Abstract
The present study investigated the effect of the treatment with the angiotensin II type 1 receptor (AT1) antagonist losartan in the depressive-like state and memory impairment evoked by exposure to either homotypic (i.e., repeated exposure to the same type of stressor) or heterotypic (i.e., exposure to different aversive stimuli) chronic stressors in rats. For this, male Wistar rats were subjected to a 10 days regimen of repeated restraint stress (RRS, homotypic stressor) or chronic variable stress (CVS, heterotypic stressor) while being concurrently treated daily with losartan (30 mg/kg/day, p.o.). Depressive-like state was evaluated by analysis of the alterations considered as markers of depression (decreased sucrose preference and body weight and coat state deterioration), whereas cognitive non-emotional performance was tested using the novel object recognition (NOR) test. Locomotor activity was also evaluated in the open field test. Both RRS and CVS impaired sucrose preference and caused coat state deterioration, whereas only CVS impaired body weight gain. Besides, RRS impaired short-term memory (but not long-term memory) in the NOR test. Neither depressive-like state nor memory impairment evoked by the chronic stressors was affected by the treatment with losartan. Nevertheless, CVS increased the locomotion, which was inhibited by losartan. Taken together, these results provide evidence that the chronic treatment with losartan does not affect the depressive-like state and memory impairment evoked by either homotypic or heterotypic chronic stress regimens in rats.
Keywords: losartan, depression, locomotion, anhedonia, memory, restraint stress, chronic variable stress, rats
Introduction
Clinical and preclinical studies have provided evidence of the emotional stress as a prominent factor predisposing to depression (Mazure, 1998; Hammen, 2005; Willner, 2005; Grippo and Johnson, 2009; Buynitsky and Mostofsky, 2009; Liu and Alloy, 2010; Carnevali et al., 2017). Exposure to adverse events is also related to learning and memory impairment (Buynitsky and Mostofsky, 2009; Wolf, 2009; Quaedflieg and Schwabe, 2018). In this sense, several studies have indicated that the impact of stress is determined by characteristics of the stressor stimulus, such as chronicity, predictability, controllability, and severity (Koolhaas et al., 2011; Crestani, 2016). Studies in rodents have explored the influence of predictability by comparing the effect of chronic stressors involving daily exposure to the same stressor (i.e., homotypic/predictable) versus different aversive stimuli (i.e., heterotypic/unpredictable) (Magariños and McEwen, 1995; Marin et al., 2007; Kopp et al., 2013; Pastor-Ciurana et al., 2014; Duarte et al., 2015; Costa-Ferreira et al., 2016). These studies have typically used protocols of repeated restraint stress (RRS) as homotypic stressor, whereas the chronic variable stress (CVS) is often employed as a heterotypic stressor (Crestani, 2016). Studies comparing CVS and RRS have demonstrated that the former evokes more severe changes on somatic parameters (e.g., adrenal hypertrophy and thymic involution) and hypothalamic–pituitary–adrenal (HPA) axis activity (Magariños and McEwen, 1995; Haile et al., 2001; Marin et al., 2007; Kopp et al., 2013; Pastor-Ciurana et al., 2014; Costa-Ferreira et al., 2016), which is possibly related to the habituation process identified in the RRS as consequence of the repeated exposure to the same stressor (Herman, 2013; Crestani, 2016; McCarty, 2016). Differences in depression- and anxiety-like behaviors and memory are less understood since a limited number of studies compared the effects of RRS versus CVS on these behaviors. For the best of our knowledge, the only study comparing RRS and CVS on behavioral responses was a recent study from our group in which we identified increase in anxiety-like behaviors in female (but not male) rats exposed to either RRS or CVS (Vieira et al., 2018). However, differences in depression-like behaviors and memory have never been evaluated.
Angiotensin II (Ang II) is an active peptide of the renin-angiotensin system (RAS) that has been historically implicated in the cardiovascular and hydroelectrolytic control (Hall, 2003; Karnik et al., 2015). Several reports, however, have demonstrated that in addition to its formation and action in the circulation as a blood-borne hormone, Ang II is also synthetized within the central nervous system (Saavedra, 2005). Indeed, RAS components and Ang II receptors (i.e., AT1 and AT2 receptors) were identified in limbic structures controlling stress responses (Wright and Harding, 2011; Bali and Jaggi, 2013). In this sense, previous studies have documented an involvement of the Ang II acting via activation of the AT1 receptor in the etiology of stress-evoked diseases (Watanabe et al., 1998; Saavedra et al., 2004; Saavedra et al., 2011; Bali and Jaggi, 2013; Ayyub et al., 2016; Fontes et al., 2016). The mechanisms related to involvement of Ang II/AT1 receptor in complications evoked by stress are not completely understood, but neuroinflammation, dysregulated hormonal and sympathetic responses, and oxidative stress might be involved (Saavedra et al., 2011; Labandeira-Garcia et al., 2017; Saavedra, 2017). Accordingly, the therapeutic use of AT1 receptor antagonists in the treatment of stress-related pathologies has been discussed (Quijano and Arango, 1979; Saavedra, 2005; Saavedra, 2017). However, the effects of AT1 receptor antagonists in behavioral responses to stress are still not fully understood.
Previous studies identified that systemic administration of AT1 receptor antagonists inhibited the anxiogenic-like effect evoked by both RRS and CVS (Pechlivanova et al., 2011; Ping et al., 2014; Ayyub et al., 2016; Wincewicz et al., 2016; Ranjbar et al., 2018). AT1 receptor antagonists also prevented the increase of immobility in the forced swimming test and tail-suspension test, as well as the decreased sucrose preference evoked by protocols of CVS in mice (Ping et al., 2014; Ayyub et al., 2016), thus indicating an antidepressant-like effect. However, depressive-like behaviors were only investigated in mice; and the effect of AT1 receptor antagonists in depressive-like effect to homotypic stressors has never been evaluated. Regarding stress-evoked memory impairment, previous studies identified that memory impairment in the novel object recognition (NOR) test and passive avoidance situation evoked by a RRS protocol were inhibited by treatment with AT1 receptor antagonists in rats (Braszko et al., 2013; Wincewicz and Braszko, 2014; Wincewicz et al., 2016). Treatment with AT1 receptor antagonist also inhibited impairment of spatial memory evoked by RRS in rats (Wincewicz and Braszko, 2015). Nevertheless, the influence of AT1 receptor antagonists in memory impairment evoked by heterotypic stressors has never been evaluated.
The results described above indicate important effects of AT1 receptor antagonists in behavioral changes evoked by chronic stress. However, some important issues regarding the effects of AT1 receptor antagonists in stress-evoked depressive-like state and memory impairment are still to be addressed, including: i) comparison of the behavioral responses evoked by homotypic versus heterotypic stressors; ii) evaluation of depressive-like responses to stress in rats, including analysis of behaviors other than anhedonia and despair (e.g., self-care); iii) investigation of depressive-like effect evoked by homotypic stressors; and iv) evaluation of memory impairment evoked by heterotypic stressors. Therefore, in the present study we attempted to investigate the effect of the systemic treatment with the AT1 receptor antagonist losartan in depressive-like state and memory impairment evoked by exposure to either the heterotypic stressor CVS or the homotypic stressor RRS. The potential influence of unspecific effects of the stressors and/or losartan treatment on locomotor activity was also evaluated.
Materials and Methods
Animals
One hundred twenty 60-day-old male Wistar rats weighing 200 ± 10 g were used in this study. The animals were obtained from the animal breeding facility of the São Paulo State University-UNESP (Botucatu, SP, Brazil), and were housed in collective plastic cages (four rats/cage). The animals remained in temperature-controlled room at 24°C with light–dark cycle 12:12 h (lights on between 7:00 a.m. and 7:00 p.m.) with free access to water and standard laboratory food in the Animal Facility of the Laboratory of Pharmacology-UNESP (Araraquara, SP, Brazil). The procedures and protocols were approved by the local Ethical Committee for Use of Animals (approval # 32/2014), which complies with Brazilian and international guidelines for animal use and welfare.
Chronic Stress Protocols
The protocols of RRS and CVS were based on previous studies from our group, which we reported behavioral, neuroendocrine, cardiovascular, and somatic changes following exposure to these chronic stressors (Marin et al., 2007; Cruz et al., 2012; Duarte et al., 2015; Costa-Ferreira et al., 2016; Vieira et al., 2018). In this sense, RRS was used as a homotypic stressor and CVS was chosen as a heterotypic stress regimen. For the RRS, animals were restrained in opaque plastic cylinders (15 cm length and 5.5 cm internal diameter) 1 h daily (starting always at 9:00 a.m.) for 10 consecutive days. For the CVS, animals were exposed to different stressors in a variable schedule for 10 consecutive days, according to protocol employed in our laboratory ( Table 1 ) (Duarte et al., 2015; Costa-Ferreira et al., 2016; Vieira et al., 2018). All stress sessions were performed in an adjacent room to the animal facility. RRS and CVS started simultaneously, and during this period, animals of the control groups were left undisturbed, except for cleaning the cages and pharmacological treatment, in the animal facility.
Table 1.
Protocol of CVS.
| Day | Stress type and schedule |
|---|---|
| 1 | 10:00 AM, restraint stress, 60 min; 7:00 PM, humid sawdust, overnight |
| 2 | 3:00 PM, cold (4°C) isolation, 60 min; 7:00 PM, lights on, overnight |
| 3 | 12:00 AM, lights off, 180 min; 3:00 PM, swim stress, 4 min |
| 4 | 7:30 AM, humid sawdust, all day; 7:00 PM, food/water deprivation, overnight |
| 5 | 1:00 PM, swim stress, 3 min; 7:00 PM, isolation housing, overnight |
| 6 | 2:00 PM cold (4°C) isolation, 15 min; 3:00 PM, lights off, 120 min |
| 7 | 7:00 PM, humid sawdust and lights on, overnight |
| 8 | 7:00 PM, isolation and food/water deprivation, overnight |
| 9 | 4:00 PM, restraint stress, 60 min; 7:00 PM, lights on, overnight |
| 10 | 8:00 AM, swim stress, 4 min; 10:00 h, restraint stress, 60 min |
Pharmacological Treatment
The selective AT1 receptor antagonist losartan was purchased from Sigma–Aldrich (St. Louis, MO, USA), and was diluted in saline solution (NaCl 0.9%). The pharmacological treatment with losartan (30 mg/kg/day) or vehicle (saline) started on the first day of the stress protocols and was continued daily for 10 consecutive days. The treatment was based on previous reports that losartan at this dose was effective in inhibiting the increase in plasma glucose, norepinephrine, epinephrine, and corticosterone levels; as well as the cardiovascular dysfunctions, evoked by stress in rats (Üresin et al., 2004; Costa-Ferreira et al., 2016). Losartan or vehicle was given once daily by gavage at 8:00 a.m. (Costa-Ferreira et al., 2016).
Sucrose Consumption Test
The sucrose preference test was used as a behavioral test for evaluation of anhedonia. The protocol did not include periods of food and water deprivation (D’Aquila et al., 1997; Papp, 2012; Antoniuk et al., 2019). The animals were housed individually, and the test consisted of two phases: i) habituation and ii) testing. The habituation phase was performed in 2 days. On the first day, the animals were exposed to two drinking bottles containing sucrose (2%, v/v), which was placed at the beginning of the dark phase of the light/dark cycle (i.e., 7:00 p.m.) and kept for a period of 24 h (habituation: day 1). Thereafter, the two bottles containing sucrose were replaced by other two containing water, and the animals had access to bottles containing water for 24 h (habituation: day 2).
After completion of the habituation phase, the animals were tested for sucrose preference (testing phase). For this, two drinking bottles were offered at the beginning of the dark phase of the light/dark cycle (i.e., 7:00 p.m.): one containing sucrose solution (2%, v/v) and one containing water. Sucrose preference was calculated by weighting the bottles (values obtained in grams) at the beginning of the exposure and after 3 h (3 h sucrose preference) and 24 h (24 h sucrose preference). The percentage of sucrose preference was calculated as the ratio of sucrose solution consumed over the total amount of fluid consumed (water + sucrose solution) × 100.
To control liquid lost by spillage or evaporation, the weight of drinking bottles placed in empty cages at the same time as the solutions were offered to animals was evaluated, and the values were discounted from the amount consumed by the animals.
Coat State Evaluation
Coat state deterioration has been described as a reliable and well-validated index of depressed-like state, which parallel symptoms identified in human depression of loss of motivation to maintain personal hygiene and self-care (Santarelli, 2003; Alonso et al., 2004; Nollet et al., 2013). Coat state was evaluated using a scale from 3 to 0, wherein 3 represents a healthy and well-cared fur while 0 represents a sick and dirty fur, with hair loss and piloerection. Intermediate states were scored as variations of 0.5 point. Coat state was evaluated in a blinded manner in the last day of the chronic stress protocols.
Open Field Test
The open field (OF) test was used for evaluation of locomotion (Prut and Belzung, 2003; Grippo et al., 2014). The OF consisted of a PLEXIGLAS chamber measuring 54 cm (width) × 54 cm (length) × 30 cm (height). A central area in the middle of the arena measuring 24 cm (width) × 24 cm (length) was defined as an exposed field and is referred as “center.” Rats were individually placed in the middle of the arena and were allowed to explore freely the OF for 5 min. Analysis included measures of the distance travelled in central (central locomotion) and peripheral area (peripheral locomotion), as well as the total distance travelled (i.e., center + periphery) (total locomotion). All sessions were videotaped (Webcam LifeCam Cinema HD 720p Microsoft® using software Microsoft LifeCam version 3.22) and analysis was realized in a blinded manner using the software ANY-maze® (Stoelting, Wood Dale, Illinois, USA).
Novel Object Recognition Test
The learning performance and non-emotional memory of the animals were examined through the NOR test, which was adapted from Carey et al. (2009). The NOR test is based on the spontaneous exploration of environment, with premise that animals spend more time exploring a new object than a familiar one (Antunes and Biala, 2012).
Initially, the animals were adapted for 5 min to the apparatus (the same apparatus described in the OF test) wherein the NOR test was performed. Twenty-four hours later, phase I started. At this stage, objects A and A’ (a pair of transparent rectangular glass bottles) were centered at the ends of the apparatus, 10 cm from the walls. In phase II, carried out 10 min later, the object A’ was replaced by a colorful cube of 7 cm height × 7 cm width, called object B, and the short-term memory (SM) was assessed. In phase III, performed 24 h after phase II, the long-term memory (LM) was evaluated on the same apparatus by changing the object B by a new object, a blue rectangle of 8 cm height × 10 cm width called object C. The same animals were evaluated for short- and long-term memory.
For each phase, the time the animals explored each object was recorded (Webcam LifeCam Cinema HD 720p Microsoft® using software Microsoft LifeCam version 3.22), and the exploration was blindly analyzed using the software X-PloRat (version 2005, 1.1.0). For each object, the interaction period was defined as the time while the animal remained in physical contact with the object. Data were presented as the recognition index, which was determined by time spent on the new object divided by the time spent on both objects.
Experimental Design
In each experiment, the rats were divided into six groups: i) control vehicle, ii) control losartan, iii) RRS vehicle, iv) RRS losartan, v) CVS vehicle, and vi) CVS losartan. The protocols of chronic stress and the pharmacological treatment with losartan started on the same day and continued for 10 consecutive days.
Experiment 1: Effects of Chronic Stress and/or Losartan Treatment in the Depression-Like Behaviors and Locomotion
The six experimental groups (n = 10/group) were subjected to the 10 days regimen of RRS or CVS while being concurrently treated daily with the selective AT1 receptor antagonist losartan (30 mg/kg/day, p.o.). Four evaluations were performed in the animals in this protocol. Coast state and body weight were evaluated on the 10th day, after the last session of stress/treatment. On the 11th day, the animals of all experimental groups were subjected to OF for evaluation of the locomotion. Lastly, the sucrose consumption test started at the night of the 11th day. At the end of the experiments, the rats were euthanized via anesthetic overdose (urethane, 250 mg/ml/200 g body weight, i.p.).
Experiment 2: Effects of Chronic Stress and/or Losartan Treatment in the Short-Term and Long-Term Memory
Such as in the previous protocol, the six experimental groups (n = 9/group) were subjected to the 10 days regimen of RRS or CVS while being concurrently treated daily with losartan (30 mg/kg/day, p.o.), and the memory was evaluated in the NOR test. On the 11th day, 24 h after the last session of stress/treatment, the animals of all experimental groups were allowed to habituate for 5 min to the apparatus wherein the NOR test was performed (the same apparatus used in the OF test). Twenty-four hours later, the animals were subjected to NOR test for evaluation of short-term memory. Long-term memory was assessed 24 h later. At the end of the experiments, the rats were euthanized via anesthetic overdose (urethane, 250 mg/ml/200 g body weight, i.p.).
Data Analysis
Data were expressed as mean ± SEM. All data were analyzed using the software GraphPad Prism version 7.0 (GraphPad Software Inc., La Jolla, CA, USA). The data were analyzed using two-way ANOVA, with stress and pharmacological treatment as independent factors. When statistical differences were identified by two-way ANOVA, Bonferroni post hoc test was performed to assess specific differences between the experimental groups. P < 0.05 was assumed as significant.
Results
Effects of Chronic Stress and/or Losartan Treatment in the Depression-like Behaviors and Locomotion
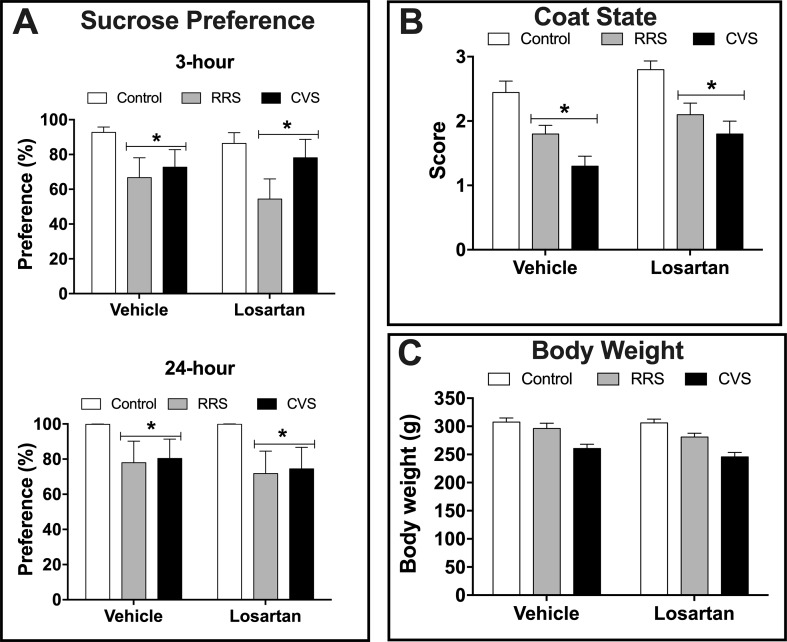
Depressive-like state—Analysis of the sucrose preference indicated effect of stress (3 h: F(2,54) = 4.93, P < 0.01; 24 h: F(2,54) = 3.87, P < 0.02) in both 3 and 24 h consumption analysis, but without influence of losartan treatment (3 h: F(1,54) = 0.33, P > 0.05; 24 h: F(1,54) = 0.24, P > 0.05) and stress × treatment interaction (3 h: F(2,54) = 0.46, P > 0.05; 24 h F(2,54) = 0.06, P > 0.05) ( Figure 1A ). Analysis of the sucrose consumption (data not shown) also indicated effect of stress (3 h: F(2,54) = 5.06, P < 0.009; 24 h: F(2,54) = 4.29, P < 0.01) in analysis of 3 and 24 h, but without influence of losartan treatment (3 h: F(1,54) = 0.07, P > 0.05; 24 h: F(1,54) = 0.40, P > 0.05) and stress × treatment interaction (3 h: F(2,54) = 0.82, P > 0.05; 24 h F(2,54) = 0.65, P > 0.05).
Figure 1.
Depressive-like state in animals treated with either vehicle or losartan control (white bars) and subjected to RRS (gray bars) or CVS (black bars). (A) Sucrose preference (%) evaluated during 3 and 24 h. *P < 0.05 versus respective control group, two-way ANOVA (n = 10/group). (B) Coat state score. *P < 0.05 versus respective control group, two-way ANOVA (n = 10/group). (C) Body weight at the 10th day of stress protocol. Two-way ANOVA (n = 10/group). The bars in all graphs represent the mean ± SEM.
Analysis of coat state deterioration indicated effect of stress (F(2,54) = 21.51, P < 0.0001) and losartan treatment (F(1,54) = 8.25, P < 0.005), but without stress × treatment interaction (F(2,54) = 0.20, P > 0.05) ( Figure 1B ). Analysis of the body weight at the last day of stress protocols (i.e., 10th day) indicated effect of stress (F(2,54) = 25.61, P < 0.0001), but without effect of losartan treatment (F(1,54) = 2.25, P > 0.05) and stress × treatment interaction (F(2,54) = 0.42, P > 0.05) ( Figure 1C ).
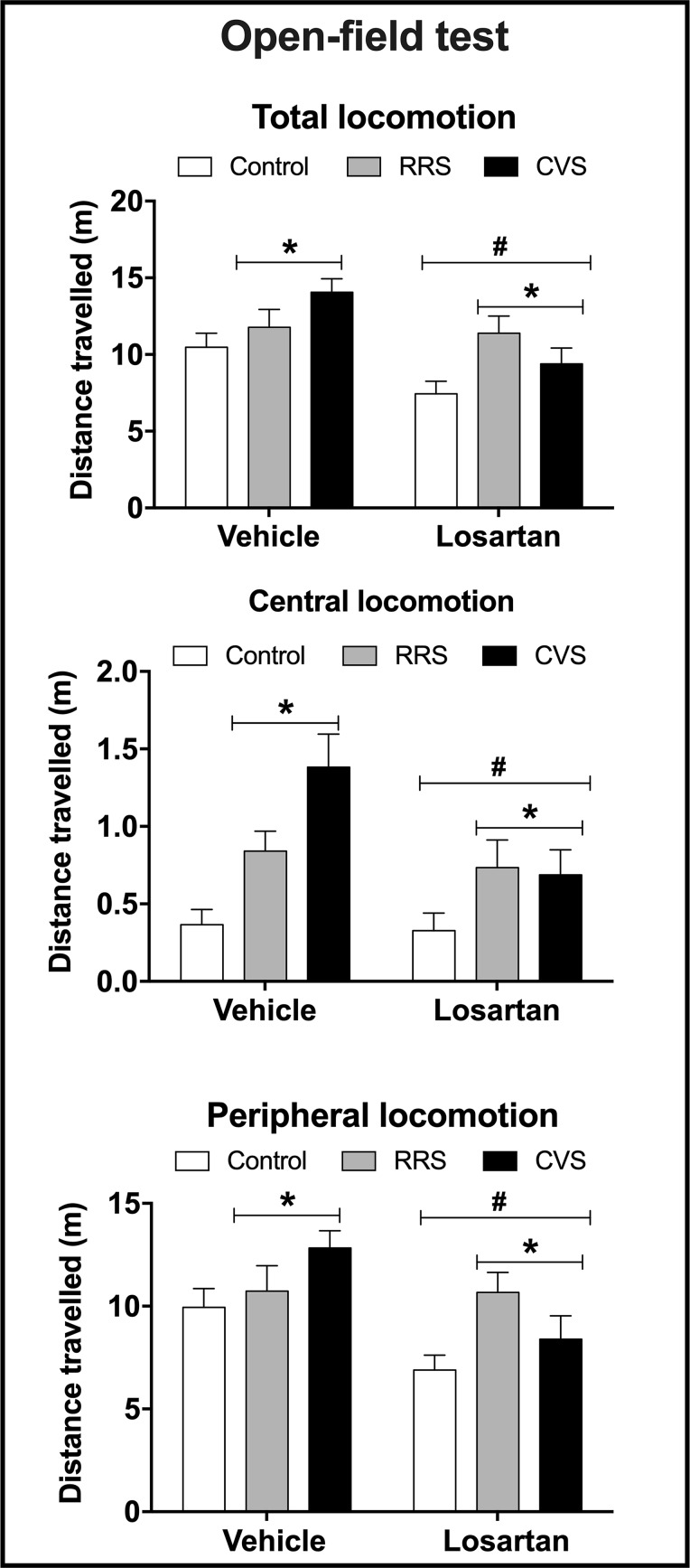
Locomotion—Analysis of the total, central, and peripheral locomotion in the OF test indicated effect of stress (total: F(2,54) = 4.77, P < 0.01; central: F(2,54) = 9.49, P < 0.0004; peripheral: F(2,54) = 3.28, P < 0.04) and losartan treatment (total: F(1,54) = 10.63, P < 0.002; central: F(1,54) = 4.75, P < 0.03; peripheral: F(1,54) = 9.56, P > 0.003), but without stress × treatment interaction (total: F(2,54) = 2.32, P > 0.05; central: F(2,54) = 2.57, P > 0.05; peripheral: F(2,54) = 2.55, P > 0.05) ( Figure 2 ).
Figure 2.
Total locomotion (distance travelled in the periphery + center, top graph) and distance travelled in the center (central locomotion, middle graph) and periphery (peripheral locomotion, bottom graph) in the open field apparatus in animals treated with either vehicle or losartan control (white bars) and subjected to RRS (gray bars) or CVS (black bars). The bars represent the mean ± SEM. *P < 0.05 vs respective control group, #P < 0.05 vs respective vehicle groups. Two-way ANOVA followed by Bonferroni post-hoc test (n = 8–10/group).
Effects of Chronic Stress and/or Losartan Treatment in the Short-Term and Long-Term Memory
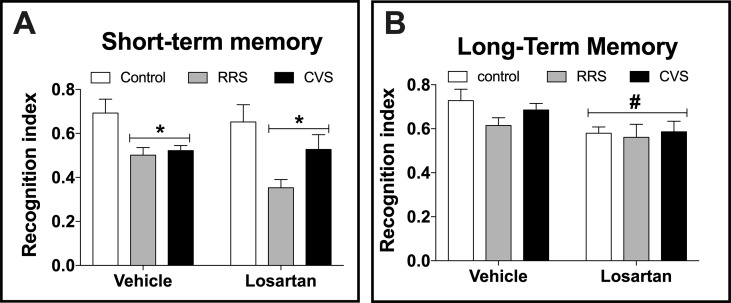
Analysis of the short-term memory indicated effect of stress (F(2,48) = 10.54, P < 0.0002), but without influence of losartan treatment (F(1,48) = 0.16, P > 0.05) and stress × treatment interaction (F(2,48) = 0.33, P > 0.05) ( Figure 3A ). Analysis of the long-term memory indicated effect of losartan treatment (F(1,48) = 7.99, P < 0.006), but without influence of stress (F(2,48) = 1.2, P > 0.05) and stress × treatment interaction (F(2,48) = 0.58, p > 0.05) ( Figure 3B ).
Figure 3.
Cognitive non-emotional performance in the novel object recognition (NOR) test in animals treated with either vehicle or losartan control (white bars) and subjected to RRS (gray bars) or CVS (black bars). (A) Object discrimination rate at day 1 of NOR test (short-term memory). *P < 0.05 versus respective control group, two-way ANOVA (n = 9/group). (B) Object discrimination rate at day 2 of NOR test (long-term memory). #P < 0.05 versus vehicle groups, two-way ANOVA (n = 9/group). The bars in all graphs represent the mean ± SEM.
Discussion
The present study is the first to compare the effects of homotypic versus heterotypic chronic stressors in depression and memory. Our results are in line with previous evidence that both CVS and RRS evoked depressive-like state and memory impairment (Willner, 2005; Buynitsky and Mostofsky, 2009; Willner, 2017). Nevertheless, as stated above, previous studies comparing RRS vs CVS have demonstrated that increase in HPA axis activity, adrenal hypertrophy, and thymic involution are mainly observed after CVS exposure, whereas RRS minimally affects these parameters (Magariños and McEwen, 1995; Haile et al., 2001; Marin et al., 2007; Kopp et al., 2013; Pastor-Ciurana et al., 2014; Costa-Ferreira et al., 2016). These differences have been proposed to be related to the habituation process of the HPA axis activation identified in RRS, which is an important adaptive response that limits the long-term impact of chronic stressors (Herman, 2013; Crestani, 2016; McCarty, 2016). However, this habituation is more consistently observed in parameters related to HPA axis than other biological responses (Crestani, 2016). For instance, several studies have indicated that habituation of cardiovascular responses upon repeated exposure to restraint stress is limited or absent (McDougall et al., 2000; Conti et al., 2001; Daubert et al., 2012; Benini et al., 2019). Accordingly, similar cardiovascular and autonomic changes were identified following exposure to either RRS or CVS (Duarte et al., 2015; Costa-Ferreira et al., 2016; Vieira et al., 2018). Our results are further supported by evidence that RRS and CVS evoke similar morphological changes in limbic structures (Magariños and McEwen, 1995). Therefore, data reported here are consistent with the idea that habituation, which limits the long-term impact of stress (Herman, 2013; McCarty, 2016), is a specific response of some biological system rather than a general body response; so that some dysfunctions (e.g., depression and memory impairment) might be similarly evoked by both homotypic and heterotypic chronic stressors.
Evaluation of the depression-like state in the present study included analysis of a series of changes that are commonly used as markers of depression in rodents, such as decreased sucrose preference and body weight gain and coat state deterioration (Willner, 2005; Nollet et al., 2013). Treatment with losartan did not affect any of the depression-like responses evaluated in the present study. Our findings contrast with previous evidence that systemic treatment (p.o.) with valsartan inhibited the decrease of sucrose preference evoked by a CVS protocol in mice (Ping et al., 2014). Systemic treatment with either valsartan or irbesartan also inhibited the CVS-evoked increase of immobility in the forced swimming test and tail-suspension test in mice (Ping et al., 2014; Ayyub et al., 2016). To the best of our knowledge, present study is the first to investigate the effect of the treatment with an AT1 receptor antagonist in stress-evoked depression-like state in rats, so that discrepancy with previous studies might be explained by the different species tested. Differences in experimental procedures and parameters evaluated might also explain the discrepancies. For instance, present study provides the first evidence of the treatment with an AT1 receptor antagonist in stress-evoked coat state deterioration. Besides, effect of these drugs in depression-like state evoked by homotypic stressors has never been investigated previously. However, differences in the AT1 receptor antagonists employed in the different studies seem not to explain the different findings. For instance, the ki values of the several AT1 receptor antagonists are equivalent (Alexander et al., 2017), and the dose of losartan used in the present study (30 mg/kg) is similar to those of valsartan (5–40 mg/kg) and irbesartan (40 mg/kg) employed previously (Ping et al., 2014; Ayyub et al., 2016). Besides, lipophilicity and brain penetration of these antagonists do not differ (Michel et al., 2013). Finally, although irbesartan and valsartan exhibited insurmountable antagonism and losartan evoked surmountable antagonist, the active losartan metabolite EXP3174 presented an insurmountable antagonism (de Gasparo et al., 2000; Michel et al., 2013). Accordingly, previous evidence confirmed the efficacy of the dose of losartan used in the present study (see discussion below). However, it is important to mention that treatment in previous studies was longer (28 days) (Ping et al., 2014; Ayyub et al., 2016) in relation to that employed in the present study (10 days), which may contribute to discrepancies.
We also identified that treatment with losartan did not affect the decreased discrimination rates in the NOR test evoked by the chronic stressors. This finding contrasts with previous evidence that systemic treatment with either telmisartan or candesartan inhibited the impairment of short memory evaluated in the NOR test evoked by RRS in rats (Braszko et al., 2013; Wincewicz and Braszko, 2014; Wincewicz et al., 2016). Treatment with telmisartan also inhibited the impairment of spatial memory evoked by RRS in rats (Wincewicz and Braszko, 2015). Although telmisartan is more lipophilic than the other antagonists [i.e., it should be better able to penetrate central nervous system (CNS)], lipophilicity and brain penetration of candesartan and losartan/EXP3174 are similar (Michel et al., 2013). Type of antagonism also seems not to explain the different findings once despite candesartan exhibited insurmountable antagonism, and telmisartan and losartan evoked surmountable antagonist (de Gasparo et al., 2000; Michel et al., 2013). Therefore, an important difference that might explain the discrepancy is treatment time, which was longer (21 days) in previous studies in relation to the present report (10 days) (Braszko et al., 2013; Wincewicz and Braszko, 2014; Wincewicz et al., 2016). Besides, previous studies employed longer protocols of RRS (21 days vs 10 days) with longer restraint sessions (2–2.5 h vs 1 h) (Braszko et al., 2013; Wincewicz and Braszko, 2014; Wincewicz et al., 2016). To the best of our knowledge, present study provides the first evidence of the treatment with an AT1 receptor antagonist in CVS-evoked memory impairment.
Previous studies reported dose-dependent binding of losartan to the AT1 receptor in brain areas within the blood–brain barrier following peripheral administration of doses ranging from 1 to 100 mg/kg (Song et al., 1991; Zhuo et al., 1994; Wang et al., 2003). These findings are further supported by functional evidence that losartan administrated peripherally (at similar doses to that used in the present study) inhibited the pressor response, water intake, and vasopressin release in the circulation evoked by intracerebroventricular administration of Ang II (Polidori et al., 1996; Culman et al., 1999). Systemic administration of losartan at the same dose used in the present study also evoked antidepressant-like effect in nonstressed animals (Diniz et al., 2018). Furthermore, stress is a condition that might promote increase in blood–brain barrier (BBB) permeability (Skultétyová et al., 1998), thus facilitating the penetration of drugs within the brain. Therefore, the absence of effect of the pharmacological treatment with losartan in depressive-like state and memory impairment evoked by RRS and CVS in the present does not seem to be due to infectivity of the pharmacological treatment. Accordingly, we reported recently that the same treatment with losartan prevented the cardiovascular and autonomic changes evoked by either RRS or CVS (Costa-Ferreira et al., 2016).
Since the behavioral tests employed in the present study (i.e., NOR and sucrose preference) might be influenced by changes in exploratory behavior, the effect of the chronic stressors and the losartan treatment on the locomotor activity in the OF test was also evaluated. Although the findings regarding the effect of CVS in the OF are controversial (Albonetti and Farabollini, 1992; Katz et al., 1981; D’Aquila et al., 2000; Song et al., 2018), our results are in line with previous evidence that this heterotypic stressor increases locomotor activity (Harris, 1997; Grønli et al., 2005). The absence of change following RRS has been proposed to be related to the habituation process (Albonetti and Farabollini, 1992). The CVS-evoked hyperlocomotion is not related to depressive-like state and memory impairment, since a decrease rather than an increase in behavioral responses was identified in sucrose preference and NOR test in animals subjected to the CVS. Interestingly, losartan treatment inhibited the increase in locomotion evoked by CVS, which is in line with previous evidence that irbesartan inhibited the CVS-evoked hypolocomotion in the OF (Ayyub et al., 2016). Besides, it confirms the efficacy of the pharmacological treatment with losartan.
The results reported in the present study also indicated that our treatment with losartan decreased the locomotor activity and impaired the long-term (but not the short-term) memory independently of the stress exposure. Previous studies have already shown that treatment with losartan for either 10 days or 4 or 9 weeks decreased the exploratory activity (Pechlivanova et al., 2011; Tchekalarova et al., 2014; Tchekalarova et al., 2016), thus supporting our findings. Regarding the effects of AT1 receptor antagonists in memory, the data are controversial, with some results indicating improvement while others did not identify effects (Braszko, 2005; Braszko et al., 2013; Wincewicz and Braszko, 2014; Wincewicz and Braszko, 2015; Wincewicz et al., 2016; ). Therefore, our data are in line with previous evidence that losartan might impair non-emotional memory.
In summary, the present findings provide evidence that chronic treatment with losartan does not affect the depressive-like state and memory impairment evoked by either homotypic or heterotypic chronic stress regimens in rats. Nevertheless, our results suggest that losartan inhibits hyperlocomotion evoked by heterotypic stressors. Importantly, this study indicates the necessity of further studies evaluating the efficacy of AT1 receptor antagonists in treatment of stress-evoked dysfunctions. Indeed, more evidence comparing species (e.g., rats vs mice), stress protocols (e.g., homotypic vs heterotypic), treatment time, and AT1 receptor antagonists (e.g., surmountable vs insurmountable antagonists) is necessary to provide more conclusive information regarding the efficacy of AT1 receptor antagonists in the treatment of stress-evoked depressive-like state and memory impairment.
Data Availability Statement
All datasets generated for this study are included in the manuscript and the supplementary files.
Ethics Statement
This study was carried out in accordance with the recommendations of Brazilian and international guidelines. The protocol was approved by the Ethical Committee for Use of Animals of the School of Pharmaceutical Sciences—UNESP.
Author Contributions
WC-F and CC contributed to the conception and design of the work. WC-F, GM-S, LG-S, MM, and CC contributed to the acquisition, analysis, and interpretation of data. WC-F and CC drafted the manuscript. GM-S, LG-S and MM critically revised the manuscript, and CC approved the final version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Elisabete Z.P. Lepera and Rosana F.P. Silva for technical assistance. This work was supported by FAPESP (grants # 2015/05922-9 and 2017/19249-0), CNPq (grant # 456405/2014-3) and Programa de Apoio ao Desenvolvimento Científico da Faculdade de Ciências Farmacêuticas da UNESP—PADC. CC is a CNPq research fellow (process # 305583/2015-8).
References
- Albonetti M. E., Farabollini F. (1992). Behavioural responses to single and repeated restraint in male and female rats. Behav. Processes 28, 97–109. 10.1016/0376-6357(92)90052-F [DOI] [PubMed] [Google Scholar]
- Alexander S. P., Christopoulos A., Davenport A. P., Kelly E., Marrion N. V., Peters J. A., et al. (2017). The concise guide to pharmacology 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R., Griebel G., Pavone G., Stemmelin J., Le Fur G., Soubrié P. (2004). Blockade of CRF1 or V1b receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol. Psychiatry 9, 278–286. 10.1038/sj.mp.4001464 [DOI] [PubMed] [Google Scholar]
- Antoniuk S., Bijata M., Ponimaskin E., Wlodarczyk J. (2019). Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci. Biobehav. Rev. 99, 101–116. 10.1016/j.neubiorev.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Antunes M., Biala G. (2012). The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110. 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyub M., Najmi A., Akhtar M. (2016). Protective effect of irbesartan an angiotensin (at1) receptor antagonist in unpredictable chronic mild stress induced depression in mice. Drug Res. (Stuttg) 67, 59–64. 10.1055/s-0042-118172 [DOI] [PubMed] [Google Scholar]
- Bali A., Jaggi A. S. (2013). Angiotensin as stress mediator: role of its receptor and interrelationships among other stress mediators and receptors. Pharmacol. Res. 76, 49–57. 10.1016/j.phrs.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Benini R., Oliveira L. A., Gomes-de-Souza L., Crestani C. C. (2019). Habituation of the cardiovascular responses to restraint stress in male rats: influence of length, frequency and number of aversive sessions. Stress 22, 151–161. 10.1080/10253890.2018.1532992 [DOI] [PubMed] [Google Scholar]
- Braszko J. J. (2005). Valsartan abolishes most of the memory-improving effects of intracerebroventricular angiotensin II in rats. Clin. Exp. Hypertens. 27, 635–649. 10.1080/10641960500298723 [DOI] [PubMed] [Google Scholar]
- Braszko J. J., Wincewicz D., Jakubów P. (2013). Candesartan prevents impairment of recall caused by repeated stress in rats. Psychopharmacology (Berl) 225, 421–428. 10.1007/s00213-012-2829-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynitsky T., Mostofsky D. I. (2009). Restraint stress in biobehavioral research: recent developments. Neurosci. Biobehav. Rev. 33, 1089–1098. 10.1016/j.neubiorev.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Carey A. N., Lyons A. M., Shay C. F., Dunton O., McLaughlin J. P. (2009). Endogenous opioid activation mediates stress-induced deficits in learning and memory. J. Neurosci. 29, 4293–4300. 10.1523/JNEUROSCI.6146-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L., Montano N., Statello R., Sgoifo A. (2017). Rodent models of depression-cardiovascular comorbidity: bridging the known to the new. Neurosci. Biobehav. Rev. 76, 144–153. 10.1016/j.neubiorev.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Conti L. H., Shannon M. H., Murry J. D., Printz M. P. (2001). Repeated restraint stress-induced increase in baroreceptor reflex sensitivity: role of corticotropin-releasing factor. Neuropeptides 35, 71–81. 10.1054/npep.2001.0847 [DOI] [PubMed] [Google Scholar]
- Costa-Ferreira W., Vieira J. O., Almeida J., Gomes-de-Souza L., Crestani C. C. (2016). Involvement of type 1 angiontensin ii receptor (at1) in cardiovascular changes induced by chronic emotional stress: comparison between homotypic and heterotypic stressors. Front. Pharmacol. 7, 262. 10.3389/fphar.2016.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani C. C. (2016). Emotional stress and cardiovascular complications in animal models: a review of the influence of stress type. Front. Physiol. 7, 251. 10.3389/fphys.2016.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F. C., Marin M. T., Leão R. M., Planeta C. S. (2012). Behavioral and neuroendocrine effects of the exposure to chronic restraint or variable stress in early adolescent rats. Int. J. Dev. Neurosci. 30, 19–23. 10.1016/j.ijdevneu.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Culman J., von Heyer C., Piepenburg B., Rascher W., Unger T. (1999). Effects of systemic treatment with irbesartan and losartan on central responses to angiotensin II in conscious, normotensive rats. Eur. J. Pharmacol. 367, 255–265. 10.1016/S0014-2999(98)00983-2 [DOI] [PubMed] [Google Scholar]
- D’Aquila P. S., Newton J., Willner P. (1997). Diurnal variation in the effect of chronic mild stress on sucrose intake and preference. Physiol. Behav. 62, 421–426. 10.1016/S0031-9384(97)00042-5 [DOI] [PubMed] [Google Scholar]
- D’Aquila P. S., Peana A. T., Carboni V., Serra G. (2000). Exploratory behaviour and grooming after repeated restraint and chronic mild stress: effect of desipramine. Eur. J. Pharmacol. 399 (1), 43–47. 10.1016/S0014-2999(00)00332-0 [DOI] [PubMed] [Google Scholar]
- Daubert D. L., McCowan M., Erdos B., Scheuer D. A. (2012). Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats. J. Physiol. 590, 4881–4895. 10.1113/jphysiol.2012.232314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. (2000). International union of pharmacology. Pharmacol. Rev. 52, 415–72. [PubMed] [Google Scholar]
- Diniz C. R. A. F., Casarotto P. C., Fred S. M., Biojone C., Castrén E., Joca S. R. L. (2018). Antidepressant-like effect of losartan involves TRKB transactivation from angiotensin receptor type 2 (AGTR2) and recruitment of FYN. Neuropharmacology 135, 163–171. 10.1016/j.neuropharm.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Duarte J. O., Cruz F. C., Leão R. M., Planeta C. S., Crestani C. C. (2015). Stress vulnerability during adolescence. Psychosom. Med. 77, 186–199. 10.1097/PSY.0000000000000141 [DOI] [PubMed] [Google Scholar]
- Fontes M. A. P., Martins Lima A., Santos R. A. S. (2016). Brain angiotensin-(1–7)/Mas axis: a new target to reduce the cardiovascular risk to emotional stress. Neuropeptides 56, 9–17. 10.1016/j.npep.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Grippo A. J., Johnson A. K. (2009). Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 12, 1–21. 10.1080/10253890802046281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A. J., Ihm E., Wardwell J., McNeal N., Scotti M.-A. L., Moenk D. A., et al. (2014). The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Psychosom. Med. 76, 277–284. 10.1097/PSY.0000000000000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønli J., Murison R., Fiske E., Bjorvatn B., Sørensen E., Portas C. M., et al. (2005). Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol. Behav. 84, 571–7. 10.1016/j.physbeh.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Haile C. N., GrandPre T., Kosten T. (2001). Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl). 154, 213–220. 10.1007/s002130000650 [DOI] [PubMed] [Google Scholar]
- Hall J. E. (2003). Historical perspective of the renin-angiotensin system. Mol. Biotechnol. 24, 27–39. 10.1385/MB:24:1:27 [DOI] [PubMed] [Google Scholar]
- Hammen C. (2005). Stress and depression. Annu. Rev. Clin. Psychol. 1, 293–319. 10.1146/annurev.clinpsy.1.102803.143938 [DOI] [PubMed] [Google Scholar]
- Harris R. (1997). Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol. Behav. 63, 91–100. 10.1016/S0031-9384(97)00425-3 [DOI] [PubMed] [Google Scholar]
- Herman J. P. (2013). Neural control of chronic stress adaptation. Front. Behav. Neurosci. 7, 61. 10.3389/fnbeh.2013.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S. S., Unal H., Kemp J. R., Tirupula K. C., Eguchi S., Vanderheyden P. M. L., et al. (2015). Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli. Pharmacol. Rev. 67, 754–819. 10.1124/pr.114.010454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. J., Roth K. A., Carroll B. J. (1981). Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci. Biobehav. Rev. 5, 247–251. 10.1016/0149-7634(81)90005-1 [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., Bartolomucci A., Buwalda B., de Boer S. F., Flügge G., Korte S. M., et al. (2011). Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 35, 1291–1301. 10.1016/j.neubiorev.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Kopp B. L., Wick D., Herman J. P. (2013). Differential effects of homotypic vs. Physiol. Behav. 122, 246–252. 10.1016/j.physbeh.2013.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Garcia J. L., Rodríguez-Perez A. I., Garrido-Gil P., Rodriguez-Pallares J., Lanciego J. L., Guerra M. J. (2017). Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front. Aging Neurosci. 9, 129. 10.3389/fnagi.2017.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. T., Alloy L. B. (2010). Stress generation in depression: a systematic review of the empirical literature and recommendations for future study. Clin. Psychol. Rev. 30 (5), 582–593. 10.1016/j.cpr.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños A. M., McEwen B. S. (1995). Stress-induced atrophy of apical dendrites of hippocampal ca3c neurons: comparison of stressors. Neuroscience 69, 83–88. 10.1016/0306-4522(95)00256-I [DOI] [PubMed] [Google Scholar]
- Marin M. T., Cruz F. C., Planeta C. S. (2007). Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol. Behav. 90, 29–35. 10.1016/j.physbeh.2006.08.021 [DOI] [PubMed] [Google Scholar]
- Mazure C. M. (1998). Life stressors as risk factors in depression. Clin. Psychol. Sci. Pract. 5, 291–313. 10.1111/j.1468-2850.1998.tb00151.x [DOI] [Google Scholar]
- McCarty R. (2016). Learning about stress: neural, endocrine and behavioral adaptations. Stress 19, 449–475. 10.1080/10253890.2016.1192120 [DOI] [PubMed] [Google Scholar]
- McDougall S. J., Paull J. R. A., Widdop R. E., Lawrence A. J. (2000). Restraint stress: differential cardiovascular responses in wistar-kyoto and spontaneously hypertensive rats. Hypertension 35, 126–129. 10.1161/01.HYP.35.1.126 [DOI] [PubMed] [Google Scholar]
- Michel M. C., Foster C., Brunner H. R., Liu L. (2013). A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol. Rev. 65, 809–848. 10.1124/pr.112.007278 [DOI] [PubMed] [Google Scholar]
- Nollet M., Guisquet A.-M., Belzung C. (2013). Models of depression: unpredictable chronic mild stress in mice. Curr. Protoc. Pharmacol. 61, 5.65.1–5.65.17. 10.1002/0471141755.ph0565s61 [DOI] [PubMed] [Google Scholar]
- Papp M. (2012). “Models of Affective Illness: Chronic Mild Stress in the Rat” in Current Protocols in Pharmacology (Hoboken, NJ, USA: John Wiley & Sons, Inc; ). 10.1002/0471141755.ph0509s57 [DOI] [PubMed] [Google Scholar]
- Pastor-Ciurana J., Rabasa C., Ortega-Sánchez J. A., Sanchís-Ollè M., Gabriel-Salazar M., Ginesta M., et al. (2014). Prior exposure to repeated immobilization or chronic unpredictable stress protects from some negative sequels of an acute immobilization. Behav. Brain Res. 265, 155–162. 10.1016/j.bbr.2014.02.028 [DOI] [PubMed] [Google Scholar]
- Pechlivanova D. M., Stoynev A. G., Tchekalarova J. D. (2011). The effects of chronic losartan pretreatment on restraint stress-induced changes in motor activity, nociception and pentylenetetrazol generalized seizures in rats. Folia Med. (Plovdiv). 53, 69–73. 10.2478/v10153-010-0040-z [DOI] [PubMed] [Google Scholar]
- Ping G., Qian W., Song G., Zhaochun S. (2014). Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol. Biochem. Behav. 124, 5–12. 10.1016/j.pbb.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Polidori C., Ciccocioppo R., Pompei P., Cirillo R., Massi M. (1996). Functional evidence for the ability of angiotensin AT1 receptor antagonists to cross the blood-brain barrier in rats. Eur. J. Pharmacol. 307, 259–267. 10.1016/0014-2999(96)00270-1 [DOI] [PubMed] [Google Scholar]
- Prut L., Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33. 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- Quaedflieg C. W. E. M., Schwabe L. (2018). Memory dynamics under stress. Memory 26, 364–376. 10.1080/09658211.2017.1338299 [DOI] [PubMed] [Google Scholar]
- Quijano J., Arango G. J. (1979). The breadfruit from colombia-a detailed chemical analysis. Econ. Bot. 33, 199–202. 10.1007/BF02858288 [DOI] [Google Scholar]
- Ranjbar H., Aghaei I., Moosazadeh M., Shabani M. (2018). Angiotensin II type 1 receptor blocker losartan attenuates locomotor, anxiety-like behavior, and passive avoidance learning deficits in a sub-chronic stress model. Iran. J. Basic Med. Sci. 21, 856–862. 10.22038/IJBMS.2018.27113.6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M. (2005). Brain angiotensin ii: new developments, unanswered questions and therapeutic opportunities. Cell. Mol. Neurobiol. 25, 485–512. 10.1007/s10571-005-4011-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M. (2017). Beneficial effects of angiotensin ii receptor blockers in brain disorders. Pharmacol. Res. 125, 91–103. 10.1016/j.phrs.2017.06.017 [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Sánchez-Lemus E., Benicky J. (2011). Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology 36, 1–18. 10.1016/j.psyneuen.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M., Ando H., Armando I., Baiardi G., Bregonzio C., Jezova M., et al. (2004). Brain angiotensin ii, an important stress hormone: regulatory sites and therapeutic opportunities. Ann. N. Y. Acad. Sci. 1018, 76–84. 10.1196/annals.1296.009 [DOI] [PubMed] [Google Scholar]
- Santarelli L. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (80-) 301, 805–809. 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Skultétyová I., Tokarev D., Jezová D. (1998). Stress-induced increase in blood-brain barrier permeability in control and monosodium glutamate-treated rats. Brain Res. Bull. 45, 175–8. 10.1016/S0361-9230(97)00335-3 [DOI] [PubMed] [Google Scholar]
- Song K., Zhuo J., Mendelsohn F. A. O. (1991). Access of peripherally administered DuP 753 to rat brain angiotensin II receptors. Br. J. Pharmacol. 104, 771–772. 10.1111/j.1476-5381.1991.tb12503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Guo Y., Jiang S., Wei L., Liu Z., Wang X., et al. (2018). Antidepressant effects of the ginsenoside metabolite compound k, assessed by behavioral despair test and chronic unpredictable mild stress model. Neurochem. Res. 43, 1371–1382. 10.1007/s11064-018-2552-5 [DOI] [PubMed] [Google Scholar]
- Tchekalarova J. D., Ivanova N., Atanasova D., Pechlivanova D. M., Lazarov N., Kortenska L., et al. (2016). Long-term treatment with losartan attenuates seizure activity and neuronal damage without affecting behavioral changes in a model of co-morbid hypertension and epilepsy. Cell. Mol. Neurobiol. 36, 927–941. 10.1007/s10571-015-0278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchekalarova J. D., Ivanova N. M., Pechlivanova D. M., Atanasova D., Lazarov N., Kortenska L., et al. (2014). Antiepileptogenic and neuroprotective effects of losartan in kainate model of temporal lobe epilepsy. Pharmacol. Biochem. Behav. 127, 27–36. 10.1016/j.pbb.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Üresin Y., Erbas B., Özek M., Özkök E., Gürol A. O. (2004). Losartan may prevent the elevation of plasma glucose, corticosterone and catecholamine levels induced by chronic stress. J. Renin-Angio-Aldo S. 5, 93–96. 10.3317/jraas.2004.017 [DOI] [PubMed] [Google Scholar]
- Vieira J. O., Duarte J. O., Costa-Ferreira W., Morais-Silva G., Marin M. T., Crestani C. C. (2018). Sex differences in cardiovascular, neuroendocrine and behavioral changes evoked by chronic stressors in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 426–437. 10.1016/j.pnpbp.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Wang J. M., Tan J., Leenen F. H. H. (2003). Central nervous system blockade by peripheral administration of at1 receptor blockers. J. Cardiovasc. Pharmacol. 41, 593–599. 10.1097/00005344-200304000-00012 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Fujioka T., Hashimoto M., Nakamura S. (1998). Stress and brain angiotensin ii receptors. Crit. Rev. Neurobiol. 12, 305–317. 10.1615/CritRevNeurobiol.v12.i4.20 [DOI] [PubMed] [Google Scholar]
- Willner P. (2005). Chronic mild stress (cms) revisited: consistency and behavioural-neurobiological concordance in the effects of cms. Neuropsychobiology 52, 90–110. 10.1159/000087097 [DOI] [PubMed] [Google Scholar]
- Willner P. (2017). The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol. Stress 6, 78–93. 10.1016/j.ynstr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincewicz D., Braszko J. J. (2014). Telmisartan attenuates cognitive impairment caused by chronic stress in rats. Pharmacol. Reports 66, 436–441. 10.1016/j.pharep.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Wincewicz D., Braszko J. J. (2015). Angiotensin II AT1 receptor blockade by telmisartan reduces impairment of spatial maze performance induced by both acute and chronic stress. J. Renin-Angio.-Aldo. S. 16, 495–505. 10.1177/1470320314526269 [DOI] [PubMed] [Google Scholar]
- Wincewicz D., Juchniewicz A., Waszkiewicz N., Braszko J. J. (2016). Angiotensin II type 1 receptor blockade by telmisartan prevents stress-induced impairment of memory via HPA axis deactivation and up-regulation of brain-derived neurotrophic factor gene expression. Pharmacol. Biochem. Behav. 148, 108–118. 10.1016/j.pbb.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Wolf O. T. (2009). Stress and memory in humans: twelve years of progress? Brain Res. 1293, 142–154. 10.1016/j.brainres.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Wright J. W., Harding J. W. (2011). Brain renin-angiotensin–a new look at an old system. Prog. Neurobiol. 95, 49–67. 10.1016/j.pneurobio.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Zhuo J., Song K., Abdelrahman A., Mendelsohn F. A. O. (1994). Blockade by intravenous losartan of at 1 angiotensin ii receptors in rat brain, kidney and adrenals demonstrated by in vitro autoradiography. Clin. Exp. Pharmacol. Physiol. 21, 557–567. 10.1111/j.1440-1681.1994.tb02555.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the manuscript and the supplementary files.