Abstract
Cadmium (Cd), a potent cardiotoxic environmental heavy metal, induces oxidative stress and membrane disturbances in cardiac myocytes. Phosphodiesterase (PDEs) retards the positive inotropic effects of β-adrenoceptor activation by decreasing levels of cAMP via degradation. Hence, PDE inhibitors sensitize the heart to catecholamine and are therefore, used as positive inotropic agents. The present study was designed to probe the potential attenuating effects of the selective PDE4 inhibitor (Roflumilast, ROF), on cardiac biomarkers, lipid profile, lipid peroxidation products, antioxidant status and histology of cardiac tissues against Cd-induced cardiotoxicity in rats. Rats were randomly distributed into four different groups: group 1, served as the normal control group. Group 2, served as the toxic control group and were administered Cd (3 mg/kg, i.p.) for next 7 days. Groups 3 and 4, served as treatment groups that received Cd with concomitant oral administration of ROF doses (0.5 and 1.5 mg/kg), respectively for 7 days. Serum samples of toxic control group rats resulted in significant (P < 0.001) increase in lactate dehydrogenase (LDH), creatine phosphokinase (CPK), total cholesterol (TC), triglycerides (TG) and low density lipoproteins (LDL) levels with concomitant decrease in high density lipoproteins (HDL) levels in serum which were found reversed with both of ROF treatment groups. Cd also causes significant increased (P < 0.001) in myocardial malondialdehyde (MDA) contents while cardiac glutathione (GSH) level, superoxide dismutase (SOD) and catalase (CAT) enzyme activities were found decreased whereas both doses of ROF, significantly reversed these oxidative stress markers and antioxidant enzymes. Cardiotoxicity induced by Cd also resulted in enhanced expression of non-phosphorylated and phosphorylated form of NF-κB p65 and decreased expression of glutathione-S-transferase (GST) and NQO1 which were found reversed with ROF treatments, comparable to normal control group. Histopathological changes were also improved by ROF administration as compared to Cd treated rats alone. In conclusion, Roflumilast exhibited attenuating effect against Cd-induced cardiac toxicity.
Keywords: Cadmium, Cardiotoxicity, CRF, GST, NF-κB, NQO1, Roflumilast
1. Introduction
Cadmium (Cd), a toxic metal, is capable to be absorbed in population at low-level chronic exposure by different ways including smoking, diet, polluted water with Cd and occupational exposure in certain industries. Due to its long half-life, which ranged from 10 to 40 years, Cd accumulates in the body (Nordberg et al., 2007, Akerstrom et al., 2013).
Besides the carcinogenic potential, Cd has been associated with nephrotoxicity, bone disease and cardiac abnormalities (ATSDR, 2012). Although, Cd causes multi-organ toxicities, heart is one of the most targeted organs that results in oxidative damage after Cd exposure (Alissa and Ferns, 2011, Mollaoğlu et al., 2006). Earlier research conducted in human’s myocardial autopsy show that the myocardial content of Cd increases up to late middle age (Borné et al., 2015). The capability of Cd to change lipid metabolism and its contribution to the progression of cardiovascular diseases (CVDs) is not only reported in earlier experimental studies but epidemiological surveys also support the association of Cd and CVDs such as: hypertension, atherosclerosis, stroke and cardiac arrest (Satarug et al., 2003, Larregle et al., 2008, Tellez-Plaza et al., 2013). Even though several mechanisms of cardiovascular toxicities mediated by Cd were proposed, including inflammation, the precise mechanism(s) is/are still unexplored (Fagerberg et al., 2013). Cd toxicity generally involves interruption of numerous cellular functions and damage to various cellular structures (Stohs et al., 2000, Nemmiche et al., 2007). The main diseases and exact biochemical changes in heart tissues as a result of Cd-induced toxicities are directly related to Cd concentration and cardiac oxidative stress (Ercal et al., 2001, Yazıhan et al., 2011).
Cardiomyopathies often lead to myocarditis and myocardial infarction which might result in elevated levels of cardiac marker enzymes, importantly lactate dehydrogenase (LDH), and creatine phosphokinase (CPK), in the circulation, and thus serve as diagnostic markers in direct myocardial endothelial injury and damage to the myocardial cells (Ansari et al., 2007, Ansari et al., 2006).
Decreased levels of antioxidant enzymes namely catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR) and glutathione peroxidase (GPx), are highly correlated with multi-organ oxidative damage because of more production of reactive oxygen species (ROS) (Blokhina et al., 2002, Ansari et al., 2008, Valdecantos et al., 2009). Findings of the study conducted by Mishra, (2004) show that increased levels of free radicals may adversely affect cell survival not only by their membrane damage potential through oxidative stress, but also by causing irreversible DNA modification in cells (Mishra, 2004). Malondialdehyde (MDA), a breakdown product of polyunsaturated fatty acids oxidation, functions as a valid indicator of lipid peroxidation (LPO) in tissues (Ayala and Muñoz, 2014). Cd ions have high affinity to sulfhydryl (-SH), carboxyl and phosphate groups containing biological structures, and can inhibit numerous enzymes like Cu, Zn-SOD, phosphatases. Thus, they would tend to mediate abnormal metabolic reactions, including lipid metabolism.
As Cd exposure ultimately leads to oxidative stress, scientists pay more attention on searching for compounds that have antioxidant potential to prevent/treat Cd-induced cardiac damage. A previously conducted study by Brandes and Kreuzer (2005), suggests that as NADPH oxidase activates ROS and causes disturbance of the antioxidant defense system, hence, ROS might be one of the reason of cardiotoxicity.
A report shows that in myocardial tissues, the formation of cytokines or ROS activating NF-κB transcription factor not only further accelerates the pathologic process of endothelial dysfunction, but also exacerbates various CVD such as unstable angina pectoris, acute myocardial infarction and heart failure (Liu and Malik, 2006, Oeckinghaus and Ghosh, 2009).
Many physiological processes such as vascular resistance (VR), cardiac output (CO), visceral motility, inflammation, immune response and reproduction depends on cyclic nucleotides (cAMP and cGMP) and intracellular second messengers for their tissue protective and anti-inflammatory characters (Ghosh et al., 2009, Houslay and Adams, 2003). PDEs, by metabolizing cAMP and cGMP to their inactive forms, are considered pivotal in the regulation of various physiological functions in cardiac myocytes including pace-making, contractility, cell growth and survival (Imam et al., 2018).
Based on the expression of PDE isoenzyme in nearly all tissue, alteration in these enzymes expression/activity and cyclic nucleotide signaling in cardiovascular system have been identified in multiple CVD (Amsallem et al., 2005). Pharmacological inhibition of PDEs could be one of the potential approaches to address some of cardiac problems, especially congestive heart failure (Imam et al., 2018).
Recently, scientists are more interested in targeting selective of PDE and developed a number of specific PDE enzymes inhibitors (Imam et al., 2016). Selective PDE type-4 (PDE4), is mainly expressed in cardiovascular tissues, inflammatory cells, airway smooth muscle and brain (Muller et al., 1996). It is well known that inhibition of PDE4 results in enhancing levels of intracellular cAMP and thus initiating intracellular signaling pathway that involves the activation of both; protein kinase A and cAMP sensitive element binding protein or family of transcription factors (CREB/ATF-1), as well as down regulation of NF-κB transcriptional activity (Baldwin, 2001). Roflumilast (ROF), one of PDE4-inhibitor has been reported to suppress inflammatory responses by activating cAMP/PKA pathways and suppressing LPO (Erdogan et al., 2007). ROF reduced release of chemokines (CCL2, CCL3, CCL4 and CXCL10) and tumor necrosis factor alpha (TNF-α) from human lung macrophages in dose-dependent fashion (Buenestado et al., 2012). Furthermore, inhibition of TNF-α-mediated pathways can reduce myocardial ischemia-reperfusion injury (Saito et al., 2012). TNF-α is directly involved in the activation of activated NF-κB which is critical for the inflammatory processes (Barnes and Karin, 1997, Brasier, 2006). Understanding of the selective targets of PDEs in cardiovascular biology and disease would further explore and facilitate their potential development as new application in treating/preventing CVDs.
To achieve the greatest possible reduction in CVD, possible strategies for treatment should be targeted at reducing the patient’s oxidative stress and elevated lipids profile. Considering the reported literature highlighting the possible beneficial effects of ROF in cardiovascular-related disorders, this study was designed to evaluate the protective efficacy of ROF in Cd-induced oxidative cardiotoxicity and dyslipidemia in Wistar albino rats.
2. Materials and methods
2.1. Chemicals and reagents
Cadmium chloride (CdCl2) salt was procured from Sigma Chemical Co. (St. Luis, Mo, USA). Antibodies (Primary and secondary) for Western blotting were purchased from Santa Cruz (Dallas, USA). Nitrocellulose membrane was obtained from Bio-Rad Laboratories (Hercules, USA). Western blot detection kits (Chemiluminescence) were supplied from GE Healthcare Life Sciences (Piscataway, USA). All used chemicals and reagents were of analytical grade.
2.2. Animals
This study was conducted using total twenty-four healthy male Wistar albino rats (200–250 g, 6–8 weeks old), housed at the Animal Care Unit, College of Pharmacy, Prince Sattam Bin Abdulaziz University (PSAU), KSA. During acclimatization period, animals were administered with water ad libitum and a standard diet consisting of (g/kg): flour 380, chokar 380, molasses 12, NaCl 5.8, nutrivet L 2.5, potassium m-bisulphate 1.2, vegetable oil 38, fish meal 170 and powdered milk 150. The study was approved by the Ethical Review Committee, College of Pharmacy, Prince Sattam Bin Abdulaziz University, KSA (approval ref no. HAP-01-KJ-050).
All the experiments performed in present study obeyed and followed the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (1996).
2.3. Experimental design
Rats were randomly separated into four groups (n = 6). Group 1 was labeled as normal control and receive normal saline only for 7 days, Group number 2 served as disease control group and was administered with CdCl2 (3 mg/Kg, IP.) daily for 7 days. Groups 3 & 4 served as treated groups and were co-administered with CdCl2 and tested drug (Roflumilast) in two increasing doses of 0.5 and 1.5 mg/Kg (PO), respectively, once a day for 7 days.
After 24 h of last dose, blood samples were collected from retro orbital plexus of all the animals under light anesthesia (Diethyl ether). Serum was separated and stored at −20 °C until further biochemical estimations of LDH, CPK and lipid profile. After successful blood collection, all rats were sacrifice by cervical dislocation and heart was isolated. Small part of heart was placed in 10% formalin solution for histopathological examination and the remaining heart preserved at −80 °C until the biochemical analysis of different parameters (MDA, SOD, CAT and GSH) and Western blot analysis.
2.4. Biochemical estimations in serum
Functions of heart were assessed by measuring the levels of LDH and CPK in serum using commercially available diagnostic kits (BioSystems S.A., Barcelona, Spain). Respective diagnostic kits were used to estimate LDH and CPK levels and expressed in IU/L (Tietz, 2005).
2.5. Lipid profile estimation
The concentrations of Triglycerides (TGs), Total Cholesterol (TC), and High Density Lipoprotein (HDL-C) in the serum were analyzed using commercial assay kits (Giesse Diagnostics S.r.l., Rome, Italy). Very Low Density Lipoprotein (VLDL-C), Low Density Lipoprotein (LDL-C), Atherosclerotic index and Cardiac Risk Factor (CRF) were calculated by given formula:
2.6. Biochemical estimations in heart tissue
Heart tissues were cut in to small pieces and homogenized (10% w/v) using homogenizer in ice–cold phosphate buffer (0.1 M, pH 7.4) followed by centrifugation for 30 min (4 °C) at 12000 rpm. Standard protocols were used to estimate myocardial MDA (Esterbauer and Cheeseman, 1990), total glutathione (GSH) (Jollow et al., 1974), SOD (Marklund, 1985) and CAT (Claiborne, 1985).
2.7. Western blot analysis
Protein isolation was performed as follows. Isolated heart tissues from rats of all groups were washed with ice-cold PBS followed by minced and homogenization in cold protein lysis buffer and protease inhibitor cocktail (Ansari et al., 2013). The cell lysates were incubate on ice for 60 min with vortex mixing after every 10 min, followed by centrifugation for 10 min (12,000 RPM, 4 °C), to obtained total cellular proteins. Total protein content was measured according to the well-established method of Lowry et al. (1951). Western blot analysis was done by following the previously described method of Ansari et al. (2013). Briefly, protein (25–50 µg) from each group was separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically moved to nitrocellulose membranes. Protein blots was incubated overnight at 4 °C with primary antibodies against GST, NF-κB p65, pNF-κB p65 and NQO1 and peroxidase-conjugated secondary antibodies at 25 °C. Bands were visualized using the enhanced chemiluminescence method (GE Health Care, Mississauga, Canada). Band intensities were calculated comparative to β-actin bands using an image analysis system (ImageJ® image processing program, National Institutes of Health, Bethesda, USA). Images were capture by using C-Digit chemiluminescent western blot scanner (LI-COR, Lincoln, USA).
2.8. Histopathological studies
Heart isolated tissue previously preserved in 10% buffered formalin were further processed for histopathology studies. The tissues were dehydrated, embedded on paraffin block and cut in to sections of about 5 µm thickness followed by staining with hematoxylin and eosin (H&E), Masson’s trichrome (MT) and Periodic Acid–Schiff (PAS) stain. Then, the samples were observed under microscope by histopathologist.
2.9. Statistical analysis
The data is presented as mean ± standard error of mean (SEM) with 95% confidence intervals (CI). Student’s t-test was used for the comparison of diseased control with control group while, one-way analysis of variance (ANOVA) followed by Tukey’s test was applied to check significant difference of treatments groups with diseased control group. P < 0.01 considered as significantly different (Dawson-Saunders and Trapp, 1990). Results were analyzed by using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Effect of ROF on serum diagnostic marker enzymes
Intraperitoneal injection of Cd (3 mg/Kg) for 7 days induced severe biochemical changes in cardiac tissues. The LDH and CPK levels were estimated in serum and showed in Table 1. In rats treated with Cd only, the serum diagnostic marker enzymes were found to be significantly (p < 0.001) increased than that for the rats in the control group. ROF treatment significantly and dose-dependently restored the elevated levels of these enzymes by 42.51% and 51.42% for LDH and by 54.05% and 65.58% for CPK, respectively.
Table 1.
Effect of ROF on LDH and CK in serum against Cd-induced cardiotoxic rats.
| Groups | LDH (IU/L) | CPK (IU/L) |
|---|---|---|
| Control | 107.93 ± 9.72 | 59.15 ± 2.52 |
| CdCl2 (3 mg/Kg) | 333.24 ± 14.08*** | 213.63 ± 5.78*** |
| ROF (0.5 mg/Kg) + CdCl2 | 191.58 ± 11.76## | 98.16 ± 4.37### |
| ROF (1.5 mg/Kg) + CdCl2 | 161.90 ± 9.57### | 73.52 ± 4.88### |
All values are expressed as Mean ± SEM (n = 6).
p < 0.001, compared with control group.
p < 0.01.
p < 0.001 compared with CdCl2 group (One-way ANOVA followed by Tukey’s test).
3.2. Effect of ROF on serum lipid profile
The serum lipid levels of Cd-treated rats were determined and depicted in Table 2. Serum total cholesterol (TC), triglycerides (TG), and low density lipoprotein (LDL-C) levels were found to be significantly (p < 0.001) higher in the Cd only treated group with concomitant decrease in high density lipoprotein (HDL-C) levels than those in the normal control group. The serum levels in ROF treated groups at both doses were significantly decreased by 22.61%, and 40.95%, for TC, by 29.81%, and 35.00% for TG and by 27.87%, and 53.63% for LDL-C (p < 0.001), respectively as compared to toxic group (Cd only). However, ROF treatment at both doses significantly improves the HDL-C levels by 25.16% (p < 0.01) and 39.05% (p < 0.001) respectively.
Table 2.
Effect of ROF on lipid profile against Cd-induced cardiotoxic rats.
| Control | CdCl2 (3 mg/Kg) | ROF (0.5 mg/Kg) + CdCl2 | ROF (1.5 mg/Kg) + CdCl2 | |
|---|---|---|---|---|
| TC (mg/dl) | 109.67 ± 3.53 | 233.06 ± 8.05*** | 180.37 ± 4.58### | 137.63 ± 5.04### |
| TG (mg/dl) | 48.70 ± 2.36 | 114.42 ± 2.35*** | 80.31 ± 1.53### | 74.37 ± 2.42### |
| HDL (mg/dl) | 41.48 ± 1.32 | 23.25 ± 1.22*** | 29.10 ± 2.14## | 32.33 ± 1.47### |
| LDL (mg/dl) | 58.45 ± 3.87 | 186.92 ± 8.68*** | 134.83 ± 4.19### | 90.42 ± 5.51### |
| VLDL (mg/dl) | 9.74 ± 0.47 | 22.88 ± 0.47*** | 16.06 ± 0.30## | 14.87 ± 0.48### |
| Atherosclerotic Index | 1.65 ± 0.11 | 9.22 ± 0.83*** | 5.13 ± 0.17### | 3.31 ± 0.28### |
| Cardiac Risk Factor | 2.65 ± 0.11 | 10.22 ± 0.83*** | 6.13 ± 0.17### | 4.31 ± 0.28### |
All values are expressed as Mean ± SEM (n = 6).
p < 0.001, compared with control group.
p < 0.01.
p < 0.001 compared with CdCl2 group (One-way ANOVA followed by Tukey’s test).
3.3. Effect of ROF on myocardial oxidative stress markers
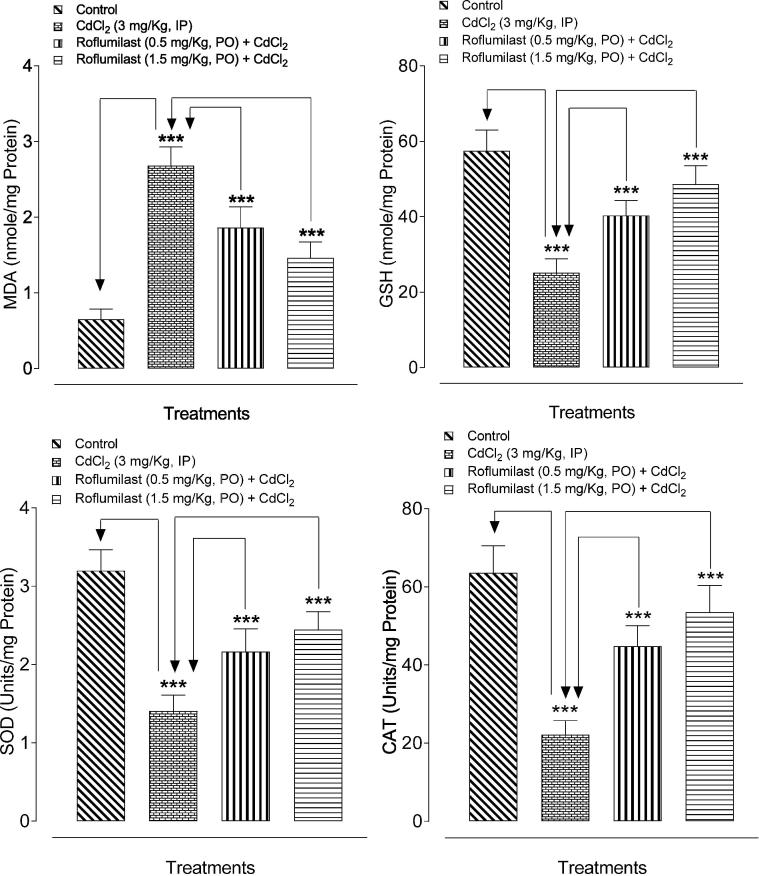
The results of Cd-induced oxidative stress are showed in Fig. 1. Cd treatment resulted in increased MDA levels and decreased GSH contents as well as SOD and CAT enzyme activity in heart tissues significantly (p < 0.001) as compared to the normal rats. ROF treatment significantly (p < 0.001) reversed the Cd-induced increase in myocardial MDA levels and decrease in GSH level as well as SOD and CAT enzyme activity.
Fig. 1.
Bar diagram showing the protective effect of ROF on myocardial oxidative stress against Cd-induced cardiotoxicity in rats. ***p < 0.001, showed comparison of CdCl2 with control (Unpaired t-test) and treated (ROF; 0.5 and 1.5 mg/kg) with CdCl2 group (One-way ANOVA followed by Tukey’s test). Each bar represents mean ± SEM (n = 6).
3.4. Effect of ROF on Western blot analysis
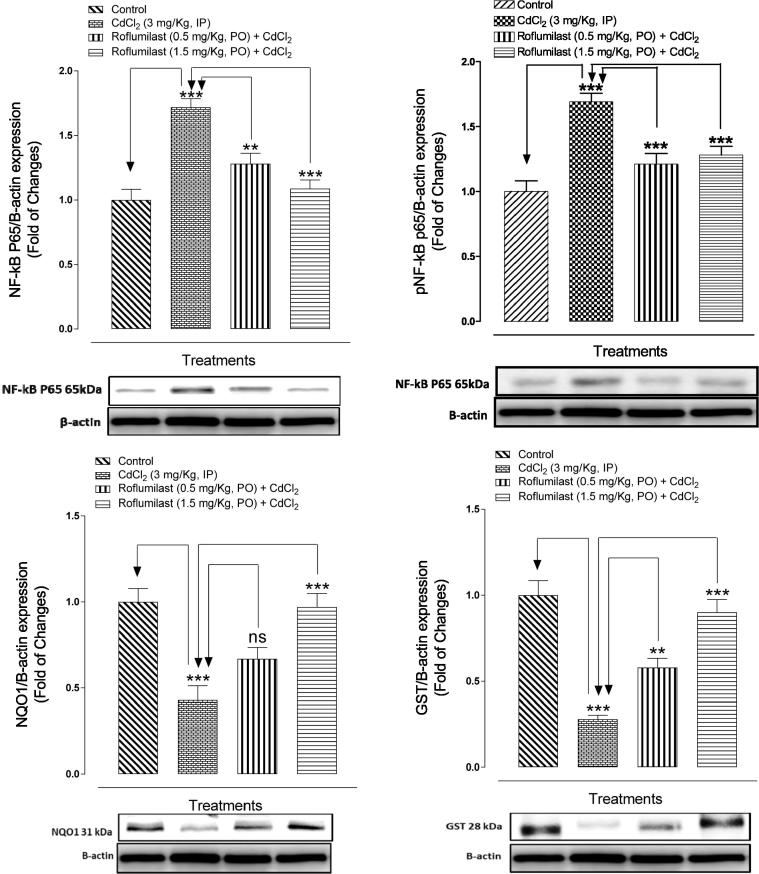
In present study, the influence of ROF on protein expression of GST, NF-κB p65, and NQO1 was studied and results were showed in Fig. 2. Western blotting was performed to investigate the expression of NF-κB p65 as activation of NF-κB plays significant role in the inflammatory mediator production. Western blotting analyses revealed that the Cd treatment found to be resulted in significantly enhanced NF-κB p65, protein expression when compared with normal control group. ROF treatment at both doses (0.5 and 1.5 mg/kg), significantly suppressed the non-phosphorylated and phosphorylated form of NF-κB p65 expression, as compared to the Cd-injected rats. Furthermore, Cd-injected rats also showed significant (p < 0.001) reduced levels of GST and NQO1 protein expression as compared to control group, while these reduced levels were significantly (p < 0.001) found to be restored in ROF treated rats at higher dose, except NQO1 at lower dose as results were non-significant.
Fig. 2.
Bar diagram showing the protective effect of ROF on NF-κB p65, GST and NQO1 protein expression in myocardial tissues of Cd-intoxicated rats. nsp > 0.05, **p < 0.01, ***p < 0.001, showed comparison of CdCl2 with control (Unpaired t-test) and treated (ROF; 0.5 and 1.5 mg/kg) with CdCl2 group (One-way ANOVA followed by Tukey’s test). Each bar represents mean ± SEM (n = 6).
3.5. Effect of ROF on histopathology of heart tissue
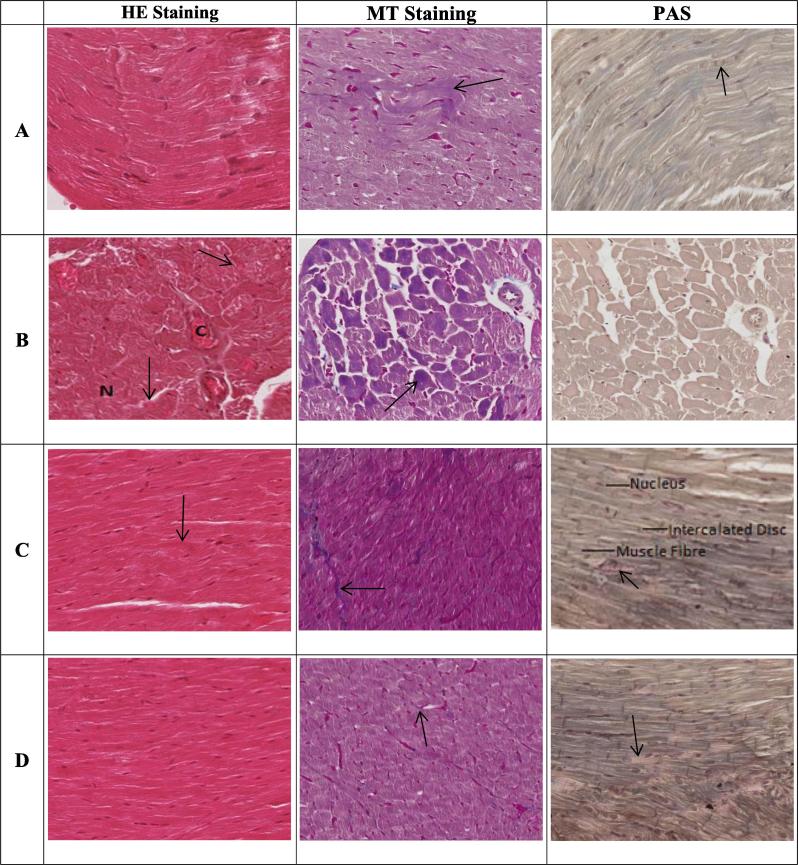
The heart tissues of the control rats showed normal heart architecture and histology using H&E staining (Fig. 3). Photomicrograph of Cd-treated group showed distorted cardiac muscle cells with the infiltration of inflammatory cells. However, the photomicrographs of ROF-treated groups showed normal myocardiocytes without cardiac hypertrophy. Masson trichrome (MT) staining showed normal distribution of collagen fibers with no evidence of fibrosis in control groups. Highly increased collagen bundles were observed in the heart tissues of Cd treated group. Somewhat normal appearance of collagen fibers was seen in ROF treated groups. Control group of PAS shows normal distribution of PAS-positive materials. Cd treated group shows very little to almost absence of PAS-positive materials. ROF treated group shows almost normal distribution of PAS-positive materials. Histological scores are presented in Table 3.
Fig. 3.
Effect of ROF on histopathological features of Cd-intoxicated heart tissues with Haematoxylin and Eosin (H & E, 100X), Masson Trichrome (MT, 100X) and Periodic Acid–Schiff (PAS, 100X) staining. Photomicrographs of heart tissues of (A) normal control rats, (B) CD- intoxicated rats, (C) ROF treated at low dose (0.5 mg/Kg), and (D) ROF treated at high dose (1.5 mg/Kg). In figures, abbreviation “C” indicates for congestion and “N” for necrosis. Arrows in H&E stained figures, indicates degenerative marks of myocardiocytes, in PAS stained figures, indicate PAS positive materials while in MT stained figures, indicate collagen fibers.
Table 3.
Effect of ROF on histopathological scoring against Cd-induced cardiotoxic rats.
| HE Staining (General histological status) | MT Staining (Collagen fibers) | PAS (PAS positive materials) | |
|---|---|---|---|
| A | 0 (Normal) | 0 (Normal) | 0 (Normal) |
| B | (−3) (3steps below normal due to three pathological features) | (−3) (highly increased collagen fibers) | (−3) almost absence of PAS positive materials |
| C | (−1) (one step below normal due to one pathological feature) | (−1) (slightly increased collagen fibers) | (−1) (slightly presence of PAS positive materials) |
| D | 0 (non-significant changes) | 0 (non-significant changes) | 0 (non-significant changes) |
4. Discussion
Cadmium (Cd) was used extensively in consumer products and exposed to major population through tobacco smoke, certain foods that contain Cd and ambient air, particularly in the industrial and hazardous waste sites (Yassin and Martonik, 2004). Among the research studies that evaluated the relationship between Cd exposure and death because of cardiac impairment, most studies confirmed positive results (Nawrot et al., 2008, Menke et al., 2009). In present study, the protective effect of ROF was evaluated against Cd-induced cardiac oxidative stress and dyslipidemia in rats. Experimental results showed that ROF treatment at both doses (0.5 and 1.5 mg/kg) could prevent the Cd -induced myocardial oxidative stress in rats when co-administered with Cd for 7 consecutive days.
Previous literature proposed that Cd exposure results in variety of toxicological effects and alterations in biochemical functions markers producing severe threat to health. Cd -induced toxicity can generate free radical ions from membrane poly unsaturated fat peroxidation and these free ions resulted in production of ROS, as a result intracellular antioxidant defense system inhibited (WHO, 1992, Casalino et al., 2002). Cd-toxicity may affect the activities of some antioxidant enzymes, increased the activities of serum diagnostic marker enzymes and reliable cardiac injury markers by inducing the expression of different stress genes (Wang and Templeton, 1998, Watjen et al., 2001, Manna et al., 2008). In present study also, Cd treatment results in increased serum diagnostic marker enzymes whereas, administration of ROF notably reduced the activities of the cardiac diagnostic marker enzymes in the serum of rats. These results proposed that ROF may decrease serum enzyme levels because of anti-lipoperoxidative, antioxidant and membrane stabilizing properties.
Previous literature suggests that Cd-induced CVD is related with the abnormalities in the lipid metabolism and elevate the contents of lipids and lipoproteins in serum (Murugavel and Pari, 2007, Larregle et al., 2008). The results from present study also, showed increased serum levels of TC, TG, LDL and VLDL with concomitant decreased in HDL levels after Cd-administration, clearly showing the impairment in lipoprotein metabolism. The changes in the HMG-CoA reductase activity (main enzyme for the synthesis of cholesterol) may reduce the LDL receptor gene expression which interferes with uptake of cholesterol from the blood by macrophages, and in turn causes more cholesterol synthesis in heart and hypercholesterolemia (Kantola et al., 1998). The uptake of TC by macrophages is impaired by Cd exposure, and causes increased serum cholesterol level (Ramirez and Gimenez, 2002). Previous literature suggested that cytokines play significant role in the increased levels of serum TG and VLDL production by enhancing lipogenesis in liver and overwhelming oxidation of fatty acids (Nachiappan et al., 1994). On the basis of observed results, it was suggested that abnormalities in lipids and lipoprotein levels might be because of Cd-induced myocardial oxidative stress.
ROF treatment in Cd-intoxicated rats significantly normalized the elevated serum lipids and lipoproteins levels through their anti-lipoperoxidative actions, and prevents the membrane lipid oxidation by preserving the membranes of cells and organelles from the Cd-induced oxidative stress. Thus, the hypolipidemic effect of ROF by inhibiting the cholesterol and oxidized lipid accumulation could diminish the prevalence of atherosclerosis upon Cd exposure or other environmental toxicants.
The principal target sites for Cd-toxicity are cell membranes followed by lipid peroxidation, proteins oxidation and thiol reduction (Stohs et al., 2000, Larregle et al., 2008). The results from present study also demonstrate the increased levels of myocardial LPO, after Cd administration which might be because of the excess generation of OFR and decreased antioxidant defense. ROF treatment in Cd treated rats successfully restores the elevated levels of LPO which is one of the oxidative stress markers, revealing the anti-lipid peroxidative and antioxidant effects of ROF.
Antioxidant enzymes prevent the cellular integrity from oxidative damage caused by OFR. SOD and CAT play important role in preventing the excess production of OFR (Casalino et al., 2002, Ansari et al., 2007). The activity of both enzymes is observed to be reduced in the heart tissue of the Cd treated rats which confirm the Cd-induced myocardial oxidative stress. ROF treatment significantly restores the activities of these enzymes showing that Cd-induced cardiac toxicity can be attenuated by ROF administration.
Glutathione (GSH) is also one of the useful indicators for myocardial injury caused by oxidative stress and plays important role in cellular defense against oxidative damage caused by OFR and other ROS by scavenging them (Kurian et al, 2016). Cd-induced toxicity prominently diminished the myocardial GSH status, as shown by significant decrease in the GSH levels which describe the increased consumption of GSH for the detoxification of Cd-induced oxidative stress. ROF treatment in Cd treated rats could prevent the depletion of non-enzymatic antioxidants (GSH), indicating ROF role in the GSH metabolism which in turn increases the GSH concentration and intracellular antioxidant power (Morales et al., 2006).
NF-κB exist in the cytosol and is activated in response to different inflammatory stimuli, atmospherically pollutants, pro-oxidants, cancer-causing agents, stress, etc (Aggarwal, 2004). Increased level of ROS may activate initial step in the signal mechanism of activation of redox-sensitive NF-κB signaling (Bar-Shai et al., 2008, Kwak et al., 2011). In previous studies, it has been reported that NF-kB activation stimulates apoptosis induced by Cd in rats (Xie and Shaikh, 2006, Yuan et al., 2014). In the present study, Cd-induced oxidative stress results in activation of NF-kB protein expression and decreased expression of GST and NQO1, whereas, ROF administration in Cd-intoxicated rats effectively suppresses the myocardial oxidative stress through the inhibition of NF-kB signals transduction pathways and increased expression of GST and NQO1. Our results are corroborated with the previous findings (Xiaoli et al., 2011). These findings suggest that the preventive effect of ROF against Cd-induced myocardial oxidative stress may be through the activation of GST and NQO1.
Photomicrographs of the heart tissues of Cd treated rats showed abnormal histological changes. There was no significant differences were observed in myofibril impairment, between control and ROF treated groups which showed that ROF is accomplished of preventing the histological changes caused by Cd-induced injury.
According to Cortijo et al. (2009) ROF possess anti-inflammatory and antioxidant effect. In present study also, ROF treatment with potentially protects the heart tissues and restores its functions impaired from Cd-induced myocardial oxidative stress. The biochemical and histological results from the present study proposed the potential effect of ROF in protecting the cardiac tissues against oxidative damages and hyperlipidemia therefore ROF could be used in future as an effective treatment against cardiotoxicity, hyperlipidemia and associated cardiovascular disorders.
5. Conclusions
On the basis of observed results from the present study ROF could be proposed as a novel target for the therapeutic strategies and prognostic biomarker in various cardiovascular pathologies, especially disorders caused by heavy metals. However, the exact mechanism of ROF is not clear therefore further detailed studies are needed to explore and confirmed the protective molecular mechanism of ROF against CVD and dyslipidemia.
Acknowledgments
Acknowledgment
This project was supported by the Deanship of Scientific Research, Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia under the research project number 2016/03/6694.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aggarwal B.B. Nuclear factor-nB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Akerstrom M., Barregard L. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol. Appl. Pharmacol. 2013;268:286–293. doi: 10.1016/j.taap.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Alissa E.M., Ferns G.A. Heavy metal poisoning and cardiovascular diseases. J. Toxicol. 2011;2011 doi: 10.1155/2011/870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsallem E., Kasparian C. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst. Rev. 2005;1:CD002230. doi: 10.1002/14651858.CD002230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.N., Bhandari U. Evaluation of antioxidant and neuroprotective effect of ethanolic extract of Embelia ribes Burm in focal cerebral ischemia/reperfusion- induced oxidative stress in rats. Fundament. Clin. Pharmacol. 2008;22:305–314. doi: 10.1111/j.1472-8206.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- Ansari M.N., Bhandari U. Ethanolic Zingiber officinale extract pretreatment alleviates isoproterenol-induced oxidative myocardial necrosis in rats. Indian J. Exp. Biol. 2006;44:892–897. [PubMed] [Google Scholar]
- Ansari M.N., Bhandari U. Protective role of curcumin in myocardial oxidative damage induced by isoproterenol in rats. Human Exp. Toxicol. 2007;26:933–938. doi: 10.1177/0960327107085835. [DOI] [PubMed] [Google Scholar]
- Ansari M.A., Maayah Z.H. The role of aryl hydrocarbon receptor signaling pathway in cardiotoxicity of acute lead intoxication in vivo and in vitro rat model. Toxicol. 2013;306:40–49. doi: 10.1016/j.tox.2013.01.024. [DOI] [PubMed] [Google Scholar]
- ATSDR, Agency for Toxic Substances and Disease Registry Toxicological Profile for Cadmium. 2012. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15 Available at: [PubMed]
- Ayala A., Muñoz M.F. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014;2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A.S., Jr. Series introduction: the transcription factor nf-κb and human disease. J. Clin. Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J., Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Bar-Shai M., Carmeli E. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-κB activation. Free Rad. Biol. Med. 2008;44:202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Blokhina O., Virolainen E. Antioxidants, oxidative damage and oxygen deprivation stress a review. Ann. Bot. 2002;91 doi: 10.1093/aob/mcf118. 179–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borné Y., Barregard L. Cadmium exposure and incidence of heart failure and atrial fibrillation: a population-based prospective cohort study. BMJ Open. 2015;5(6) doi: 10.1136/bmjopen-2014-007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes R.P., Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc. Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Brasier A.R. The NF-κB regulatory network. Cardiovasc. Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- Buenestado A., Grassin-Delyle S. Roflumilast inhibits the release of chemokines and TNF-α from human lung macrophages stimulated with lipopolysaccharide. British J. Pharmacol. 2012;165:1877–1890. doi: 10.1111/j.1476-5381.2011.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino E., Calzaretti G. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicol. 2002;179:37–50. doi: 10.1016/s0300-483x(02)00245-7. [DOI] [PubMed] [Google Scholar]
- Claiborne A.L. Assay of catalase. In: Greenwald R.A., editor. Handbook of Methods of Oxygen Radical Research. CRC Press; London (UK), Bacoraton: 1985. pp. 283–285. [Google Scholar]
- Cortijo J., Iranzo A. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. British J. Pharmacol. 2009;156:534–544. doi: 10.1111/j.1476-5381.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Saunders B., Trapp R.G. Prentice-Hall International; East Norwalk: 1990. Basic and Clinical Biostatistics; pp. 100–121. [Google Scholar]
- Ercal N., Gurer-Orhan H. Toxic metals and oxidative stress. Part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Erdogan S., Celik S. Elevated cAMP levels reverse Brucella melitensis-induced lipid peroxidation and stimulate IL-10 transcription in rats. Res. Vet. Sci. 2007;82:181–186. doi: 10.1016/j.rvsc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Fagerberg B., Bergstrom G. Cadmium exposure, intercellular adhesion molecule-1 and peripheral artery disease: a cohort and an experimental study. BMJ Open. 2013;3(3) doi: 10.1136/bmjopen-2012-002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Sawant O. Phosphodiesterase inhibitors: their role and implications. Int. J. PharmTech. Res. 2009;1:1148–1160. [Google Scholar]
- Houslay M.D., Adams D.R. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O. Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-B signaling pathways. Pharmacol. Rep. 2018;70:993–1000. doi: 10.1016/j.pharep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O. Apremilast reversed carfilzomib-induced cardiotoxicity through inhibition of oxidative stress, NF-kB and MAPK signaling in rats. Toxicol. Mech. Methods. 2016;26:700–708. doi: 10.1080/15376516.2016.1236425. [DOI] [PubMed] [Google Scholar]
- Jollow D.J., Mitchell J.R. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene as the hepatic metabolite. Pharmacol. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kantola T., Kivisto K.T. Grapefruit juice greatly increases serum concentration of lovastatin and lovastatin acid. Clin. Pharmacol. Ther. 1998;63:397–402. doi: 10.1016/S0009-9236(98)90034-0. [DOI] [PubMed] [Google Scholar]
- Kurian G.A., Rajagopal R. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxid. Med. Cell Longev. 2016;2016:1656450. doi: 10.1155/2016/1656450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.H., Jung J.K. Nuclear factor-κ B inhibitors; a patent review (2006–2010) Expert Opin. Therap. Patents. 2011;21:1897–1910. doi: 10.1517/13543776.2011.638285. [DOI] [PubMed] [Google Scholar]
- Larregle E.V., Varas S.M. Lipid metabolism in liver of rat exposed to cadmium. Food Chem. Toxicol. 2008;46:1786–1792. doi: 10.1016/j.fct.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Liu S.F., Malik A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosenburg N.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manna P., Sinha M. Amelioration of cadmium-induced cardiac impairment by taurine. Chem. Biol. Interact. 2008;174:88–97. doi: 10.1016/j.cbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Marklund S.L. Pyrogallol autooxidation. In: Greenwald R.A., editor. Handbook of Methods for Oxygen Radical Research. CRC Press; London (UK), Bacoraton: 1985. pp. 243–247. [Google Scholar]
- Menke A., Muntner P. Cadmium levels in urine and mortality among U.S. adults. Environ. Health Perspect. 2009;117:190–196. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K.P. Cell membrane oxidative damage induced by gammaradiation and apoptotic sensitivity. J. Environ. Pathol. Toxicol. Oncol. 2004;23:61–66. doi: 10.1615/jenvpathtoxoncol.v23.i1.60. [DOI] [PubMed] [Google Scholar]
- Mollaoğlu H., Gokcimen A. Caffeic acid phenethyl ester prevents cadmium-induced cardiac impairment in rat. Toxicol. 2006;227:15–20. doi: 10.1016/j.tox.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Morales A.I., Vincente-Sanchez C. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006;44:2092–2100. doi: 10.1016/j.fct.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Muller T., Engels P. Subtypes of the type 4 cAMP phosphodiesterases: structure, regulation and selective inhibition. Trends Pharmacol. Sci. 1996;17:294–298. doi: 10.1016/0165-6147(96)10035-3. [DOI] [PubMed] [Google Scholar]
- Murugavel P., Pari L. Diallyl tetrasulfide protects cadmium-induced alterations in lipids and plasma lipoproteins in rats. Nutr. Res. 2007;27:356–361. doi: 10.1016/j.nutres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Nachiappan V., Curtiss D. Cytokines inhibit fatty acid oxidation in isolated rat hepatocytes. Synergy among TNF, IL-6 and IL-1. Shock. 1994;1:123–129. doi: 10.1097/00024382-199402000-00007. [DOI] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington: 1996. Guide for the Care and Use of Laboratory Animals; pp. 1–7. [Google Scholar]
- Nawrot T.S., Van Hecke E. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ. Health Perspect. 2008;116:1620–1628. doi: 10.1289/ehp.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmiche S., Chabane-Sari D. Role of α-tocopherol in cadmium induced oxidative stress in Wistar rat’s blood, liver and brain. Chem. Biol. Interact. 2007;170:221–230. doi: 10.1016/j.cbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Nordberg G.F., Nogawa K. Cadmium. In: Nordberg G.F., Fowler B.A., Nordberg M., Friberg L.T., editors. Handbook on the toxicology of Metals. 2007. pp. 445–486. [Google Scholar]
- Oeckinghaus A., Ghosh S. The NF-kB family of transcription factors and its regulation. Cold Spring Harbor Perspect. Biol. 2009;1:1–14. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez D.C., Gimenez M.S. Lipid modification in mouse peritoneal macrophages after chronic cadmium exposure. Toxicol. 2002;172:1–12. doi: 10.1016/s0300-483x(01)00560-1. [DOI] [PubMed] [Google Scholar]
- Saito Y., Watanabe K. Disruption of group IVA cytosolic phospholipase A2 attenuates myocardial ischemia-reperfusion injury partly through inhibition of TNF-α-mediated pathway. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2018–2030. doi: 10.1152/ajpheart.00955.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S., Baker J.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003;137:65–83. doi: 10.1016/s0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2000;19:201–213. [PubMed] [Google Scholar]
- Tellez-Plaza M., Guallar E. Cadmium exposure and incident cardiovascular disease. Epidemiol. 2013;24:421–429. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz N. fourth ed. WB Saunders Co.; Philadelphia, U.S.A: 2005. Textbook of Clinical Chemistry and Molecular Diagnostics. [Google Scholar]
- Valdecantos P., Matute P. Obesity and oxidative: role of antioxidants supplementation. Rev. Invest. Clin. 2009;61 127–127. [PubMed] [Google Scholar]
- Wang Z., Templeton D.M. Induction of c-fos proto oncogene in mesengial cells by cadmium. J. Biol. Chem. 1998;273:73–79. doi: 10.1074/jbc.273.1.73. [DOI] [PubMed] [Google Scholar]
- Watjen W., Benters J. Zn2+ and Cd2+ increase the cyclic GMP level in PC12 cells by inhibition of the cyclic nucleotide phosphodiesterase. Toxicol. 2001;157:167–175. doi: 10.1016/s0300-483x(00)00370-x. [DOI] [PubMed] [Google Scholar]
- WHO . WHO; Geneva: 1992. Environmental Health Criteria 134, Cadmium. International Programme on Chemical Safety (IPCS) [Google Scholar]
- Xiaoli L., Xiaola Y. Proanthocyanidins from grape seeds modulate the NF-kB signal transduction pathways in rats with TNBS-induced ulcerative colitis. Molecules. 2011;16:6721–6731. doi: 10.3390/molecules16086721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Shaikh Z.A. Cadmium-induced apoptosis in rat kidney epithelial cells involves decrease in nuclear factor-κB activity. Toxicol. Sci. 2006;91:299–308. doi: 10.1093/toxsci/kfj131. [DOI] [PubMed] [Google Scholar]
- Yassin A.S., Martonik J.F. Urinary cadmium levels in the U S working population, 1988–1994. J. Occup. Environ. Hyg. 2004;1:324–333. doi: 10.1080/15459620490445499. [DOI] [PubMed] [Google Scholar]
- Yazıhan N., Koçak M.K. Involvement of galectin-3 in cadmium-induced cardiac toxicity. Anadolu Kardiyol Derg. 2011;11(6):479–484. doi: 10.5152/akd.2011.130. [DOI] [PubMed] [Google Scholar]
- Yuan G., Dai S. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int. J. Clin. Exp. Pathol. 2014;7:2905–2914. [PMC free article] [PubMed] [Google Scholar]