Abstract
This study was the first to detect the presence of the two compounds momilactone A (MA) and momilactone B (MB) in rice bran using liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS). By in vitro assays, both MA and MB exhibited potent inhibitory activities on pancreatic α-amylase and α-glucosidase which were significantly higher than γ-oryzanol, a well-known diabetes inhibitor. Remarkably, MA and MB indicated an effective inhibition on trypsin with the IC50 values of 921.55 and 884.03 µg/mL, respectively. By high-performance liquid chromatography (HPLC), quantities of MA (6.65 µg/g dry weight) and MB (6.24 µg/g dry weight) in rice bran were determined. Findings of this study revealed the α-amylase, α-glucosidase and trypsin inhibitors MA and MB contributed an active role to the diabetes inhibitory potential of rice bran.
Keywords: Momilactones A and B, Rice bran, Anti-diabetes, α-amylase, α-glucosidase, Trypsin, HPLC and LC-ESI-MS methods
1. Introduction
Diabetes mellitus is a chronic disease that appears at all ages and leads to many serious complications including cardiovascular diseases, stroke, kidney failure, blindness, and nerve damage (Steppan et al. 2001). Among diabetic types, type 2 is the most common in adults due to the impaired diet and exercise regimens. The type 2 diabetes may commence from a combination of factors including genetic predisposition, environment, and pancreatic beta-cell dysfunction (Leahy, 2005). In these patients, their body cannot efficiently use insulin - a hormone that regulates glucose uptake into the cells. Without a proper control, this process will progressively lead the body to chronic insulin resistance, hyperglycemia, fatigue, weight loss, and other severe consequences. In fact, therapeutic interventions were proposed tended to control those pathogenic factors. However, the most beneficial method is keeping blood glucose at normal levels after a meal (Abesundara et al., 2004). In human, the initial ingested foods are diverse, and they are complicatedly digested by multiple hydrolytic enzymes. Among these, α-amylase and α-glucosidase play a vital role in the hydrolysis of polysaccharides to liberate more suitable glucose for absorption (Ercan and El, 2016). The earlier study indicated that the hyperglycemia in type 2 diabetes patients was attributed to starch and glucose metabolism by pancreatic α-amylase and intestinal α-glucosidase (Apostolidis et al., 2007). On the other consideration, trypsin, an important proteolytic enzyme, takes part in many metabolic processes of human. Previous studies proved that trypsin inhibition might attenuate protein digestibility (Quesada et al.,1996) and indirectly reduce blood glucose level by enhancing insulin absorption (Morishita et al., 1992, Park et al., 2007). Recent researches also demonstrated that trypsin inhibitors could restrain food intake by promoting satiety impression and therefore, prevented several metabolic diseases as diabetes and obesity (Nakajima et al., 2011, Komarnytsky et al., 2011, Serquiz et al., 2016, Carvalho et al., 2016). For those reasons, the discovery of new potent inhibitors from natural origins which can simultaneously inhibit the activities of α-amylase, α-glucosidase and trypsin are a potential approach for the control of risks from type 2 diabetes.
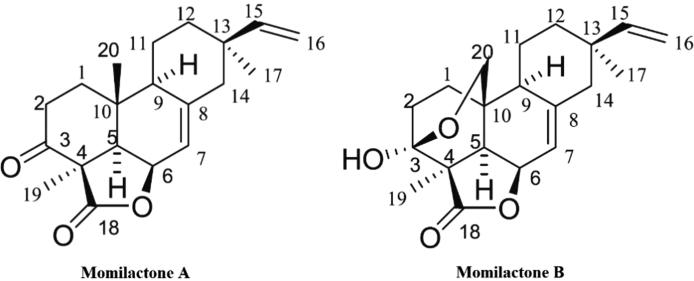
Momilactones A (MA) and B (MB) (Fig. 1) have been known as authoritative allelochemicals against several pathogens of crops (Kato-Noguchi, 2011, Toyomasu et al., 2008, Kato-Noguchi et al., 2010, Chung et al., 2006, Cartwright et al., 1977, Obara et al., 2002, Fukuta et al., 2007). In addition, MA and MB might have a certain role to support tolerance of rice to salinity and drought stresses (Xuan et al., 2016, Quan and Xuan, 2018). MA and MB also exhibited several beneficially bioactivities including antioxidant (Fukuta et al., 2007), cytotoxic (Chung et al., 2005a), antitumor (Kim et al., 2007) and anticancer activities (Joung et al., 2008, Park et al., 2014). Of which, MB is commonly determined in lower quantity in rice plant parts but exerted greater biological activities than MA. Nevertheless, the isolation and purification of MA and MB, chiefly from rice husk, are not straightforward. Currently, very few laboratories in the world can successfully isolate and purify MA and MB. As a result, the commercial price of pure standards MA and MB is not affordable, and therefore, studies on biological activities of the two compounds has been limited. Hitherto, the antidiabetic property of MA and MB has not been fully studied.
Fig. 1.
Chemical structures of momilactones A and B (Quan et al., 2019).
On the other hand, rice is the staple food for more than half of the world population. Besides using white rice as a main food source owing to its well-known nutritional values, rice by-products have had an increasing interest from food processing industry and pharmaceutical fields (Esa et al., 2013, Sohail et al., 2016). Theoretically, paddy-field produces approximately 70% white rice, 20% husk and 10% bran (Esa et al.,2013). The diabetes inhibitory potential of crude extracts from rice by-products was sorely investigated (Yang et al., 2010, Yehia and Saleh, 2012). Among rice by-products, rice bran versus diabetes was the most thoroughly studied. In which, tocotrienol, γ-oryzanol, fiber and functional peptides including amino acid sequences and protein hydrolysates were prerequisite compounds that could combat diabetes (Esa et al., 2013, Ghatak and Panchal, 2012, Uraipong and Zhao, 2016, Tashiro et al., 1987, Liu et al., 2017, Boonloh et al., 2015). However, the antidiabetic role of individual compounds in rice bran has been sporadically studied. Recently, although MA and MB have been reported to be α-amylase and α-glucosidase inhibitors and appeared in different rice plant parts including leaf, husk, root as well as refined rice grain (Quan et al., 2019), the presence of MA and MB and their contribution to the antidiabetic property in rice bran has not been revealed yet. Thus, in this study, we at first documented and compared the diabetic inhibitory potential of MA and MB in rice bran and γ-oryzanol (an outstanding diabetes inhibitor in rice bran) through in vitro assays of pancreatic α-amylase and trypsin inhibitions. The protocol to integrate HPLC and LC-ESI-MS to identify and quantify MA and MB in rice bran was also described.
2. Materials and methods
2.1. Reagents
The extraction and isolation solvents were purchased from Junsei Chemical Co., Ltd., Tokyo, Japan. Acetonitrile used for LC analyses was obtained from Fisher Scientific company, Hampton, NH, USA. Iodine solution, acarbose, and γ-oryzanol were procured from Fujifilm Wako Pure Chemical Corporation, Osaka, Japan. Silica gel, α-amylase from porcine pancreas (type VI-B), α-glucosidase from Saccharomyces cerevisiae, trypsin from porcine pancreas, soluble starch, p-nitrophenyl-α-D-glucopyranoside (pNPG), Nα-benzoyl-L-arginine 4-nitroanilide hydrochloride (BAPNA) and all buffer components were acquired from Sigma-Aldrich, St. Louis, MO, USA.
2.2. Collection and preparation of samples
Rice husk and rice bran of Koshihikari variety (Japonica subtype) were collected from rice mills allocated near Hiroshima University, Higashi-Hiroshima Campus, Japan in July 2017. After drying by sunlight for one week, samples were deliberately sifted and winnowed to remove impurities. The samples were then dried in an oven at 50 °C for 3 days and kept in a container for further extractions.
2.3. Extraction and isolation process of momilactones A and B from rice husk
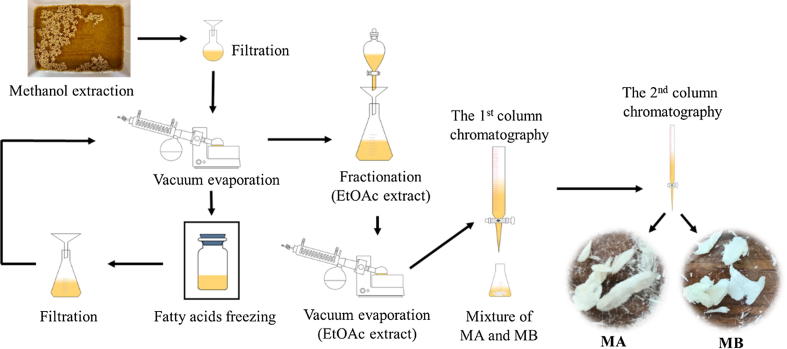
Extraction and isolation procedures were described previously (Quan et al., 2019). Briefly, from 7 kg of rice husks, 52 mg of MA and 44 mg MB were isolated by repeated column chromatography (Fig. 2). The identification and confirmation of MA and MB were conducted by high performance liquid chromatography (HPLC), thin-layer chromatography (TLC), and gas chromatography-mass spectrometry (GC-MS) techniques. The structures of MA and MB were re-confirmed by 1H and 13C nuclear magnetic resonance (NMR) analyses and compared with those in the previous research (Minh et al., 2018b). The purified MA and MB were used for biological activities and quantification of these compounds in rice bran.
Fig. 2.
The extraction and isolation process of momilactones A and B.
2.4. Quantification and confirmation of MA and MB in rice bran by HPLC and LC-ESI-MS
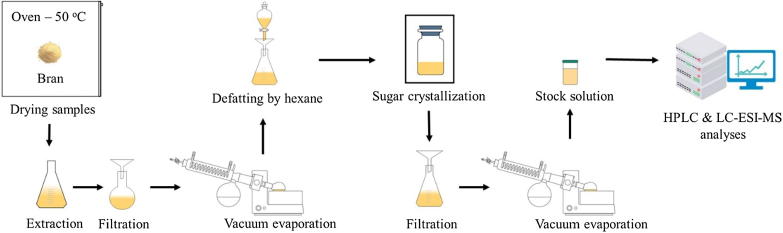
Rice bran (100 g) was extracted by 400 mL of MeOH for 1 week. After filtering, crude methanolic extract was obtained and mixed with an equal volume of hexane in a separatory funnel. After 2 h, the hexane layer with fatty compounds was removed, the methanol layer was filtered and kept in a fridge (4 °C) for 1 day. The methanol-soluble part was separated from crystallized sugars by another filtration and evaporated to yield a defatted bran extract (DBE) with the stock concentration of 100 mg/mL (Fig. 3). The HPLC analysis was done in a similar method as described previously (Quan et al., 2019). The contents of MA and MB in rice bran were compared and calculated in consonance with the retention times (RT) and peak areas of the standards MA and MB with the samples.
Fig. 3.
Preparation of rice bran for HPLC and LC-ESI-MS analyses.
The DBE sample was analyzed by LC-ESI-MS technique. The analytical system included an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, CA, USA) equipped with a source of electrospray ionization (ESI). The column Waters Spherisorb ODS2 (10 µm, 250 mm × 4.6 mm i.d.) and mobile phase comprising of 0.1% trifluoroacetic acid in 70% acetonitrile were used in LC phase. The operation time was 30 min with a flow rate of 0.4 mL/min under the room temperature. ESI condition was set up as follows: ion spray voltage (4.5 kV), sheath gas flow rate (60 arb) and aux gas flow rate (20 arb). MS analysis was run by a positive Fourier transform mass spectrometer (FTMS) at a resolution of 60,000 with a scan range of m/z 100–1000. An amount of 10 µL of DBE sample (50 mg/mL) and standard momilactones (0.5 mg/mL) were separately injected to the system by an autosampler. The presence of MA and MB in DBE was confirmed by comparing their extracted ion chromatograms (EIC) and mass spectra with those of standard momilactones.
2.5. α-Amylase and α-glucosidase inhibition assays
A modified model of the starch-iodine method described by Al-Dabbas et al., 2006 was used to assess the porcine pancreatic α-amylase (PPA) inhibition of momilactones A and B, DFE, and γ-oryzanol. Concisely, in each well of a microplate (U-shape, Greiner Bio-one, NC, USA), 20 µL of sample were pre-incubated with 20 µL of PPA solution (2 mg/mL in 20 mM phosphate buffer containing 6 mM sodium chloride, pH 6.9) at 37 °C for 10 min. The reaction was activated by pipetting 30 µL of soluble starch (0.5%). After 6 min of incubation, an aliquot of 20 µL of hydrochloric acid (1 M) were added to stop the reaction, followed by 100 µL of 0.25 mM iodine solution. The absorbance at 565 nm was read by a microplate reader (MultiskanTM Microplate Spectrophotometer, Thermo Fisher Scientific, Osaka, Japan). The inhibition percentage of samples on PPA was calculated by the following formula:
where S is the absorbance of reaction with presence of samples, C is the absorbance of reaction without enzyme, W is the absorbance of reaction without samples. Acarbose was used as a positive reference. The IC50 value was determined to exhibit 50% inhibitory capacity of reaction at a certain concentration.
The α-glucosidase inhibitory assay was conducted, adhered to the earlier described protocol (Quan et al., 2019). In brief, 40 µL of samples including momilactones A and B, DBE, and γ-oryzanol which were two-times diluted by 0.1 M potassium phosphate buffer (pH 7), were incubated with 40 µL of α-glucosidase enzyme solution (0.5 U/mL in the buffer) at 25 °C. After 6 min, 20 µL of 5 mM pNPG substrate were added to inaugurate the reaction. The mixture was incubated for another 8 min and eventually terminated by adding 100 µL of 0.1 M Na2CO3. The absorbance was recorded at 405 nm by a microplate reader. The inhibition percentage was calculated by the following equation:
where A is absorbance of reaction with either MA or MB or positive controls (acarbose or quercetin) and B is absorbance of reaction with 50% methanol in buffer. IC50 value was obtained by the same way as above.
2.6. Trypsin inhibition assay
The trypsin inhibition was assayed using an adapted protocol of method reported previously (Balisteiro et al., 2013). Initially, the stock solution of samples was diluted two times by 50 mM Tris buffer (containing 20 mM CaCl2, pH 8.2). In each test sample, 50 µL of trypsin and 100 µL of BAPNA substrate were added, followed by incubation at 37 °C for 12 min. Finally, the reaction was stopped by pipetting 25 µL of acetic acid 30% into the mixture. Then absorbance was measured at 410 nm by a microplate reader. The inhibition percentage was calculated by the formula (ii) and IC50 value was achieved by the same way as mentioned above. Positive controls were caffeic acid and tannic acid. Final concentrations of enzyme and BAPNA substrate in the reaction were 40 and 160 µg/mL, respectively.
2.7. Statistical analysis
Data were elaborated on the Minitab 16.0 software (Minitab Inc., State College, PA, USA). All analyses were in a complete randomization with three replications, and results were displayed as mean ± standard error (SE). Significant differences were determined by one-way ANOVA using Tukey’s test at p < 0.05.
3. Results
3.1. Isolation and confirmation of momilactones A and B
By using the repeated column chromatography, MA and MB were successfully isolated from rice husk extract. The confirmation of MA and MB by HPLC, TLC, GC-MS, and NMR was reported previously (Minh et al., 2018b, Ahmad et al., 2019, Quan et al., 2019). The results can be seen in supplementary data (Figs. A1–A3).
3.2. Inhibition of α-amylase, α-glucosidase and trypsin
In the previous study, we reported the inhibitory effects of MA and MB on α-amylase and α-glucosidase from bacteria (Quan et al., 2019). In the present research, we confirmed such activity on porcine pancreatic α-amylase (PPA) together with a comparison of inhibitory activities of momilactones, defatted bran extract, and a well-known diabetic inhibitor γ-oryzanol. Additionally, to the extent of our knowledge, this is the first study that investigates inhibitory activities of MA and MB on trypsin, the enzyme linked to both diabetes and obesity.
Data present means ± standard errors. Means within a column followed by different letters are significantly different at p < 0.05 level. -: not calculated. MA: momilactone A; MB: momilactone B; DBE: defatted bran extract; GO: γ-oryzanol
As shown in Table 1, both MA and MB obtained inhibitory activities against PPA (IC50 = 132.56 and 129.02 µg/mL, respectively) and α-glucosidase (IC50 = 991.95 and 612.03 µg/mL, respectively (Quan et al., 2019)). These effects were also relatively compared with the well-known commercial diabetic inhibitor (acarbose: IC50 = 80.26 µg/mL for α-amylase and 2549.00 µg/mL for α-glucosidase). By comparing the IC50 values, it might conclude that MA and MB are asymptotic with the antidiabetic level of acarbose. On the other hand, DBE performed an IC50 value of 779.03 µg/mL for PPA inhibition while γ-oryzanol showed a negligible activity which was recorded as 18.65% inhibition at a concentration of 4 mg/mL. However, in α-glucosidase assay, γ-oryzanol (IC50 = 17.54 mg/mL) evinced a higher inhibitory activity than DBE (16.65 mg/mL). To sum up, MA and MB remarkably exhibited stronger suppressive effects than γ-oryzanol and DBE on both PPA and α-glucosidase assays.
Table 1.
IC50 values of pancreatic α-amylase and α-glucosidase inhibitory activities of momilactones A and B, defatted bran extract, and γ-oryzanol.
| Sample | IC50 value of α-amylase inhibition (µg/mL) | IC50 value of α-glucosidase inhibition (µg/mL) |
|---|---|---|
| MA | 132.56 ± 0.51 b | 991.95 ± 0.96 b |
| MB | 129.02 ± 0.09 b | 612.03 ± 0.39 a |
| DBE | 779.03 ± 3.87 c | 16653.00 ± 59.00 e |
| GO | – | 1754.20 ± 6.38 c |
| Acarbose | 80.26 ± 0.24 a | 2549.00 ± 5.15 d |
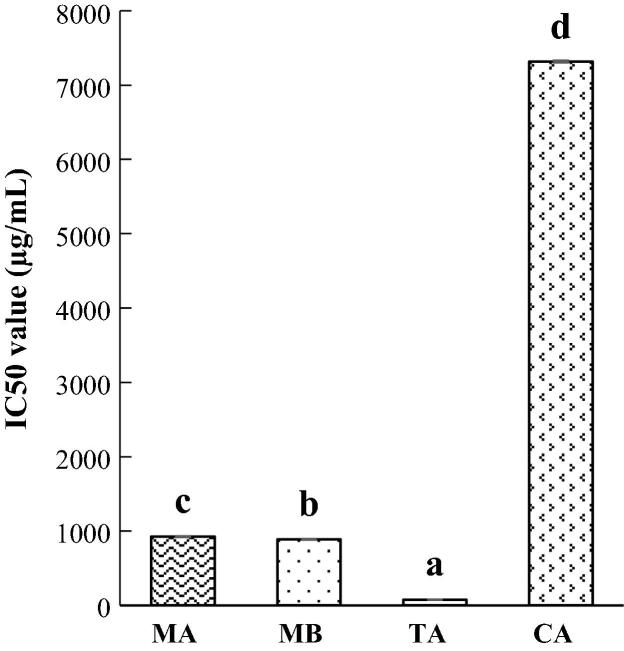
The inhibitory effect of MA and MB on trypsin activity was delineated by IC50 value (µg/mL) in Fig. 4. In particular, trypsin inhibition of MA (921.55 µg/mL) was significantly lower than that of MB (884.03 µg/mL). Both MA and MB comparably exhibited the inhibitory level as two phenolic inhibitors tannic acid (75.66 µg/mL) and caffeic acid (7.31 mg/mL). Conspicuously, trypsin inhibitory activity of MA was 7.9-folds and of MB was 8.2-folds stronger than caffeic acid. Meanwhile, DBE showed a trivial inhibition of 11.94% at a concentration of 20 mg/mL. γ-oryzanol did not offer an inhibition on trypsin activity.
Fig. 4.
Trypsin inhibitory effect of momilactones A and B. MA: momilactone A; MB: momilactone B; TA: tannic acid; CA: caffeic acid; Dissimilar letters indicate significant differences (p < 0.05).
3.3. Contents of MA and MB in rice bran
Results of HPLC and LC-ESI-MS showed that MA and MB contents in rice bran can be reliably identified and quantified. By HPLC, MA and MB in bran were determined by comparing the similarity of retention time and peak areas between sample and standards (Fig. A.4). As shown in Table 2, quantity of MA and MB in rice bran was similar to each other (6.65 and 6.24 µg/g dry weight, respectively). This is the first study so far to detect and quantify MA and MB contents in rice bran.
Table 2.
Peak information of momilactones A and B detected in rice bran by HPLC.
| Sample | Peak estimation | Retention time | Area |
|---|---|---|---|
| DBE50 + Std (3:1) | MB | 14.53 | 2,322,546 |
| MA | 17.73 | 3,138,205 | |
| DBE50 | MB | 14.53 | 291,048 |
| MA | 17.74 | 164,757 |
DBE50 was defatted bran extract at 50 mg/mL; DBE50 + Std0.5 (3:1) was defatted bran extract (50 mg/mL) mixed with pure MA and MB (0.5 mg/mL) at ratio 3:1 (v/v)
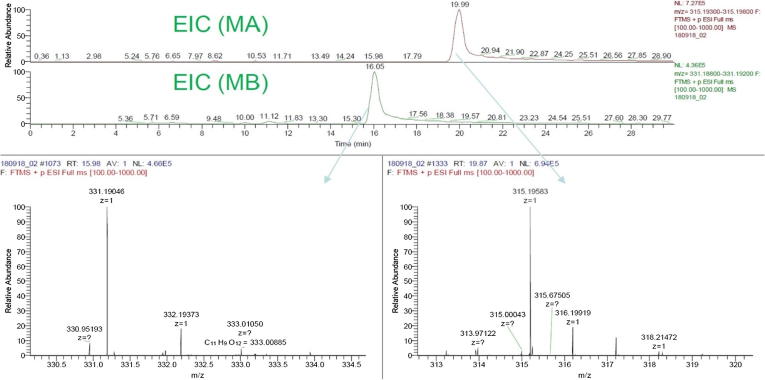
LC-ESI-MS method was introduced to be able to confirm the presence of MA and MB in rice grain (Quan et al., 2019). In this research, by integration of a specific extraction and LC-ESI-MS methods, we certified the existence of these bioactive compounds in rice bran extract (Fig. 3). The use of a positive FTMS mode and certain mass range scans resulted in an extracted ion chromatogram (EIC) of the sample which illustrated two major peaks. The retention time and fragmentation patterns from peaks of DBE sample were confirmed as MA (RT∼19.87, 315.196) and MB (RT∼15.98, 331.190) which were totally coincided with those of standards MA and MB (Fig. A.5). Fig. 5 illustrates LC-ESI-MS results, which first confirms the presence of MA and MB in rice bran.
Fig. 5.
Extracted ion chromatograms and mass spectra of momilactones A and B detected in rice bran.
4. Discussion
Nowadays, the use of available antidiabetic drugs may bring undesirable and severe side effects (Arulselvan et al.,2014). The quest of new antidiabetic compounds is indispensable to overcome diabetic problems worldwide. Nonetheless, the isolation and identification of active compounds with either no or minimal adverse effects are greater challenges to biomedical and scientific researches. To date, compounds with diterpene lactone structure possessing antidiabetic property have been released intermittently. An exhaustive search of related literature brought only one result which was andrographolide, a diterpenoid lactone isolated from Andrographis paniculata, was potent for diabetic control (Yu et al., 2003, Subramanian et al., 2008, Brahmachari, 2017). Hence, MA and MB regarded as a new chemical class performing antidiabetic ability in this study should be attached more special importance.
MA and MB have been principally detected in rice husk (Kato-Noguchi et al., 2010, Chung et al., 2006, Minh et al., 2018a, Minh et al., 2018b; Ahmad et al., 2019), leaf (Xuan et al., 2016, Lee et al., 1999), root and root exudates (Kato-Noguchi, 2011, Kato-Noguchi et al., 2010). The content of MA and MB varied among rice cultivars and growing stage (Xuan et al., 2016, Lee et al., 1999). This research highlighted the antidiabetic activity of pure MA and MB, detected and quantified the two compounds in rice bran. Among enzymatic assays, MA and MB presented the most remarkable inhibition on porcine pancreatic α-amylase activity (IC50 = 132.56 and 129.02 µg/mL for MA and MB, respectively) which were closely in line with the activity of the standard inhibitor acarbose (IC50 = 80.26 µg/mL). These inhibitory activities might stem from the presence of lactone ring in the structure of MA and MB, which was reported to play a potent role in anti-α-glucosidase activity (Yin et al., 2014). Additionally, diterpenoid component was convinced to be involved in trypsin deduction (Xing et al., 2003, Kang et al., 2006, Kim et al., 2006). Moreover, the appearance of hydroxyl group at C-3 in the diterpenoid part of MB might increase the antidiabetic competence as compared to MA (Quan et al., 2019). The influence of number and position of the hydroxyl groups of natural compounds on α-glucosidase and trypsin activities was substantiated by earlier studies (Reddy et al., 2009, Yin et al., 2014, Rohn et al., 2002). Among plant-derived compounds, the phenolic group was most intensively elaborated due to its abundance and availability. Tan et al. (2013) reported the α-glucosidase inhibitory activity of kaempferol and chlorogenic acid isolated from Gynura medica leaf with the IC50 value of higher than 2 mg/mL. In research on trypsin inhibition of phenolics from extracts of pears, lentils and cocoa beans by Quesada et al. (1996), gallic acid and catechin were potential inhibitors with IC50 values of 4.8 and higher than 10 mg/mL, respectively. By comparing with results of the present study, we documented that MA and MB were noteworthy diabetes inhibitors in term of α-amylase, α-glucosidase, and trypsin inhibitions.
Although previous studies introduced several techniques to isolate and purify MA and MB (Chung et al., 2006; Cartwright et al., 1981; Minh et al., 2018b, Chung et al., 2005a, Chung et al., 2005b), none of them proposed a detailed process that can be extensively applicable for isolation these diabetic inhibitors as our study. Furthermore, we successfully developed a simple method that helped precisely detect MA and MB in rice bran for the first time. Results from the advanced technique LC-ESI-MS were reliable (Fig. 5), nevertheless, the key of achievements might emanate from the sample processing (Fig. 3). Particularly, after withdrawing fatty and low polarity components by hexane, we proceeded with a sugar abolishment based on the crystallization of sugars at low temperature. Basically, momilactones are minor constituents in rice and the productivity of MA and MB isolation may be accelerated by various factors as UV-irradiation (Cartwright et al., 1981, Kodama et al., 1988), temperature and extracting solvents (Minh et al., 2018b). The rejection of compounds with high molecular weight or lower polarity may enhance the sensitivity in detecting MA and MB, which has not been mentioned in the earlier researches. Though contents of MA and MB quantified in rice bran were 6.65 and 6.24 µg/g dry weight, respectively, their individual activity on the suppression of hydrolytic enzymes linked to diabetes was considerable. Therefore, the contribution of MA and MB to the anti-diabetic capacities of rice bran should be further endorsed by in vivo models as well as clinical trials.
In addition, γ-oryzanol, a commercially-important bioactive phytochemical of rice bran, is a mixture of ferulic acid esters of triterpene alcohols and sterols, which possesses a wide spectrum of health-beneficial effects, including anticarginogenic, anti-inflammatory, antihyperlipidemic, antidiabetic, and neuroprotective (Lemus et al. 2014). Most of the evidence about antidiabetic effect of γ-oryzanol was from in vivo assays, but no in vitro study on inhibitions of the key enzymes linked to diabetes was investigated. This current study for the first time resolved this concern. Results from in vitro assays pointed out that the inhibitory effect of MA and MB on α-amylase, α-glucosidase and trypsin were more significantly potent than that of γ-oryzanol and defatted bran extract. As a result, along with quantification results, MA and MB can be considered as new members of diabetic inhibitors and might contribute an active role to the diabetes inhibition of rice bran. Trends in the use of rice bran as a source of anti-diabetes, therefore, have been more fortified by this study.
5. Conclusion
By in vitro assays, for the first time, the present study manifested the decisive function of MA and MB in the inhibition of key enzymes related to diabetes. The first identification and quantification of MA and MB in rice bran using advanced techniques may launch a new direction for further isolations of these diabetic inhibitors in larger scales. Findings of this research highlighted that MA and MB contributed an important role in anti-diabetes property of rice bran, although in vivo trials on MA and MB should be further explored. Given that quantities of MA and MB are largely varied among rice cultivars, the breeding of new rice cultivars with high amounts of MA and MB may be useful and economical to help control diabetes.
Acknowledgments
Acknowledgements
The authors expressed thanks to Do Tan Khang for his assistance in isolation of MA and MB to this research. We thank Japanese government (Monbukagakusho) to provide Nguyen Van Quan a scholarship.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2019.03.006.
Contributor Information
Tran Dang Xuan, Email: tdxuan@hiroshima-u.ac.jp.
Hoang-Dung Tran, Email: thdung@ntt.edu.vn.
Tran Dang Khanh, Email: tdkhanh@vaas.vn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abesundara K.J.M., Matsui T., Matsumoto K. α-Glucosidase inhibitory activity of some Sri Lanka plant extracts, one of which, Cassia auriculata, exerts a strong antihyperglycemic effect in rats comparable to the therapeutic drug acarbose. J. Agric. Food Chem. 2004;52:2541–2545. doi: 10.1021/jf035330s. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Xuan T.D., Minh T.N., Siddiqui N.A., Quan N.V. Comparative extraction and simple isolation improvement techniques of active constituents’ momilactone A and B from rice husks of Oryza sativa by HPLC analysis and column chromatography. Saudi Pharm. J. 2019;27:17–24. doi: 10.1016/j.jsps.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dabbas M.M., Kitahara K., Suganuma T., Hashimoto F., Tadera K. Antioxidant and α-amylase inhibitory compounds from aerial parts of Varthemia iphionoides Boiss. Biosci Biotechnol Biochem. 2006;70:2178–2184. doi: 10.1271/bbb.60132. [DOI] [PubMed] [Google Scholar]
- Apostolidis E., Kwon Y.I., Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007;8:46–54. [Google Scholar]
- Arulselvan P., Ghofar H.A.A., Karthivashan G., Halim M.F.A., Ghafar M.S.A., Fakurazi S. Antidiabetic therapeutics from natural source: a systematic review. Biomed. Prev. Nutr. 2014;4:607–617. [Google Scholar]
- Balisteiro D.M., Rombaldi C.V., Genovese M.I. Protein, isoflavones, trypsin inhibitory and in vitro antioxidant capacities: Comparison among conventionally and organically grown soybeans. Food Res. Int. 2013;51:8–14. [Google Scholar]
- Boonloh K., Kukongviriyapan V., Kongyingyoes B., Kukongviriyapan U., Thawornchinsombut S., Pannangpetch P. Rice bran protein hydrolysates improve insulin resistance and decrease pro-inflammatory cytokine gene expression in rats fed a high carbohydrate-high fat diet. Nutrients. 2015;7 doi: 10.3390/nu7085292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari G. Andrographolide: A molecule of antidiabetic promise. In: Brahmachari G., editor. Discovery and Development of Antidiabetic Agents from Natural Products. Elsevier; Amsterdam: 2017. pp. 1–27. [Google Scholar]
- Cartwright D.W., Langcake P., Pryce R.J., Leworthy D.P., Ride J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry. 1981;20:535–537. [Google Scholar]
- Cartwright D.W., Langcake P., Pryce R.J., Leworthy D.P., Ride J.P. Chemical activation of host defence mechanisms as a basis for crop protection. Nature. 1977;267:511–513. [Google Scholar]
- Carvalho F.M.C., Lima V.C.O., Costa I.S., Medeiros A.F., Serquiz A.C., Lima M.C.J.S., Serquiz R.P., Maciel B.L.L., Uchôa A.F., Santos E.A., Morais A.H.A. A trypsin inhibitor from tamarind reduces food intake and improves inflammatory status in rats with metabolic syndrome regardless of weight loss. Nutrients. 2016;8:544. doi: 10.3390/nu8100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I.M., Ali M., Hahn S.J., Siddiqui N.A., Lim Y.H., Ahmad A. Chemical constituents from the hulls of Oryza sativa with cytotoxic activity. Chem. Nat. Compd. 2005;41:182–189. [Google Scholar]
- Chung I.M., Hahn S.J., Ahmad A. Confirmation of potential herbicidal agents in hulls of rice, Oryza sativa. J. Chem. Ecol. 2005;31:1339–1352. doi: 10.1007/s10886-005-5290-5. [DOI] [PubMed] [Google Scholar]
- Chung I.M., Jung T.K., Kim S.H. Evaluation of allelopathic potential and quantification of momilactone A, B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food Chem. 2006;54:2527–2536. doi: 10.1021/jf052796x. [DOI] [PubMed] [Google Scholar]
- Ercan P., El S.N. Inhibitory effects of chickpea and Tribulus terrestris on lipase, α-amylase and α-glucosidase. Food Chem. 2016;205:163–169. doi: 10.1016/j.foodchem.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Esa N.M., Ling T.B., Peng L.S. By-products of rice processing: An overview of health benefits and applications. J. Rice Res. 2013;1:107. [Google Scholar]
- Fukuta M., Xuan T.D., Deba F., Tawata S., Khanh T.D., Chung I.M. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interact. 2007;2:245–251. [Google Scholar]
- Ghatak S.B., Panchal S.S. Anti-diabetic activity of oryzanol and its relationship with the anti-oxidant property. Int. J. Diabetes Dev. Ctries. 2012;32:185–192. [Google Scholar]
- Joung Y.H., Lim E.J., Kim M.S., Lim S.D., Yoon S.Y., Lim Y.C., Yoo Y.B., Ye S.K., Park T., Chung I.M., Bae K.Y., Mang Y.M. Enhancement of hypoxia-induced apoptosis of human breast cancer cells via STAT5b by momilactone B. Int. J. Oncol. 2008;33:477–484. [PubMed] [Google Scholar]
- Kang O.H., Choi Y.A., Park H.J., Kang C.S., Song B.S., Choi S.C., Nah Y.H., Yun K.J., Cai X.F., Kim Y.H., Bae K.H., Lee Y.M. Inhibition of trypsin-induced mast cell activation by acanthoic acid. J. Ethnopharmacol. 2006;105:326–331. doi: 10.1016/j.jep.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H. Convergent or parallel molecular evolution of momilactone A and B: potent allelochemicals, momilactones have been found only in rice and the moss Hypnum plumaeforme. J. Plant Physiol. 2011;168:1511–1516. doi: 10.1016/j.jplph.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H., Hasegawa M., Ino T., Ota K., Kujime H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010;167:787–791. doi: 10.1016/j.jplph.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Park H.R., Park E., Lee S.C. Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007;55:1702–1706. doi: 10.1021/jf062020b. [DOI] [PubMed] [Google Scholar]
- Kim S., Na M., Oh H., Jang J., Sohn C.B., Kim B.Y., Oh W.K., Ahn J.S. PTP1B inhibitory activity of kaurane diterpenes isolated from Siegesbeckia glabrescens. J. Enzyme Inhib. Med. Chem. 2006;21:379–383. doi: 10.1080/14756360600741560. [DOI] [PubMed] [Google Scholar]
- Kodama O., Suzuki T., Miyakawa J., Akatsuka T. Ultraviolet-induced accumulation of phytoalexins in rice leaves. Agric. Biol. Chem. 1988;52:2469–2473. [Google Scholar]
- Komarnytsky S., Cook A., Raskin I. Potato protease inhibitors inhibit food intake and increase circulating cholecystokinin levels by a trypsin-dependent mechanism. Int. J. Obes. 2011;35 doi: 10.1038/ijo.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy J.L. Pathogenesis of type 2 diabetes mellitus. Arch. Med. Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Yoneyama K., Takeuchi Y., Konnai M., Tamogami S., Kodama O. Momilactones A and B in rice straw harvested at different growth stages. Biosci Biotechnol Biochem. 1999;63:1318–1320. doi: 10.1271/bbb.63.1318. [DOI] [PubMed] [Google Scholar]
- Lemus C., Apostolis A., Maria H., Alexios L.S. γ-Oryzanol: An attractive bioactive component from rice bran. In: Ronald R.W., Victor R.P., Sherma Z., editors. Wheat and Rice in Disease Prevention and Health. Academic Press; San Diego: 2014. pp. 409–430. [Google Scholar]
- Liu Y.Q., Strappe P., Shang W.T., Zhou Z.K. Functional peptides derived from rice bran proteins. Crit. Rev. Food Sci. Nutr. 2017;8398:1–8. doi: 10.1080/10408398.2017.1374923. [DOI] [PubMed] [Google Scholar]
- Minh T.N., Xuan T.D., Ahmad A., Elzaawely A.A., Teschke R., Van T.M. Efficacy from different extractions for chemical profile and biological activities of rice husk. Sustainability. 2018;10:1356. [Google Scholar]
- Minh T.N., Xuan T.D., Ahmad A., Elzaawely A.A., Teschke R., Van T.M. Momilactones A and B: optimization of yields from isolation and purification. Separations. 2018;5:28. [Google Scholar]
- Morishita I., Morishita M., Takayama K., Machida Y., Nagai T. Hypoglycemic effect of novel oral microspheres of insulin with protease inhibitor in normal and diabetic rats. Int. J. Pharm. 1992;78:9–16. [Google Scholar]
- Nakajima S., Hira T., Tsubata M., Takagaki K., Hara H. Potato extract (Potein) suppresses food intake in rats through inhibition of luminal trypsin activity and direct stimulation of cholecystokinin secretion from enteroendocrine cells. J. Agric. Food Chem. 2011;59:9491–9496. doi: 10.1021/jf200988f. [DOI] [PubMed] [Google Scholar]
- Obara N., Hasegawa M., Kodama O. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci. Biotechnol. Biochem. 2002;66:2549–2559. doi: 10.1271/bbb.66.2549. [DOI] [PubMed] [Google Scholar]
- Park C., Jeong N.Y., Kim G.Y., Han M.H., Chung I.M., Kim W.J., Yoo Y.H., Choi Y.H. Momilactone B induces apoptosis and G1 arrest of the cell cycle in human monocytic leukemia U937 cells through downregulation of pRB phosphorylation and induction of the cyclin-dependent kinase inhibitor p21Waf1/Cip1. Oncol. Rep. 2014;31:1653–1660. doi: 10.3892/or.2014.3008. [DOI] [PubMed] [Google Scholar]
- Park S.H., Kwon J.H., Lim S.H., Park H.W., Kim C.W. Characterization of human insulin microcrystals and their absorption enhancement by protease inhibitors in rat lungs. Int. J. Pharm. 2007;339:205–212. doi: 10.1016/j.ijpharm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Quan N.T., Xuan T.D. Foliar application of vanillic and p-hydroxybenzoic acids enhanced drought tolerance and formation of phytoalexin momilactones in rice. Arch. Agron. Soil Sci. 2018:1–16. [Google Scholar]
- Quan N.V., Hoang-Dung T., Xuan T.D., Ahmad A., Dat T.D., Khanh T.D., Teschke R. Momilactones A and B are α-amylase and α-glucosidase inhibitors. Molecules. 2019;24:482. doi: 10.3390/molecules24030482. https://10.3390/molecules24030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada C., Bartolomé B., Nieto O., Gómez-Cordovés C., Hernández T., Estrella I. Phenolic inhibitors of α-amylase and trypsin enzymes by extracts from pears, lentils, and cocoa. J. Food Prot. 1996;59:185–192. doi: 10.4315/0362-028X-59.2.185. [DOI] [PubMed] [Google Scholar]
- Reddy P.P., Tiwari A.K., Rao R.R., Madhusudhana K., Rao V.R.S., Ali A.Z., Babu K.S., Rao J.M. New labdane diterpenes as intestinal α-glucosidase inhibitor from antihyperglycemic extract of Hedychium spicatum (Ham. Ex Smith) rhizomes. Bioorganic Med. Chem. Lett. 2009;19:2562–2565. doi: 10.1016/j.bmcl.2009.03.045. [DOI] [PubMed] [Google Scholar]
- Rohn S., Rawel H.M., Kroll J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002;50:3566–3571. doi: 10.1021/jf011714b. [DOI] [PubMed] [Google Scholar]
- Serquiz A.C., Machado R.J.A., Serquiz R.P., Lima V.C.O., de Carvalho F.M.C., Carneiro M.A.A., Maciel B.L.L., Uchôa A.F., Santos E.A., Morais A.H.A. Supplementation with a new trypsin inhibitor from peanut is associated with reduced fasting glucose, weight control, and increased plasma CCK secretion in an animal model. J. Enzyme Inhib. Med. Chem. 2016;31:1261–1269. doi: 10.3109/14756366.2015.1103236. [DOI] [PubMed] [Google Scholar]
- Sohail M., Rakha A., Butt M.S., Iqbal M.J., Rashid S. Rice bran nutraceutics: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2016;57:3771–3780. doi: 10.1080/10408398.2016.1164120. [DOI] [PubMed] [Google Scholar]
- Steppan C.M., Shannon T.B., Savitha B., Elizabeth J.B., Ronadip R.B., Christopher M.W., Hiralben R.P., Rexford S.A., Mitchell A.L. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Subramanian R., Asmawi M.Z., Sadikun A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008;55:391–398. [PubMed] [Google Scholar]
- Tan C., Wang Q., Luo C., Chen S., Li Q., Li P. Yeast α-glucosidase inhibitory phenolic compounds isolated from Gynura medica leaf. Int. J. Mol. Sci. 2013;14:2551–2558. doi: 10.3390/ijms14022551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Hashino K., Shiozaki M., Ibuki F., Maki Z. The complete amino acid sequence of rice bran trypsin inhibitor. J. Biochem. 1987;102:297–306. doi: 10.1093/oxfordjournals.jbchem.a122054. [DOI] [PubMed] [Google Scholar]
- Toyomasu T., Kagahara T., Okada K., Koga J., Hasegawa M., Mitsuhashi W., Sassa T., Yamane H. Diterpene phytoalexins are biosynthesized in and exuded from the roots of rice seedlings. Biosci. Biotechnol. Biochem. 2008;72:562–567. doi: 10.1271/bbb.70677. [DOI] [PubMed] [Google Scholar]
- Uraipong C., Zhao J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase, β-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2016;96:1101–1110. doi: 10.1002/jsfa.7182. [DOI] [PubMed] [Google Scholar]
- Xing F.C., Shen G., Nguyen T.D., Ok H.K., Young M.L., Jung J.L., Young H.K. Inhibitory effect of kaurane type diterpenoids from Acanthopanax koreanum on TNF-α secretion from trypsin-stimulated HMC-1 cells. Arch. Pharmacal Res. 2003;26:731–734. doi: 10.1007/BF02976683. https://doi.org/ 12.1007/BF02976683. [DOI] [PubMed] [Google Scholar]
- Xuan T.D., Minh T.N., Anh L.H., Khanh T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016;36:52. [Google Scholar]
- Yang J.Y., Kang M.Y., Nam S.H., Friedman M. Antidiabetic effects of rice hull smoke extract in alloxan-induced diabetic mice. J. Agric. Food Chem. 2010;60:87–94. doi: 10.1021/jf2035077. [DOI] [PubMed] [Google Scholar]
- Yehia R.S., Saleh A.M. Antifungal activity of rice straw extract on some phytopathogenic fungi. Afr. J. Biotechnol. 2012;11:13586–13590. [Google Scholar]
- Yin Z., Zhang W., Feng F., Zhang Y., Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness. 2014;3:136–174. [Google Scholar]
- Yu B.C., Hung C.R., Chen W.C., Cheng J.T. Antihyperglycemic effect of andrographolide in streptozotocin-induced diabetic rats. Planta Med. 2003;69:1075–1079. doi: 10.1055/s-2003-45185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.