of Imp E (92.73% of Imp E (1), 0.70% of sulfide (4), and 0.85% of sulfoxide (5), respectively) (Fig. 2 B). In this method, the purification of Imp E was accomplished in one day without degradation. The same procedure was followed for the purification of Imp E in four days in order to check the effect of time on the separation and elution of Imp E. However, the methanol fraction showed only 25.85% of Imp E, indicating that extended purification resulted in the degradation of dimer.
Silica gel proved to be efficacious in order to get better separation than alumina for Imp E (1) as silica phases tend to be acidic, less polar and retain basic samples while alumina phases tend to be basic, more polar and retain acids better. In the present work, potential dimer impurity of pantoprazole has been isolated in excellent HPLC purity (92.73%) through a combination of enrichment and chromatographic isolation (Scheme 2). The structure of impurity was confirmed through mass, IR and NMR analyses (Table 4). The 1H-NMR and 13C-NMR were further supported by 2D 1H-13C HSQC correlation spectrum.
Scheme 2.
Isolation of Imp E (1) through progressive enrichment and column chromatography.
Table 4.
General properties and structure assignments of PAN (6) and Imp E (1).
| Entry | Assignments | PAN (6) | Imp E (1) |
|---|---|---|---|
| 1 | Description | Almost white powder | Brown powder |
| 2 | Molecular weight | 432.4 | 764.72 |
| 3 | RT | 9.66 | 11.98 |
| 4 | RRT | 1.0 | 1.24 |
| 5 | Mass spectral data | 384.18 (M + H)+, 382.20 (M-H)- | 765.17 (M + H)+, 763.22 (M-H)- |
| 6 | IR absorption bands (cm−1) | 3555, 3483, 3368, 3198, 2997, 2941, 2845, 1653, 1589, 1568, 1491, 1464, 1449, 1427, 1375, 1362, 1306, 1277, 1229, 1211, 1171, 1121, 1088, 1074, 1042, 986, 961, 937, 837, 816, 806, 797, 775, 752, 710, 679, 644, 631, 583, 554, 525 | 3424, 3107, 3005, 2945, 2843, 2791, 2600, 1722, 1707, 1690, 1659, 1628, 1584, 1549, 1491, 1464, 1447, 1427, 1381, 1337, 1302, 1277, 1234, 1172, 1157, 1125, 1069, 1040, 993, 935, 878, 847, 822, 797, 758, 731, 712, 660, 627, 567, 519 |
| 7 | 1H-NMR (δH (J)) | 7.95 (d, J = 5.6 Hz, 1H, Ar-CH), 7.50 (d, J = 8.8 Hz, 1H, Ar-CH), 7.31 (d, J = 1.6 Hz, 1H, Ar-CH), 6.89 (dd, J = 2.0 Hz, 1H, Ar-CH), 6.83 (d, J = 6.0 Hz, 1H, Ar-CH), 6.65 (t, J = 74.8 Hz, 1H, -OCHF2), 4.67 (d, J = 12.8 Hz, 1H, -CH2), 4.48 (d, J = 12.8 Hz, 1H, -CH2), 3.73 (s, 3H, -OCH3), 3.55 (s, 3H, -OCH3) | 12.82 (bs, 2H, -NH); 8.03 (d, J = 5.2 Hz, 2H, Ar-CH), 7.20–7.66 (m, 4H, Ar-CH), 6.67 (d, J = 5.2 Hz, 2H, Ar-CH), 6.35 (t, J = 74.4 Hz, 2H, -OCHF2), 4.72–4.84 (dd, J = 12.8, 12.8 Hz, 4H, -CH2), 3.77 (s, 6H, -OCH3), 3.72 (s, 6H, -OCH3) |
| 8 | 13C-NMR (δC (J)) | 164.1, 158.4, 146.9, 146.7, 145.9, 144.6, 144.5, 144.3, 117.6, 117.6 (t, J = 256.0 Hz, -OCHF2), 111.2, 108.0, 107.6, 57.14, 56.0 | 158.9, 155.3, 146.1, 145.6, 143.7, 141.2, 132.3, 126.4, 122.9, 116.8 (t, J = 257.0 Hz, -OCHF2), 115.3, 109.9, 108.2, 102.9, 61.4, 57.8, 55.8 |
| 9 | 19F-NMR (δF (J)) | −79.39 (d, J = 75.3 Hz) | −80.64 (d, J = 478.22 Hz) |
RT: Retention time.
RRT: Relative retention time.
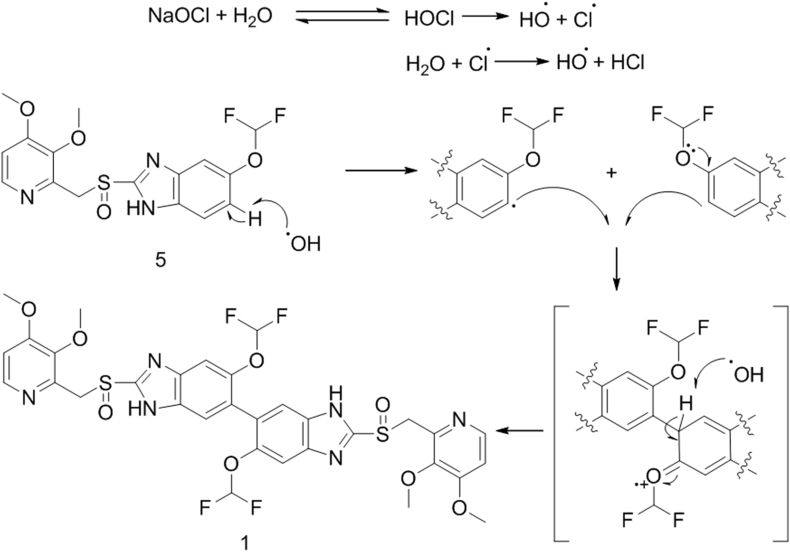
3.6. Plausible mechanism
The plausible mechanism of dimer (1) formation via ortho–ortho coupling of pantoprazole sulfoxide (5) during the oxidation of sulfide (4) to sulfoxide (5) using NaOCl as an oxidizing agent in presence of alkaline medium is depicted in Scheme 3. The mechanism could follow a free radical process which leads to the dimerization of radical species to form the dimerized product Imp E (1).
Scheme 3.
Plausible mechanism for the formation of Imp E (1).
4. Conclusions
It can be concluded from the present study that the oxidation of sulfide (4) to sulfoxide (5) in the synthesis of PAN (6) can be carried out using water as a reaction medium. The sodium salt formation of pantoprazole sulfoxide (5) has been carried out in ACN to afford API of international standards (yield: 84% and HPLC purity: 99.92%) having dimer impurity ≤0.03%. A detailed investigation of formation and control of Imp E (1) in the synthesis of pantoprazole API has been reported for the first time.
Acknowledgments
The authors acknowledge the management of Micro Labs Ltd., API R&D Centre, Bangalore for providing research and analytical facilities.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2019.02.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Electronic Supplementary Information (ESI) available: The supporting information includes HPLC chromatograms, 1H NMR, 13C NMR, 19F NMR, 2D 1H-13C HSQC, IR and MS spectra of PAN (6) and Imp E (1), respectively.
References
- 1.B. Kohl, E. Sturm, G. Rainer, Fluoroalkoxy substituted benzimidazoles useful as gastric acid secretion inhibitors, U.S. Pat. 4758579 (July 19, 1988).
- 2.B. Kohl, E. Sturm, K. Klemm, et al., Dialkoxyridines, process for their preparation, their application and medicaments containing them, Eur. Pat. 0166287 (January 2, 1986).
- 3.Kohl B., Sturm E., Bilfinger J.S. (H+, K+)-ATPase inhibiting 2-[(2-pyridylmethyl)sulfinyl]benzimidazoles. 4. A novel series of dimethoxypyridyl-substituted inhibitors with enhanced selectivity. The selection of pantoprazole as a clinical candidate. J. Med. Chem. 1992;35:1049–1057. doi: 10.1021/jm00084a010. [DOI] [PubMed] [Google Scholar]
- 4.Richardson P., Hawkey C.J., Stack W.A. Proton pump inhibitors. Drugs. 1998;56:307–335. doi: 10.2165/00003495-199856030-00002. [DOI] [PubMed] [Google Scholar]
- 5.R.A. Murlidhar, S.B. Bhaskar, B.P. Gopinathan, et al., Process for the preparation of pantoprazole sodium, PCT Pat. Appl. WO 2009066317 (July 23, 2009).
- 6.P.N. Francisco, M.P. Andres, Process for the preparation of pantoprazole, PCT Pat. Appl. WO 2006100243 (September 28, 2006).
- 7.Mathad V.T., Govindan S., Kolla N.K. An improved and single-pot process for the production of pantoprazole substantially free from sulfone impurity. Org. Process Res. Dev. 2004;8:266–270. [Google Scholar]
- 8.W. Kyu-Jung, K. Young-Deuck, Process for preparing pantoprazole sodium sesquihydrate, PCT Pat. Appl. WO 2009075516 (June 18, 2009).
- 9.I. Avrutov, M. Mendelovici, Processes for the production of substituted 2-(2-pyridylmethyl) sulfinyl-1H-benzimidazoles, U.S. Pat. 7129358 B2 (October 31, 2006).
- 10.I. Avrutov, M. Mendelovici, Processes for the Production of substituted 2-(2-pyridylmethyl) sulfinyl-1H-benzimidazoles, U.S. Pat. Appl. US 20080091024 A1 (April 17, 2008).
- 11.F. Chin-Tsai, Method for preparing 2-(2-pyridinylmethylsulfinyl) benzimidazoles, U.S. Pat. 7531666 B2 (May 12, 2009).
- 12.P.C. Alberto, A process for the preparation of pantoprazole and intermediates thereof, PCT Pat. Appl. WO 2002028852 A1, (April 11, 2002).
- 13.R.B. Maimo, J.C. Pardo, L. Coppi, Method for oxidizing a thioether group into a sulfoxide group, U.S. Pat. 6603009 B2 (August 5, 2003).
- 14.J. Patrick, A.T. Timothy, Chemical process for the production of sulphinyl derivatives by oxidation of the corresponding co-derivatives with perborates, PCT Pat. Appl. WO 1999047514 A1 (September 23, 1999).
- 15.9th ed. vol. 1. 2017. pp. 3264–3265. (European Pharmacopoeia-National Formulary). [Google Scholar]
- 16.United States Pharmacopeia-National Formulary . vol. 33. 2016. ([USP 32 NF 27]). pp. 3200. [Google Scholar]
- 17.Reddy G.M., Bhaskar B.V., Reddy P.P. Structural identification and characterization of potential impurities of pantoprazole sodium. J. Pharmaceut. Biomed. Anal. 2007;45:201–210. doi: 10.1016/j.jpba.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Pandey S., Pandey P., Mishra D. A validated stability indicating HPLC method for the determination of process-related impurities in pantoprazole bulk drug and formulations. Braz. J. Pharm. Sci. 2013;49:175–184. [Google Scholar]
- 19.Raman N.V.V.S.S., Reddy K.R., Prasad A.V.S.S. Validated chromatographic methods for the determination of process related toxic impurities in pantoprazole sodium. Chromatographia. 2008;68:481–484. [Google Scholar]
- 20.United States Pharmacopeial Convention USP Reference Standards. http://static.usp.org/doc/referenceStandards/dailycatalog.pdf
- 21.International Council for harmonisation of technical requirements for pharmaceuticals for human use (ICH), draft revised guidance on impurities in new drug substances, Q3A(R) Fed. Regist. 2000;65(140):45085. [Google Scholar]
- 22.International Council for harmonisation of technical requirements for pharmaceuticals for human use (ICH), draft revised guidance on impurities in new drug substances, Q3B(R) Fed. Regist. 2000;65(139):44791. [Google Scholar]
- 23.International Council for harmonisation of technical requirements for pharmaceuticals for human use (ICH), impurities guidelines for residual solvents, Q3(C) Fed. Regist. 1997;62(247):67377. [Google Scholar]
- 24.International Council for harmonisation of technical requirements for pharmaceuticals for human use (ICH), revised guidance on impurities in new drug substances, Q3A(R), availability. Notice. Fed. Regist. 2003;68:6924. [PubMed] [Google Scholar]
- 25.Awasthi A.K., Kumar B., Aga M.A. An efficient and facile synthesis of D-cycloserine substantially free from potential impurities. Chem. Heterocycl. Comp. 2017;53:1248–1253. [Google Scholar]
- 26.Awasthi A.K., Kumar B., Aga M.A. An efficient, facile synthesis of etoricoxib substantially free from impurities: isolation, characterization and synthesis of novel impurity. ChemistrySelect. 2017;2:9722–9725. [Google Scholar]
- 27.Venumanikanta K., Kumar P., Reddy B.M. A simple and commercially viable process for improved yields of metopimazine, a dopamine D2-receptor antagonist. Org. Process Res. Dev. 2017;21:720–731. [Google Scholar]
- 28.Sankareswaran S., Mannam M., Chakka V. Identification and control of critical process impurities: an improved process for the preparation of dolutegravir sodium. Org. Process Res. Dev. 2016;20:1461–1468. [Google Scholar]
- 29.Kumar N., Devineni S.R., Gajjala P.R. Four process-related potential new impurities in ticagrelor: identification, isolation, characterization using HPLC, LC/ESI-MSn, NMR and their synthesis. J. Pharmaceut. Biomed. Anal. 2016;120:248–260. doi: 10.1016/j.jpba.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Kumar N., Devineni S.R., Singh G. Identification, isolation and characterization of potential process-related impurity and its degradation product in Vildagliptin. J. Pharmaceut. Biomed. Anal. 2016;119:114–121. doi: 10.1016/j.jpba.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 31.Kumar N., Devineni S.R., Dubey S.K. Potential impurities of anxiolytic drug, clobazam: identification, synthesis and characterization using HPLC, LC-ESI/MSn and NMR. J. Pharmaceut. Biomed. Anal. 2017;137:268–278. doi: 10.1016/j.jpba.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 32.Neelamegam R., Palatnik M.T., Rini J.F. Dimerization of phenols and naphthols using an aqueous sodium hypochlorite. Tetrahedron Lett. 2010;51:2497–2499. [Google Scholar]
- 33.Awasthi A.K., Kumar L., Tripathi P. Environmentally benign and facile process for the synthesis of pantoprazole sodium sesquihydrate: phase transformation of pantoprazole sodium heterosolvate to pantoprazole sodium sesquihydrate. ACS Omega. 2017;2:5460–5469. doi: 10.1021/acsomega.7b00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.