Abstract
Background
Origanum syriacum (O. syriacum) is a very popular edible and medicinal plant in the East Mediterranean countries. The aims of the current study were to use microwave-ultrasonic assisted hydrodistillation (MUAHD) method to produce essential oils (EOs) from wild O. syriacum samples collected from four different geographical areas in The West Bank using water as a solvent, determine the phytochemical profile using GC-MS analysis and assess their antioxidant and antibacterial potential.
Methods
Essential oils were produced using MUAHD method. Gas chromatography coupled with mass spectrometer detector (GC-MS) was employed for phytochemical analysis. In vitro antibacterial and antioxidant potentials were carried out.
Results
Differences in the EOs yield among the four Origanum samples were observed. GC-MS analysis of EOs revealed terpenes as the major constituents; monoterpenes (22–56%) and oxygenated monoterpenes (28–57%). Thymol, α-terpinene and carvacrol represent the bulk of all phytochemicals detected by GC-MS analysis. γ-Terpinene-rich EOs, exhibited the highest antioxidant capacity. Thymol-rich EOs were found to be most effective against Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus (MRSA) (MIC 390 µg/mL). Alpha-terpinene-rich chemotype EOs exhibited the highest inhibitory effect of Pseudomonas aeruginosa (MIC of 1560 µg/mL). Interestingly, γ-terpinene-rich EO showed promising antibacterial properties against Enterococcus faecium (MIC 97 µg/mL) and a powerful anti-oxidant effect (91.45% ±2.30).
Conclusion
The current study supports the use of MUAHD as a time-saving, cost-effective, environment-friendly method for production of high quality O. syriacum EO for potential use as a natural complementary treatment and in the prevention of bacterial infections as well as oxidation by free radicals without compromising the quantity.
Keywords: Antibacterial, Antioxidant, Essential oil, GC-MS, Microwave-ultrasonic assisted hydrodistillation, Origanum syriacum
1. Introduction
Natural products from plants, animals and minerals have been used for medicinal purposes since antiquity and have been the most integral source of drugs (Rates, 2001). Human knowledge about herbal potential curing effect was documented since 18,000 B.C.E. (De Sousa Araújo et al., 2016, Geneste and Mauriac, 2014). Ancient civilizations, modern pharmaceutical, food and cosmetic industries relied on essential oils (EOs) as a flavoring, antibacterial, antifungal and antioxidant agents (Sharifi-Rad et al., 2017). For example, essential oils EOs isolated from phytogenic sources were utilized in cosmetics, perfumes, food, religious ceremonies and medicines. Moreover, aromatic oils were incorporated into a number of pharmaceutical dosage forms including suppositories, ointments, creams powders, pills and tinctures (Başer and Buchbauer, 2015, Lawless, 2013, Rose, 1999). Furthermore, some EOs were found to possess pesticidal and herbicidal activity (Chaimovitsh et al., 2017, Tworkoski, 2002). In recent years, EOs played a primary role in many of Complementary and Alternative Medicine modalities such as aromatherapy, ayurveda, flower essence therapy, massage therapy, naturopathic, unani and traditional Arabic Islamic medicine (Prakashan, 2009, WHO, 2001).
Origanum syriacum L. (O. syriacum), commonly known as white oregano, Syrian oregano, za'atar, zatar or zather, is a perennial bushy aromatic herbaceous plant belongs to the Lamiaceae (Labiatae) family and is native to the Mediterranean region (Seidemann, 2005). O. syriacumis a wild herb grows in the mountains and valleys. It is about 40 cm high, with ovate-oblong slightly hairy leaves with a strong pungent taste, and white aromatic flowers. The Arabic name za'atar is widely used in many countries of the Arab world which could refer to other plant species from the Lamiaceae family, such as Coridothymus capitatus, Thymbra spicata and Satureja thymbra, all of which share similar uses and aromatic flavor profile. Za'atar is strongly associated with Palestine and it is one the most popular folkloric culinary and medicinal herb (Ali-Shtayeh et al., 1998). Every year, tons of the plant material are collected and consumed and therefore considered of a high economic value (Boyd, 2016). In Palestinian folk medicine, O. syriacum is used for the treatment of skin fungal diseases, abdominal pain, throat infection and cough. Similar therapeutic uses of Origanum have been reported from neighboring countries as Jordan (Shehadeh et al., 2017, Shehadeh et al., 2014), Syria and Lebanon (Aburjai et al., 2007, Ali-Shtayeh et al., 2000, Husein et al., 2014).
This work aims were, to use microwave-ultrasonic assisted hydrodistillation (MUAHD) method as a new and green technology to extract the EOs from dried wild O. syriacum collected from four different regions (Jerusalem, Bethlehem, Qalqilya and Tulkarem) of the West Bank/Palestinian Authority. The chemical composition of the produced EOs and the percentage yield obtained will be compared with previously reported for EOs produced by the conventional hydrodistillation technique. Moreover, EOs will be assessed for their antibacterial and antioxidant properties in vitro.
2. Materials and methods
2.1. Chemical reagents
Dimethyl sulfoxide (DMSO) was purchased from Riedeldehan, Germany. DPPH (2,2-Diphenyl-1-picrylhydrazyl) and Trolox were purchased from Sigma-Aldrich, Germany. Methanol and n-hexane were purchased from Lobachemie, India. Mueller Hinton broth was purchased from Himedia, India.
2.2. Instrumentations
Varian Chrompack CP-3800 gas chromatograph connected to Saturn 2200 GC-MS-MS was used for gas GC-MS analysis (Saturn, Netherlands). Ultrasonic-microwave cooperative extractor (CW-2000, China) was used to extract the EOs. UV visible spectrophotometer (Jenway-7315, England) was used to assess the antioxidant activity. Dried plant material was powdered using Moulinex model (Uno China) incubator (Nuve, Turkey), 96-well plates (Greiner Bioone, North America), syringe filter 0.45 µm pore size (Microlab, China) and micropipettes (Finnpipette, Finland) were used for experimental work. Purified water was obtained using Millie Q (Zalion, Jerusalem).
2.3. Collection and preparing plant materials
O. syriacum herb was collected from four regions of the West Bank area of Palestine (Jerusalem, Qalqilya, Tulkarem and Bethlehem) during the flowering phase at the end of July 2016. The leaves were separated from the stems, carefully washed using purified water and dried in the shade at room temperature. Voucher specimens (Pharm-PCT-A1729-1732) were deposited in the herbarium at the laboratory of harmacognosy and Phytochemistry of the Faculty of Medicine and Health Sciences, Department of Pharmacy, An-Najah National University, Nablus, West Bank.
2.4. Essential oil preparation
The EOs of the four samples of O. syriacum plant were extracted by using a microwave-ultrasonic method which was described by Jaradat et al., 2016 with modifications (Jaradat et al., 2016). In brief, the extraction apparatus used in this study consists of a microwave oven combined with an ultrasonic extractor. The plant powder (100 g) suspended in water in a round bottom flask connected to the Clevenger-type apparatus was exposed to ultrasonic and microwaves. Microwave-ultrasonic power was adjusted at 1000 W; the ultrasonic power of the apparatus was adjusted at its maximum (50 W and frequency of 40 kHz) at 100 °C for 10 min, repeated three times. The obtained EO was collected into a clean beaker, dried over anhydrous sodium sulfate (Na2SO4) using 0.90–1.00 g and stored in the refrigerator at 2–8 °C.
2.5. Bacterial isolates
Staphylococcus aureus (ATCC 25923), resistant Staphylococcus aureus (MRSA) and Enterococcus faecium (ATCC 700221), Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) were used in the study.
2.6. Gas chromatography-Mass spectrometry
Varian Chrompack (CP-3800 Gas Chromatograph) connected to Saturn 2200 GC-MS-MS- equipped with split-splitless injector and Agilant-DB-5 GC column (5% diphenyl 95% dimethyl polysiloxane), (30 m × 0.25 mm ID, 0.25 μm film thicknesses) was used. The injector temperature was set at 250 °C with a split ratio of 1:10. The detector and transfer-line temperatures were 160 °C and 230 °C respectively.
The MS ionization source was 180 °C and the ionization voltage was 70 eV. The column oven temperature was kept at 60 °C for 1 min (isothermal), and rammed from 60 °C to 250 °C at a rate of 3 °C/min, and held constant at 250 °C for 1 min to a total time of 65 min. The mass detector was set to scan ions between 40 and 400 m/z using full scan mode and electron impact (EI, 70 eV). Essential oil samples were subjected to GC/MS analysis, after diluting 1 μL aliquot of the sample to 10 μL in GC grade n-hexane. A hydrocarbon mixture of n-alkanes (C8-C20) was analyzed separately by GC-MS using the same column (Agilant-DB-5) and under the same chromatographic conditions. Each sample was analyzed twice.
A linear temperature program was used to separate the different oil components. For each component (peak) separated by GC-MS, the linear retention index (arithmetic) was calculated according to Adams and Van den Dool and Kratz (Van den Dool and Kratz, 1963, Adams, 2007). Identification of oil components was performed by matching their spectra with mass spectra data bank libraries including WILEY, NIST and ADAMS-2007 and comparing their linear retention indices with reported values in literature, mainly Adam’s library (Adams, 2007, Tawaha and Hudaib, 2012).
2.7. Identification of EOs constituents
The EOs constituents were identified by matching their recorded spectra with the data bank mass spectra (NIST, WILEYand ADAMS-2007 libraries) provided by the instrument's software also by comparing their linear retention indices with reported values in the literature (Adam's library 2007).
2.8. Antioxidant activity
DPPH methanolic solution was prepared at a concentration of 0.002% w/v. Serial dilutions of Trolox (standard) and the extracted O. syriacum EOs (1, 2, 3, 5, 7, 10, 20, 30, 40, 50, and 80 μg/mL) were tested for DPPH reducing capacity (Alma et al., 2003). Antioxidant potential was assessed using ultraviolet spectrophotometer at a wavelength of 517 nm using methanol as the blank solution. Percentage inhibition of DPPH was calculated using the following equation:
where, A: the absorbance of the blank and B: the absorbance of the sample.
Concentration necessary to induce 50% inhibition of DPPH (IC50) for each of the studied O. syriacum EOs and Trolox (standard) were calculated by using Bio Data Fit edition 1.02 (data fit for biologist).
2.9. Determination of MIC
Broth microdilution method was used to assess antibacterial activities of O. syriacum EOs. The applied procedure was conducted according to Forbes et al., methods (Jorgensen and Ferraro, 1998, Forbes et al., 2007). A volume of 100 µL of EO stock solution (50 × 103 µg/mL) was added to 90 µL of Mueller Hinton broth, followed by subsequent dilution. An aliquot of 10 µL of bacterial suspension (5 × 107 CFU/mL) was added. Positive and negative controls were also included. Plates were incubated at 35 °C for 24 h. Experiments were carried out in triplicates. The least concentration of EOs that inhibited visible bacterial growth was considered as the minimum inhibitory concentration (MIC).
3. Results
3.1. EOs analysis
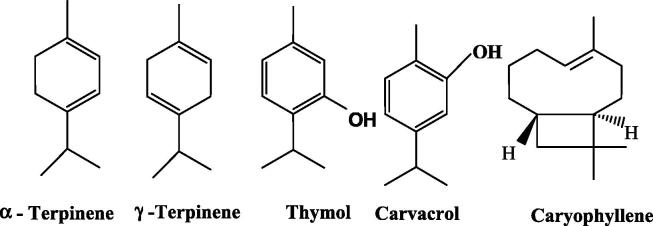
Differences in the yield of EOs produced by MUAHD technique were observed (Table 1). Qalqilya's sample yield was the highest (3.28% v/w); Bethlehem 2.74% v/w, Tulkarem 2.2% v/w and the lowest was recorded for Jerusalem sample of 1.7% v/w. GC/MS analysis of the produced EOs revealed that terpenes were the major components in all samples as shown in Table 2. EOs from Qalqilya and Tulkarem samples were rich in oxygenated monoterpenes. However, monoterpene hydrocarbons were the major phytochemicals in the EOs obtained from Bethlehem and Jerusalem samples. In addition, distinct differences were noted in the percentages and nature of the detected terpenes. α-Terpinene and γ-terpinene were the major detected unsaturated monoterpene hydrocarbon, while thymol and carvacrol were the major oxygenated monoterpenes. In addition, caryophyllene and its oxide derivative were the major detected sesquiterpenes (Fig. 1).
Table 1.
O. syriacum EOs yield obtained by conventional and microwave ultrasonic assisted hydrodistillation.
| Origin | Yield (% V/W) |
Reference | |
|---|---|---|---|
| HD | MUAHD | ||
| Palestine | 1.1 – 1.3 | 1.7–3.3* | (Khoury et al., 2016) |
| Jordan | 0.6–1.6 | NA | (Afifi et al., 2017) |
| Lebanon | 1.3 – 4.4 | NA | (Awada et al., 2012) |
| Turkey | 0.8–3.5 | NA | (Toncer et al., 2010) |
| Iran | 0.9–1.7 | NA | (Pirigharnaei et al., 2011) |
| Morocco | 0.8.2–2.61 | NA | (Fadel et al., 2010, Aboukhalid et al., 2017) |
HD: Hydrodistillation.
MUAHD: Microwave Ultrasonic Assisted Hydrodistillation.
: Current study.
Table 2.
GC-MS analysis of wild O. syriacum leaves EO samples from The West Bank/Palestinian Authority.
| No | Compound† | Molecular Formula | Molecular Weight | Retention Time | RI†† | Relative Content (%) of EOs |
|||
|---|---|---|---|---|---|---|---|---|---|
| Q* | J* | T* | B* | ||||||
| 1 | α-Thujenea | C10H16 | 136 | 5.893 | 930 | 1.04 | 0.74 | 1.35 | 2.86 |
| 2 | α- Pinenea | C10H16 | 136 | 6.115 | 939 | 1.37 | 1.35 | 1.15 | 2.00 |
| 3 | β-Pinenea | C10H16 | 136 | 7.740 | 979 | 2.57 | 2.11 | 1.18 | 2.16 |
| 4 | 1-Phellandrenea | C10H16 | 136 | 8.352 | 1003 | 0.68 | 0.55 | 0.31 | 0.60 |
| 5 | α-Terpinenea | C10H16 | 136 | 8.718 | 1017 | 12.97 | 27.95 | 21.97 | 36.80 |
| 6 | tert-Butyl benzenee | C10H14 | 134 | 9.225 | – | 1.36 | 0.97 | 0.68 | 1.27 |
| 7 | γ-Terpinenea | C10H16 | 136 | 10.237 | 1060 | 3.05 | 21.14 | 6.70 | 10.51 |
| 8 | α-Terpinolenea | C10H16 | 136 | 11.290 | 1086 | 0.47 | 0.93 | 0.75 | 0.94 |
| 9 | 4-Terpineolb | C10H18O | 154 | 15.281 | 1177 | 2.10 | 0.99 | 0.62 | 0.83 |
| 10 | Thymoquinoneb | C10H12O2 | 164 | 18.274 | 1252 | 2.17 | 1.28 | 1.24 | 2.10 |
| 11 | Thymolb | C10H14O | 150 | 20.104 | 1290 | 19.99 | 11.74 | 39.87 | 5.90 |
| 12 | Carvacrolb | C10H14O | 150 | 20.461 | 1299 | 18.99 | 13.64 | 15.44 | 21.40 |
| 13 | Caryophyllenec | C15H24 | 204 | 25.382 | 1419 | 12.89 | 6.38 | 1.93 | 2.20 |
| 14 | α-Amorphenec | C15H24 | 150 | 28.130 | 1483 | 0.64 | 0.37 | 0.21 | – |
| 15 | delta-Cadinenec | C15H24 | 204 | 29.389 | 1522 | – | 0.39 | – | – |
| 16 | Caryophyllene oxided | C15H24O | 220 | 31.861 | 1583 | 2.19 | 0.92 | 0.43 | 0.50 |
| 17 | Octahydro-tetramethyl- phenanthrenee | C18H26 | 242 | 43.799 | – | 0.82 | 0.37 | 0.45 | 0.51 |
| Identified Phytochemical Groups | |||||||||
| Monoterpene hydrocarbonsa | 22.15 | 54.77 | 33.41 | 55.87 | |||||
| Oxygenated monoterpenesb | 43.25 | 27.65 | 57.17 | 30.23 | |||||
| Sesquiterpene hydrocarbonsc | 13.53 | 7.14 | 2.11 | 2.20 | |||||
| Oxygenated sesquiterpenesd | 2.19 | 0.92 | 0.43 | 0.50 | |||||
| Non terpene hydrocarbonse | 2.18 | 1.34 | 1.13 | 1.78 | |||||
| Total of phytochemical groups | 83.3 | 91.82 | 94.28 | 90.58 | |||||
Q*: Qalqilya, J*: Jerusalem, T*: Tulkarem, B*: Bethlehem.
Compounds are listed based on their elution order identified according to WILEY, NIST and ADAMS-2007 libraries.
= Monoterpene.
= Oxygynated monoterpene.
= Sesquiterpene.
= Oxygenated sesquiterpene.
= Non terpene hydrocarbons.
RI: (Retention Indices) linear (arithmetic) retention index calculated on a DB-5 equivalent column.
Fig. 1.
Major terpenes detected in O. syriacum EOs.
3.2. Antioxidant activity
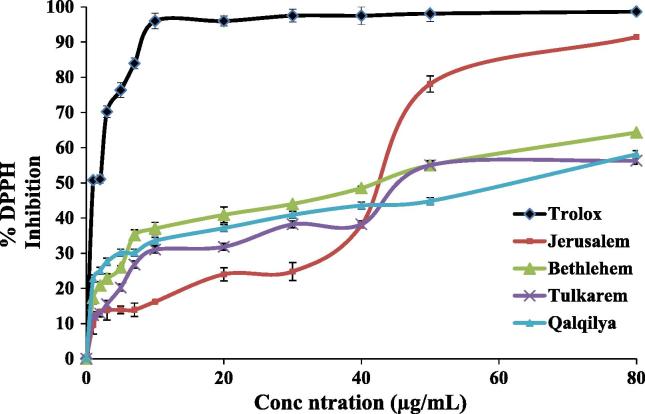
All O. syriacum EOs under investigation exhibited inhibitory effect on 1,1-diphenyl-2-picrylydrazyl (DPPH) free radical production in a dose-dependent pattern as shown in Table 2. At the highest tested concentration 80 µg/mL, Jerusalem EO displayed the strongest free radical scavenging ability of all tested samples, while Bethlehem EO was the second. Tulkarem and Qalqilia EOs displayed the weakest free radical inhibition activity (56.3% and 58.1% respectively). IC50 values of antioxidant capacity are listed in Table 3.
Table 3.
In vitro antioxidant activity of O. syriacum leaves EOs and the standard Trolox.
| Concentration (µg/mL) | DPPH Percentage Inhibition Induced by |
||||
|---|---|---|---|---|---|
| Standard |
O. syriacum EO from |
||||
| Trolox | Jerusalem | Bethlehem | Tulkarem | Qalqilya | |
| 1 | 50.72 ± 0.29 | 9.65 ± 1.77 | 17.30 ± 0.00 | 12.35 ± 2.34 | 22.80 ± 0.76 |
| 2 | 51.00 ± 0.58 | 13.40 ± 2.60 | 20.80 ± 1.67 | 12.85 ± 0.85 | 25.00 ± 1.25 |
| 3 | 70.20 ± 1.22 | 13.80 ± 0.00 | 22.80 ± 1.09 | 15.50 ± 1.45 | 27.56 ± 2.50 |
| 5 | 76.34 ± 1.62 | 13.90 ± 2.80 | 25.90 ± 1.34 | 20.22 ± 0.00 | 30.14 ± 1.66 |
| 7 | 83.95 ± 2.10 | 13.90 ± 1.06 | 35.12 ± 0.55 | 26.75 ± 2.42 | 30.14 ± 2.34 |
| 10 | 95.99 ± 1.52 | 16.20 ± 1.90 | 37.00 ± 1.50 | 31.01 ± 1.14 | 33.50 ± 1.08 |
| 20 | 96.00 ± 2.20 | 24.00 ± 0.00 | 40.90 ± 1.75 | 31.81 ± 2.22 | 37.14 ± 0.00 |
| 30 | 97.50 ± 1.44 | 24.80 ± 1.85 | 44.00 ± 2.21 | 38.20 ± 1.85 | 40.90 ± 1.99 |
| 40 | 97.50 ± 1.82 | 38.00 ± 2.52 | 48.50 ± 0.00 | 38.20 ± 2.12 | 43.56 ± 0.85 |
| 50 | 98.10 ± 2.50 | 78.10 ± 0.75 | 55.00 ± 0.57 | 55.00 ± 1.23 | 44.80 ± 0.57 |
| 80 | 98.66 ± 2.25 | 91.45 ± 2.30** | 64.30 ± 1.34 | 56.30 ± 0.00 | 58.12 ± 0.57 |
| *IC50 | 1.90 ± 1.59 | 39.80 ± 1.60 | 31.60 ± 1.09 | 63.10 ± 1.42 | 61.70 ± 1.23 |
Values are means ± SD of three experiments carried out in triplicates.
IC50 were calculated by using Bio Data Fit edition 1.02.
Maximum DPPH inhibitory effect.
3.3. Antibacterial activity
In this study, EOs of O. syriacum leaves, collected from Jerusalem, Bethlehem, Qalqilya and Tulkarem regions of Palestine exhibited antibacterial activity against all tested bacterial strains. However, the growth inhibition varied according to the source of the plant species as shown in Table 4. EO from Jerusalem species showed the highest antibacterial activity especially against S. aureus and E. faecium with MIC of 97 µg/mL. The effect against P. aeruginosa and E. coli growth was less pronounced with MIC range of 1560- ≥25,000 µg/mL.
Table 4.
In vitro antibacterial activity of EOs of O. syriacum leaves, collected from Jerusalem, Bethlehem, Qalqilya and Tulkarem regions.
| Bacterial isolate | MIC (µg/mL) |
|||
|---|---|---|---|---|
| Jerusalem | Bethlehem | Qalqilya | Tulkarem | |
| Staphylococcus aureus | 97 | 930 | 390 | 390 |
| MRSA | 6250 | 780 | 780 | 390 |
| Enterococcus faecium | 97 | 190 | 780 | 780 |
| Pseudomonas aeruginosa | >25,000 | 1560 | 3130 | ≥25,000 |
| Escherichia coli | ≥25,000 | 3130 | 3130 | 6250 |
Values are means for three experiments carried out in triplicates.
4. Discussion
The West Bank of Palestine ecosystem is considered among the highest in plant biodiversity despite its small size. O. syriacum is one of the most popular edible and folkloric plants in Palestine (Ali-Shtayeh et al., 1998, Ali-Shtayeh et al., 2000, Feinbrunn, 1938, Halim et al., 1991). It has been reported as a remedy for many ailments, an antidote for poisons and as a food preservative (Al-Antaki, 1935, Plessner, 1962).
As shown in Table 1, higher EOs yield (1.7–3.3 %v/w) was obtained applying MUAHD technique compared to conventional hydrodistillation (HD) method. Where the highest yield (3.28% v/w) was obtained from Qalqillya (the lowest land area of 45–125 m above sea level (ASL)); and the least yield (1.7% v/w) was obtained from Jerusalem (the highest land area (546–852 m ASL)). This is in agreement with other finding were variations in the yield of O. syriacum leaves EOs obtained by conventional hydrodistillation method (HD)was region-specific (Khoury et al., 2016, Afifi et al., 2017, Awada et al., 2012, Toncer et al., 2010, Pirigharnaei et al., 2011, Fadel et al., 2010, Aboukhalid et al., 2017) as summarized in Table 1.
GC-MS analysis of Zaatar EOs produced from the harvested wild plants representing four geographical regions in Palestine, showed similar chemical profile to that reported in the literature (Aboukhalid et al., 2017, Afifi et al., 2017, Farjam et al., 2014, Lukas et al., 2009). Terpenes were the most dominant components (83–94%) as shown in Table 2. Phytochemicals were discriminated into two major groups; mono- and sesquiterpenes. However, the nature of EOs' terpenoidal compounds varied according to the location of O. syriacum collection land area. Monoterpene hydrocarbons were abundant in EOs from dry (40% humidity), high altitude mild Mediterranean climate (temperature 19–28 °C) collection points as Jerusalem and Bethlehem (54.77, 55.87% respectively). Furthermore, the highest content of oxygenated monoterpenes was detected in EOs from humid (70%) hot subtropical Mediterranean (25–32 °C) and low altitude (45–500 m ASL) samples collected from Qalqilya and Tulkarem areas (43.25, 57.17% respectively) as shown in Table 2. Essential oil derived from Qalqilya's (low altitude, humid and hot region) sample was the richest in sesquiterpene hydrocarbons (13.53%). Climacteric factors were reported to affect the composition and the yield of EOs from Lamiaceae herbs (Aboukhalid et al., 2017, Ibrahim et al., 2014) (Table 1).
In addition, MUAHD-derived EOs showed that α-terpineneand thymol (Fig. 1) were dominant in our samples (36.8 and 39.87% respectively). To the best of our knowledge, thymol and carvacrol were the dominant oxygenated monoterpenes components in most previously studied Origanum EOs. This is the first report of α-terpinene (unsaturated monoterpene, Fig. 1) domination in O. syriacum EO which was absent in Egyptian, Lebanese, Jordanian and Turkish species (Afifi et al., 2017, Arslan, 2016, El Gendy et al., 2015, Ibrahim et al., 2012).
All O. syriacum EOs analyzed in the current study indicated that thymol was the dominant phenolic monoterpene (5.9–39.87%) followed by α-terpinene representing unsaturated monoterpene hydrocarbons (12.97–36.8%). GC-MS analysis (Table 2) established similarity in the EOs chemical composition from the neighboring hot, humid and low altitude regions Qalqilya and Tulkarem, being rich in thymol (19.99 and 39.87%)and carvacrol (18.99 and 15.44%) respectively. Qalqilya's EO was the richest in caryophyllene (sesquiterpene) 21.4%. On the other hand, α-terpinene (27.95 and 36.80%) and γ-terpinene (21.14 and 10.51%) were dominant in the mild Mediterranean, dry and high altitude Jerusalem and Bethlehem respectively. Carvacrol content (21.40%) was highest in the coldest, dry and elevated area of Bethlehem. Compositional percentage differences in the four EOs could be attributed to the nature of the soil, humidity, climate, light intensity and temperature of the native geographical region (Isaac and Rishmawi, 2015). These findings were in agreement with previously published reports of the domination of monoterpenes (carvacrol, thymol, α-terpinene and γ-terpinene) in plants studied from the neighboring countries (Abu-Lafi et al., 2007, Al-Kalaldeh et al., 2010, Alma et al., 2003, El Gendy et al., 2015, Farhat et al., 2012, Figuérédo et al., 2005, Fleisher and Fleisher, 1991). In comparison to the HD method used in the previous reports, EOs produced by MUAHD method, utilized in the present study, were significantly rich in oxygenated monoterpenes (27.7–57.2%) and sesquiterpene hydrocarbons (2.1–13.5%). Moreover, MUAHD is a quick (10 min compared to 2–4 h using hydrodistillation) and a green method for EO production without compromising the quantity and quality.
Free radical scavenging capacity of MUAHD-produced EOs was relatively high (IC50 of 31.6–63.1 µg/mL) and dose-dependent (Table 3, Fig. 2) with differences in regard to the geographic area. Bethlehem, carvacrol and α-terpinene richest, and Jerusalem EOs, γ-terpinene and α-terpinene rich, were the most active as antioxidants (IC50 of 31.6–39.8 μg/mL respectively). However, at the highest tested concentration (80 μg/mL), Jerusalem EO induced almost equivalent DPPH inhibition activity of the standard (Trolox). Both Qalqilya and Tulkarem EOs showed similar antioxidant capacity (IC50 of 61.7 and 63.1 μg/mL, respectively) at the maximum tested concentration. The weakest antioxidant activity was noted for the thymol-rich (39.9%) EO (IC50 of 63.1 μg/mL). It is noteworthy that as low as 31.7 μg/mL of the EO was able to induce 50% inhibition of DPPH. The free radical scavenging capacity of all four studied EOs was higher than previously reported results for O. syriacum by Tepe's group (IC50 134 μg/mL) as shown in Fig. 2.
Fig. 2.
In vitro antioxidant capacity of Trolox (standard) and the four O. syriacum EOs values shown represent the means ± SD for three experiments carried out in triplicates.
In general, the Palestinian Origanum species showed superior antioxidant capacity when compared to the reported activity from Turkey and Egypt IC50 0.021 – 0.027 and 6.6 µg/mL respectively (Tepe et al., 2004, Viuda-Martos et al., 2010).
Our findings indicate that EOs with higher unsaturated terpenes (γ-terpinene and α-terpinene) induced higher DPPH inhibition compared to the oxygenated (thymol and carvacrol) Fig. 2. This might suggest a role for unsaturated hydrocarbons in free radical scavenging ability.
Assessment of the antimicrobial activity of the produced EOs against five standard bacterial strains revealed that O. syriacum EOs had moderate antimicrobial activity with minimum inhibitory concentrations (MICs) varying from 97 to >25,000 µg/mL as shown in Table 4. The antimicrobial activities of the EOs were higher against Gram-positive bacteria species as compared to Gram-negative. The observed antibacterial effects were in accordance with previous study results regarding Turkish Origanum EOs activity against S. aureus, E. coli and P. aeruginosa strains with MICs values of 26,500 µg/mL, 26.25 mg/mL and 10,500 µg/mL, respectively (Tepe et al., 2004). Comparable results were reported by Hussein et al., against similar bacterial strains (Husein et al., 2014, Jaradat et al., 2016). Methicillin resistance Staphylococus aureus (MRSA) was more susceptible to growth inhibitory effect by thymol and carvacrol-rich EOs from Tulkarem (MIC 390 µg/mL), Bethlehem and Qalqilya (MIC 780 µg/mL). The higher amount of unsaturated non-oxygenated terpene hydrocarbons in Jerusalem EO was associated with low activity against MRSA (MIC 6250 µg/mL) compared to that of the oxygenated terpene-rich Eos (Table 4). P. aeruginosa was the most resistant tested bacteria to thymol-rich EOs from Tulkarem and Jerusalem districts with MIC value >25,000 µg/mL. However, P. aeruginosa was more susceptible to α-terpinene chemo-type EO of Bethlehem (MIC 1560 µg/mL). Carvacrol rich EOs of Bethlehem and Qalqilya induced the highest growth inhibitory effect on P. aeruginosa with MIC values of 1560 and 3130 µg/mL respectively. E. faecium was most sensitive to α-terpinene and γ-terpinene rich Jerusalem and BethlahemEOs (MIC 100–190 µg/mL respectively). On the other hand, phenols-rich EOs from Qalqilya and Tulkarem samples required at least four times the concentration of nonhydroxylated terpenes-rich EOs to inhibit the growth of the same micro-organism (Table 4).
In summary, low content of carvacrol in Jerusalem EO was associated with lack of inhibitory ability on both E. coli and P. aeruginosa (MIC > 25,000 µg/mL). The high content of carvacrol in Bethlehem and Qalqilya O. syriacum EOs was correlated with higher susceptibility of E. coli (MIC 3130 µg/mL) compared to the reported MIC of 12,500 µg/mL for neighbouring Syrian Origanum (Lukas et al., 2009). Furthermore, γ-terpenine-rich O. syriacum EO from Jerusalem district was found to be the most active against both Enterococcus faecium (E. faecium) and S. aureus (MIC 97 µg/mL).
5. Conclusion
In conclusion, the phytochemical composition of O. syriacum EOs produced by the new MUAHD technique was distinctive from those obtained with conventional HD procedure. MUAHD method afforded higher yield of EOs with less effort, lower cost and shorter time. Thus, MUAHD can be considered as a rapid, cost-effective, green environment-friendly method for EOs production without compromising the quality or the quantity.
Phytochemical profile of the investigated EOs affected biological activity. The higher the lipophilic content, the higher the antioxidant activity. Whereas, the higher the phenolic (hydrophilic) occurrence, the lower the antioxidant capacity. Moreover, increased lipophilic content (e.g. Jerusalem sample) was noticeably associated with increased activity against Gram-positive bacteria. On the other hand, thymol-rich EOs were more active against Gram-positive bacteria. Furthermore, carvacrol-rich EOs were active against both Gram-positive and Gram-negative bacteria.
Acknowledgment
This work was financially supported by the Deanship of Academic Research at The University of Jordan [grant numbers 1541] and Hamdi Mango Center for Scientific Research [grant number 2073] to whom authors are thankful for.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2019.03.001.
Contributor Information
Mayadah Shehadeh, Email: m.shehadeh@ju.edu.jo.
Nidal Jaradat, Email: nidaljaradat@najah.edu.
Motasem Al-Masri, Email: motasemm@najah.edu.
Abdel Naser Zaid, Email: anzaid@najah.edu.
Fatima Hussein, Email: f.huseen@najah.edu.
Ghadeer Suaifan, Email: gh.suaifan@ju.edu.jo.
Rula Darwish, Email: rulad@ju.edu.jo.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
GC-MS for EO of O. syriacum from Bethlehem
Supplementary figure 2.
GC-MS for EO of O. syriacum from Jerusalem
Supplementary figure 3.
GC-MS for O. syriacum from Qalqilya
Supplementary figure 4.
GC-MS for O. syriacum from Tulkarem
References
- Aboukhalid K., Alfaiz C., Douaik A., Bakha M., Kursa K., Agacka-Moldoch M., Machon N., Tomi F., Lamiri A. Influence of environmental factors on essential oil variability in origanum compactum BENTH. Growing wild in Morocco. Chem. Biodiv. 2017;14(9):1–17. doi: 10.1002/cbdv.201700158. e1700158. [DOI] [PubMed] [Google Scholar]
- Abu-Lafi S., Odeh I., Dewik H., Qabajah M., Imam A., Dembitsky V.M., Hanus L.O. Natural compounds of Palestine flora. Comparison analysis by static headspace and steam distillation GC-MS of semivolatile secondary metabolites from leaves of cultivated Palestinian Majorana syriaca. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2007;151(1):21–29. doi: 10.5507/bp.2007.004. [DOI] [PubMed] [Google Scholar]
- Aburjai T., Hudaib M., Tayyem R., Yousef M., Qishawi M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007;110(2):294–304. doi: 10.1016/j.jep.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Adams R.P. Academic Press; San Diego: 2007. Identification of essential oil components by gas chromatography/mass spectrometry. [Google Scholar]
- Afifi F.U., Kasabri V., Beltran S., Abuhammad A., Abaza I.F., Ganado O., Al-Gabbiesh A.H. Comparison of different methods in determination of essential oil composition of Origaum syriacum from Jordan and its modulation of pancreatic enzymes. Rev. Roum. Chim. 2017;62(1):15–21. [Google Scholar]
- Al-Antaki D. Al-Maktaba Al-Thaqafiya; Cairo: 1935. Tadhkirat Ula li-'Lbabwa'l-Jami'al-'Ujab al-'Ujab (Arabic) [Google Scholar]
- Al-Kalaldeh J.Z., Abu-Dahab R., Afifi F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010;30(4):271–278. doi: 10.1016/j.nutres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Ali-Shtayeh M., Yaghmour R.M.-R., Faidi Y., Salem K., Al-Nuri M. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998;60(3):265–271. doi: 10.1016/s0378-8741(97)00153-0. [DOI] [PubMed] [Google Scholar]
- Ali-Shtayeh M.S., Yaniv Z., Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J. Ethnopharmacol. 2000;73(1):221–232. doi: 10.1016/s0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- Alma M.H., Mavi A., Yildirim A., Digrak M., Hirata T. Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. Growing in Turkey (Pharmacognosy) Biol. Pharm. Bull. 2003;26(12):1725–1729. doi: 10.1248/bpb.26.1725. [DOI] [PubMed] [Google Scholar]
- Arslan M. Herbage yield, essential oil content and components of cultivated and naturally grown Origanum syriacum. Scientific papers-series A. Agronomy. 2016;59:178–182. [Google Scholar]
- Awada F., Kobaissi A., Chokr A., Hamze K., Hayar S., Mortada A. A factors affecting quantitative and qualitative variation of thyme (Origanum syriacum L.) essential oil in Lebanon. Adv. Environ. Biol. 2012:1509–1515. [Google Scholar]
- Başer K., Buchbauer G. CRC Press - Taylor & Francis Group; New York: 2015. Handbook of essential oils: science, technology, and applications. [Google Scholar]
- Chaimovitsh D., Shachter A., Abu-Abied M., Rubin B., Sadot E., Dudai N. Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci. 2017;65(1):19–30. [Google Scholar]
- De Sousa Araújo T.A., de Melo J.G., Júnior W.S.F., Albuquerque U.P. Medicinal plants. In: Albuquerque U., Nóbrega Alves R., editors. Introduction to Ethnobiology. Springer; Cham: 2016. pp. 143–149. [Google Scholar]
- El Gendy A.N., Leonardi M., Mugnaini L., Bertelloni F., Ebani V.V., Nardoni S., Mancianti F., Hendawy S., Omer E., Pistelli L. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai. Egypt. Ind. Crops Prod. 2015;67:201–207. [Google Scholar]
- Fadel O., Ghazi Z., Mouni L., Benchat N., Ramdani M., Amhamdi H., Wathelet J.-P., Asehraou A., Charof R. Comparison of microwave-assisted hydrodistillation and traditional hydrodistillation methods for the Rosmarinus eriocalyx essential oils from eastern Morocco. J. Mater. Environ. Sci. 2010;2(2):112–117. [Google Scholar]
- Farhat M., Tóth J., Héthelyi B.É., Szarka S. Sz., Czigle S. Sz. Analysis of the essential oil compounds of Origanum Syriacum L., European. Pharm. J. 2012;59(2):6–14. [Google Scholar]
- Feinbrunn N. New data on some cultivated plants and weeds of the early bronze age in Palestine. Palestine J. Botany, Series J. 1938;1:238–240. [Google Scholar]
- Figuérédo G., Cabassu P., Chalchat J.C., Pasquier B. Studies of mediterranean oregano populations—V. Chemical composition of essential oils of oregano: Origanum syriacum L. var. bevanii (Holmes) Ietswaart, O. syriacum L. var. sinaicum (Boiss.) Ietswaart and O. syriacum L. var. syriacum from Lebanon and Israel. Flavour Fragr. J. 2005;20(2):164–168. [Google Scholar]
- Fleisher A., Fleisher Z. Chemical composition of Origanum syriacum L. essential oil. Aromatic plants of the Holy Land and the Sinai, Part V. J. Essent. Oil Res. 1991;3(2):121–123. [Google Scholar]
- Forbes B., Sahm D., Weissfeld A. twelves ed. Mosby Elsevier; USA: 2007. Study Guide for Bailey & Scott's Diagnostic Microbiology. [Google Scholar]
- Geneste J.-M., Mauriac M. The conservation of Lascaux cave, France. In: Saiz-Jimenez C., editor. The Conservation of Subterranean Cultural Heritage. CRC Press; New York: 2014. pp. 165–172. [Google Scholar]
- Halim A., Mashaly M., Zaghloul A., El-Fattah H.A., De Pooter H. Chemical constituents of the essential oils of Origanum syriacum and Stachys aegyptiaca. Int. J. Pharmacognosy. 1991;29(3):183–187. [Google Scholar]
- Husein A.I., Ali-Shtayeh M.S., Jamous R.M., Zaitoun S.Y.A., Jondi W.J., Zatar N.A.-A. Antimicrobial activities of six plants used in traditional Arabic Palestinian herbal medicine. Afr. J. Microbiol. Res. 2014;8(38):3501–3507. doi: 10.3109/13880209.2014.886274. [DOI] [PubMed] [Google Scholar]
- Ibrahim L., Karaky M., Ayoub P., El Ajouz N., Ibrahim S. Chemical composition and antimicrobial activities of essential oil and its components from Lebanese Origanum syriacum L. J. Essent. Oil Res. 2012;24(4):339–345. [Google Scholar]
- Ibrahim M.E., Mohamed M.A., Khalid K.A. Effect of growing locations on the essential oil content and compositions of lemon verbena shrubs under the conditions of Egypt. J. Ess. Oil Bear. Plant. 2014;17(2):288–294. [Google Scholar]
- Isaac J., Rishmawi K. Applied Research Institute; Jerusalem: 2015. Status of the environment in the State of Palestine. [Google Scholar]
- Jaradat N.A., Zaid A.N., Abuzant A., Shawahna R. Investigation the efficiency of various methods of volatile oil extraction from Trichodesma africanum and their impact on the antioxidant and antimicrobial activities. J. Intercult Ethnopharmacol. 2016;5(3):250–257. doi: 10.5455/jice.20160421065949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J.H., Ferraro M.J. Antimicrobial susceptibility testing: general principles and contemporary practices. Clin. Infect. Dis. 1998;26:973–980. doi: 10.1086/513938. [DOI] [PubMed] [Google Scholar]
- Khoury M., Stien D., Eparvier V., Ouaini N., El Beyrouthy M. Report on the medicinal use of eleven Lamiaceae species in lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. -Based Complementary Altern. Med. 2016;2016:1–17. doi: 10.1155/2016/2547169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless J. Conari Press; Massachusetts: 2013. The encyclopedia of essential oils: the complete guide to the use of aromatic oils in aromatherapy, herbalism, health, and wellbeing. [Google Scholar]
- Lukas B., Schmiderer C., Franz C., Novak J. Composition of essential oil compounds from different Syrian populations of Origanum syriacum L. (Lamiaceae) J. Agric. Food. Chem. 2009;57(4):1362–1365. doi: 10.1021/jf802963h. [DOI] [PubMed] [Google Scholar]
- Pirigharnaei M., Zare S., Heidary R., Khara J., EmamaliSabzi R., Kheiry F. The essential oils compositions of Iranian oregano (Origanum vulgare L.) populations in field and provenance from Piranshahr district, West Azarbaijan province, Iran. Avicenna J Phytomed. 2011;1(2):106–114. [Google Scholar]
- Plessner M. Daud al-Antaki's 16th century encyclopedia on medicine, natural history and occult sciences. Int. Congress History Sci. (10th, Ithaca) 1962:26-VIII. 2-IX 635-637. [Google Scholar]
- Prakashan N. Nirali Prakashan; India: 2009. Pharmacognosy. [Google Scholar]
- Rates S.M. Plants as source of drugs. Toxicon. 2001;39(5):603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Rose J. Frog Books; USA: 1999. 375 Essential oils and hydrosols. [Google Scholar]
- Sharifi-Rad J., Sureda A., Tenore G.C., Daglia M. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules. 2017;22(1):1–55. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehadeh M., Suaifan G., Darwish R. Complementary and alternative modalities; a new vein in weight control and reduction interventions. a pilot study in Jordan. Int. J. Biol. Biomed. 2017;2:1–5. [Google Scholar]
- Shehadeh M., Silvio S., Ghadeer A., Darwish R.M., Giangaspero A., Vassallo A., Lepore L., Oran S.A., Hammad H., Tubaro A. Topical anti-inflammatory potential of six Salvia species grown in Jordan. Jordan J. Pharm. Sci. 2014;7(2):153–161. [Google Scholar]
- Seidemann J. Springer-Verlag; Berlin Heidelberg New York, European Union: 2005. World spice plants: economic usage, botany, taxonomy. [Google Scholar]
- Tawaha K.A., Hudaib M.M. Chemical composition of the essential oil from flowers, flower buds and leaves of Thymus capitatus Hoffmanns & Link from Jordan. J. Essent. Oil Bear Pl. 2012;15(6):988–996. [Google Scholar]
- Tepe B., Daferera D., Sökmen M., Polissiou M., Sökmen A. The in vitro antioxidant and antimicrobial activities of the essential oil and various extracts of Origanum syriacum L var bevanii. J. Sci. Food Agric. 2004;84(11):1389–1396. doi: 10.1021/jf049859g. [DOI] [PubMed] [Google Scholar]
- Toncer O., Karaman S., Diraz E. An annual variation in essential oil composition of Origanum syriacum from Southeast Anatolia of Turkey. J. Med. Plants Res. 2010;4(11):1059–1064. [Google Scholar]
- Tworkoski T. Herbicide effects of essential oils. Weed Sci. 2002;50(4):425–431. [Google Scholar]
- Van den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M., El Gendy A.E.-N.G., Sendra E., Fernandez-Lopez J., Abd El Razik K., Omer E.A., Pérez-Alvarez J.A. Chemical composition and antioxidant and anti-Listeria activities of essential oils obtained from some Egyptian plants. J. Agric. Food. Chem. 2010;58(16):9063–9070. doi: 10.1021/jf101620c. [DOI] [PubMed] [Google Scholar]
- WHO, 2001. Legal status of traditional medicine and complementary/alternative medicine: a worldwide review. Geneva. (WHO/EDM/TRM/2001.2).
Website
- Boyd B. A political ecology of Za’atar. EnviroSociety. 2016 www.envirosociety.org/2016/06/a-political-ecology-of-zaatar 15 June. [Google Scholar]
- Farjam M.H., Kavoosifar A., Joukar M. Salvia Aegyptiaca oil extracted by microwave-assisted and normal hydrodistillation: composition and antibacterial activity. Free Library (October, 1) 2014 https://www.thefreelibrary.com/Salvia Aegyptiaca oil extracted by microwave-assisted and normal...-a0417895211 (accessed June 18 2018) [Google Scholar]