Abstract
Surface-coated nanocarriers have been extensively used to enhance the delivery of anticancer drugs and improve their therapeutic index. In this study, chitosan (CS)-coated flexible liposomes (chitosomes) containing 5-fluorouracil (5-FU) were designed and characterized for use as a novel approach to target colon cancer cells. 5-FU-loaded flexible liposomes (F1, F2, and F3) and 5-FU-loaded chitosomes (F4, F5, and F6) were prepared using film hydration and electrostatic deposition techniques, respectively. The particle size, polydispersity index (PDI), zeta potential, entrapment efficiency (EE%), morphology, and in vitro drug release ability, and cytotoxicity of the formulations were determined. The results revealed that the size of chitosomes ranged from 212 to 271 nm with a positive surface charge of 6.1 to 14.7 mV, whereas the particle size of liposomes ranged from 108 to 234 nm with negative surface charges of −2.3 to −16.3. F3 and F6 had a spherical shape with a rough surface structure. The in vitro drug release study revealed that chitosomes retard 5-FU release as opposed to the 5-FU solution and liposomes. The cytotoxicity study using a colon cancer cell line (HT-29) showed that 5-FU-loaded chitosomes were more effective in killing cancer cells in a sustained manner than liposomes and the 5-FU solution. Chitosomes were therefore successfully developed as nanocarriers of 5-FU, with potential cytotoxicity for colorectal cancer cells.
Keywords: Liposomes, Chitosomes, 5-fluorouracil, Colorectal cancer
1. Introduction
Colorectal cancer (CRC) is considered one of the most commonly detected cancers that causes mortality (Siegel et al., 2017). Different approaches have been applied to control or eradicate CRC such as surgery, radiotherapy, and chemotherapy. Chemotherapy is one of the mainly used approaches to treat cancer. Unfortunately, the major cause for the marginal effect elicited by some chemotherapeutic agents result from poor physicochemical properties, low bioavailability, and poor tissue selectivity (Nie et al., 2007). Therefore, high dose chemotherapy is necessary to overcome such limitations, which usually increases the risk of adverse effects (Nie et al., 2007).
5-fluorouracil (5-FU) is commonly used alone or in combination with other chemotherapeutic drugs to treat CRC (Wen et al., 2016). It is a pyrimidine analogue anticancer agent that functions as an antimetabolite to inhibit cell proliferation. 5-FU is effective against numerous tumors, including colorectal, breast, liver and pancreatic cancer (Tseng et al., 2015). As it is associated with a short half-life in the body (<30 min), high dose 5-FU over a long period is recommended to achieve the desired effect (Karmi et al., 2011). Unfortunately, this magnitude and duration of administration may aggravate the risk of adverse effect such as cardiotoxicity, myelosuppression, neurotoxicity and gastrointestinal upset (Wen et al., 2016).
Numerous approaches have been proposed to minimize the risk of 5-FU side effects and increase its anticancer activity. Nano-sized drug delivery system is one of the best approaches to improve the therapeutic index of 5-FU (Karmi et al., 2011). Nano-size carriers that target cancer tissues could passively or actively increase selectivity and reduce the side effects of anticancer agents (Kim et al., 2018). Liposomes are considered one of the most promising carriers for anticancer drugs (Han et al., 2016), and their vesicular structure allows them to accommodate both hydrophilic and lipophilic drugs. Despite the advantages of liposomes, further improvements are still required to increase their efficiency as drug delivery systems. The pH and enzymes of the GIT were found to disassemble and disrupt liposomes, liberating the encapsulated drug (Taira et al., 2004). Coating liposomal surface with polymers is an approach used to stabilize liposomes in normal body milieus, retard drug release, and deliver the loaded drug to the intended target site (Mady et al., 2009). Living cells usually carry a negative charge; therefore, nano-particles with positively charged surfaces are expected to interact with these cells by electrostatic interaction, facilitating the cellular uptake of the drug (Sunderland et al., 2006, Laye et al., 2008). Therefore, for more efficient delivery of liposomes to the target sites, surface coating of liposomes has been employed (Kim et al., 2018). Chitosan (CS) is a common polymer that possesses cationic, bioadhesive, biocompatible, biodegradable, and absorption-enhancing properties (Cho et al., 2016). Many researchers have investigated the ability of CS to improve the properties of liposomes. CS-coated liposomes “chitosomes” have therefore been employed as promising cargos in drug delivery. CS-coated liposomes have therefore gained mucoadhesive characteristics, which facilitate drug absorption with prolonged residence time (Chen et al., 2016, Zhou et al., 2018).
However, adequate data regarding CS-coated liposomes as a delivery system for 5-FU have not yet been presented in the literature. Therefore, the characterization of CS-coated liposomes containing 5-FU is urgently warranted. CS-coated liposomes may extend the residence time and increase the effectiveness of 5-FU. In the current study, 5-FU was loaded into CS-coated liposomes, and the resulting formulations were characterized via particle size, zeta potential, polydispersity index (PDI), drug entrapment, and stability studies. In addition, in vitro drug release, hemocompatibility, and cytotoxicity were investigated using these systems and compared to those of uncoated liposomes. The 5-FU-loaded chitosomes system is expected to be a potential approach to increase the cytotoxic efficacy of 5-FU.
2. Materials and methods
2.1. Materials
5-fluorouracil (5-FU), low molecular weight chitosan (viscosity 20,000 cps) degree of deacetylation (DD of 92%), cholesterol, dicetyl phosphate (DCP) and MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide) were purchased from Sigma-Aldrich Chemical Co. Ltd. (St. Louis, MO, USA). Sodium tripolyphosphate (TPP), tween 80 and glacial acetic acid were obtained from BDH Organic (Poole, Dorset, UK). Lipoid S100 (PC, soybean lecithin, >94% phosphatidylcholine) was purchased from Lipoid GmbH, (Ludwigshafen, Germany). Methanol and acetonitrile (HPLC grade) were obtained from Fisher Scientific Co., (Loughborough, UK). PBS (pH 7.4) solution (8 g NaCl (137 mM), 0.2 g KCl (2.7 mM), 1.15 g Na2HPO4·7H2O (8.1 mM), and 0.2 g KH2PO4 (1.47 mM) in 1000 mL deionized water) was selected as the release medium. All other chemicals were of analytical grade.
2.2. Preparation of 5-FU-loaded liposomes and chitosomes
5-FU loaded liposomes in the presence and absence of tween 80 and DCP were prepared by using the thin-film hydration technique (Table 1). In brief, PC, cholesterol, tween 80, and DCP were dissolved in a mixture of organic solvents (chloroform and methanol; 2:1, v/v). The organic solvents were removed under vacuum and at 60 °C using a rotary evaporator (Buchi Rotavapor R-200, Büchi Labortechnik, Flawil, Switzerland). The resultant dried, thin film was purged with nitrogen gas to remove the possible traces of organic solvents. Liposomes were formed by hydrating the dried lipid film with PBS (pH 7.4) containing 5-FU. The resultant liposomal dispersion was then probe-sonicated (Sonopuls HD70, Badnelin, Berlin, Germany) for 2 min at an amplitude of 60% to retrieve liposomes in the nano-size range (Tsumoto et al., 2009). An ice bath was used to control the heat that was generated during sonication. For CS-coated liposomes, the system was prepared by dissolving CS in 0.5% v/v acetic acid solution (adjusted pH 5.5–6.0) to prepare the 1% w/v CS solution. The prepared CS solution (1% w/v) was added dropwise to an equal volume of liposomal dispersion containing 5-FU with continuous sonication (40% amplitude) for 5 min using a probe-sonicator (Bian et al., 2015). The formed dispersion was kept under constant stirring for 2 h at room temperature. Moreover, the CS-coated liposomes were produced by electrostatic interaction between negatively charged liposomes and positively charged CS. 5-FU-loaded liposomes were mixed with an equal volume of 0.5% (w/v) CS solution (0.5% v/v of acetic acid, pH 5.5–6.0). The obtained suspensions were then magnetically stirred for 60 min at room temperature to obtain the final formulation of 0.25% w/v CS (Table 1).
Table 1.
The composition of 5-fluorouracil (5-FU) loaded liposomes and chitosan coated liposomes (chitosomes) and CSNPs.
| Codes/Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | CSNPs |
|---|---|---|---|---|---|---|---|
| Lipoid S100 | 1.0 | 0.9 | 0.9 | 1.0 | 0.9 | 0.9 | – |
| Tween 80 | – | 0.1 | 0.1 | – | 0.1 | 0.1 | – |
| Cholesterol | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | – |
| DCP | – | – | 0.1 | – | – | 0.1 | – |
| CS | – | – | – | 0.25% | 0.25% | 0.25% | 0.25% |
| TPP | – | – | – | – | – | – | 0.5 |
| 5-FU | 1% | 1% | 1% | 1% | 1% | 1% | 1% |
The liposomes were prepared by molar ratios.
Dicetyl phosphate; DCP; CS: chitosan, %w/v; TPP: tripolyphosphate; 5-FU: 5-fluorouracil, % w/v.
F1: conventional liposomes; F2: flexible liposomes containing tween 80; F3: flexible liposomes containing tween 80 and DCP.
FL4, F5 andFL6: chitosan coated all these liposomes; CSNPs: chitosan nanoparticles.
For comparison, 5-FU-loaded CS nanoparticles (CSNPs) were prepared. TPP solution (0.25%w/v) was slowly added to the CS solution with proper stirring for 2 h. CSNPS were collected by centrifuging the formed suspension at 13,000 rpm and 25 °C for 30 min. The particles were then washed thrice to remove free 5-FU and were stored in distilled water until the day of use. All formulations were filled in vials and stored in the refrigerator at 4–8 °C until use.
2.3. Physicochemical characterization of FU-loaded liposomes and chitosomes
2.3.1. Particle size distribution and zeta potential measurements
Particle size, PDI and zeta potential of liposomes, chitosomes, and CSNPs were measured using the Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, Worcestershire, UK). The samples were adequately diluted with deionized water to prevent the multi-scattering phenomena, and equilibrated at 25 °C. All samples were run in triplicate.
2.3.2. Determination of encapsulation efficiency and drug loading
The encapsulation efficiency (EE%) and drug loading (DL%) of the 5-FU-loaded liposomes, chitosomes, and CSNPS were determined by the indirect method (Badran et al., 2016). One mL of each sample was placed in an Eppendorf tube and centrifuged at 30,000 rpm for 30 min using Optima™ Max-E, Ultra Cooling Centrifuge (Beckman Coulter, Pasadena, CA, USA). The un-entrapped 5-FU (free) in the supernatant portion was analyzed by HPLC. The obtained liposomes were diluted with an appropriate volume of methanol, and the liposomes were broken down to determine the total amount of 5-FU. EE% and DL% were calculated using the following equations:
| (1) |
| (2) |
5-FUtotal represents the amount of 5-FU used to prepare the systems, while 5-FUfree was the amount of 5-FU in the supernatant after centrifugation.
2.3.3. Morphological characterization
Transmission electron microscopy (TEM) was employed to visualize the shape and surface morphology of selected liposome and chitosome formulations. The samples were mounted on a grid and negatively stained with a 2% (w/v) aqueous solution of uranyl acetate. Excess uranyl acetate solution was removed using a filter paper, and the samples were left to dry in a dust-free environment. The dried samples were then observed by TEM (JEM-1011, JEOL, Tokyo, Japan) at 60 kV.
2.3.4. Physical stability studies
The physical stability of the investigated formulations, and their particle size and PDI were determined after storage at 4 °C for 1 month. All experiments were performed in triplicate.
2.4. In vitro 5-FU release studies
The in vitro release profiles of 5-FU from liposomes, chitosomes and CSNPS were evaluated using the dialysis method (Song et al., 2015). An amount of sample containing 5 mg of 5-FU was poured into dialysis bags (MWCO 12–14 kDa), firmly sealed and immersed in 50 mL of phosphate buffer, pH 7.4. The samples were kept in a water bath shaker (JULABO GmbH, Seelbach, Germany) at 37 ± 0.5 °C and 100 rpm. The sink condition for in vitro release study was maintained since the aqueous solubility of 5-FU has been reported as 10–12 mg/mL (Ashour et al., 2016). At predetermined time intervals, 3 mL was withdrawn from the receptor solution and replaced with a fresh phosphate buffer solution. The amount of 5-FU in each sample was quantified using HPLC, and the cumulative amount of 5-FU released from the sample calculated. All experiments were performed in triplicate.
2.5. Hemocompatability studies
The compatibility of the investigated nano-systems (liposomes, chitosomes and CSNPs) with normal cells was estimated using a hemocompatability test (Table 4). For this test, the protocol by Huang et al. (2016) was adopted with minor modifications. In brief, erythrocyte suspension (15 μL) and 1500 µL phosphate buffer solution were placed in a test tube followed by the addition of 200 μL of the investigated formulation. After gentle mixing and standing at room temperature for 2 h, the sample was centrifuged at 1500 rpm for 5 min. Phosphate buffered saline solution was used as the negative control (0% hemolysis of erythrocytes) and 10% Triton-X-100 used as the positive control (100% hemolysis of erythrocytes).
Table 4.
The protocol of hemocompatabilty studies of 5-FU loaded liposomes and chitosomes and CSNPs.
| Tube | Tubes | Test (µl) | PBS (µl) | Erythrocyte (µl) |
|---|---|---|---|---|
| 1 | PBS | 200 | 1500 | 15 |
| 2 | Triton | 200 | 1500 | 15 |
| 3 | F1 | 200 | 1500 | 15 |
| 4 | F2 | 200 | 1500 | 15 |
| 5 | F3 | 200 | 1500 | 15 |
| 6 | F4 | 200 | 1500 | 15 |
| 7 | F5 | 200 | 1500 | 15 |
| 8 | F6 | 200 | 1500 | 15 |
| 9 | CSNPs | 200 | 1500 | 15 |
2.6. In vitro cytotoxicity studies
The cytotoxicity of 5-FU-loaded liposomes, chitosomes, and CSNPs were evaluated using human colon cancer (HT-29) cells. The viability of the cells in the presence and absence of 5-FU-loaded nanocarriers was evaluated by MTT assay. Cells were passaged at sub-confluency, and all cells were used at late passage. At sub-confluency, the cells were seeded into 96-well plates at a density of 7 × 103 cells/well and incubated at 37 °C in a humidified atmosphere of 5% CO2. The cultures were then incubated with the 5-FU formulations and pure 5-FU at 3.1, 6.2, 12.5, 25, 50, 100, 200, and 400 μg/mL for 24 h. Treatment was stopped by carefully washing with fresh DMEM. A 10-μL volume of MTT solution (5 mg/mL in PBS) was added and incubated for another 4 h at 37 °C, and the reaction product was solubilized in 50 μL DMSO; absorbance was then measured at 540 nm. Negative controls including the untreated cells (media alone) were used in all studies. H2O2 was chosen as a positive control as it has been demonstrated to induce apoptosis in cancer cells. The viability of untreated controls was normalized to 100%. Absorbance was measured at 540 nm using a microplate reader (ELX 800; Bio-Tek Instruments, Winooski, VT, USA), and cell viability was calculated using the following equation:
| (3) |
2.7. HPLC analysis
The amount of 5-FU in the investigated samples was quantified using an HPLC method (Alanazi et al., 2009) by injecting a 20-μL sample into the HPLC Waters™ system (Waters™, Milford, MA, USA). Phosphate buffer was used as a mobile phase (40 mM phosphate buffer adjusted to pH 7.0 using 10% w/v potassium hydroxide) and was pumped through a C18 column (µ-Bondapak™, 4.6 × 150 mm, 10 μm particle size) at a rate of 1 mL/min. The UV/Vis detector was set at 260 nm to detect 5-FU in each sample. All operations were carried out at room temperature.
2.8. Statistical data analysis
Data analysis was carried out using Microsoft Excel, Version 2010 and origin software, version 6.1. Results are expressed as mean ± standard error.
3. Results and discussion
3.1. Particle size distribution and zeta potential measurements
The physicochemical parameters of the prepared liposomes, CS-coated liposomes and CSNPS are presented in Table 2. All formulations displayed particle sizes in the nanometer range. Mean particle size of conventional liposomes (F1), liposomes containing tween 80 (F2) and liposomes containing tween80/DCP (F3) were 234, 146, and 108 nm, with PDI values of 0.26, 0.23, and 0.31, respectively. It is clear that the presence of an edge activator (tween 80) in F2 and F3 resulted in a smaller liposomal size than conventional liposomes (F1). This reduction in size was attributed to the action of the edge activator (tween 80) on the membrane of liposomes to reduce the surface tension of the media, leading to phospholipid arrangement in small vesicles (Alomrani et al., 2015). CSNPs exhibited particle size in the nanometer range (194 nm) with a PDI value of 0.27, and a large particle size of the CS-coated liposomes was found when compared to that of the corresponding uncoated liposomes (Table 2). Particle size of the CS-coated liposomes F4, F5, and F6 were 212, 226 and 271 nm, with PDI values of 0.42, 0.33, and 0.40, respectively. This enlargement in particle size was attributed to the coating of the CS layer on the outer surface of liposomes (Soo et al., 2014, Zhao et al., 2015).
Table 2.
The physicochemical characteristics of 5-FU loaded liposomes and chitosomes and CSNPs.
| Codes | Particle size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| F1 | 234 ± 9 | 0.26 ± 0.03 | −2.3 ± 0.6 |
| F2 | 146 ± 19 | 0.23 ± 0.06 | −7.5 ± 1.4 |
| F3 | 108 ± 11 | 0.31 ± 0.05 | −16.3 ± 1.5 |
| F4 | 212 ± 21 | 0.42 ± 0.06 | 6.1 ± 0.5 |
| F5 | 236 ± 16 | 0.33 ± 0.019 | 8.3 ± 1.1 |
| F6 | 271 ± 13 | 0.40 ± 0.03 | 14.7 ± 0.9 |
| CSNPs | 194 ± 4 | 0.27 ± 0.01 | 28.8 ± 7.4 |
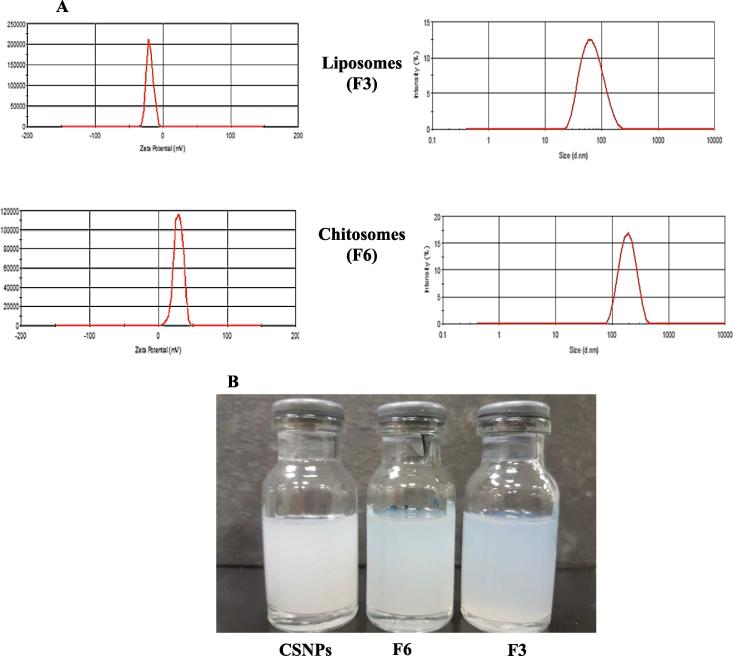
Zeta potential is often used to identify the nature of a particle's surface charge. Table 2 displays the zeta potentials of liposomes, CS-coated liposomes and CSNPs. F1 showed a slight negative zeta potential of −2.3. The negative value of the liposomes was increased by the presence of tween 80 (F2) and tween 80/DCP (F3), −7.5 and −16.3 mV, respectively; these findings align with that found in other studies (Song et al., 2012, Alomrani et al., 2015). The electronegativity of the oxygen atoms present in the ethylene oxide moieties of tween 80 is believed to be the cause of the negative surface charge of the particles (Marzio et al., 2013). A further increase in the negative surface charge of the liposome (F3) could be a result of DCP as an anionic surfactant (Mehanna et al., 2017). In contrast, CS-coated liposomes exhibited positive zeta potential values of 6.1, 8.3, and 14.7 mV for F4, F5 and F6, respectively. This conversion of the surface charge resulted from the electrostatic interaction between liposomes and CS to form chitosomes (Paolina et al., 2006). Therefore, the high zeta potential of F6 was attributed to the adsorption of a high amount of CS around the negatively charged liposomes (F3). F1 had a very weak negative surface charge as it was close to neutral, indicating no strong electrostatic interaction between F1 and CS. However, coating F1 with CS successfully occurred with the formation of F4 due to hydrogen bonding between phospholipids and CS (Perugini et al., 2000, Chen et al., 2016). CSNPs showed positive zeta potential (28.8 mV), which is expected as CS is a positively charged molecule (see Fig. 1).
Fig. 1.
Particle size and zeta potential of F3 and F6 (A), and images of F3, F6, and CSNPs (B).
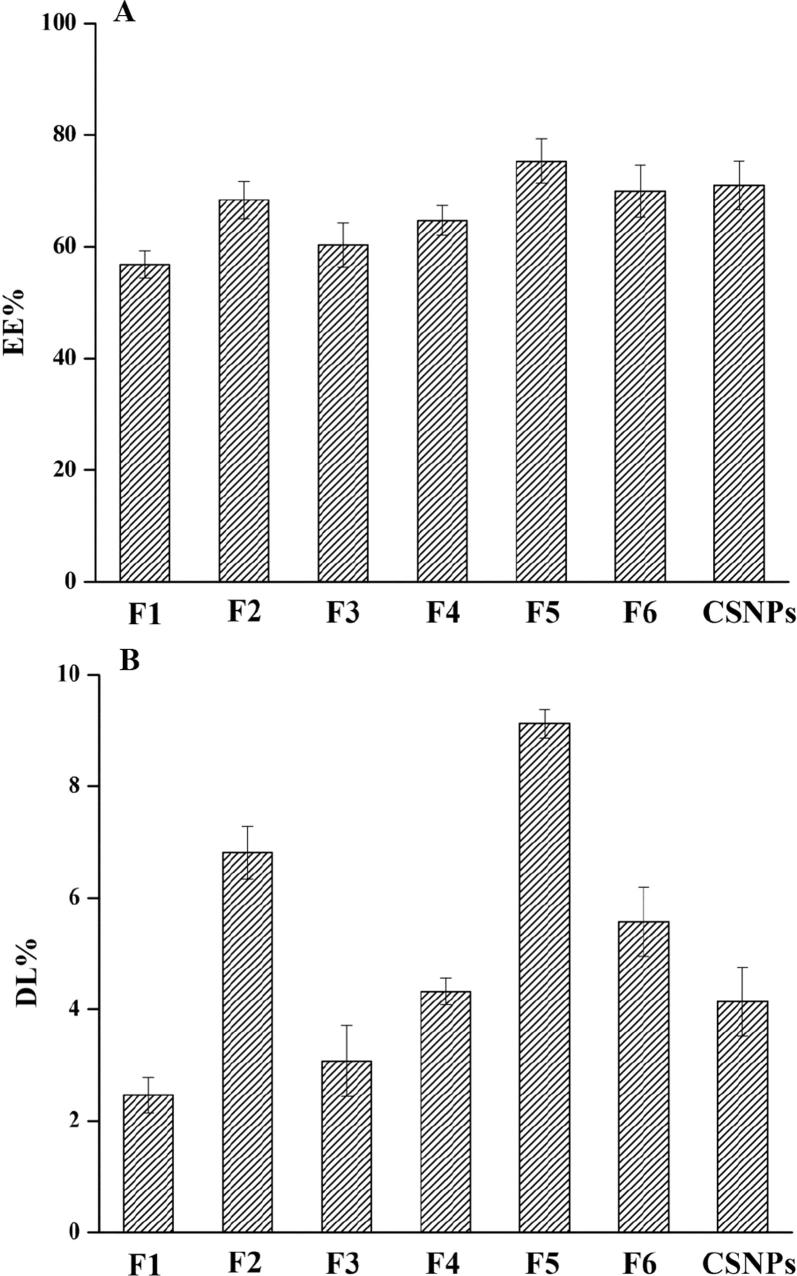
3.2. Encapsulation efficiency (EE%) and drug loading (DL%)
EE% and DL% are important parameters that affect the efficiency of drug delivery systems (Fig. 2). The liposome formulations F1, F2, and F3 had EE% of 37, 46, and 42% and DL% of 2.5, 4.8 and 3.1%, respectively. These results demonstrate that the presence of tween 80 (F2) enhanced EE% and DL% of liposomes, aligning with the results found by El Zaafarany et al., 2010, Alomrani et al., 2015.
Fig. 2.
Entrapment efficiency (EE%) and drug loading (DL%) of 5-FU-loaded liposomes, chitosomes, and CSNPs (Mean ± SD, n = 3).
The liposomes containing CS relatively improved EE% and DL% of 5-FU as F4, F5, and F6 had EE% of 45, 55, and 51%, and DL% values of 4.2, 6.1, and 5.6, respectively. Earlier studies confirmed that chitosomes hold the drug due to CS adsorption on the surface of liposomes (Nguyen et al., 2014); EE% and DL of CSNPs were 61% and 7.3, respectively. As 5-FU is a hydrophilic molecule, it is expected to accumulate in the hydrophilic core of liposomes. Therefore, the increased EE and DL of coated liposomes could be attributed to the interaction between positively charged CS and negatively charged 5-FU, leading to more 5-FU molecules on the surface of chitosomes (Wang et al., 2013).
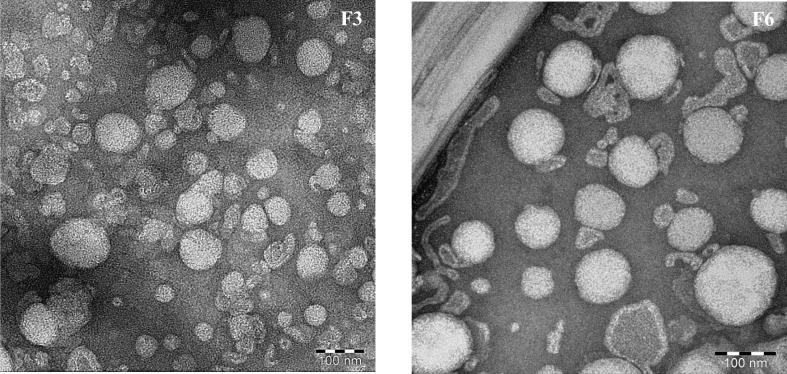
3.3. Morphological characterization
The morphology of the prepared liposomes and chitosomes was investigated using TEM. F3 was chosen to represent liposomes and F6 as the corresponding coated liposome. Fig. 3 displays the TEM images of F3 and F6; a spherical shape with rough surface structure is revealed for these formulations. The image of F6 depicts the presence of hollow particles, proving that CS generated a film layer around liposomal particles.
Fig. 3.
Transmission electron microscopy micrographs of liposomes (F3) and chitosomes (F6).
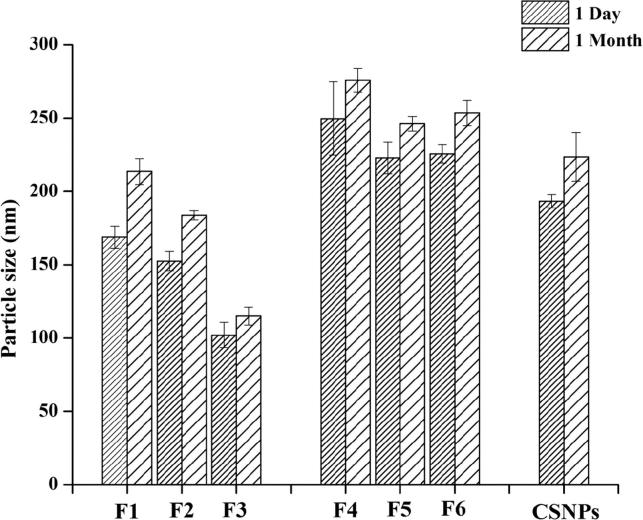
3.4. Physical stability studies
The physical stability of liposomes and coated liposomes when stored at 4 °C for one month was assessed (Fig. 4). These liposomes displayed a slight change in particle size after one month of storage (Fig. 4), and a remarkable increase was observed with F2. Such effect could be attributed to the flexibility of the liposomal bilayer due to the presence of the edge activator. Membrane flexibility facilitates the merging of two vesicles and the formation of larger particles.
Fig. 4.
Variation in particle size of 5-FU-loaded liposomes, chitosomes, and CSNPs during storage at 4 °C (Mean ± SD, n = 3).
In contrast, the chitosomes showed slight but non-significant change in particle size after one month of storage, suggesting that the presence of CS on the outer surface of liposomes could improve the physical stability of liposomes (Chen et al., 2016, Zhou et al., 2018).
3.5. In vitro 5-FU release studies
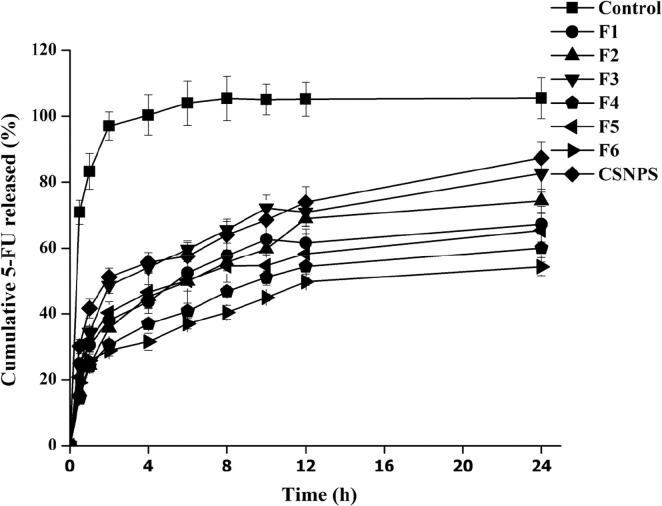
In vitro release studies are used to evaluate entrapment, membrane flexibility and integrity, and affinity of the drug to the carrier systems (Wang et al., 2017). In this study, the in vitro release of 5-FU from liposomes, CS-coated liposomes, and CSNPs carrier systems was examined and compared to that of the drug solution (control) using the dialysis bag technique. As expected, the aqueous solution of 5-FU showed fast release as approximately 70% of 5-FU was released within the first hour and almost 100% was released in the 2 h (Fig. 5). This mode of drug release is expected for small hydrophilic molecules (Tan et al., 2017, Hardiansyah et al., 2015). On the other hand, the release rate of 5-FU from the prepared systems exhibited a biphasic mode: an initial fast rate of drug release followed by a slow rate of drug release.
Fig. 5.
In vitro release profile of 5-FU from 5-FU solution, 5-FU-loaded liposomes, chitosomes, and CSNPs in PBS (pH 7.4) at 37 ± 0.5 °C (Mean ± SD, n = 3).
The 5-FU-loaded liposome formulations F1, F2, and F3 respectively released 43.6%, 49.8%, and 54.1% of the loaded 5-FU within the first 2 h. After 12 h, 61.6%, 68.7%, and 70.9% of 5-FU was released from F1, F2, and F3, respectively. The amount of drug detected in the donor compartment during the first 2 h was mainly due to un-entrapped drug. This assumption correlates with the EE% data presented in Table 3 which revealed that 54–63% of 5-FU in F1, F2, and F3 was present on the exterior of the liposomal particles. 5-FU is a small and hydrophilic molecule; therefore, it can easily leach from the lipid membrane. Furthermore, sustained release of 5-FU from liposomes could be attributed to the enclosed lipid shell, which permits slow release of 5-FU from the lipid matrix (Perez et al., 2015). F2 exhibited a 5-FU release pattern due to its high EE% and flexibility of the membrane containing tween 80 (Chen et al., 2016). Flexible liposomes have the ability to modify their shape for transportation into smaller openings with a hydrophilic surface (Dragicevic-Curic et al., 2010). By using DCP in F3, more 5-FU was detected in the release media. This effect resulted from the repulsion between 5-FU and DCP, increasing 5-FU release (Dragicevic-Curic et al., 2010). When liposomes were coated with CS, the release of 5-FU extended. In this context, CS improved the stability and sustained release behavior of liposomes (Beenken et al., 2014). Over a 4 h period, the values of 5-FU release from chitosomes were 40.7%, 46.5% and 31.4% from F4, F5 and F6, respectively. Moreover, cumulative release of 5-FU from F4, F5 and F6 was 53.4%, 50.1% and 44.6%, respectively, after 12 h (Fig. 5). The slowest release values of 5-FU were due to the presence of CS, which hindered the diffusion of 5-FU into the release medium (Chen et al., 2016). The initial drug release was attributed to the leaching of un-entrapped 5-FU adsorbed on the outer surface of the carriers, while the later slow release phase could be due to drug entrapment in the lipid membrane and/or CS layer. This would result in increased diffusion time of 5-FU into the dissolution media (Panwar et al., 2010). Over 4 h, drug release from CSNPs was 57.5%, and cumulative release reached 73.9% and 87.4% after 12 and 24 h, respectively. This initial fast release was due to the diffusion of 5-FU from the surface of CSNPS, while the slow release was a matrix effect (Badran et al., 2016).
Table 3.
The EE% and DL% of 5-FU loaded 5-FU loaded liposomes and chitosomes and CSNPs.
| Codes | EE% | DL% |
|---|---|---|
| F1 | 37 ± 2.5 | 2.5 ± 0.32 |
| F2 | 46 ± 3.3 | 4.8 ± 0.47 |
| F3 | 42 ± 4.1 | 3.1 ± 0.64 |
| F4 | 45 ± 5.8 | 4.2 ± 0.24 |
| F5 | 55 ± 6.9 | 6.1 ± 0.25 |
| F6 | 51 ± 5.6 | 5.6 ± 0.62 |
| CSNPs | 61 ± 4.3 | 7.3 ± 0.29 |
3.6. Hemocompatability
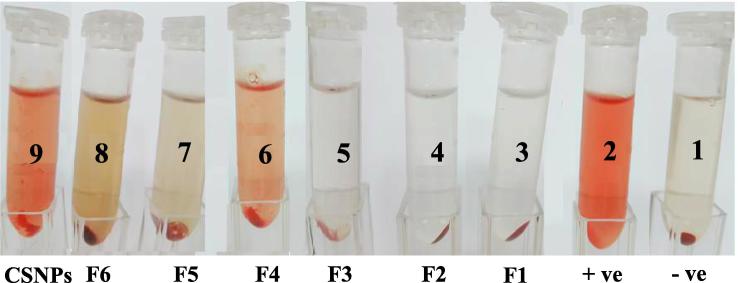
The biocompatibility of the prepared carriers was evaluated using the erythrocyte hemolysis test (Yang et al., 2015) with isotonic solution and deionized water serving as the negative and positive controls, respectively. The results of the test (Fig. 6) showed that erythrocytes had to be incubated in isotonic solution to maintain their integrity, with no occurrence of hemolysis (negative control) (Fig. 6). Conversely, hemolysis was observed when erythrocyte was incubated in deionized water (positive control). For the liposomal formulations F1, F2, and F3, no hemolysis was observed, indicating good hemocompatabilty. Minor to moderate hemolysis was observed with the chitosome formulations F4, F5, and F6. The positively charged surface of these formulations may be the cause of such action. Significant hemolysis was observed with CSNPs, and the results of the hemocompatability test revealed that erythrocyte hemolysis was influenced by the surface charge of the formulations. Neutral and negatively charged particles caused no effect and were safe toward erythrocytes, while positively charged particles induced hemolysis. The greater positive zeta potential of CSNPS is therefore a possible cause of hemolysis.
Fig. 6.
Hemolysis test results obtained using the naked eye (6, negative control; 2, positive control; 3–9, test samples).
3.7. In vitro cytotoxicity studies
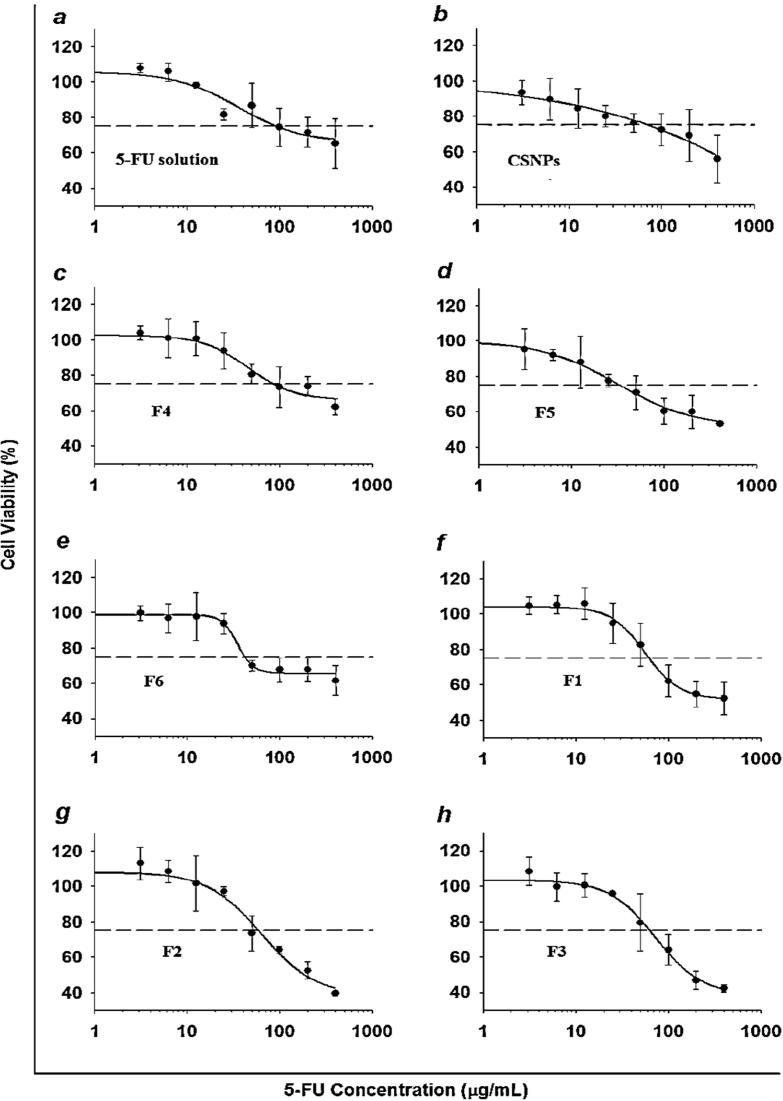
The cytotoxicity of the selected liposomes, CS-coated liposomes, and CSNPs with or without 5-FU compared to that of the drug solution was evaluated by performing an MTT (Fig. 7) assay at different 5-FU concentrations (3.1, 6.2, 12.5, 25, 50, 100, 200, and 400 μg/mL) in HT-29 cells.
Fig. 7.
Cytotoxicity of 5-FU from 5-FU-loaded liposomes, chitosomes and CSNPs in the HT-29 cell line (Mean ± SD, n = 6).
The results reveal that the unloaded formulations and PBS did not display significant cytotoxicity, and >90% of HT-29 cells survived when the same concentration range was used as the 5-FU formulations (Fig. 7). The % cell viability declined as 5-FU concentration increased in all formulations. At low concentrations (3.1, 6.2, 12.5 and 25 μg/mL), free 5-FU was more efficient in preventing cell growth than liposomes, chitosomes, and CSNPs, which could be attributed to the low amounts of 5-FU released. By increasing the 5-FU concentration (50, 100, 200 and 400 μg/mL), liposomes displayed a more rapid decrease in cell growth than coated liposomes and CSNPS, which aligns with the results of in vitro release studies. By using chitosomes, cell viability was reduced in a sustained manner due to the CS layer, which enhanced the cytotoxic effect of 5-FU against HT-29. Coated liposomes possess high cell affinity, which increases the cellular uptake of the drug due to the positive surface charge (CS), leading to enhanced absorption on the negatively charged cell membrane in cells (Yang et al., 2015).
4. Conclusion
Novel chitosomes containing 5-FU have been successfully molded as nano-cargoes for use in the treatment of colon cancer. Both 5-FU loaded liposomes and chitosomes showed desirable physicochemical properties. The chitosomes exhibited valuable EE% and DL% with good physical stability, and sustained 5-FU release. In addition, the negatively charged liposomes did not produce any hemolysis and were safe toward erythrocytes. But in case of CS coated liposomes, the erythrocyte hemolysis was increased depending on the values of positive zeta potential. The in vitro cytotoxicity studies showed that chitosomes improved the cytotoxicity of 5-FU with a sustained effect unlike liposomes and the 5-FU solution. These results suggest that chitosomes could be considered as promising carriers for 5-FU delivery in cancer therapy. The obtained chitosomes will be the subject of future studies that aim to improve the anticancer effectiveness of 5-FU.
Acknowledgement
The authors would like to extend their sincere appreciation to the College of Pharmacy Research Center and the Deanship of Scientific Research at King Saud University for financial and logistic support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alanazi F.K., Yassin A.E., El-Badry M. Validated high performance liquid chromatographic technique for determination of 5- fluorouracil: applications to stability studies and simulated colonic media. J. Chromatogr. Sci. 2009;47(7):558–563. doi: 10.1093/chromsci/47.7.558. [DOI] [PubMed] [Google Scholar]
- Alomrani A.H., Gamal A.S., Amro A.A. Itraconazole-hydroxypropyl-β-cyclodextrin loaded deformable liposomes: in vitro skin penetration studies and antifungal efficacy using Candida albicans as model. Colloids Surf. B Biointerf. 2015;121:74–81. doi: 10.1016/j.colsurfb.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Ashour A.E., Badran M.M., Ashok K. Di-block PLCL and tri-block PLCLG matrix polymeric nanoparticles enhanced the anticancer activity of loaded 5-fluorouracil. EEE Trans. NanoBiosci. 2016;15(7):739–747. doi: 10.1109/TNB.2016.2612340. [DOI] [PubMed] [Google Scholar]
- Badran M.M., Gamaleldin I.H., Saeed A.A. Pravastatin-loaded chitosan s: Formulation, characterization and cytotoxicity studies. J. Drug Deliv. Sci. Technol. 2016;32:1–9. [Google Scholar]
- Beenken K.E., Smith J.K., Skinner R.A. Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J. Biomater. Appl. 2014;29:514–523. doi: 10.1177/0885328214535452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Y., Gao D., Liu Y. Preparation and study on anti-tumor effect of chitosan-coated oleanolic acid liposomes. RSC Adv. 2015;5:18725–18732. [Google Scholar]
- Chen H., Hao P., Panpan L. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf. B: Biointerf. 2016;143:455–462. doi: 10.1016/j.colsurfb.2016.03.061. [DOI] [PubMed] [Google Scholar]
- Cho I.S., Park C.G., Huh B.K. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016;39:124–132. doi: 10.1016/j.actbio.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Dragicevic-Curic N., Gräfe S., Gitter B. Surface charged temoporfin-loaded flexible vesicles: In vitro skin penetration studies and stability. Int. J. Pharmaceut. 2010;384:100–108. doi: 10.1016/j.ijpharm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- El Zaafarany G.M., Gehanne A.S., Samar A.M. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skindelivery. Int. J. Pharm. 2010;397:164–172. doi: 10.1016/j.ijpharm.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Han N.-K., Shin D.H., Kim J.S. Hyaluronan-conjugated liposomes encapsulating gemcitabine for breast cancer stem cells. Int. J. Nanomed. 2016;11:1413–1425. doi: 10.2147/IJN.S95850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiansyah L.-Y., Huang M.-C., Yang B.S. Novel pH-sensitive drug carriers of carboxymethyl-hexanoyl chitosan (Chitosonic® acid) modified liposomes. RSC Adv. 2015;5:23134–23143. [Google Scholar]
- Huang H., Lai W., Cui M. An evaluation of blood compatibility of silver nanoparticles. Sci. Rep. 2016;5(6,):25518. doi: 10.1038/srep25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmi A., Husseini G.A., Faroun M. Multifunctional nanovehicles for combined 5-fluorouracil and gold s based on the nanoprecipitation method. J. Nanosci. Nanotechnol. 2011;11:4675–4683. doi: 10.1166/jnn.2011.4156. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Shin D.H., Kim J.S. Preparation, characterization, and pharmacokinetics of liposomal docetaxel for oral administration. Arch. Pharm. Res. 2018;41(7):765–775. doi: 10.1007/s12272-018-1046-y. [DOI] [PubMed] [Google Scholar]
- Laye C., McClements D.J., Weiss J. Formation of biopolymer-coated liposomes by electrostatic deposition of chitosan. J. Food Sci. 2008;73(5):N7–N15. doi: 10.1111/j.1750-3841.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- Mady M.M., Darwish M.M., Khalil S. Biophysical studies on chitosan coated liposomes. Eur. Biophys. J. 2009;38:1127–1133. doi: 10.1007/s00249-009-0524-z. [DOI] [PubMed] [Google Scholar]
- Marzio L.D., Esposito S., Rinaldi F. Polysorbate 20 vesicles as oral delivery system: in vitro characterization. Colloids Surf. B:Biointerf. 2013;104:200–206. doi: 10.1016/j.colsurfb.2012.10.036. [DOI] [PubMed] [Google Scholar]
- Mehanna M.M., Abd El-Kader N., Magda W.S. Liposomes as potential carriers for ketorolac ophthalmic delivery: formulation and stability issues. Braz. J. Pharm. Sci. 2017;53(2):16127–16137. [Google Scholar]
- Nguyen T.X., Lin H., Li L. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B. 2014;2:7149–7159. doi: 10.1039/c4tb00876f. [DOI] [PubMed] [Google Scholar]
- Nie S., Xing Y., Kim G.J. Nanotechnology applications in cancer. Ann. Rev. Biomed. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- Panwar P., Pandey B., Lakhera P. Preparation, characterization, and in vitro release study of albendazole-encapsulated nanosize liposomes. Int. J. Nanomed. 2010;5:101. doi: 10.2147/ijn.s8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino, D., Fresta, M., Sinha, P., Ferrari, M. 2006. In: Webester, J.G. (Ed.). Encyclopedia of Medical Devices and Instrumentation, second ed., vol. 437. John Wiley and Sons, New York.
- Perez A.P., Altube M.J., Schilrreff P. Topical amphotericin B in ultradeformable liposomes: formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerf. 2015;139:190–198. doi: 10.1016/j.colsurfb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Perugini P., Genta I., Pavanetto F. Study on glycolic acid delivery by liposomes and microspheres. Int. J. Pharm. 2000;196(1):51–61. doi: 10.1016/s0378-5173(99)00439-1. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Stacey A.F., William F.A. Colorectal cancer incidence patterns in the United States. J. Natl. Cancer. Inst. 2017;109(8):1–6. doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C.K., Prabagar B., Chang-Koo S. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf. B: Biointerf. 2012;92:299–304. doi: 10.1016/j.colsurfb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Song J., Yang X., Jacobson O. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultra-small gold nano-rod vesicles for cancer therapy. ACS Nano. 2015;9:9199–9209. doi: 10.1021/acsnano.5b03804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo N.P., Na Rae J., So H.J. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J. I. Eng. Chem. 2014;20:1481–1485. [Google Scholar]
- Sunderland C.J., Steiert M., Talmadge J.E. Targeted nanoparticles for detecting and treating cancer. Drug Dev. Res. 2006;67:70–93. [Google Scholar]
- Taira M.C., Chiaramon N.S., Pecuch K.M. Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Deliv. 2004;11:123–128. doi: 10.1080/10717540490280769. [DOI] [PubMed] [Google Scholar]
- Tan G., Shihui Y., Hao P. Bioadhesive chitosan-loaded liposomes: A more efficient and higher permeable ocular delivery platform for timolol maleate. I. J. Biol. Macromol. 2017;94:355–363. doi: 10.1016/j.ijbiomac.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Tseng C.L., Chen J.C., Wu Y.C. Development of lattice-inserted 5-Fluorouracil-hydroxyapatites as a chemotherapeutic delivery system. J. Biomater. Appl. 2015;30:388–397. doi: 10.1177/0885328215588307. [DOI] [PubMed] [Google Scholar]
- Tsumoto K., Matsuo H., Tomita M. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids Surf. B: Biointerf. 2009;68(1):98–105. doi: 10.1016/j.colsurfb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Wang M., Tingting Z., Yanping L. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C. 2017;71:1231–1240. doi: 10.1016/j.msec.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Wang Y., Puwang L., Lingxue K. Chitosan-modified PLGA with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013;14:585–592. doi: 10.1208/s12249-013-9943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Junxiong C., Di Z. Pseudolaric acid B induces mitotic arrest and apoptosis in both 5-fluorouracil-sensitive and -resistant colorectal cancer cells. Cancer Lett. 2016;383:295–308. doi: 10.1016/j.canlet.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Yang Z., Junli L., Jinhua G. Chitosan coated vancomycin hydrochloride liposomes: Characterizations and evaluation. Int. J. Pharm. 2015;495:508–515. doi: 10.1016/j.ijpharm.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Zhao G.D., Sun R., Ni S.L. Development and characterisation of a novel chitosan-coated antioxidant liposome containing both coenzyme Q10 and alpha-lipoic acid. J. Microencapsul. 2015;32:157–165. doi: 10.3109/02652048.2014.973072. [DOI] [PubMed] [Google Scholar]
- Zhou F., Tao X., Yajing Z. Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocoll. 2018;83:17–24. [Google Scholar]